Abstract
OBJECTIVES
To examine frailty transitions in Mexican American (MA) and European American (EA) older adults.
DESIGN
Longitudinal, observational cohort study.
SETTING
Socioeconomically diverse neighborhoods in San Antonio, Texas.
PARTICIPANTS
312 MA and 285 EA community-dwelling older adults (65+) with frailty information at baseline (1992–96) and transition information at follow-up (2000–01) in the San Antonio Longitudinal Study of Aging (SALSA).
MEASUREMENTS
Five frailty characteristics (weight loss, exhaustion, weakness, slowness, and low physical activity), frailty score (0–5), and overall frailty state (non-frail = 0 characteristics, pre-frail = 1 or 2, frail = 3+) were assessed at baseline. Transitions (progressed, regressed, or no change) were assessed for frailty score and state. Odds ratios (OR) of progression and regression in individual characteristics were estimated using generalized estimating equations, adjusting for age, sex, ethnic group, socioeconomic status, comorbidity, diabetes, and follow-up interval.
RESULTS
Diabetes with macrovascular complications (OR=1.84, 95%CI: 1.02–3.33), fewer years of education (OR=0.96, 95%CI: 0.93–1.0) and follow-up interval (OR=1.3, 95%CI: 1.17–1.46) were significant predictors of progression in any frailty characteristic. Mortality increased by frailty state, and pre-frail individuals were more likely than frail to regress.
CONCLUSION
Diabetes with macrovascular complications and fewer years of education are important predictors of progression in any frailty characteristic. Because of increased risk of death compared with the non-frail state and the increased likelihood of regression compared with the frail state, the pre-frail state may be an optimal target for intervention.
Keywords: frailty, older adults, transitions
INTRODUCTION
Frailty has been hypothesized to be a geriatric syndrome that is recognized by clinicians and characterized by decreased resilience to stressors, causing increased risk for age-related complications and outcomes.1 The syndrome has been operationalized as a research construct by validated criteria developed in the Cardiovascular Health Study (CHS), and defined as the presence of three or more of five characteristics: weight loss, exhaustion, low physical activity, weakness, and slowness.2 Frail individuals have been shown to be at risk for adverse outcomes, such as falls, disability, institutionalization, and death.2, 3 A pre-frail state is defined as the presence of one or two of these characteristics, and pre-frail are at higher risk of adverse outcomes compared to non-frail.2
Previous studies report that transitions between frailty states (non-frail, pre-frail, frail) are fairly common, with individuals either worsening or improving over time.4, 5 Gill et al.4 studied frailty transitions over 4.5 years in 754 predominantly European American (EA) participants. They reported that 57% of participants had at least one transition over approximately four and a half years (although the most common pattern was to remain in the baseline frailty state).4 In addition, while it was more common for individuals to worsen in frailty state (rates up to 43%), improvement to a lesser frailty state did occur (rates up to 23%). Frail individuals were unlikely to improve, and were more likely to remain frail. In contrast, Ottenbacher et al.,5 who followed Mexican American (MA) participants for 10 years in the Hispanic Established Populations for the Epidemiologic Study of the Elderly (H-EPESE), found that the majority of frail individuals were deceased at follow-up. Transitions in individual frailty characteristics were not the primary focus of these prior studies, and neither study was able to make direct comparisons between MAs and EAs.
The purpose of this study was to characterize change in both individual frailty characteristics and overall frailty state in a longitudinal, bi-ethnic cohort of community-dwelling older adults. The unique San Antonio Longitudinal Study of Aging (SALSA) cohort, comprised of approximately equal numbers of MAs and EAs, allows for direct ethnic comparisons. Thus, study results may not only help to identify frailty states and individual frailty characteristics that are optimal targets for intervention to prevent or delay worsening across frailty states but also potential health disparities in frailty transitions.
