Abstract
Angiogenesis and vascular regression are critical for the female ovulatory cycle. They enable progression and regression of follicular development, and corpora lutea formation and regression. Angiogenesis in the ovary occurs under the control of the vascular endothelial growth factor-A (VEGFA) family of proteins, which are generated as both pro-(VEGF165) and anti(VEGF165b)-angiogenic isoforms by alternative splicing. To determine the role of the VEGF165b isoforms in the ovulatory cycle, we measured VEGF165b expression in marmoset ovaries by immunohistochemistry and ELISA, and used transgenic mice over-expressing VEGF165b in the ovary. VEGF165b was expressed in the marmoset ovaries in granulosa cells and theca, and the balance of VEGF165b:VEGF165 was regulated during luteogenesis. Mice over-expressing VEGF165b in the ovary were less fertile than wild-type littermates, had reduced secondary and tertiary follicles after mating, increased atretic follicles, fewer corpora lutea and generated fewer embryos in the oviduct after mating, and these were more likely not to retain the corona radiata. These results indicate that the balance of VEGFA isoforms controls follicle progression and luteogenesis, and that control of isoform expression may regulate fertility in mammals, including in primates.
Introduction
Formation of new blood vessels (angiogenesis) has a critical role in the female reproductive system, by affecting, for example, the cyclic changes that occur in the ovary during the ovulatory cycle (Charnock-Jones et al. 1993), ovarian follicular development (Yan et al. 1993), corpus luteum (CL) and endometrium formation (Kamat et al. 1995), and remodelling of mammary gland to enable lactation (Pepper et al. 2000). Specifically, studies in which the principal angiogenic factor, vascular endothelial growth factor-A (VEGFA), was inhibited in vivo in the mouse, marmoset or macaque established that angiogenesis is required throughout follicular development, as ovarian follicles progress during the cycle (Zimmermann et al. 2001, 2002, 2003, Wulff et al. 2002), after ovulation for the formation of the CL (Wulff et al. 2001, 2002, Zimmermann et al. 2001, 2002) and for normal endometrial development (Fan et al. 2008, Fraser et al. 2008).
VEGFA expression has been localised within the different ovarian and endometrial compartments using immunohistochemistry, PCR and in situ hybridisation (Charnock-Jones et al. 1993, Shweiki et al. 1993). However, VEGF is alternatively spliced to generate many isoforms that differ in the heparin-binding activity and receptor activation potential. Alternative splicing of exons 6 and 7 generates different-length isoforms (e.g. VEGF121, VEGF165, VEGF189) and alternative splice site selection in exon 8 generates two families of isoforms, the pro-angiogenic VEGFxxx family (where xxx is the number of amino acids in the peptide monomer, e.g. VEGF165) and the anti-angiogenic VEGFxxxb family (e.g. VEGF121b, VEGF165b; Harper & Bates 2008). The two families differ in the choice of splice site in the terminal exon, exon 8, resulting in differing C termini, and differing activities (Bates et al. 2002). Many commercial antibodies and probes do not distinguish between the VEGFxxxb and VEGFxxx families, and even fewer between the isoforms within each family. It has previously been shown that VEGF165b, the most widely studied form of the VEGFxxxb family, inhibits VEGF165-mediated angiogenesis in the rabbit cornea, chick chorioallantoic membrane, rat mesentery and mouse dorsal skin chamber (Cebe Suarez et al. 2006), and during physiological angiogenesis in the mammary fat pad (Qiu et al. 2008). It also inhibits pathological angiogenesis in the retina due to ischaemia (Magnussen et al. 2010) or inflammation (Hua et al. 2010), and in cancer (Rennel et al. 2008a, 2008b). Switching the splice site from proximal, VEGF165 encoding, to distal, VEGF165b encoding, results in reduced angiogenesis in cancer (Varey et al. 2008) and the retina (Nowak et al. 2010). Analysis of VEGF165b expression in multiple human tissues has shown that the levels of VEGF165b vary substantially from <15% of total VEGF in placenta (Bates et al. 2006), to >95% in normal human colon (Varey et al. 2008), and that this ratio varies greatly between tissues (Woolard et al. 2009) in physiological and pathological conditions (Schumacher et al. 2007), including those where pathological angiogenesis is occurring (Pritchard-Jones et al. 2007).
As VEGFA has a critical role in ovarian angiogenesis, we investigated the expression pattern of VEGFxxxb in the ovary of the marmoset monkey, a species in which the contribution of VEGF to ovarian function has been comprehensively studied (Fraser & Duncan 2009), and examined whether expression levels are altered during the ovulatory cycle. To investigate the effects of manipulation of VEGF165b in the ovary, we used a transgenic (TG) mouse that over-expresses VEGF165b. We show that VEGFxxxb is expressed in primate ovaries, is downregulated during CL formation, and that over-expression of VEGF165b in mice reduces fertility by inhibiting follicular development and CL formation.
Results
VEGF165b is expressed in primate ovaries
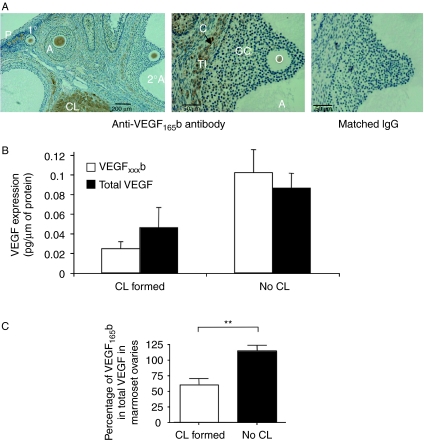
Sections of marmoset ovaries were stained for VEGFxxxb using an antibody raised against the nine amino acid C terminus of human VEGF165b (Woolard et al. 2004; Fig. 1A). Immunohistochemical analysis showed clear VEGF165b expression in the theca layer of follicles and in the granulosa cell layer of primary and secondary follicles (Fig. 1A), while it was absent from the granulosa cells of tertiary follicles. Localisation was also present in the CL.
Figure 1.
Expression of VEGF165b in primate ovary. (A) Endogenous expression of VEGF165b in marmoset ovary using an anti-VEGF165b specific antibody. TI, theca interna; GC, granulosa cells; C, capillary; O, oocyte; CL, Corpus luteum; 2°A, secondary antral follicle; 1°, primary; A, antral; P, primordial. (B) ELISA for VEGFxxxb and total VEGF on protein extracted from marmoset ovary. (C) Percentage of VEGF that is VEGFxxxb in marmoset ovary. **P<0.01 t-test with Welch's correction. n=7 total.
