Abstract
Burkholderia cepacia is an important pathogen in cystic fibrosis (CF) and an infrequent cause of nosocomial infection in non-CF patients. This report describes a large hospital outbreak that appeared to involve both patient groups, a previously unrecognized phenomenon. Ribotype restriction fragment length polymorphism (RFLP) profiles and pulsed-field gel electrophoresis–resolved macrochromosomal RFLPs were analyzed, a ribotype-based phylogenic tree was constructed, and case-control and cohort studies were performed. A single dominant clone was found in both CF and non-CF groups. Phylogenic analysis suggests that it has evolved independently and that such highly transmissible strains can emerge rapidly and randomly. Acquisition risk in the CF patients was linked to hospitalization (odds ratio = 5.47, P = .0158, confidence interval = 1.28–26.86) and was associated with significantly increased mortality rates. Infection control policies must now consider this threat of transmission between non-CF and CF patients.
Burkholderia (Pseudomonas) cepacia is a nutritionally versatile, gram-negative organism, commonly found in soil and water. It was first described in 1950 by Burkholder [1] as the phytopathogen responsible for “slippery skin,” a bacterial rot of onions. This microorganism has become an important opportunistic human pathogen, with a particular predilection for the lung in patients with cystic fibrosis (CF). One risk factor for acquisition appears to be hospitalization [2]. Acquisition of B. cepacia by patients with CF may be associated with a rapid decline in pulmonary function with increased morbidity and mortality [3–5]. Unlike Pseudomonas aeruginosa in CF, acquisition of B. cepacia is associated with increased mortality at all levels of pulmonary function [4]. The threat posed by B. cepacia has led to stringent infection control measures in the CF community [5].
Analysis by ribotype (ribosomal RNA operon-associated) restriction fragment length polymorphisms (RFLPs) and pulsed-field gel electrophoresis (PFGE)–resolved chromosomal RFLP profiles confirm that several distinct highly transmissible CF-associated B. cepacia clones exist [6, 7]. There are also numerous strains of negligible transmissibility [8]. The precise mode of spread of highly transmissible strains has not been determined, but social contact between CF patients appears to be an important factor [9].
Two distinctly different categories of B. cepacia outbreaks have been reported: outbreaks that occur and persist in the CF community due to cross-spread of highly transmissible CF-associated strains [7, 9, 10] and the small, short-lived focal outbreaks that occur in non-CF patients due to nosocomial acquisition from a contaminated common source [11–13]. In this report, we describe the emergence of an alarming third category of outbreak that involves both CF and non-CF populations.
We used DNA-based epidemiologic typing methods to characterize the genetic relatedness of the involved isolates. Case-control and cohort studies were carried out to investigate the potential risk factors for acquisition and the mortality associated with acquisition. Environmental microbiologic studies were performed to identify potential reservoirs and modes of transmission. Ribotype RFLP patterns of isolates from this outbreak were compared with those of other B. cepacia isolates in our database, permitting phylogenic analysis and a graphical representation of the evolution of this ubiquitous environmental organism that has emerged as a human pathogen.
Methods
Study site and patient population
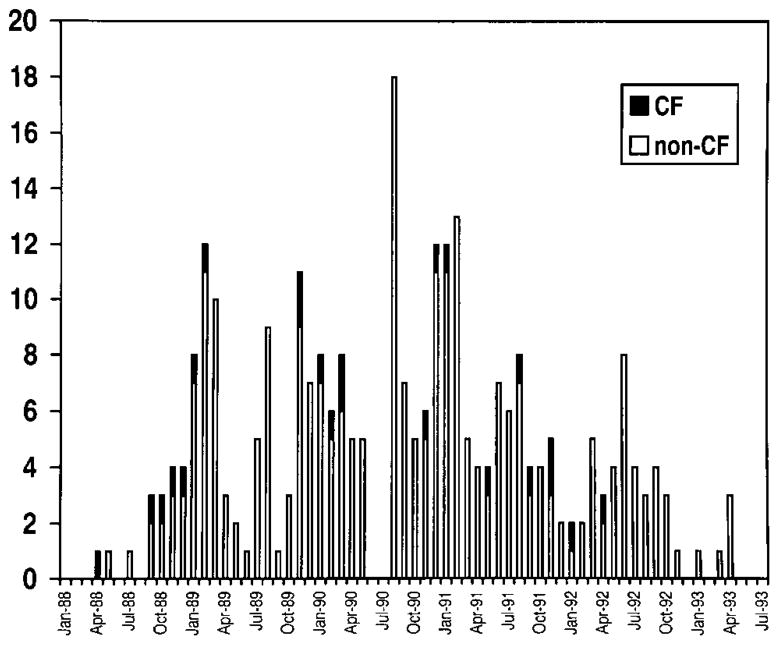
The outbreak occurred at a university hospital in Mississippi. The hospital has 352 adult beds and three intensive care units (ICUs): a medical ICU (MICU), a surgical ICU (SICU), and a coronary care unit (CCU). The pediatric hospital is in a separate wing with 226 beds, a pediatric ICU (PICU), and a nursery ICU. The pediatric pulmonology department has an active practice caring for children with CF. From 1988 to 1993, admissions to the adult hospital averaged 16,361 annually; admissions to the pediatric hospital averaged 4311 annually, and the average annual CF patient registry for the hospital was 152. Beginning in April 1988, there was a significant increase in B. cepacia isolation (figure 1) among SICU, MICU, PICU, and CCU patients. This occurred simultaneously with an increase in isolation of B. cepacia from patients with CF. Between April 1988 and April 1993, 23 patients with CF had sputum cultures positive for B. cepacia, and 1 had an isolate from blood as well. In total, 245 non-CF patients harbored B. cepacia. The first isolate associated with the outbreak was recovered from the sputum of a 27-year-old man with CF in April 1988. By July 1988, isolates appeared in sputum specimens from non-CF patients receiving mechanical ventilation in the SICU. MICU, PICU, and CCU patients were affected soon after. Of the 245 non-CF patients, isolates were obtained from sputum (228), intravenous catheters (11), urine (10), wounds (10), chest tube drainage (2), pleural (4), and miscellaneous sites (5). Isolates from 90 of the 245 non-CF patients were banked.
