Abstract
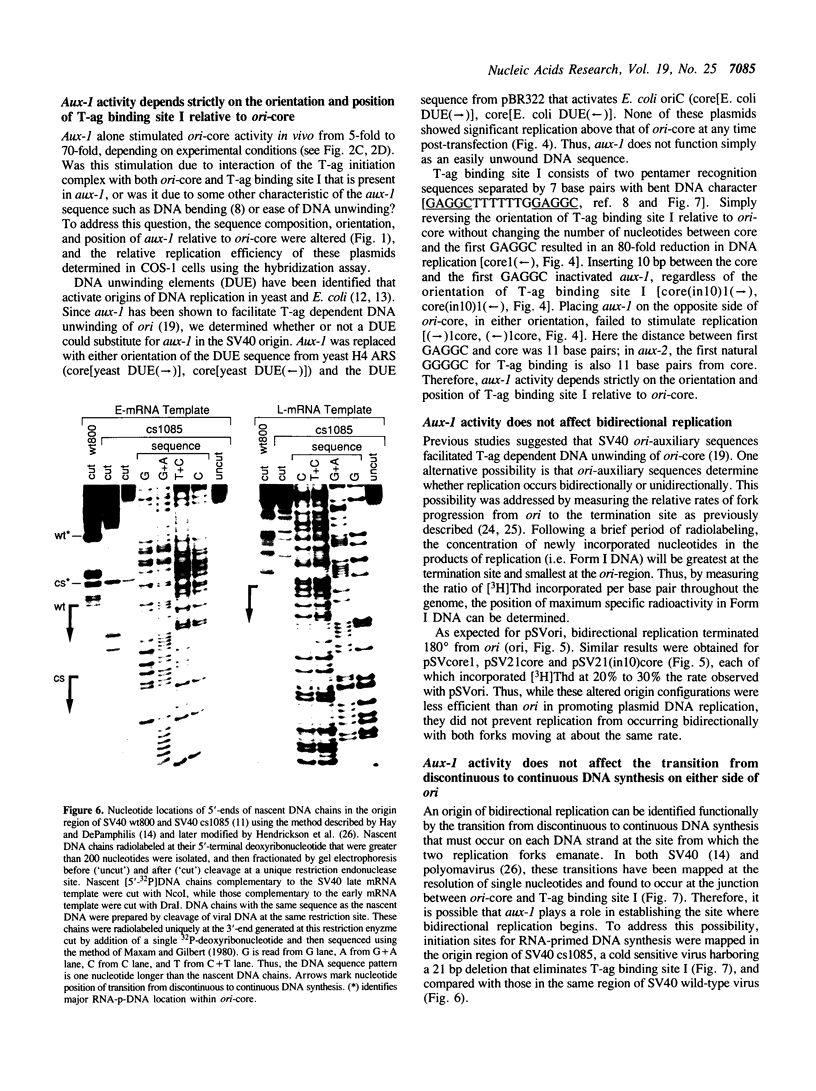
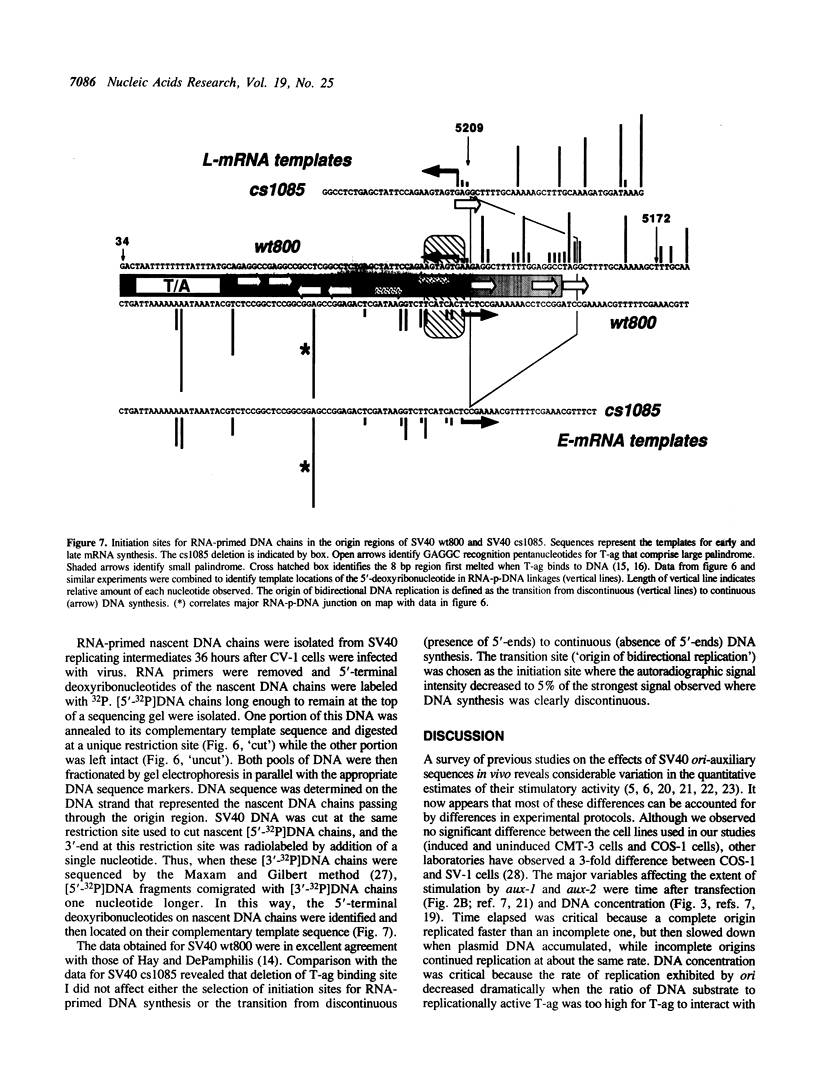
SV40 origin auxiliary sequence 1 (aux-1) encompasses T-antigen (T-ag) binding site I and facilitates origin core (ori-core) activity in whole cells or cell extracts. Aux-1 activity depended completely upon its sequence, orientation and spacing relative to ori-core. Aux-1 activity was lost either by inserting 10 base pairs between aux-1 and ori-core or by placing either orientation of aux-1 on the opposite side of ori-core. Reversing the orientation of aux-1 in its normal position actually inhibited replication. Easily unwound DNA sequences that stimulate yeast or E. coli origins of replication could not replace aux-1. Aux-1 did not affect bidirectional replication. Replication remained bidirectional even when aux-1 was inactivated, and deletion of aux-1 did not affect selection of RNA-primed DNA synthesis initiation sites in the origin region: the transition from discontinuous to continuous DNA synthesis that marks the origin of bidirectional replication occurred at the same nucleotide locations in both wild-type and aux-1 deleted origins. These results support a model for initiation of SV40 DNA replication in which T-ag binding to aux-1 (T-ag binding site I) facilitates the efficiency with which T-ag initiates replication at ori-core (T-ag binding site II) without affecting the mechanism by which initiation of DNA replication occurs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergsma D. J., Olive D. M., Hartzell S. W., Subramanian K. N. Territorial limits and functional anatomy of the simian virus 40 replication origin. Proc Natl Acad Sci U S A. 1982 Jan;79(2):381–385. doi: 10.1073/pnas.79.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Hurwitz J. Localized melting and structural changes in the SV40 origin of replication induced by T-antigen. EMBO J. 1988 Oct;7(10):3149–3158. doi: 10.1002/j.1460-2075.1988.tb03182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher J., Robinson E. C., Hay R. T. Interactions between the adenovirus type 2 DNA polymerase and the DNA binding domain of nuclear factor I. New Biol. 1990 Dec;2(12):1083–1090. [PubMed] [Google Scholar]
- Chandrasekharappa S. C., Subramanian K. N. Effects of position and orientation of the 72-base-pair-repeat transcriptional enhancer on replication from the simian virus 40 core origin. J Virol. 1987 Oct;61(10):2973–2980. doi: 10.1128/jvi.61.10.2973-2980.