Protein was extracted from whole ovaries and total VEGF and VEGFxxxb levels were measured using either an ELISA for total VEGF or one specific for the VEGFxxxb isoforms (Fig. 1B and C). Protein quantification showed that in ovaries that contained no corpora lutea, the measured VEGFxxxb levels were similar to the total VEGF levels (Fig. 1B), suggesting that the majority of VEGF in these quiescent ovaries is VEGF165b (not different from 100%). In contrast, in ovaries in which corpora lutea were found there was less VEGF165b than total VEGF, and the percentage of total VEGF attributable to VEGF165b was significantly smaller, 65±11% (P<0.05 compared to no CL, Fig. 1C, n=7).
VEGF165b over-expression in the ovary reduces fertility in TG mice
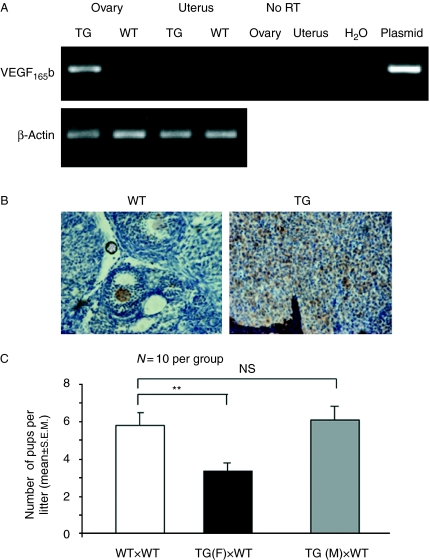
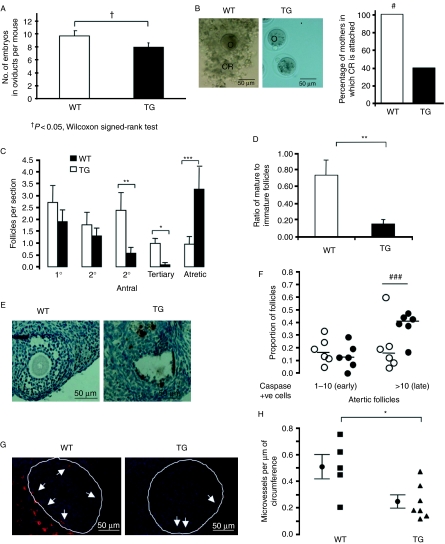
We generated TG mice over-expressing human VEGF165b under control of the mouse mammary tumour virus (MMTV) promoter (Qiu et al. 2008). These mice showed strong expression of VEGF165b mRNA in the ovary of virgin animals, and weak expression in the uterus (Fig. 2A). Immunohistochemical analysis of mouse ovaries using a human specific anti-VEGF antibody showed strong expression of VEGF in the TG ovaries, but not in the wild-type (WT) ovaries (Fig. 2B). When TG females were mated with WT mice, the numbers of pups born to these mothers was significantly lower per litter (3.3±0.4 compared with 5.9±0.7 in the WT littermates). In contrast, WT females mated with TG males produced normal-sized litters (6.1±0.7 pups per litter, Fig. 2C). To help determine whether the reduction in pup number was due to reduced ovulatory function or due to developmental defects, we determined pups birth weight and their genotype. Pups were born at a Mendelian frequency (50% of offspring were TG), and with normal birthweight (Supplementary Figure 1, see section on supplementary data given at the end of this article) indicating no offspring-dependent developmental defect. To determine whether ovulation was normal, we opened the oviducts of 13 TG and 9 WT females 0.5 days post coitus (dpc) and collected the embryos. The TG mothers had 82±6% of the number of embryos found in the oviduct of the WT mothers (P<0.05; Fig. 3A). Moreover, strikingly, the embryos in the oviducts from the TG lacked adherent corona radiata in 60% of the mothers (Fig. 3B). Embryos were cultured for 4 days, and survival was measured by determining the rate of progression to blastocyst stage. There was no difference in the percentage of embryos reaching blastocyst stage when comparing embryos from TG and WT mothers (Supplementary Figure 2, see section on supplementary data given at the end of this article). This suggested a defect in folliculogenesis rather than developmental defects. We, therefore, collected ovaries from six TG and six WT female mice at 0.5 dpc and sectioned through the ovaries to determine follicle number. There was no statistical difference in the total number of follicular elements (follicles and corpora lutea) between WT (12±2.6 per section) and TG (8.6±1.3 per section, P>0.1 t-test) mice sampled per section. However, as shown in Fig. 3C, there was a significant reduction in secondary, tertiary and mature follicle numbers in the TG compared with the WT mice. There was no statistical difference in primordial or primary follicles per section between WT and TG animals. Furthermore, there was a significant reduction in the percentage of tertiary to secondary follicles (Fig. 3D), suggesting not only a reduction in follicular development, but also a further defect in follicular maturation to the tertiary stage. Interestingly, there were more atretic follicles in the TG mice than those in the WT. The mechanisms for increased atresia were investigated by staining for apoptotic cells in the ovaries (Fig. 3E). Activated caspase-3-positive cells have been used as an indicator of early and late atresia (Utsunomiya et al. 2008). Atretic follicles with fewer than 10 cleaved caspase-3-positive cells have previously been regarded as early atresia and those with more than 10 as late atresia. There was no increase in the proportion of follicles in early atresia in TG mice (11%) compared with WT (13%), but a statistically significant increase in the proportion of late atretic follicles in TG (40%, n=6 mice; P<0.01, Fisher's exact test) compared with WT (24%, n=6, Fig. 3F). To determine whether this reduction was associated with reduced vascular density around follicles, we stained the ovaries with isolectin B4, and determined the number of vessels covering the area (Fig. 3G). There was a significant reduction in blood vessels surrounding follicles from TG mothers (Fig. 3H, P<0.05).
Figure 2.
Over-expression of VEGF165b in mouse ovary reduces litter size. (A) Mouse ovaries from wild-type or transgenic MMTV-VEGF165b mice as shown were taken and RNA was extracted. RT-PCR was performed using primers specific for the human transgene. (B) Mouse tissues were fixed and stained using an anti-human VEGF antibody that detects all human isoforms but does not detect mouse VEGF. (C) Measurement of litter size in 10 transgenic (TG) or wild-type (WT) females mated to wild-type males. **P<0.01 paired t-test, n=10 compared with littermate control. Wild-type females were also mated to TG males. NS, not significant compared with wild-type (unpaired t-test).
Figure 3.
