Abstract
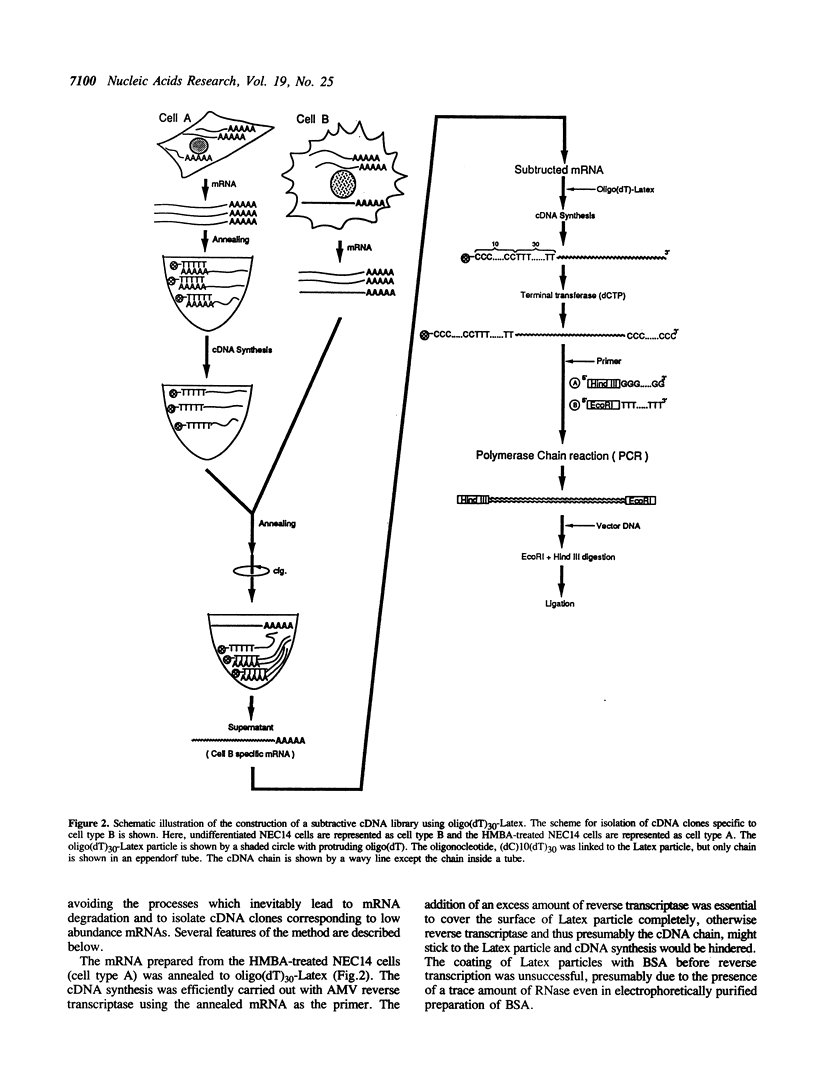
The human embryonal carcinoma cell line NEC14 can be induced to differentiate by the addition of 10(-2)M N,N'-hexamethylene-bis-acetamide (HMBA). A subtractive cDNA library specific to undifferentiated NEC14 cells was constructed using oligo(dT)30-Latex and polymerase chain reaction (PCR). The method was designed to improve the efficiency of subtraction and the enrichment of cDNA clones corresponding to low abundance mRNAs. The single strand of cDNA was made from mRNA prepared from the HMBA-treated NEC14 cells using an oligo(dT)30 primer covalently linked to Latex particles. After removal of the mRNA template by heat-denaturation and centrifugation, the subtractive hybridization was carried out between the cDNA-oligo(dT)30-Latex and mRNA from untreated NEC14 cells. Unhybridized mRNA collected by centrifugation was hybridized repeatedly to the cDNA-oligo(dT)30-Latex and subtractive mRNA was converted to cDNA. The subtractive cDNA was then amplified by PCR and cloned into pBluescript II KS-. The cDNA library thus constructed consisted of approximately 10,000 independent clones with cDNA inserts of 1.7 Kb on average. Differential hybridization of these transformants indicated that approximately 3% of them contained cDNA inserts specific to the undifferentiated EC cells, some of which were derived from low abundance mRNAs.