Figure 1.
Frequency of first culture positive for B. cepacia at any site, April 1988–April 1993, in CF and non-CF patients.
In outpatient clinics, patients with CF known to have B. cepacia were isolated from CF patients without B. cepacia. Hospitalized CF patients with B. cepacia were placed in respiratory isolation in single rooms. CF patients without B. cepacia were cautioned to avoid contact with them. Non-CF patients harboring B. cepacia were not isolated, and these patients were admitted to wards with young adults with CF. The majority of the non-CF patients with B. cepacia were or had been critically ill and had required mechanical ventilation in the ICUs.
Bacterial isolation
B. cepacia was identified by standard methods [14]. Sputum samples were plated onto blood agar, chocolate agar, eosin-methylene blue agar, and PC agar (prepared plated medium for isolation of Pseudomonas [sic] cepacia; BBL Microbiology Systems, Gaithersburg, MD). B. cepacia was identified by automated susceptibility testing (MSII; Abbott Laboratories Diagnostic Division, Santa Clara, CA).
To investigate the potential role of the environment or environmental contamination as a source of acquisition, 124 cultures of ICU environments were performed between September 1990 and February 1991. Specimens were obtained from mechanical ventilators, ambu bags, blood pressure cuffs, sink drains, handles and faucets, laryngoscope blades, hand soaps, mouthwash, antiseptics, irrigant solutions, and wheelchairs by sterile swabs streaked onto PC agar. Hand cultures of personnel were not studied, as survival of B. cepacia on hands is extremely limited and variable [15–17]. Between November 1990 and September 1992, 316 B. cepacia isolates from 104 patients were saved (14 with CF) at −70°C.
Molecular epidemiology
Isolation of chromosomal DNA and EcoRI ribotype analysis were done as previously described [6–8]. All 14 CF patients with banked B. cepacia had their isolates ribotyped, and isolates were typed from 35 of the 90 non-CF patients chosen at random. SpeI-based PFGE macrochromosomal RFLP analysis was performed on a subset by methods previously described [6–8].
Standard criteria were used for comparing PFGE and ribotype RFLP patterns [6–8, 18]. For rrn-associated RFLPs, given that B. cepacia strains typically display 7–10 distinct hybridizing bands, a shared ribotype would correspond to an index of similarity, D ≥ 0.790 [6–8].
Phylogenic analysis
For the phylogenic analysis, ribotype RFLPs were entered into our computerized database (Scanalytics/CSPI, Fairfax, VA), which contains ribotype RFLPs of a wide variety of B. cepacia strains, including epidemic, nonepidemic, and environmental strains. By use of EcoRI rrn-RFLPs from this database, an rrn-based phylogenic tree was inferred by Dollo parsimony methods [7, 19].
Epidemiology
Case-control studies of CF patients and a descriptive study of non-CF patients were done to investigate risk factors for acquisition. Of 268 patients who had ≥1 B. cepacia isolate, 23 had CF. A retrospective chart review case-control study was performed on 20 of these patients for whom charts could be obtained. A retrospective chart review was also done on 90 of the 245 patients without CF. Selection of these 90 patients was based on the availability of a clinical isolate in our collection. For patients who died, contribution of B. cepacia to mortality was determined by criteria of the Centers for Disease Control (CDC) National Nosocomial Infections Surveillance System [20].
For the non-CF analysis, data collected included age, sex, stay in an ICU, exposure to nebulized medications, oxygen therapy, prior administration of intravenous antibiotics, and mechanical ventilation.
For the CF case-control study, a case was defined as any patient who had ≥1 B. cepacia isolate obtained between 1988 and 1993. A control was defined as a patient appearing on the CF registry during the same year. Three controls, matched for sex and age were sought for each case. Data collected included age, sex, duration of prior hospitalization, number of treatments with nebulized medications, duration of prior antibiotic therapy in the hospital, use of supplemental oxygen, admission to ICU, and ward on which hospitalization occurred. To compare severity of CF, Shwachman-Kulczycki scores [21] were retrospectively estimated at the time of initial isolation of B. cepacia in cases and for the nearest date available in matched controls. Time period of analysis began 3 years prior to isolation of B. cepacia and continued through the epidemic period or until death of the case-patients, as previous studies have provided circumstantial evidence that CF patients may harbor B. cepacia for as long as 2 years prior to its isolation from sputum [22]. Analysis of data was repeated on the subset of 10 patients known to harbor the epidemic strain. Of these 10 patients, 1 died in an accident and was excluded from all analysis of mortality.
For statistical analysis, we used Epi Info version 6.02 software (CDC, Atlanta), analysis of variance, the Kruskal-Wallis test, and Yates’ corrected χ2 or Fisher’s exact tests where appropriate. All tests were two-tailed. We also did logistic regression (version 6.01 for Windows; SAS Institute, Cary, NC).
Results
Environmental microbiology
Of 125 environmental specimens, only 3 grew B. cepacia; these were taken from ventilator tubing of a non-CF patient who harbored B. cepacia in the respiratory tract. This patient had required prolonged mechanical ventilation before B. cepacia was isolated. No B. cepacia was found in the neighboring ventilator.