Changes in the embryos of the TG female mice over-expressing VEGF165b. (A) Mice were culled at 0.5 dpc and embryos were collected from the oviducts. VEGF165b over-expression led to a reduced number of embryos in the oviducts. †P<0.05, Wilcoxon signed-rank test. (B) Images of embryos showing that those from TG females lack the corona radiata (CR) structure. #P<0.05 Fisher's exact test. (C) Ovaries from these mice were fixed, sectioned through and stained with H&E to count follicles. P<0.001 one-way ANOVA, *P<0.05, **P<0.01, ***P<0.001 Bonferroni post-hoc test. n=6 per group. (D) The number of tertiary follicles as a percentage of secondary follicles was determined. **P<0.01 Mann–Whitney t-test. (E) Ovaries were stained with cleaved caspase-3 antibody and the number of positive (apoptotic cells) was determined. (F) The proportion of follicles with between 1 and 10 and >10 positive cells were compared (###P<0.001, Fisher's exact test on follicle frequency, n=6 animals per group, 274 follicles WT, 234 TG) (G). Ovaries taken at 0.5 dpc were fixed and stained using isolectin B4 to detect endothelial cells, and counterstained with Hoechst. Images of follicles from WT and TG female ovaries stained with IB4 to show MVD. (H) Microvessels were counted and expressed as microvessel covering area per unit perimeter of the follicle. *P<0.05, Mann–Whitney t-test.
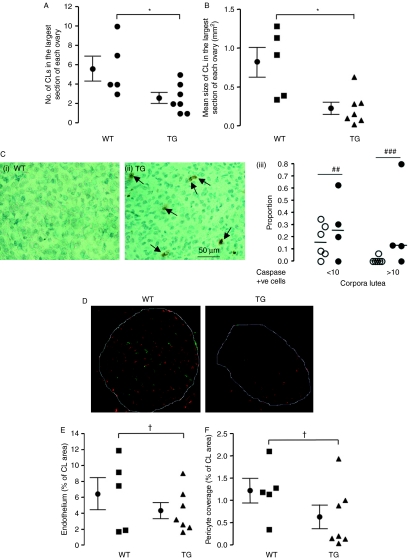
Angiogenesis is also required for corpora lutea formation. As VEGF165b levels are reduced in marmoset ovaries containing CL, it is possible that a downregulation occurs, to allow the intense angiogenesis that takes place in this organ. We, therefore, measured the size, number and vascularity of the CL in the largest cross section of ovaries from seven TG and five WT mice, to determine whether VEGF165b over-expression inhibits CL formation. The number of CL per ovary was reduced in TG animals, consistent with reduced follicular development (Fig. 4A). In addition, the size of the CL was also reduced (Fig. 4B) from 0.82±0.19 mm2 in the WT to 0.23±0.08 mm2 in the TG (P<0.01, t-test). The proportion of CL with both between 1 and 10 cleaved caspase-3-positive cells (early CL regression) was also increased in TG (31% compared with 18% in WT) and with more than 10 cleaved caspase-3-positive cells (26% compared with 1%, Fig. 4C). These results indicate that regression of the CL is more rapid in the TG mice. Figure 4D shows microvascular (red) and pericyte (green) staining in the CL. Both the microvascular density (MVD; Fig. 4E) and the mean pericyte coverage (Fig. 4F) were reduced in the TG mice (both P values <0.05, t-test with Welch's correction).
Figure 4.
Over-expression of VEGF165b in the mouse ovary results in changes in the corpus luteum (CL). Ovaries collected from mice at 0.5 dpc were fixed and stained by H&E to investigate CL structure. (A) The number of CL in the largest cross section of each ovary was counted. *P<0.05, t-test. (B) The total size of the CL in the largest cross section of each ovary was measured with ImageJ. *P<0.05, t-test. (C) Ovaries stained for cleaved caspase-3 were analysed for corpus luteum staining in WT (i) and TG (ii). Numbers of CL were counted (iii) according to the proportion with 1–10 (early regression) and >10 cleaved caspase-3-positive cells (late regression; ##P<0.01, ###P<0.001, Fisher's exact test on CL frequency). n=6 mice per group, 90 CL in WT, 45 in TG). (D) Ovaries collected from mice at 0.5 dpc were fixed and stained for microvessels using isolectin B4 (red) and pericytes with anti-NG2 antibody (green). (E) Blood vessel density was counted and expressed as microvascular density (microvessel covering area per mm2 of CL). (F) Pericytes covering area per unit area of CL were counted.,†P<0.05, t-test with Welch's correction.
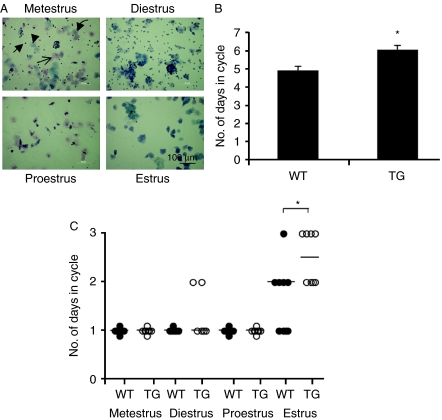
To determine whether the reduced follicular development resulted in, or was associated with, altered ovarian cyclicity, we staged mice by daily vaginal smearing (Fig. 5A). Figure 5B shows that the TG mice had a 23% increase in ovulatory cycle length (6.0±0.25 days, n=6) compared with WT littermates (4.85±0.26 days, n=7, P=0.022, Mann–Whitney U-test). Closer examination showed that the increase in the length of the estrous cycle was due to extension of estrus (Fig. 5C) from 1.6±0.24 to 2.5±0.19 days (P<0.05, Mann–Whitney U-test).
Figure 5.
VEGF165b over-expression alters estrous cycling. Estrus cycle was determined in MMTV-VEGF165b transgenic mice, expressing VEGF165b in the ovary, and littermate sisters, by composition of cells in vaginal smears. Arrow, neutrophils; arrow head, parabasal cells; curved arrow, intermediate cells and *cornified superficial cells. (B) The TG mice had a significantly longer cycle time than the littermate controls. (C) Determination of the lengths of the cycles of each mice showed that estrus was longer by a day in the TG compared with WT mice. *P<0.05, Mann–Whitney U-test.