Full text
PDF



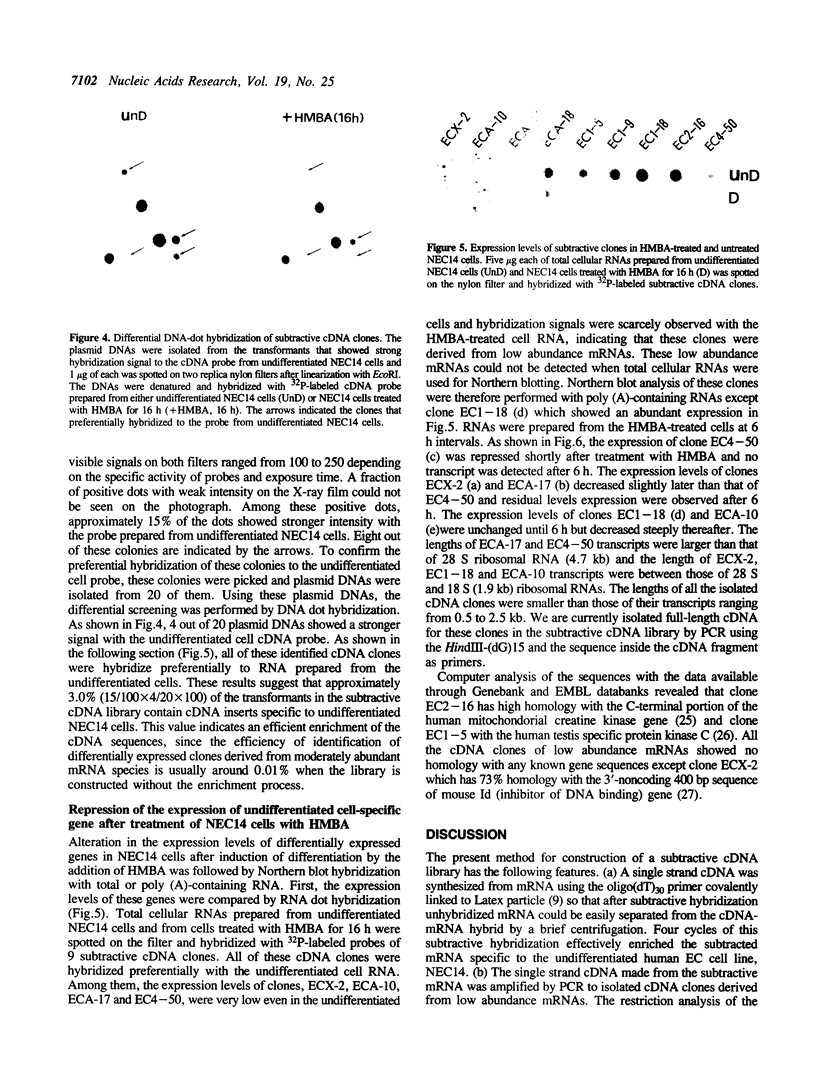
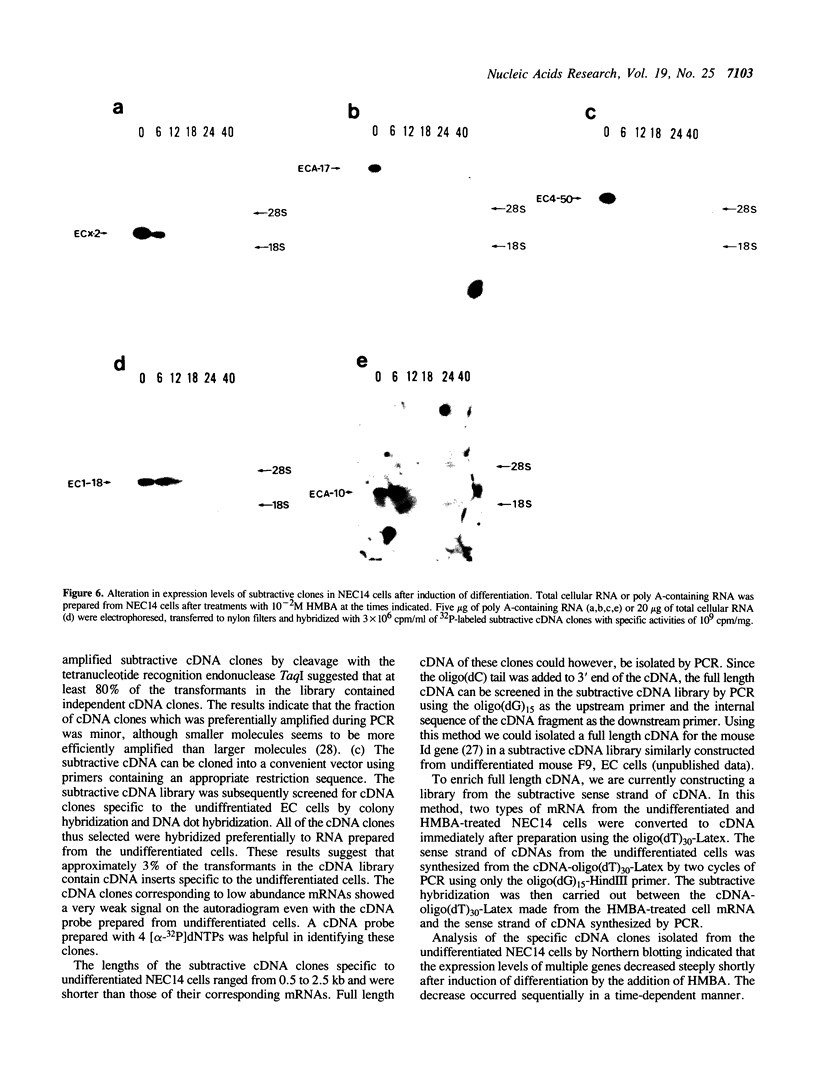

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe S. J., Oyen O., Sandberg M., Frøysa A., Hansson V., Jahnsen T. Molecular cloning of a tissue-specific protein kinase (C gamma) from human testis--representing a third isoform for the catalytic subunit of cAMP-dependent protein kinase. Mol Endocrinol. 1990 Mar;4(3):465–475. doi: 10.1210/mend-4-3-465. [DOI] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Haas R. C., Strauss A. W. Separate nuclear genes encode sarcomere-specific and ubiquitous human mitochondrial creatine kinase isoenzymes. J Biol Chem. 1990 Apr 25;265(12):6921–6927. [PubMed] [Google Scholar]
- Hara E., Nakada S., Takehana K., Nakajima T., Iino T., Oda K. Molecular cloning and characterization of cellular genes whose expression is repressed by the adenovirus E1a gene products and growth factors in quiescent rat cells. Gene. 1988 Oct 15;70(1):97–106. doi: 10.1016/0378-1119(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Hara E., Takehana K., Nakada S., Oda K., Kawata M., Kimura H., Sekiya S. A transient decrease in N-myc expression and its biological role during differentiation of human embryonal carcinoma cells. Differentiation. 1991 Jul;47(2):107–117. doi: 10.1111/j.1432-0436.1991.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Nakada S., Nakajima T., Oda K., Kawata M., Kimura H., Sekiya S. Expression of various viral and cellular enhancer-promoters during differentiation of human embryonal carcinoma cells. Differentiation. 1990 Feb;42(3):191–198. doi: 10.1111/j.1432-0436.1990.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Inoue H., Nojima H., Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990 Nov 30;96(1):23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Neumann R., Keyte J. Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res. 1988 Dec 9;16(23):10953–10971. doi: 10.1093/nar/16.23.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribayashi K., Hikata M., Hiraoka O., Miyamoto C., Furuichi Y. A rapid and efficient purification of poly(A)-mRNA by oligo(dT)30-Latex. Nucleic Acids Symp Ser. 1988;(19):61–64. [PubMed] [Google Scholar]