METHODS
Sample
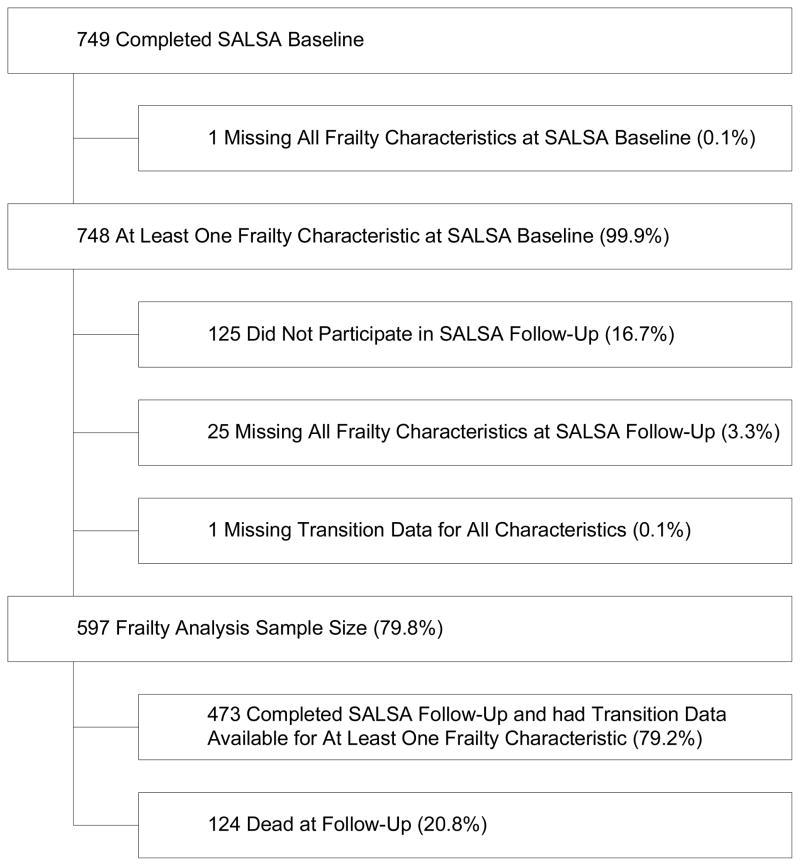
Subjects were 597 participants in the SALSA baseline examination (1992–1996) for whom data was available to characterize change in at least one frailty characteristic from baseline to follow-up. Follow-up examination occurred in 2000–2001. This sample has been described previously. 6, 7 Ethnic group was classified using a validated, standardized algorithm.8 749 participants completed the baseline examination for a response rate of 70.5%. At the follow-up exam, 474 of 599 surviving participants completed the study examination for a response rate of 79.1%.
The SALSA baseline and follow-up examination consisted of a comprehensive home-based assessment, conducted in the participant’s home, and a performance-based assessment, conducted at a clinical research center. Trained, bilingual staff administered assessments in English or Spanish, according to participants’ preference. The study was approved by the Institutional Review Board of The UT Health Science Center San Antonio, and all subjects gave informed consent.
Frailty Characterization
Validated CHS criteria and standardization procedures2 were applied to the pooled SALSA sample; standardized cutpoints have been published previously.6
Walking Speed
Subjects were timed in seconds as they walked 10 feet, at their usual pace, starting from a standing position. Walking speed was standardized based on median height and sex. Participants in the slowest quintile for each sex group were considered slow. If a participant was unable to walk, he or she was considered slow.
Grip Strength
Grip strength was measured in kilograms using a handheld dynamometer in the dominant hand, and was standardized based on body mass index (BMI) quartiles and sex. Participants in the lowest quintile for each sex group were considered weak.
Physical Activity
Self-reported physical activity over the previous year was assessed using the Minnesota Leisure Time Physical Activity Questionnaire, which yields average energy expenditure in kilocalories per week,9 and was standardized based on sex. Participants in the lowest quintile for each sex group were considered to have low energy expenditure.
Exhaustion
Exhaustion was measured by the Geriatric Depression Scale10 question, “Do you feel full of energy?” Subjects who responded “no” to this question were considered exhausted.
Weight Loss
Weight loss was assessed by response to the question, “In the last year have you gained or lost more than 10 pounds?” Response choices were: gained only, lost only, both gained and lost, or neither. Intentionality was not assessed. Only those participants who reported that they had lost but not gained weight were considered as having weight loss.