Discussion
Follicular development in the ovary has been shown to be dependent on angiogenesis, in particular on blood vessel growth driven by the angiogenic isoforms of VEGF. VEGF165 is upregulated in the follicle during development (Ravindranath et al. 1992, Shweiki et al. 1993, Taylor & Mueller 2004) and inhibition of VEGF by administration of VEGF-TRAP (Wulff et al. 2002), antibodies to VEGF (Zimmermann et al. 2001) and VEGFR tyrosine kinase inhibitors (McFee et al. 2009) results in inhibition of follicular development. However, all these inhibitors target both the pro-angiogenic and the anti-angiogenic isoforms of VEGF (Varey et al. 2008). VEGF165b has been shown to be anti-angiogenic in both pathological circumstances, such as ischemic retinopathy (Konopatskaya et al. 2006), models of age-related macular degeneration (Hua et al. 2010), prostate (Rennel et al. 2008a), lung (Merdzhanova et al. 2010), renal (Rennel et al. 2008a), skin (Pritchard-Jones et al. 2007) and colon (Varey et al. 2008) cancers, and in physiological angiogenesis including gonadogenesis (Artac et al. 2009) and mammary gland formation (Qiu et al. 2008). The results shown here indicate that the regulation of follicular development and hence fertility are under control of differential splicing of VEGF. We show that VEGF165b is localised within different cell types in the marmoset ovary and levels decrease in ovaries containing corpora lutea. Crucially, the over-expression of VEGF165b in the mouse ovary, driven by the MMTV promoter, demonstrated a functional role for this isoform, causing delayed follicular and corpus luteal development that contributed to a fertility defect in these mice. The MMTV promoter drives expression in tissues in response to glucocorticoid hormones, and specifically progesterone (Otten et al. 1988). It is therefore expressed in a variety of tissues during pregnancy and the ovarian cycle, but the strongest expression is in the mammary gland and the ovary in healthy mice (Wang & Greenwald 1993, Wagner et al. 2001).
From the results described above it appears that VEGF-mediated angiogenesis, inhibited by VEGF165b expression, is important for multiple components of the ovulatory cycle. However, we did not discern an alteration in the total number of follicles in these mice, indicating that any effect of VEGF165b over-expression on oogenesis was not detectable. It is the maturation of the follicle from primary to secondary and to mature follicles that was inhibited by the anti-angiogenic VEGF165b isoform over-expression, as it is by VEGF inhibition (Zimmermann et al. 2003). Importantly, we show here that the quality of the progression is also inhibited, as the relationships between the cumulus cells and the ovum appear to be disrupted, resulting in release of an ovum lacking the cumulus cell–oocyte complex. The percent reduction in embryos in the oviduct was not as great as the reduction in pup number, suggesting that there was a reduction in the ability of the embryos to implant in the uterus, in addition to the reduction in embryos released. However, culture of the embryos did not show any evidence of developmental defects in the embryos themselves, which together with the normal birth weight indicate that the reduction in litter size is dependent on ovarian function.
The increase in the length of the estrous cycle, due to an increase in the length of estrus, could be a result of, or lead to, the delay in follicular development and defective CL formation as has been shown when VEGFA is inhibited (Fraser & Duncan 2009). Normally, the breakdown of the basement membrane in the mature follicle results from intense invasive angiogenesis across the theca interna that is stimulated by an increase in VEGF165 expression by the granulosa cells (Shweiki et al. 1993). The over-expression of VEGF165b in the granulosa cells driven by the MMTV promoter appears to be able to delay this vessel growth with resulting reduction in the size of the CL. This is likely to affect steroid production by the CL (Fraser et al. 2000), but the impact of VEGF165b on progesterone secretion has yet to be determined.
Generation of the different variants of VEGF results from a process of alternative splicing that is tightly regulated in normal conditions. Splice site selection is normally under the control of a co-ordinated and regulated set of splice factors, including SRSF6 (SRp55; Nowak et al. 2008), ASF/SF2 (Nowak et al. 2010) and SC35 (Merdzhanova et al. 2010), which themselves are regulated by kinases such as SRPK1 (Nowak et al. 2010). Very little is known about the regulation of splice factors in the ovary, but the results of the present study add to findings of other mRNAs that are alternatively spliced in the ovary (e.g. LH receptor, Mandai et al. 1997; FGFR2, Parrott & Skinner 1998; FSHR, Kraaij et al. 1998 and oestrogen receptor, Chu et al. 2000) to suggest that regulation of ovarian function may be controlled not only by regulation of gene transcription, but also by regulation of alternative splicing.
In conclusion, we show that alternative splicing of VEGF occurs in the primate ovary, and can regulate ovarian function and fertility in mice. These results raise the intriguing possibility that, in humans, the balance of pro- vs anti-angiogenic isoforms may regulate follicular development, menstrual cycle length, and ultimately, fertility.
Materials and Methods
Animal maintenance: mice
The TG MMTV-VEGF165b (TG) line was generated on C57BL6×CBA/CA background and back-crossed with C57BL6 mice as described (Qiu et al. 2008). All experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act, 1986, and associated guidelines and with approval of the University of Bristol Ethical Review Panel. These animals have three copies of the MMTV-VEGF165b construct (Qiu et al. 2008). For ovary experiments, 2- to 4-month-old F5–7 generation female TG mice and WT littermate controls were used. For experiments in the oviduct and ovary, TG or WT females were mated with WT male mice. Once plugged, female mice were culled and oviducts and ovary were dissected.
Marmoset tissue
Immunohistochemical localisation of VEGF165b was carried out on ovaries obtained from normal adult marmoset monkeys (Callithrix jacchus), which had been fixed in neutral buffered formalin and stored in paraffin blocks, having been generated from experiments described previously (Duncan et al. 2008). Two pairs of ovaries from the mid-follicular and mid-luteal phase of the ovulatory cycle were used. For measurement of levels of VEGF and VEGFxxxb within the marmoset ovary, ovaries were obtained from adult marmosets being killed as a result of health problems not related to the reproductive system. Four marmosets were sedated and killed as described previously (Wulff et al. 2002). Their ovaries were removed, weighed and rapidly frozen in dry ice before being stored at −70 °C until required. Four of the ovaries contained follicles, while four consisted predominantly luteal tissue.
RT-PCR
Briefly, total RNA was isolated with TRIzol (Invitrogen) extraction and DNase I (Invitrogen) was digested as per the manufacturer's instructions to prevent gDNA contamination. DNase-treated RNA (1 μg) was reverse transcribed into cDNA with avian myeloblastosis virus (AMV) reverse transcriptase using method described by the manufacturer (Promega). Both cDNA and RNA treated with DNase I were subjected to PCR with forward primer 5′-ACA AGA TCC GCA GAC GTG TA-3′ and reverse primer 5′-ACA GAT GGC TGG CAA CTA GA-3′ for transgene detection. PCR amplification was initiated at 94 °C for 4 min, 35 cycles of 94 °C for 30 s, 50 °C for 30 s and 72 °C for 30 s, followed by final extension at 72 °C for 10 min. A band at 199 bp indicated VEGF165b transgene expression.