RFLP analysis
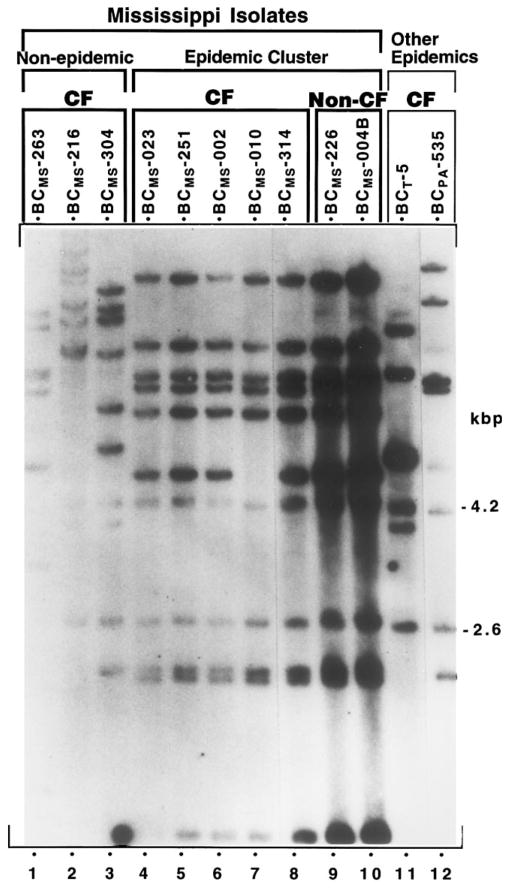
Ribotype RFLP analysis demonstrated that a single dominant clone of B. cepacia was present in both CF and non-CF patients. This was confirmed by PFGE (figure 2, figure 3). Of 23 CF patients with B. cepacia, 14 had isolates available. Of these 14, 10 (71%) had the epidemic strain. Of the 90 non-CF patients with B. cepacia, 35 had isolates typed, and 30 (86%) had the epidemic strain (table 1). The ribotype of the epidemic strain had a characteristic banding pattern (see lanes 4–10, figure 2). A non-CF pediatric patient with perinatal asphyxia and ventilator dependence, hospitalized between 2 January 1987 and 2 March 1994, had B. cepacia isolated from sputum from August 1989 to December 1992. RFLP analysis of sequential isolates from this patient (banked from 1991) demonstrated persistence of the epidemic strain (data not shown).
Figure 2.
EcoRI ribotype profiles of B. cepacia isolates from Mississippi hospital outbreak (lanes 1–10) and profiles of epidemic strains from centers elsewhere (lanes 11, 12). Origin of isolates by lane: 1–3, CF patients who independently acquired B. cepacia; 4–8, CF patients who harbored epidemic strain; 9 and 10, non-CF patients who harbored epidemic strain; lane 11, BCT-5 clone from Toronto/Edinburgh CF center epidemic [7] and member of same clonal lineage as Toronto isolate BCT-7 (shown in figure 4); 12, BCPA-535 Philadelphia CF center epidemic [23].
Figure 3.
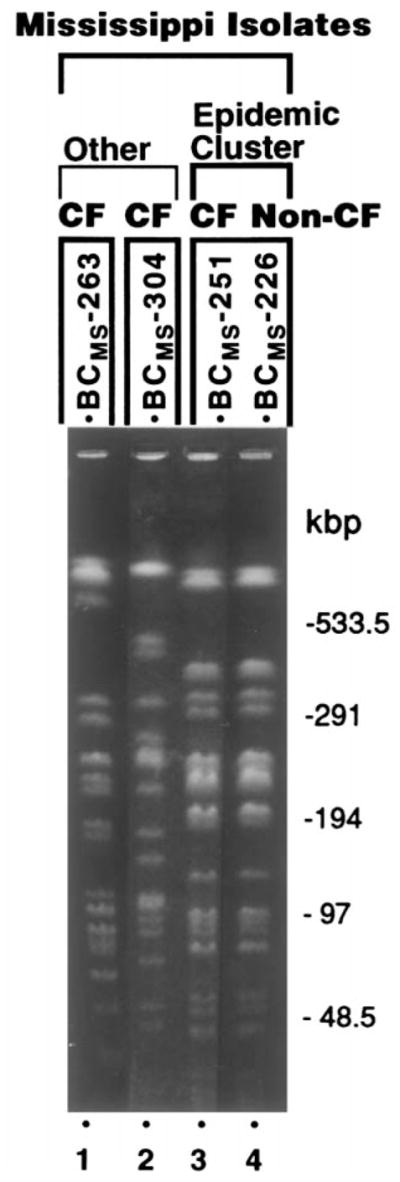
SpeI-based pulsed-field gel electrophoresis (PFGE) characterization of B. cepacia isolates from Mississippi hospital outbreak. Lanes 1 and 2, isolates from CF patients who independently acquired B. cepacia; PFGE restriction fragment length polymorphism (RFLP) patterns differ from epidemic strain (lanes 3, 4) and from each other. Lanes 3 and 4 represent epidemic cluster and demonstrate prototypic PFGE RFLP pattern of epidemic strain. Lanes 3 and 4, respectively, isolates from CF and non-CF patients.
Table 1.
Characteristics of CF patients with B. cepacia and non-CF patients with B. cepacia.
| CF group (n = 14) | Non-CF group (n = 90) | |
|---|---|---|
| Mean age (years) | 18 | 46 |
| Males | 10 (71) | 57 (63) |
| Females | 4 (29) | 33 (37) |
| Intensive care unit patients | 0 | 77 (85.5) |
| Ventilated patients | 0 | 76 (84.4) |
| Patients’ isolates typed | 14 | 35 |
| No. with epidemic B. cepacia strain | 10 (71) | 30 (86) |
| No. who died | 9 (45) | 43 (47.8) |
| Relationship of B. cepacia to mortalitya | ||
| Causal | 5 | 1 |
| Contributory | 1 | 34 |
| Not related | 2 | 8 |
| Unknown | 1 | 0 |
NOTE. Data are no. (%).
By criteria described by CDC National Nosocomial Infections Surveillance System [20].