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Kelly T. J. Transcriptional activator nuclear factor I stimulates the replication of SV40 minichromosomes in vivo and in vitro. Cell. 1989 Nov 3;59(3):541–551. doi: 10.1016/0092-8674(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Cohen G. L., Wright P. J., DeLucia A. L., Lewton B. A., Anderson M. E., Tegtmeyer P. Critical spatial requirement within the origin of simian virus 40 DNA replication. J Virol. 1984 Jul;51(1):91–96. doi: 10.1128/jvi.51.1.91-96.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia A. L., Deb S., Partin K., Tegtmeyer P. Functional interactions of the simian virus 40 core origin of replication with flanking regulatory sequences. J Virol. 1986 Jan;57(1):138–144. doi: 10.1128/jvi.57.1.138-144.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia A. L., Lewton B. A., Tjian R., Tegtmeyer P. Topography of simian virus 40 A protein-DNA complexes: arrangement of pentanucleotide interaction sites at the origin of replication. J Virol. 1983 Apr;46(1):143–150. doi: 10.1128/jvi.46.1.143-150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L. Transcriptional elements as components of eukaryotic origins of DNA replication. Cell. 1988 Mar 11;52(5):635–638. doi: 10.1016/0092-8674(88)90398-4. [DOI] [PubMed] [Google Scholar]
- Deb S., DeLucia A. L., Baur C. P., Koff A., Tegtmeyer P. Domain structure of the simian virus 40 core origin of replication. Mol Cell Biol. 1986 May;6(5):1663–1670. doi: 10.1128/mcb.6.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Nathans D. Regulatory mutants of simian virus 40. Effect of mutations at a T antigen binding site on DNA replication and expression of viral genes. J Mol Biol. 1982 Apr 15;156(3):531–548. doi: 10.1016/0022-2836(82)90265-0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Guo Z. S., Gutierrez C., Heine U., Sogo J. M., Depamphilis M. L. Origin auxiliary sequences can facilitate initiation of simian virus 40 DNA replication in vitro as they do in vivo. Mol Cell Biol. 1989 Sep;9(9):3593–3602. doi: 10.1128/mcb.9.9.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C., Guo Z. S., Roberts J., DePamphilis M. L. Simian virus 40 origin auxiliary sequences weakly facilitate T-antigen binding but strongly facilitate DNA unwinding. Mol Cell Biol. 1990 Apr;10(4):1719–1728. doi: 10.1128/mcb.10.4.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T., DePamphilis M. L. Initiation of SV40 DNA replication in vivo: location and structure of 5' ends of DNA synthesized in the ori region. Cell. 1982 Apr;28(4):767–779. doi: 10.1016/0092-8674(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Hendrickson E. A., Fritze C. E., Folk W. R., DePamphilis M. L. The origin of bidirectional DNA replication in polyoma virus. EMBO J. 1987 Jul;6(7):2011–2018. doi: 10.1002/j.1460-2075.1987.tb02465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz G. Z., Mertz J. E. Bidirectional promoter elements of simian virus 40 are required for efficient replication of the viral DNA. Mol Cell Biol. 1986 Oct;6(10):3513–3522. doi: 10.1128/mcb.6.10.