For mouse β-actin control, forward primer 5′-AGC CAT GTA CGT AGC CAT CC-3′ and reverse primer 5′-CTC TCA GCT GTG GTG GTG AA-3′ give a 228 bp PCR product, after amplification under the same cycle conditions as for transgene.
ELISA of VEGFxxxb
Tissue protein lysate was prepared from seven marmoset ovaries in RIPA buffer. Protein concentration was determined by Bio-Rad assay (Bio-Rad) and the amount of VEGF165b was determined by ELISA as previously described (Varey et al. 2008) with a specific detection antibody against VEGFxxxb isoforms with the operator masked to luteal content.
Briefly, 0.08 μg goat anti-VEGF polyclonal IgG (AF293-NA; R&D Systems, Abingdon, UK) diluted in 1×PBS (pH 7.4) was adsorbed onto each well of a 96-well plate (Immulon 2HB; Thermo Life Sciences, Basingstoke, UK) overnight at room temperature. The plate was washed three times between each step with 1×PBS–Tween (0.05%). After blocking with 100 μl of 5% BSA in PBS for 1 h at 37 °C, 100 μl recombinant human VEGF165b (R&D Systems) diluted in 1% BSA in PBS (ranging from 62.5 pg/ml to 4 ng/ml) or protein samples was added to each well. After incubation for 1 h at 37 °C with shaking and three washes, 100 μl mouse monoclonal anti-VEGFxxxb biotinylated IgG (clone 264610/1; R&D Systems) at 0.4 μg/ml was added to each well, and the plate was left for 1 h at 37 °C with shaking. Streptavidin–HRP (100 μl; R&D Systems) at 1:200 dilution in 1% BSA in PBS was added, the plate was left at room temperature for 20 min and 100 μl/well O-phenylenediamine dihydrochloride solution (substrate reagent pack DY-999; R&D Systems) was added, protected from light and incubated for 20 min at room temperature. The reaction was stopped with 50 μl/well 1 M H2SO4, and absorbance was read immediately in the Opsys MR 96 well plate reader (Dynex Technologies, Chantilly, VA, USA) at 492 nm, with control reading at 460 nm.
ELISA of pan-VEGF
Pan-VEGF detection was followed with the manufacturer's suggestion (Duoset human VEGF from R&D, cat. no. DY293). Capture antibody (0.08 μg) diluted in 1×PBS (pH 7.4) was adsorbed onto each well of the 96-well plate (Immulon 2HB; Thermo Life Sciences) overnight at room temperature. The plate was washed three times between each step with 1×PBS–Tween (0.05%). After blocking with 100 μl of 1% BSA in PBS for 1 h at 37 °C, 100 μl recombinant human VEGF165 diluted in 1% BSA in PBS (ranging from 62.5 pg/ml to 4 ng/ml) or protein samples was added to each well. After incubation for 1 h at 37 °C with shaking and three washes, 100 μl detection antibody at 0.05 μg/ml was added to each well, and the plate was left for 1 h at 37 °C with shaking. Streptavidin–HRP (100 μl; R&D Systems) at 1:200 dilution in 1% BSA in PBS was added, the plate was left at room temperature for 20 min and 100 μl/well O-phenylenediamine dihydrochloride solution (substrate reagent pack DY-999; R&D Systems) was added, protected from light and incubated for 20 min at room temperature. The reaction was stopped with 50 μl/well 1 M H2SO4, and absorbance was read immediately in the Opsys MR 96 well plate reader at 492 nm, with control reading at 460 nm.
H&E staining and follicle counting
Mouse ovaries were fixed in Bouin solution and embedded in paraffin wax, and 5 μm sections were subjected to H&E staining using standard procedure. Every fifth section for each ovary was selected for follicle counting, and for atretic follicle number, sections were sampled. Primary, secondary and tertiary follicles were categorised by using a follicle classification developed by Pedersen & Peters (1968) and Myers et al. (2004).
Immunohistochemistry
Ovary samples from WT, TG mice were fixed with Bouin solution, while marmoset ovaries were fixed in neutral buffered formalin, and embedded in paraffin. Sections (5 μm) were mounted onto gelatin/poly-l-lysine-coated glass slides. The sections were dried onto the slides in a 37 °C incubator overnight. Sections were dewaxed in xylene for 15 min and rehydrated through graded ethanol solutions (100, 90 and 70%, v/v). Microwave antigen retrieval was performed in 0.01 M sodium citric buffer (pH 6.0) for 7 min at 95 °C at 800 W followed by 9 min at 120 W. Sections were cooled to room temperature before being washed twice in deionised water for 5 min each time. Sections were incubated with freshly prepared 3% (v/v) hydrogen peroxide (BDH, Poole, UK) diluted in 1×PBS for 5 min, then washed twice for 5 min with 1×PBS and blocked with 5% (w/v) BSA (Sigma) followed by 1.5% (w/v) normal goat serum (Vector Laboratories, Peterborough, UK) in 5% (w/v) BSA for 30 min. The sections were washed twice with 0.05% (v/v) PBS–Tween at room temperature for 5 min, then incubated with the primary antibody diluted in 1.5% w/v normal goat serum in 1×PBS. A polyclonal rabbit VEGF antibody (A20 sc152; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was used. Tissue sections were treated with a matched concentration of normal, affinity-purified rabbit IgG (Sigma), used as a negative control. The sections were washed twice in 0.05% (v/v) PBS–Tween, for 5 min each time. The blocking step was repeated as before, followed by two 5-min washes in 0.05% (v/v) PBS–Tween. All sections, including the controls, were incubated with biotinylated goat anti-rabbit IgG (Vector Laboratories) diluted in 1.5% (w/v) normal goat serum for 1 h in a humid chamber at room temperature. Sections were washed twice with 0.05% (v/v) PBS–Tween, 5 min/wash, then incubated with a pre-prepared avidin–biotinylated enzyme complete kit (Vector Laboratories) for 45 min in a humid chamber at room temperature. Again, the sections were washed twice with 0.05% (v/v) PBS–Tween, 5 min each time, followed by incubation with 3,3′-diaminobenzidine substrate (Vector Laboratories) to yield a brown-coloured product. The reaction was stopped by washing twice with deionised water for 5 min. Sections were counterstained with Mayer's haematoxylin (BDH) for 5 min, then differentiated in water. Sections were dehydrated by passing through increasing concentrations of ethanol (70, 90 and 100%, v/v) for at least 2 min each, cleared in xylene for at least 10 min, and permanently mounted in DPX Mountant for histology. Staining was examined with a Nikon Eclipse E-400 microscope; images were captured using a DCN-100 digital imaging system (Nikon Instruments).