Phylogenic analysis
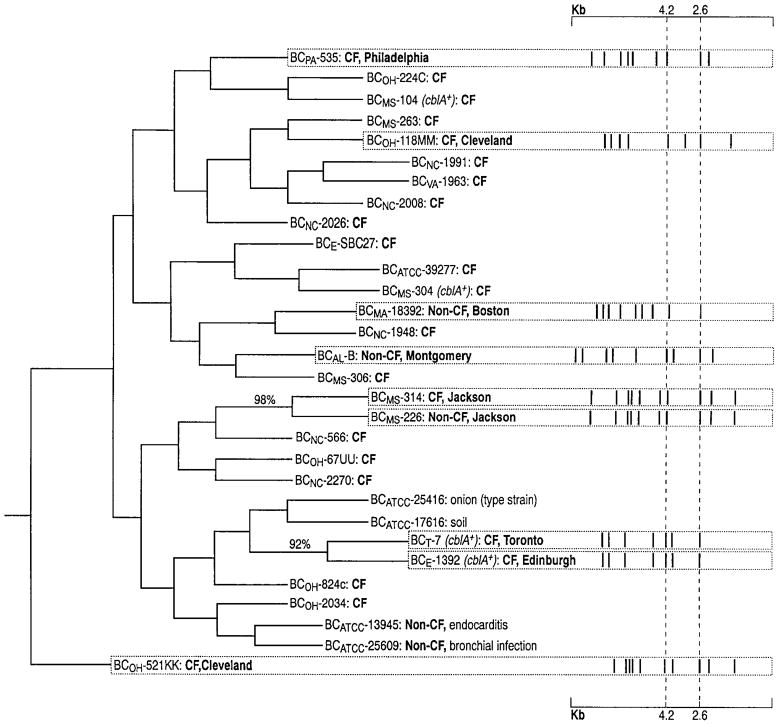
Characterized isolates from elsewhere and a variety of isolates from the Mississippi hospital population were entered into the analysis. These included a prototypic epidemic CF isolate (BCMS-314) and a prototypic epidemic non-CF isolate (BCMS-226). The resulting phylogenic tree (see Methods) is shown in figure 4. The number at a node indicates the percentage of time each branch was joined together under bootstrap analysis. Values are given for those that fell together in >50% of bootstraps. This occurred only twice, on both occasions with values >90%, indicating clonal lineages. These two clonal lineages are the previously reported Edinburgh/Toronto CF-associated epidemic [7] (isolates BCE-1392 and BCT-7) and the Mississippi hospital CF/non-CF epidemic (isolates BCMS-314 and BCMS-226). This Mississippi epidemic clone and other CF-associated epidemic clones from CF centers elsewhere (Toronto, Edinburgh, Philadelphia, Cleveland-1, Cleveland-2) [7] (A.H., L. Sun, R-Z.J., and R.G., unpublished data) do not share the same ribotypes or PFGE macrochromosomal RFLPs (figures 2, 3). Neither do they share polymorphism patterns with other isolates responsible for nosocomial non-CF out-breaks from Boston (L. Sun, R-Z.J. and R.G., unpublished data) and Alabama [12] (figure 4, figure 5).
Figure 4.
EcoRI rrn restriction fragment length polymorphism (RFLP)–based unrooted phylogenic tree of isolates from patients at 6 North American CF centers (Chapel Hill, NC; Jackson, MS; Norfolk, VA; Cleveland, Philadelphia, and Toronto) and 1 in Europe (Edinburgh) plus environmental and clinical non-CF sources. No. at dendogram node indicates % of time each branch was joined under bootstrap analysis (500 replicates) [19]. Only values >50% are given. BCMS-314 and BCMS-226 are prototypic Mississippi epidemic outbreak isolates from CF and non-CF patients, respectively. Isolate no. is followed by cblA genotype, source (CF, non-CF, environmental), and geographic location for epidemic isolates. Epidemic lineages have characteristic ribotype patterns displayed as bar codes. Fingerprint bar code markers, cepacia species-specific, conserved EcoRI bands at 2.6 and 4.2 kb, are indicated by vertical dashed lines.
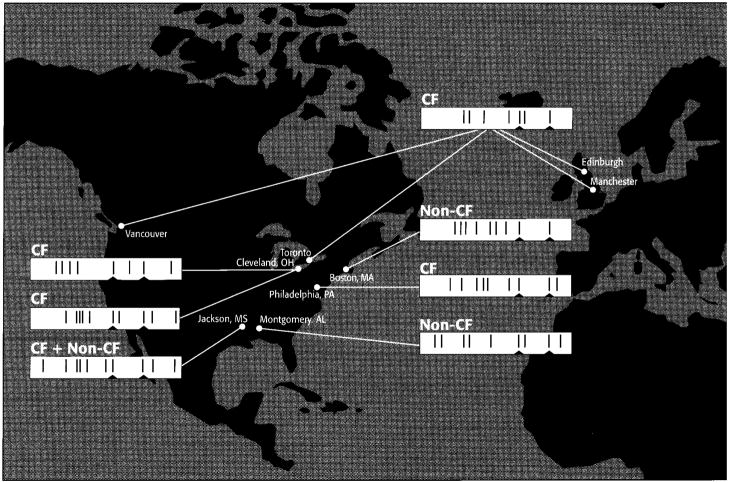
Figure 5.
Geographic distribution of epidemic strains of B. cepacia [7, 9, 12, 23–26] (unpublished data). Bar codes of prototypic ribotype patterns are displayed (see Results). Conserved cepacia species-specific, EcoRI ribotype bands at 4.2 and 2.6 kb are indicated by small notches.