- Lawyer F. C., Stoffel S., Saiki R. K., Myambo K., Drummond R., Gelfand D. H. Isolation, characterization, and expression in Escherichia coli of the DNA polymerase gene from Thermus aquaticus. J Biol Chem. 1989 Apr 15;264(11):6427–6437. [PubMed] [Google Scholar]
- Oda K., Masuda-Murata M., Shiroki K., Handa H. Mitogenic activity of the adenovirus type 12 E1A gene induced by hormones in rat cells. J Virol. 1986 Apr;58(1):125–133. doi: 10.1128/jvi.58.1.125-133.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Kawaichi M., Brownstein M., Lee F., Yokota T., Arai K. High-efficiency cloning of full-length cDNA; construction and screening of cDNA expression libraries for mammalian cells. Methods Enzymol. 1987;154:3–28. doi: 10.1016/0076-6879(87)54067-8. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Dawid I. B. Differential gene expression in the gastrula of Xenopus laevis. Science. 1983 Oct 14;222(4620):135–139. doi: 10.1126/science.6688681. [DOI] [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Sekiya S., Kawata M., Iwasawa H., Inaba N., Sugita M., Suzuki N., Motoyama T., Yamamoto T., Takamizawa H. Characterization of human embryonal carcinoma cell lines derived from testicular germ-cell tumors. Differentiation. 1985;29(3):259–267. doi: 10.1111/j.1432-0436.1985.tb00325.x. [DOI] [PubMed] [Google Scholar]
- Sekiya S., Kimura H., Yamazawa K., Kera K., Kawata M., Takamizawa H., Oda K. Induction of human embryonal carcinoma cell differentiation using N,N'-hexamethylene bisacetamide in vitro. Gynecol Oncol. 1990 Jan;36(1):69–78. doi: 10.1016/0090-8258(90)90111-w. [DOI] [PubMed] [Google Scholar]
- St John T. P., Davis R. W. Isolation of galactose-inducible DNA sequences from Saccharomyces cerevisiae by differential plaque filter hybridization. Cell. 1979 Feb;16(2):443–452. doi: 10.1016/0092-8674(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Timblin C., Battey J., Kuehl W. M. Application for PCR technology to subtractive cDNA cloning: identification of genes expressed specifically in murine plasmacytoma cells. Nucleic Acids Res. 1990 Mar 25;18(6):1587–1593. doi: 10.1093/nar/18.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel R. J., Nevins J. R. Adenovirus infection of differentiated F9 cells results in a global shut-off of differentiation-induced gene expression. Nucleic Acids Res. 1990 Oct 25;18(20):6107–6112. doi: 10.1093/nar/18.20.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]