For staining of microvessels, 5 μm ovary sections were dried thoroughly on SuperFrost slides (Fisher Scientific, Loughborough, UK). Non-specific binding was blocked with 5% normal goat serum in 0.5% PBS–Triton 100 for 1 h and avidin/biotin blocking reagent (Vector Laboratories) was applied for 15 min each on sections. Vasculature staining was performed with incubation with 5 μg/ml biotinylated isolectin B4 (Life Technologies, Paisley, UK) at 4 °C overnight and washing in 0.5% PBS–Triton 100 four times each for 5 min followed by 1 h incubation with 2 μg/ml AlexaFluor 555-conjugated streptavidin (Vector Laboratories). After washing, sections were counter stained with 5 μg/ml Hoechst for 30 min and coverslipped with Vectashield (Vector Laboratories). Images were analysed with Leica confocal microscope using a ×40 oil objective. Microvessels stained in red were selected in Photoshop and MVD (microvessel covering area per unit perimeter of the follicle or microvascular coverage area/total area % for corpus lutea) was calculated in ImageJ.
For pericyte staining, antigen retrieval was performed in 0.01 M sodium citrate buffer (pH 6.0), for 7 min at 95 °C at 800 W followed by 9 min at 120 W. Avidin/biotin blocking reagent (Vector Laboratories) was applied for 15 min each on sections and non-specific binding was blocked with 1% BSA, 2% normal horse serum in 0.05% PBS–Tween 20 (pH 7.4) for 1 h. Pericyte staining was performed with incubation with 1:200 anti-NG2 antibody in blocking solution (Molecular Probe) at 4 °C overnight and washing in 0.05% PBS–Tween 20 four times each for 5 min followed by 2 h incubation with 2 μg/ml AlexaFluor 488-conjugated goat anti-rabbit IgG (Vector Laboratories) in blocking solution. After washing, sections were counterstained with 5 μg/ml Hoechst for 30 min and coverslipped with Vectashield (Vector Laboratories). Images were analysed with Leica confocal microscope using a ×40 oil objective. Pericytes stained in green were selected in Photoshop and pericyte density (pericyte coverage area/total area %) was calculated in ImageJ (Abramoff et al. 2004).
For cleaved caspase-3 staining, antigen retrieval was performed by bringing slides to a boil in 10 mM sodium citrate buffer (pH 6.0) followed by maintaining at a sub-boiling temperature for 10 min. Sections were incubated with freshly prepared 3% (v/v) hydrogen peroxide in 1×PBS for 5 min. Non-specific blocking was achieved by incubation of sections for 1 h with 5% normal goat serum diluted in TBS–0.1% Tween 20 and with avidin D and biotin solution each for 15 min. A polyclonal rabbit cleaved caspase-3 antibody (1:400; (New England Biolabs, Hitchin, UK)) diluted in blocking solution was applied on sections and incubated overnight at 4 °C. Biotinylated goat anti-rabbit IgG diluted in blocking solution was then applied for 30 min in a humid chamber at room temperature. After washing, sections were incubated with a pre-prepared avidin–biotinylated enzyme complete kit followed by incubation with 3,3′-diaminobenzidine substrate (Vector Laboratories) to yield a brown-coloured product. The reaction was stopped by washing with deionised water for 5 min. Sections were counterstained with Mayer's haematoxylin (BDH) for 2 min and then differentiated in water. Sections were dehydrated by passing through increasing concentrations of ethanol (70, 90 and 100%, v/v) for at least 2 min each, cleared in xylene for at least 10 min and permanently mounted in DPX Mountant for histology. Staining was examined with a Nikon Eclipse E-400 microscope; images were captured using a DCN-100 digital imaging system (Nikon Instruments).
Collection of embryos
Both oviducts from each female mouse at 0.5 dpc were dissected and transferred into M2 medium in Petri dish. The ampulla was torn with fine forceps and embryos were gently squeezed out. Embryos were collected with a mouth pipette and released into M2 medium with hyaluronidase (10 mg/ml). The number of embryos was counted. Embryos were washed four times in M2 medium and transferred into well-equilibrated M16 medium in an incubator. Cells were maintained in M16 medium in the incubator for 4 days to blastocyst stage, checked each day and the number of alive and dead cells was recorded.
Cycle determination
Ten microlitre of 0.9% NaCl was injected into a female mouse vagina with a pipette. After pipetting, the solutions were collected, pipetted onto a clean slide and pulled to form a smear. After drying at room temperature, a smear was stained with 10% Giemsa for 5 min, rinsed with tap water, and dried and viewed under a light microscope. Female cyclic stage was determined, as shown in Fig. 5. Cycle determinations were repeated every day for at least two cycles and the number of days for each cycle was calculated. Four mice were excluded (two WT and two TG) as cycle time in diestrus was >3 days, indicating a missed cycle.
Statistical analysis
Figures are given as mean±s.e.m. unless stated; P<0.05 was regarded as significant. Methods of statistical analysis are included in relevant figure legend. For single comparisons t-tests were used with Welch's correction where variances were significantly different, and data normally distributed, or paired t-test when samples were matched to littermates. Integral data (e.g. number of embryos), or non-normally distributed data (determined by Kolmogorov–Smirnoff test) were tested by non-parametric statistics (Wilcoxon paired or Mann–Whitney unpaired U-test). For comparison purposes, VEGF expression, litter size, number of embryos, follicle number and proportions, vessel densities and luteal sizes, and days in cycles were considered as independent variables. The experimental units were numbers of mice in TG or WT groups. Frequencies were compared by a χ2-test (Fisher's exact test for 2×2 tables). Power analysis for non-significant values was carried out with G.Power using 80% power as a minimum cut-off.
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1530/REP-11-0091.
Declaration of interest
D O Bates and S J Harper are inventors on the VEGF165b patent.