Study of non-CF cases
Mean age of the 90 non-CF patients with banked isolates was 46 years. There were 57 males (63%) and 33 females (37%) (table 1). Of the 90 non-CF cases, 87 (97%) were hospitalized when the first isolate was obtained, and 77 (85.5%) were in an ICU when B. cepacia was first isolated (30 [39%] SICU, 19 [24.7%] MICU, 15 [19.5%] PICU, and 13 [16.8%] CCU). Eighty-two (91%) had received prior supplemental oxygen therapy. Seventy-six (84.4%) had received prior mechanical ventilation. Eighty-three (92.2%) had previously received intravenous antibiotics. Of these 90 patients, 43 (47.8%) died during hospitalization. Among those who died, 35 (81.4%) had B. cepacia isolated near the time of death. An attempt to assess the contribution of B. cepacia to mortality was made using criteria described by the National Nosocomial Infection Surveillance System [20]. Although this method is limited and prone to overestimates, it suggested that B. cepacia contributed to the deaths of 35 and was the cause of death in 1 (i.e., B. cepacia contributed to the deaths of 39% of the non-CF patients who acquired it).
CF case-control study
We compared 20 CF patients with B. cepacia with 57 controls matched for sex and age (table 2). Mean Shwachman-Kulczycki scores at the time of isolation for cases were significantly worse than for controls (66 vs. 77, P = .004). Of the 20 case-patients, 16 had B. cepacia first isolated as outpatients, and 17 of the 20 case-patients were hospitalized during the epidemic period compared with 29 of 57 controls (odds ratio [OR] = 5.47, 95% confidence interval [CI] = 1.28–26.86, P = .0158). Cases had significantly more days of hospitalization in the 3 years before B. cepacia was isolated (41.1 vs. 18.2 days, P = .0158) and during the epidemic period (22.4 vs. 12.1 days, P = .005). There were no significant differences in days of intravenous antibiotic administration, aminoglycoside therapy, oxygen therapy, or number of aerosol treatments during hospitalization. Neither analysis of these variables for both the epidemic period and for 3 years prior to isolation nor exclusion of cases and controls who were never hospitalized changed these results.
Table 2.
Case-control study comparing CF patients with B. cepacia with CF controls without B. cepacia.
| CF cases (n = 20) | CF controls (n = 57) | Odds ratio (95% confidence interval) | P | |
|---|---|---|---|---|
| Mean age (years) | 14.5 | 14.2 | ||
| Male | 14 (70) | 41 (72) | ||
| Female | 6 (30) | 16 (28) | ||
| Hospitalized during epidemic period | 17 (85) | 29 (51) | 5.47 (1.28–26.86) | .007 |
| Days of hospitalization during epidemic period | 22.4 | 12.1 | .0019 | |
| Mean (median) no. of treatments with nebulised medicationsa | 34.6 (28) | 34.0 (19.0) | .38 | |
| Mortality rate | 9 (45) | 2 (4) | 22.5 (3.6–179.7) | .00004 |
| Strain type in patients who died | ||||
| Epidemic | 4 | |||
| Nonepidemic | 4 | |||
| Not available | 1 | |||
| Relationship of B. cepacia to mortalityb | ||||
| Causal | 5c | |||
| Contributory | 1 | |||
| Not related | 2 | |||
| Unknown | 1 | |||
NOTE. Data are no. (%) unless noted otherwise.
In 3 years prior to case acquisition of B. cepacia.
By criteria described by CDC National Nosocomial Infections Surveillance System [20].
3 of were epidemic strain.
All risk factors found to be statistically significant on univariate analysis were included in the logistic regression model. These were admission to hospital, severity of illness scale, and days hospitalized during the epidemic period and total days hospitalized in the epidemic period and in the 3 years prior. Patients with higher Shwachman-Kulczycki scores (i.e., less severe CF) were less likely to acquire B. cepacia (P = .0145; OR, 0.945), and those with prior hospital admission were more likely to acquire B. cepacia (P = .0288; OR, 5.101). Days in hospital at any time was not statistically significant. Analysis excluding cases and controls not previously hospitalized demonstrated a tendency toward patients with less severe lung disease being less likely to acquire B. cepacia, but this was not statistically significant. When the analysis was repeated excluding the severity of illness score, hospital admission remained a significant risk factor for acquisition (P = .0268; OR, 4.877).
Nine of 20 case-patients died compared with 2 of 57 controls. Of the 9 persons who died, 4 harbored the epidemic strain, 4 had a nonepidemic strain, and 1 had no specimen available. B. cepacia was considered the cause of death in 5, contributory in 1, unrelated in 2, and unknown in 1.
For 20 CF patients with the epidemic strain of B. cepacia, clinical isolates were available from 14. We defined cases as the 10 patients (of 14) who harbored the epidemic strain. We matched 27 controls for age and sex. Mean Shwachman-Kulczycki scores at the time of isolation were not significantly different in the 2 groups (66 for cases, 77 for controls). All 10 case-patients were admitted to the hospital during the epidemic period compared with 14 of 27 controls (P = .0067). Cases had significantly more days of hospitalization than controls in the 3 years prior to isolation (mean, 61.4 vs. 17.8 days; P = .0067) and during the epidemic period (mean, 38.4 vs. 15.9 days; P = .0048). There were no significant differences in days of prior intravenous antibiotic administration, aminoglycoside therapy, days of oxygen therapy, or number of aerosol treatments received during hospitalization. Four of 9 evaluable case-patients died compared with 2 of 24 controls. In 3 patients, B. cepacia was considered the cause of mortality but was unrelated to death in the fourth.
Discussion
The B. cepacia outbreak described clearly involved transmission of a single strain of B. cepacia between non-CF patients and CF patients, a phenomenon not previously described. Potential environmental or common source reservoirs could not be found, and there was no evidence that nebulizer therapy [27] or respiratory therapists contributed to this epidemic. However, although CF patients with B. cepacia were placed in respiratory isolation in separate rooms on wards shared by CF and non-CF patients, they were repeatedly seen to breach isolation protocol. Furthermore, non-CF patients with B. cepacia were not isolated from either group of patients. This strongly suggests that person-to-person transmission played a major role in this outbreak involving >200 patients. In addition, our analysis of this outbreak makes apparent for the first time that transmission from non-CF patients may account for the known risk of B. cepacia acquisition in CF that is associated with hospitalization [3].
