Abstract
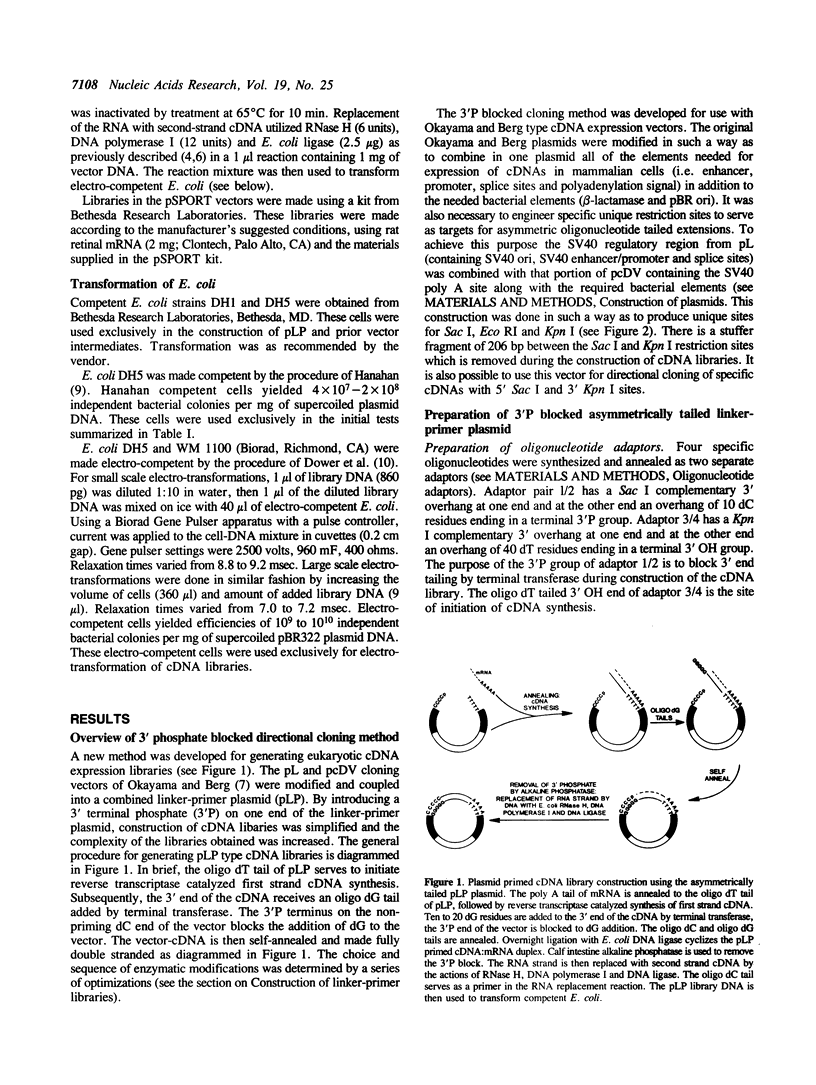
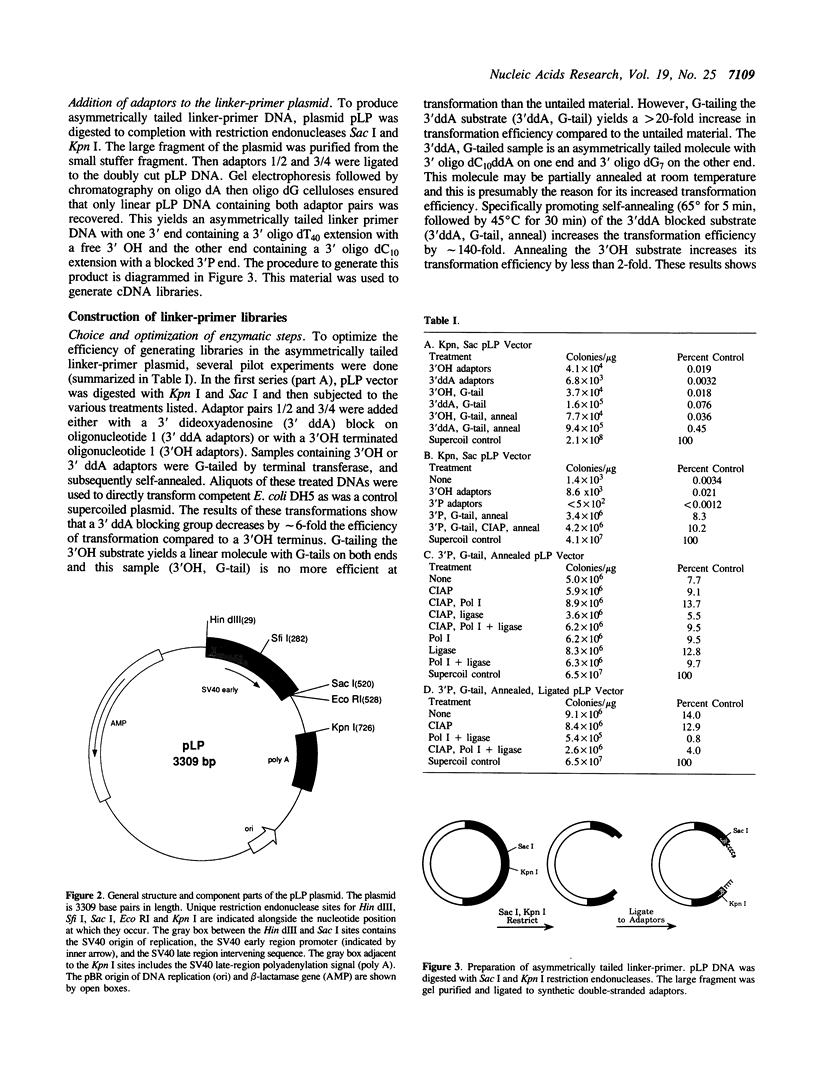
A new procedure using an asymmetrically tailed linker-primer plasmid has been developed to prepare extremely high complexity cDNA libraries. This procedure yields plasmid primed libraries with a final form equivalent to those made by the procedure of Okayama and Berg. However, the number of steps involved in library preparation is decreased. The form of the vector is such that one end of the linearized linker-primer plasmid has a 3' terminal extension of 40 deoxythymidylate residues (the dT end). The other end has a 3' terminal extension of 10 deoxycytidylate residues (the dC end). The dC end of the plasmid is blocked to further 3' extension by a 3' phosphate group. This configuration enables one to prime first strand cDNA synthesis at the dT end, tail the 3' end of the cDNA with deoxyguanylate residues without tailing the dC end (due to the 3' phosphate block). The plasmid primed cDNA can then be self-annealed and the 3' phosphate blocking group removed during the synthesis of double stranded cDNA. The efficiency of this procedure is significantly higher than other methods (including phage based libraries): linker-primer libraries have 15 to 900-fold higher complexity than libraries prepared by other methods. A cloning efficiency of 9 x 10(8) colonies per microgram of linker-primer DNA was achieved. This method should be useful for the cloning of cDNAs corresponding to extremely rare mRNAs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. C., McKnight T. D., Williams B. G. A simplified and efficient vector-primer cDNA cloning system. Gene. 1984 Nov;31(1-3):79–89. doi: 10.1016/0378-1119(84)90197-5. [DOI] [PubMed] [Google Scholar]
- Böttger E. C. High-efficiency generation of plasmid cDNA libraries using electro-transformation. Biotechniques. 1988 Oct;6(9):878–880. [PubMed] [Google Scholar]