Funding
This work was funded by a BHF grant (PG/08/054/25272) to D O Bates and LFD and BS/06/005 (to D O Bates), and Wellcome Trust grant 69029 (to D O Bates and S J Harper). H M Fraser was funded by a core grant (U.1276.00.002.00003.01) to the Medical Research Council Reproductive Sciences Unit.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Artac RA, McFee RM, Smith RA, Baltes-Breitwisch MM, Clopton DT, Cupp AS. Neutralization of vascular endothelial growth factor antiangiogenic isoforms is more effective than treatment with proangiogenic isoforms in stimulating vascular development and follicle progression in the perinatal rat ovary. Biology of Reproduction. 2009;81:978–988. doi: 10.1095/biolreprod.109.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Research. 2002;62:4123–4131. [PubMed] [Google Scholar]
- Bates DO, MacMillan PP, Manjaly JG, Qiu Y, Hudson SJ, Bevan HS, Hunter AJ, Soothill PW, Read M, Donaldson LF, et al. The endogenous anti-angiogenic family of splice variants of VEGF, VEGFxxxb, are down-regulated in pre-eclamptic placentae at term. Clinical Science. 2006;110:575–585. doi: 10.1042/CS20050292. [DOI] [PubMed] [Google Scholar]
- Cebe Suarez S, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, Manlius C, Wood J, Ballmer-Hofer K. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cellular and Molecular Life Sciences. 2006;63:2067–2077. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnock-Jones DS, Sharkey AM, Rajput-Williams J, Burch D, Schofield JP, Fountain SA, Boocock CA, Smith SK. Identification and localization of alternately spliced mRNAs for vascular endothelial growth factor in human uterus and estrogen regulation in endometrial carcinoma cell lines. Biology of Reproduction. 1993;48:1120–1128. doi: 10.1095/biolreprod48.5.1120. [DOI] [PubMed] [Google Scholar]
- Chu S, Mamers P, Burger HG, Fuller PJ. Estrogen receptor isoform gene expression in ovarian stromal and epithelial tumors. Journal of Clinical Endocrinology and Metabolism. 2000;85:1200–1205. doi: 10.1210/jc.85.3.1200. [DOI] [PubMed] [Google Scholar]
- Duncan WC, van den Driesche S, Fraser HM. Inhibition of vascular endothelial growth factor in the primate ovary up-regulates hypoxia-inducible factor-1alpha in the follicle and corpus luteum. Endocrinology. 2008;149:3313–3320. doi: 10.1210/en.2007-1649. [DOI] [PubMed] [Google Scholar]
- Fan X, Krieg S, Kuo CJ, Wiegand SJ, Rabinovitch M, Druzin ML, Brenner RM, Giudice LC, Nayak NR. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB Journal. 2008;22:3571–3580. doi: 10.1096/fj.08-111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser HM, Duncan WC. SRB Reproduction, Fertility and Development Award Lecture 2008. Regulation and manipulation of angiogenesis in the ovary and endometrium. Reproduction, Fertility, and Development. 2009;21:377–392. doi: 10.1071/RD08272. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Dickson SE, Lunn SF, Wulff C, Morris KD, Carroll VA, Bicknell R. Suppression of luteal angiogenesis in the primate after neutralization of vascular endothelial growth factor. Endocrinology. 2000;141:995–1000. doi: 10.1210/en.141.3.995. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Wilson H, Silvestri A, Morris KD, Wiegand SJ. The role of vascular endothelial growth factor and estradiol in the regulation of endometrial angiogenesis and cell proliferation in the marmoset. Endocrinology. 2008;149:4413–4420. doi: 10.1210/en.2008-0325. [DOI] [PubMed] [Google Scholar]
- Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nature Reviews. Cancer. 2008;8:880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Spee C, Kase S, Rennel ES, Magnussen AL, Qiu Y, Varey A, Dhayade S, Churchill AJ, Harper SJ, et al. Recombinant human VEGF165b inhibits experimental choroidal neovascularization. Investigative Ophthalmology & Visual Science. 2010;51:4282–4288. doi: 10.1167/iovs.09-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat BR, Brown LF, Manseau EJ, Senger DR, Dvorak HF. Expression of vascular permeability factor/vascular endothelial growth factor by human granulosa and theca lutein cells. Role in corpus luteum development. American Journal of Pathology. 1995;146:157–165. [PMC free article] [PubMed] [Google Scholar]
- Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA. VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Molecular Vision. 2006;12:626–632. [PubMed] [Google Scholar]
- Kraaij R, Verhoef-Post M, Grootegoed JA, Themmen AP. Alternative splicing of follicle-stimulating hormone receptor pre-mRNA: cloning and characterization of two alternatively spliced mRNA transcripts. Journal of Endocrinology. 1998;158:127–136. doi: 10.1677/joe.0.1580127. [DOI] [PubMed] [Google Scholar]
- Magnussen A, Rennel ES, Hua J, Bevan HS, Beazley-Long N, Lehrling C, Gammons M, Floege J, Harper SJ, Agostini HT, et al. VEGF-A165b is cytoprotective and anti-angiogenic in the retina. Investigative Ophthalmology & Visual Science. 2010;51:4273–4281. doi: 10.1167/iovs.09-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M, Konishi I, Kuroda H, Fukumoto M, Komatsu T, Yamamoto S, Nanbu K, Rao CV, Mori T. Messenger ribonucleic acid expression of LH/hCG receptor gene in human ovarian carcinomas. European Journal of Cancer. 1997;33:1501–1507. doi: 10.1016/S0959-8049(97)00166-4. [DOI] [PubMed] [Google Scholar]
- McFee RM, Artac RA, Clopton DT, Smith RA, Rozell TG, Cupp AS. Inhibition of vascular endothelial growth factor receptor signal transduction blocks follicle progression but does not necessarily disrupt vascular development in perinatal rat ovaries. Biology of Reproduction. 2009;81:966–977. doi: 10.1095/biolreprod.109.078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdzhanova G, Gout S, Keramidas M, Edmond V, Coll JL, Brambilla C, Brambilla E, Gazzeri S, Eymin B. The transcription factor E2F1 and the SR protein SC35 control the ratio of pro-angiogenic versus antiangiogenic isoforms of vascular endothelial growth factor-A to inhibit neovascularization in vivo. Oncogene. 2010;29:5392–5403. doi: 10.1038/onc.2010.281. [DOI] [PubMed] [Google Scholar]
- Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127:569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, Ladomery MR, Harper SJ, Bates DO. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by known splicing and growth factors. Journal of Cell Science. 2008;121:3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DG, Amin EM, Rennel ES, Hoareau-Aveilla C, Gammons M, Damodoran G, Hagiwara M, Harper SJ, Woolard J, Ladomery MR, et al. Regulation of vascular endothelial growth factor (VEGF) splicing from pro-angiogenic to anti-angiogenic isoforms: a novel therapeutic strategy for angiogenesis. Journal of Biological Chemistry. 2010;285:5532–5540. doi: 10.1074/jbc.M109.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten AD, Sanders MM, McKnight GS. The MMTV LTR promoter is induced by progesterone and dihydrotestosterone but not by estrogen. Molecular Endocrinology. 1988;2:143–147. doi: 10.1210/mend-2-2-143. [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Developmental and hormonal regulation of keratinocyte growth factor expression and action in the ovarian follicle. Endocrinology. 1998;139:228–235. doi: 10.1210/en.139.1.228. [DOI] [PubMed] [Google Scholar]
- Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. Journal of Reproduction and Fertility. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Baetens D, Mandriota SJ, Di Sanza C, Oikemus S, Lane TF, Soriano JV, Montesano R, Iruela-Arispe ML. Regulation of VEGF and VEGF receptor expression in the rodent mammary gland during pregnancy, lactation, and involution. Developmental Dynamics. 2000;218:507–524. doi: 10.1002/1097-0177(200007)218:3%3C507::AID-DVDY1012%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Pritchard-Jones RO, Dunn DB, Qiu Y, Varey AH, Orlando A, Rigby H, Harper SJ, Bates DO. Expression of VEGF(xxx)b, the inhibitory isoforms of VEGF, in malignant melanoma. British Journal of Cancer. 2007;97:223–230. doi: 10.1038/sj.bjc.6603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Bevan H, Weeraperuma S, Wratting D, Murphy D, Neal CR, Bates DO, Harper SJ. Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. FASEB Journal. 2008;22:1104–1112. doi: 10.1096/fj.07-9718com. [DOI] [PubMed] [Google Scholar]
- Ravindranath N, Little-Ihrig L, Phillips HS, Ferrara N, Zeleznik AJ. Vascular endothelial growth factor messenger ribonucleic acid expression in the primate ovary. Endocrinology. 1992;131:254–260. doi: 10.1210/en.131.1.254. [DOI] [PubMed] [Google Scholar]
- Rennel E, Waine E, Guan H, Schuler Y, Leenders W, Woolard J, Sugiono M, Gillatt D, Kleinerman E, Bates D, et al. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. British Journal of Cancer. 2008a;98:1250–1257. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennel ES, Hamdollah-Zadeh MA, Wheatley ER, Magnussen A, Schuler Y, Kelly SP, Finucane C, Ellison D, Cebe-Suarez S, Ballmer-Hofer K, et al. Recombinant human VEGF165b protein is an effective anti-cancer agent in mice. European Journal of Cancer. 2008b;44:1883–1894. doi: 10.1016/j.ejca.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher VA, Jeruschke S, Eitner F, Becker JU, Pitschke G, Ince Y, Miner JH, Leuschner I, Engers R, Everding AS, et al. Impaired glomerular maturation and lack of VEGF165b in Denys-Drash syndrome. Journal of the American Society of Nephrology. 2007;18:719–729. doi: 10.1681/ASN.2006020124. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Neufeld G, Gitay-Goren H, Keshet E. Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. Journal of Clinical Investigation. 1993;91:2235–2243. doi: 10.1172/JCI116450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RN, Mueller MD. Anti-angiogenic treatment of endometriosis: biochemical aspects. Gynecologic and Obstetric Investigation. 2004;57:54–56. [PubMed] [Google Scholar]
- Utsunomiya T, Tanaka T, Utsunomiya H, Umesaki N. A novel molecular mechanism for anticancer drug-induced ovarian failure: Irinotecan HCl, an anticancer topoisomerase I inhibitor, induces specific FasL expression in granulosa cells of large ovarian follicles to enhance follicular apoptosis. International Journal of Oncology. 2008;32:991–1000. [PubMed] [Google Scholar]
- Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, Dixon AR, Paraskeva C, Zaccheo O, Hassan AB, et al. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. British Journal of Cancer. 2008;98:1366–1379. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, McAllister K, Ward T, Davis B, Wiseman R, Hennighausen L. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Research. 2001;10:545–553. doi: 10.1023/A:1013063514007. [DOI] [PubMed] [Google Scholar]
- Wang XN, Greenwald GS. Hypophysectomy of the cyclic mouse. II. Effects of follicle-stimulating hormone (FSH) and luteinizing hormone on folliculogenesis, FSH and human chorionic gonadotropin receptors, and steroidogenesis. Biology of Reproduction. 1993;48:595–605. doi: 10.1095/biolreprod48.3.595. [DOI] [PubMed] [Google Scholar]
- Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Research. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- Woolard J, Bevan HS, Harper SJ, Bates DO. Molecular diversity of VEGF-A as a regulator of its biological activity. Microcirculation. 2009;16:572–592. doi: 10.1080/10739680902997333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff C, Wiegand SJ, Saunders PT, Scobie GA, Fraser HM. Angiogenesis during follicular development in the primate and its inhibition by treatment with truncated Flt-1-Fc (vascular endothelial growth factor Trap(A40)) Endocrinology. 2001;142:3244–3254. doi: 10.1210/en.142.7.3244. [DOI] [PubMed] [Google Scholar]
- Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology. 2002;143:2797–2807. doi: 10.1210/en.143.7.2797. [DOI] [PubMed] [Google Scholar]
- Yan Z, Weich HA, Bernart W, Breckwoldt M, Neulen J. Vascular endothelial growth factor (VEGF) messenger ribonucleic acid (mRNA) expression in luteinized human granulosa cells in vitro. Journal of Clinical Endocrinology and Metabolism. 1993;77:1723–1725. doi: 10.1210/jc.77.6.1723. [DOI] [PubMed] [Google Scholar]
- Zimmermann RC, Xiao E, Husami N, Sauer MV, Lobo R, Kitajewski J, Ferin M. Short-term administration of antivascular endothelial growth factor antibody in the late follicular phase delays follicular development in the rhesus monkey. Journal of Clinical Endocrinology and Metabolism. 2001;86:768–772. doi: 10.1210/jc.86.2.768. [DOI] [PubMed] [Google Scholar]
- Zimmermann RC, Xiao E, Bohlen P, Ferin M. Administration of antivascular endothelial growth factor receptor 2 antibody in the early follicular phase delays follicular selection and development in the rhesus monkey. Endocrinology. 2002;143:2496–2502. doi: 10.1210/en.143.7.2496. [DOI] [PubMed] [Google Scholar]
- Zimmermann RC, Hartman T, Kavic S, Pauli SA, Bohlen P, Sauer MV, Kitajewski J. Vascular endothelial growth factor receptor 2-mediated angiogenesis is essential for gonadotropin-dependent follicle development. Journal of Clinical Investigation. 2003;112:659–669. doi: 10.1172/JCI18740. [DOI] [PMC free article] [PubMed] [Google Scholar]