Retrospective chart review confirmed that there were numerous instances where the potential for transmission of B. cepacia between the CF and non-CF patients existed. At least four occasions were documented between March 1989 and October 1992 when a non-CF patient with B. cepacia shared a ward with an uninfected CF patient, who subsequently had B. cepacia isolated from sputum. Of the 4 CF patients involved, 3 had their B. cepacia typed, and it was the epidemic strain; 2 of the 4 non-CF patients involved had their isolates typed and both were the epidemic strain. Likewise, there was evidence that transmission could have occurred in the opposite direction: On at least 7 occasions CF patients with B. cepacia shared wards with non-CF patients from whom B. cepacia was later recovered, 3 of these were in the PICU. Although isolates were available from only 1 of the CF patients and from 2 of the non-CF patients involved, all carried the epidemic strain.
CF patients with B. cepacia were placed in isolation to prevent cross-spread of the organism. Despite these measures, illicit social contact between patients was noted repeatedly. Once 3 adolescent CF patients were simultaneously hospitalized on the same ward: 1 harbored the epidemic strain of B. cepacia and was isolated in a separate room. Shortly after the hospitalization of the infected CF patient, the epidemic strain of B. cepacia was isolated from 1 of the previously uninfected CF patients. The third CF patient died not long after the admission; B. cepacia was isolated at autopsy. Social contact outside the health care setting has also been implicated in the spread of B. cepacia [9] between CF patients but was unlikely to have played a major role in this outbreak. CF patients at this institution did not attend common exercise classes or support groups, and none were known to see each other socially. There was a summer camp for CF patients, but those known to harbor B. cepacia were routinely excluded.
A neurologically devastated ventilator-dependent child who persistently harbored B. cepacia may have served as a reservoir of infection fueling the outbreak. He was admitted to the PICU in January 1987 and shared the unit with a CF child infected with B. cepacia (not available for typing) between November 1988 and April 1989. From August 1989 until December 1992, B. cepacia was isolated from his sputum. Ribotype analysis of sequential isolates banked from 1991 demonstrated persistence of the epidemic strain (data not shown). This child remained on mechanical ventilation in the PICU until discharge in March 1994. Clearance of B. cepacia from this child’s sputum may have contributed to the decline of the outbreak. This child and other non-CF patients could have served as a source for dissemination of the epidemic strain. If so, infection control policies directed at CF patients alone were doomed to failure.
CF case-control studies indicated that acquisition of B. cepacia was associated with hospitalization and with mortality. In the non-CF patients, acquisition of B. cepacia also appeared to be associated with mortality. Although by conventional epidemiologic means it may be impossible to identify the precise chain of events or mode of transmission that gave rise to this B. cepacia epidemic, the results of our molecular epidemiologic studies clearly demonstrate that non-CF patients were involved in a CF-associated epidemic. Thus, the study demonstrates for the first time that the same clone can be shared by both patient groups. While the study cannot exclude the possibility of a common source contributing to the outbreak, it would appear that person-to-person transmission within the hospital played a major role as documented in other cases solely involving CF patients [7, 9, 10, 15, 16, 18, 22].
Acquisition of B. cepacia by the non-CF patients was a temporary phenomenon except for the ventilator-dependent PICU patient mentioned. Loss of B. cepacia commonly followed ICU discharge. In contrast, B. cepacia infection persisted in the CF patients. We previously reported that CF patients typically remain persistently infected solely with their own unique strain of B. cepacia [8]. In this study, 2 interesting CF cases were identified who retained their unique B. cepacia strains (BC MS-104 and BCMS-304; figure 4) but transiently harbored the Mississippi epidemic strain. In both persons, the retained unique strain of B. cepacia carried the cblA pilin subunit gene [6, 7]. This gene encodes giant cable mucin-binding adhesin pili, which give rise to a 300-fold increase in binding capacity to CF airway epithelium (J. Yankaskas, P. Gilligan, and R. Goldstein, unpublished data). The Mississippi epidemic strain did not possess this cblA pilin subunit gene (A.H. and R.G., unpublished data), which may account for its failure to displace the resident cblA+ B. cepacia strains in the 2 patients described.
It is clear that different B. cepacia strains have extraordinarily different capacities for transmission among the CF population [7–9]. It is possible that the epidemic B. cepacia strain at this Mississippi hospital evolved in the ventilated non-CF lung into a highly transmissible organism with a predilection for the CF lung, which then spread into and among the CF community. The hypothesis that this epidemic strain recently evolved outside the CF lung is supported by the finding that it is prototrophic for amino acid biosynthesis (unpublished data), indicating that it has not yet fully adapted to the CF lung. In contrast, strains that are highly adapted to growth in the CF lung have typically become auxotrophic [28], dependent on the amino acid–rich milieu of the CF lung.
Our phylogenic analysis (figure 4) indicates that B. cepacia isolates are very closely related, displaying a panmictic population structure (i.e., freely recombining) [29–31]. It also reveals that the prototypic epidemic isolates from CF (e.g., BCMS-314) and non-CF (e.g., BCMS-226) patients in the described Mississippi outbreak are clonal derivatives, as evidenced by the significant branching pattern that clusters these strains as a distinct lineage (bootstrap value, 98%). Further, the scattered distribution of the different epidemic clones in the tree implies that genetic changes that confer high transmissibility occur at random and that the transformation to an epidemic strain does not require relatively long periods of evolution. Thus, it appears that epidemic strains are emerging randomly, independently, and rapidly, and that no single reservoir exists (figure 5). The Mississippi hospital outbreak suggests that the human lung may act as one such reservoir, providing an environment in which B. cepacia can evolve into a highly transmissible microbe. The capacity to emerge rapidly as a highly transmissible pathogen is particularly worrisome since B. cepacia is intensively being developed as a biologic control agent for widespread use in agriculture based on its remarkable capacity to repress soil-borne pathogens while also degrading herbicides and pesticides [15, 32]. Anticipated comprehensive commercial application of this agricultural practice may therefore pose a significant threat to human health [32].