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis J. W., Scott W. A. DNA replication and chromatin structure of simian virus 40 insertion mutants. Mol Cell Biol. 1984 Aug;4(8):1499–1507. doi: 10.1128/mcb.4.8.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J. SV40 DNA replication. J Biol Chem. 1988 Dec 5;263(34):17889–17892. [PubMed] [Google Scholar]
- Kowalski D., Eddy M. J. The DNA unwinding element: a novel, cis-acting component that facilitates opening of the Escherichia coli replication origin. EMBO J. 1989 Dec 20;8(13):4335–4344. doi: 10.1002/j.1460-2075.1989.tb08620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski D., Natale D. A., Eddy M. J. Stable DNA unwinding, not "breathing," accounts for single-strand-specific nuclease hypersensitivity of specific A+T-rich sequences. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9464–9468. doi: 10.1073/pnas.85.24.9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Chen G. J., Woodworth-Gutai M. Simian virus 40 DNA replication: functional organization of regulatory elements. Mol Cell Biol. 1986 Sep;6(9):3086–3093. doi: 10.1128/mcb.6.9.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Peden K. W., Dixon R. A., Kelly T. Functional organization of the simian virus 40 origin of DNA replication. Mol Cell Biol. 1986 Apr;6(4):1117–1128. doi: 10.1128/mcb.6.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Salas E., Cupo D. Y., DePamphilis M. L. The need for enhancers is acquired upon formation of a diploid nucleus during early mouse development. Genes Dev. 1988 Sep;2(9):1115–1126. doi: 10.1101/gad.2.9.1115. [DOI] [PubMed] [Google Scholar]
- Martínez-Salas E., Linney E., Hassell J., DePamphilis M. L. The need for enhancers in gene expression first appears during mouse development with formation of the zygotic nucleus. Genes Dev. 1989 Oct;3(10):1493–1506. doi: 10.1101/gad.3.10.1493. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mul Y. M., Verrijzer C. P., van der Vliet P. C. Transcription factors NFI and NFIII/oct-1 function independently, employing different mechanisms to enhance adenovirus DNA replication. J Virol. 1990 Nov;64(11):5510–5518. doi: 10.1128/jvi.64.11.5510-5518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Tjian R. Construction and analysis of simian virus 40 origins defective in tumor antigen binding and DNA replication. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6491–6495. doi: 10.1073/pnas.77.11.