- Canfield V., Emanuel J. R., Spickofsky N., Levenson R., Margolskee R. F. Ouabain-resistant mutants of the rat Na,K-ATPase alpha 2 isoform identified by using an episomal expression vector. Mol Cell Biol. 1990 Apr;10(4):1367–1372. doi: 10.1128/mcb.10.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger P. L. Full-length cDNA clones: vector-primed cDNA synthesis. Methods Enzymol. 1987;152:371–389. doi: 10.1016/0076-6879(87)52044-4. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Messing J. A method for cloning full-length cDNA in plasmid vectors. Methods Enzymol. 1987;154:28–41. doi: 10.1016/0076-6879(87)54068-x. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kimmel A. R., Berger S. L. Preparation of cDNA and the generation of cDNA libraries: overview. Methods Enzymol. 1987;152:307–316. doi: 10.1016/0076-6879(87)52035-3. [DOI] [PubMed] [Google Scholar]
- Margolskee R. F., Kavathas P., Berg P. Epstein-Barr virus shuttle vector for stable episomal replication of cDNA expression libraries in human cells. Mol Cell Biol. 1988 Jul;8(7):2837–2847. doi: 10.1128/mcb.8.7.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R. J., Lai C., Nave K. A., Lenoir D., Ogata J., Sutcliffe J. G. Nucleotide sequences of two mRNAs for rat brain myelin proteolipid protein. Cell. 1985 Oct;42(3):931–939. doi: 10.1016/0092-8674(85)90289-2. [DOI] [PubMed] [Google Scholar]
- Oberbäumer I. New pUC-derived expression vectors for rapid construction of cDNA libraries. Gene. 1986;49(1):81–91. doi: 10.1016/0378-1119(86)90387-2. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Kawaichi M., Brownstein M., Lee F., Yokota T., Arai K. High-efficiency cloning of full-length cDNA; construction and screening of cDNA expression libraries for mammalian cells. Methods Enzymol. 1987;154:3–28. doi: 10.1016/0076-6879(87)54067-8. [DOI] [PubMed] [Google Scholar]
- Pruitt S. C. Expression vectors permitting cDNA cloning and enrichment for specific sequences by hybridization/selection. Gene. 1988 Jun 15;66(1):121–134. doi: 10.1016/0378-1119(88)90230-2. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