A capacity for pulsed evolution, involving a relatively small number of genetic changes rendering a microbe highly adapted for pulmonary habitation, might be predicted for this unusual organism. Rather than having its genes organized within a single chromosome, B. cepacia is unusual in that isolates contain one to four chromosomes, with genes divided among these independent, rrn-encoding replicons [33, 34] (unpublished data). Such a division of genomic content gives the organism a heightened recombinogenic capacity, allowing it to adapt rapidly to radical changes in environmental growth conditions [35], such as from soil to the CF lung. We have observed such pulsed evolutionary changes at the molecular genetic level by characterizing serial isolate sets from infected CF patients (unpublished data). Involved large chromosomal rearrangements and deletions leaving B. cepacia adapted to the CF lung typically also render the microbe incapable of infecting the plant host from which its Latin name was derived, i.e., cepacia (L., “of an onion”).
CDC guidelines regarding infection control in hospitals [36] state that for CF patients with B. cepacia the “cohorting or placement in the same room with a CF patient who is not infected or colonized with B. cepacia” should be avoided. Although the guidelines also state that patients harboring multiresistant organisms require isolation, the risk of B. cepacia transmission to or from non-CF patients is not specifically addressed. Given the poor prognosis associated with the acquisition of this microbe in CF [2–5] and the results of this investigation, health care workers involved in infection control and those involved in the care of CF patients should recognize the potential danger of B. cepacia transmission and consider applying these guidelines both to patients with and without CF.
Acknowledgments
Grant support: Cystic Fibrosis Foundation (to R.G.); NIH (DK-50838 [to R.G.]); Trustees of Health and Hospitals of the City of Boston biomedical research support grant (to R.G. and A.H.); Glaxo Fellowship in Infectious Diseases (to A.H.); National Science Foundation (DEB 9458247 [to M.R.]); and Mississippi State Medical Association Auxiliary (to R.T.).
We acknowledge R. Stern, P. Gilligan, D. Shapiro, J. LiPuma, and T. Stull for providing bacterial isolates; R. Beall, A. Chobanian, P. Rice, J. Govan, and H. Corwin for encouragement to initiate these studies; M. Andrew for biostatistics advice; S. Miller for clinical information; and D. Mislan from CSPI/Scanalytics for help with software. Resultant insight is dedicated to the memory of the microbial geneticist Bernard Davis.
Footnotes
Presented in part: fifth annual meeting of the Society of Healthcare Epidemiology of America, San Diego, April 1995; North American Cystic Fibrosis Conference, Dallas, October 1995.
References
- 1.Burkholder W. Sour skin, a bacterial rot of onion bulbs. Phytopathology. 1950;40:115–8. [Google Scholar]
- 2.Tablan O, Chorba T, Schidlow D, et al. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J Pediatr. 1985;107:382–7. doi: 10.1016/s0022-3476(85)80511-4. [DOI] [PubMed] [Google Scholar]
- 3.Isles A, Maclusky I, Corey M, Gold R, Prober C, Fleming P, Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104:206–10. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 4.Corey M, Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970–1989. Am J Epidemiol. 1996;143:1007–17. doi: 10.1093/oxfordjournals.aje.a008664. [DOI] [PubMed] [Google Scholar]
- 5.Hearst J, Elliott K. Identifying the killer in cystic fibrosis. Nat Med. 1995;1:626–7. doi: 10.1038/nm0795-626. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein R, Sun L, Jiang RZ, Sajjan U, Forstner J, Campanelli C. Structurally variant classes of pilus appendage fibers coexpressed from Burkholderia (Pseudomonas) cepacia. J Bacteriol. 1995;177:1039–52. doi: 10.1128/jb.177.4.1039-1052.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Jiang RZ, Steinbach S, et al. The emergence of a highly transmissible lineage of cbl+Pseudomonas (Burkholderia) cepacia causing CF centre epidemics in North America and Britain. Nat Med. 1995;1:661–6. doi: 10.1038/nm0795-661. [DOI] [PubMed] [Google Scholar]
- 8.Steinbach S, Sun L, Jiang R, et al. Transmissibility of Pseudomonas cepacia infections in clinic patients and lung-transplant recipients with cystic fibrosis. N Engl J Med. 1994;331:981–7. doi: 10.1056/NEJM199410133311504. [DOI] [PubMed] [Google Scholar]
- 9.Govan J, Brown P, Maddison J, et al. Evidence for transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet. 1993;342:15–9. doi: 10.1016/0140-6736(93)91881-l. [DOI] [PubMed] [Google Scholar]
- 10.LiPuma J, Dasen S, Nielson D, Stern R, Stull T. Person-to-person transmission of P. cepacia between patients with cystic fibrosis. Lancet. 1990;336:1094–6. doi: 10.1016/0140-6736(90)92571-x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson DJ, Kuhns JS, Vasil ML, Gerding DN, Janoff EN. DNA finger-printing by pulsed field gel electrophoresis and ribotyping to distinguish Pseudomonas cepacia isolates from a nosocomial outbreak. J Clin Microbiol. 1991;29:648–9. doi: 10.1128/jcm.29.3.648-649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pegues D, Carson L, Anderson R, et al. Outbreak of Pseudomonas cepacia in oncology patients. Clin Infect Dis. 1993;16:407–14. doi: 10.1093/clind/16.3.407. [DOI] [PubMed] [Google Scholar]