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons R., Anderson M. E., Tegtmeyer P. Three domains in the simian virus 40 core origin orchestrate the binding, melting, and DNA helicase activities of T antigen. J Virol. 1990 Feb;64(2):509–518. doi: 10.1128/jvi.64.2.509-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Murakami Y., Kern F. G., Folk W., Basilico C., Hurwitz J. DNA sequence requirements for replication of polyomavirus DNA in vivo and in vitro. Mol Cell Biol. 1987 Oct;7(10):3694–3704. doi: 10.1128/mcb.7.10.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Vakalopoulou E., Mertz R., Mastrangelo I., Hough P., Tegtmeyer P., Fanning E. Seventeen base pairs of region I encode a novel tripartite binding signal for SV40 T antigen. Cell. 1985 Sep;42(2):539–548. doi: 10.1016/0092-8674(85)90111-4. [DOI] [PubMed] [Google Scholar]
- Scheller A., Prives C. Simian virus 40 and polyomavirus large tumor antigens have different requirements for high-affinity sequence-specific DNA binding. J Virol. 1985 May;54(2):532–545. doi: 10.1128/jvi.54.2.532-545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B., Gerard R. D., Guggenheimer R. A., Gluzman Y. T antigen and template requirements for SV40 DNA replication in vitro. EMBO J. 1985 Nov;4(11):2933–2939. doi: 10.1002/j.1460-2075.1985.tb04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., Anderson S., DePamphilis M. L. Maturation of replicating simian virus 40 DNA molecules in isolated nuclei by continued bidirectional replication to the normal termination region. Biochim Biophys Acta. 1979 Nov 22;565(1):84–97. doi: 10.1016/0005-2787(79)90084-4. [DOI] [PubMed] [Google Scholar]
- Umek R. M., Kowalski D. The ease of DNA unwinding as a determinant of initiation at yeast replication origins. Cell. 1988 Feb 26;52(4):559–567. doi: 10.1016/0092-8674(88)90469-2. [DOI] [PubMed] [Google Scholar]
- Weaver D. T., Fields-Berry S. C., DePamphilis M. L. The termination region for SV40 DNA replication directs the mode of separation for the two sibling molecules. Cell. 1985 Jun;41(2):565–575. doi: 10.1016/s0092-8674(85)80029-5. [DOI] [PubMed] [Google Scholar]
- Wiekowski M., Miranda M., DePamphilis M. L. Regulation of gene expression in preimplantation mouse embryos: effects of the zygotic clock and the first mitosis on promoter and enhancer activities. Dev Biol. 1991 Oct;147(2):403–414. doi: 10.1016/0012-1606(91)90298-h. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., DePamphilis M. L. DNA binding site for a factor(s) required to initiate simian virus 40 DNA replication. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1646–1650. doi: 10.1073/pnas.83.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]