- 13.Goldmann D, Klinger J. Pseudomonas cepacia: biology, mechanisms of virulence, epidemiology. J Pediatr. 1986;108:806–12. doi: 10.1016/s0022-3476(86)80749-1. [DOI] [PubMed] [Google Scholar]
- 14.Carson L, Table O, Cassock L, Jars W, Favor M, Bland L. Comparative evaluation of selective media for isolation of Pseudomonas cepacia from cystic fibrosis patients and environmental sources. J Clin Microbiol. 1988;26:2096–100. doi: 10.1128/jcm.26.10.2096-2100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Govan J, Hughes J, Vandamme P. Burkholderia cepacia: medical, taxonomic and ecological issues. J Med Microbiol. 1996;45:395–407. doi: 10.1099/00222615-45-6-395. [DOI] [PubMed] [Google Scholar]
- 16.Nelson J, Doherty C, Brown P, Greening A, Kauffman M, Govan J. Pseudomonas cepacia in inpatients with cystic fibrosis. Lancet. 1991;338:1525. doi: 10.1016/0140-6736(91)92342-y. [DOI] [PubMed] [Google Scholar]
- 17.Burdge D, Nakielna E, Noble M. Case-control and vector studies of nosocomial acquisition of Pseudomonas cepacia in adult patients with cystic fibrosis. Infect Control Hosp Epidemiol. 1993;14:127–30. doi: 10.1086/646697. [DOI] [PubMed] [Google Scholar]
- 18.Rabkin C, Jarvis W, Anderson R, Stull T, Woods D. P. cepacia typing systems: collaborative study to assess their potential in epidemiologic investigations. Rev Infect Dis. 1989;11:600–7. doi: 10.1093/clinids/11.4.600. [DOI] [PubMed] [Google Scholar]
- 19.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–91. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 20.National nosocomial infections surveillance system manual, USA. Atlanta: Centers for Disease Control; 1988. [Google Scholar]
- 21.Shwachman H, Kulczycki L. Long-term study of one hundred five patients with cystic fibrosis. Am J Dis Children. 1958;96:8–15. doi: 10.1001/archpedi.1958.02060060008002. [DOI] [PubMed] [Google Scholar]
- 22.LiPuma J, Marks-Austin K, Holsclaw D, Winnie G, Gilligan P, Stull T. Inapparent transmission of Pseudomonas (Burkholderia) cepacia among patients with cystic fibrosis. Pediatr Infect Dis J. 1994;13:716–9. doi: 10.1097/00006454-199408000-00007. [DOI] [PubMed] [Google Scholar]
- 23.LiPuma J, Mortensen J, Dasen S, Stull T. Ribotype analysis of P. cepacia from cystic fibrosis treatment centers. J Pediatr. 1988;113:859–62. doi: 10.1016/s0022-3476(88)80018-0. [DOI] [PubMed] [Google Scholar]
- 24.Mahenthiralingam E, Campbell M, Henry D, Speert D. Typing of Pseudomonas aeruginosa and Burkholderia cepacia isolates from cystic fibrosis patients using random amplified polymorphic DNA [abstract 257] Pediatr Pulmonol Suppl. 1994;18(Suppl 10):250. [Google Scholar]
- 25.Mahenthiralingum E, Cambell M, Henry D, Speert D. Distribution of Burkholderia cepacia RAPD types. Slide no. 19. Programs and abstracts: eighth annual North American Cystic Fibrosis Conference (Dallas); Bethesda, MD: Cystic Fibrosis Foundation; 1994. [Google Scholar]
- 26.Johnson W, Tyler S, Rozee K. Linkage analysis of geographic and clinical clusters in Pseudomonas cepacia infections by multilocus enzyme electrophoresis and ribotyping. J Clin Microbiol. 1994;32:924–30. doi: 10.1128/jcm.32.4.924-930.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamill R, Houston E, Georghiou P, et al. An outbreak of Burkholderia (formerly Pseudomonas) cepacia respiratory tract colonization and infection associated with nebulized albuterol therapy. Ann Intern Med. 1995;122:762–6. doi: 10.7326/0003-4819-122-10-199505150-00005. [DOI] [PubMed] [Google Scholar]
- 28.Barth A, Pitt T. Auxotrophy of Burkholderi (Pseudomonas) cepacia from cystic fibrosis patients. J Clin Microbiol. 1995;33:2192–4. doi: 10.1128/jcm.33.8.2192-2194.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strickberger MW. Genetics. New York: Macmillan; 1968. [Google Scholar]
- 30.Smith JM, Smith NH, O’Rourke M, Spratt BG. How clonal are bacteria? Proc Natl Acad Sci USA. 1993;90:4384–8. doi: 10.1073/pnas.90.10.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wise M, Shimkets L, McArthur J. Genetic structure of a lotic population of Burkholderia (Pseudomonas) cepacia. Appl Environ Microbiol. 1995;61:1791–8. doi: 10.1128/aem.61.5.1791-1798.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes A, Govan J, Goldstein R. Agricultural use of Burkholderia (Pseudomonas) cepacia: a threat to human health? Emerg Infect Dis. 1998;4:221–7. doi: 10.3201/eid0402.980209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng H, Lessie T. Multiple replicons constituting the genome of Pseudomonas cepacia 17616. J Bacteriol. 1994;176:4034–2. doi: 10.1128/jb.176.13.4034-4042.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodley P, Romling U, Tummler B. A physical map of the Burkholderia cepacia type strain. Mol Microbiol. 1995;17:57–67. doi: 10.1111/j.1365-2958.1995.mmi_17010057.x. [DOI] [PubMed] [Google Scholar]
- 35.Holloway B. Genetics for all bacteria. Annu Rev Microbiol. 1993;47:659–84. doi: 10.1146/annurev.mi.47.100193.003303. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Draft guideline for isolation precautions in hospitals; notice. Fed Register. 1994;59:55564–8. [Google Scholar]