Frailty state was classified as an ordinal trichotomous variable (non-frail = 0 characteristics, pre-frail = 1 or 2, frail = 3+). Frailty score was calculated as the total number of frailty characteristics, ranging from 0 to 5 at the baseline exam and 0 to 6 at follow-up, with a score of 6 indicating death.
Worsening in frailty state from baseline to follow-up was defined as change from non-frail to either pre-frail or frail, or from pre-frail to frail. Improvement in frailty status was defined as change from frail to pre-frail or non-frail, or from pre-frail to non-frail. Worsening and improvement were also measured for individual frailty characteristics. For example, worsening in walking speed was defined as being classified as slow at follow-up if the baseline classification was not slow. Improvement was defined as being classified as not slow at follow-up if the baseline classification was slow.
Missing data
The analytic cohort excluded individuals who had missing data for all five frailty characteristics at baseline or at follow-up, as well as those whose change (worsened, improved, or unchanged) in at least one frailty characteristics from baseline to follow-up could be determined. Some data for individual frailty characteristics was missing in the analytic cohort at baseline and follow-up for each frailty characteristic. At baseline, 92.3% of the analytic cohort had no missing frailty data, 6.4% were missing data for only 1 characteristic, and the remaining 1.3% were missing data for 2 or 3 characteristics. At follow-up, 83.6% had no missing frailty information, 15.1% were missing information for only 1 characteristic, and 1.3% were missing information for either 2 or 3 characteristics.
Vital status
Death was ascertained by regular review of local newspaper obituaries, San Antonio Metropolitan Health District vital statistics records, search of the Social Security Death Index, and search of the National Death Index.
Covariates
Chronic Disease
Chronic diseases measured at baseline were used as covariates in the longitudinal analyses. Diabetes was assessed using American Diabetes Association criteria based on fasting plasma glucose ≥ 126 mg/dl or currently taking anti-diabetic medication(s).11 Blood pressure was measured using a random-zero sphygmomanometer with the participant seated following a 5-minute rest. Three measures were taken and blood pressure was calculated as the average of the second and third readings. Hypertension was assessed using guidelines from the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure 6 guidelines.12 Ischemic heart disease (IHD) was assessed by evaluation of a 12-lead electrocardiograph (ECG) for the presence of ischemic ECG abnormalities. These included presence of Q-waves of at least 0.04 seconds in duration in leads II, III, and aVF, and/or Q-waves in the precordial leads (V1–V6).13 Self-reported IHD, or angina pectoris, was assessed using the validated and standardized Rose questionnaire.14 Diabetes with macrovascular complications was defined as the presence of diabetes with IHD and/or stroke. For the purposes of this paper, uncomplicated diabetes was defined as presence of diabetes without IHD and/or stroke.
Chronic obstructive pulmonary disease was assessed by Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria.15 Arthritis, cancer (non-skin), congestive heart failure, and stroke were assessed by self-report of physician-diagnosed disease. Comorbid disease was calculated as presence of two or more of the above diseases excluding diabetes, which was considered separately because of its three-fold greater prevalence in MAs compared with EAs and prior evidence that diabetes is a risk factor for frailty.3, 5
Cognitive impairment
Cognitive impairment was assessed using the Folstein Mini-Mental State Examination.16 Those with a score of less than 18 were classified as cognitively impaired.
Socioeconomic Status (SES)
Monthly household income and number of years of formal education were assessed by self-report.
Follow-up interval
Time to follow-up or death (years) was included as a covariate in the analyses. The average follow-up period in the overall frailty analytic cohort was 6.4 years (range: 0.2 – 9.7). Among those who completed the follow-up examination, the average follow-up interval was 7.0 years (range: 4.4 – 9.7). Among those who died, the average follow-up interval was 4.4 years (range: 0.2 – 9.4).
Statistical Analysis
Ethnic differences in demographic, SES, disease variables, and frailty characteristics were compared using the chi-squared statistic for categorical variables and two sample t-tests for continuous variables that follow a normal distribution. The Wilcoxon two-sample test was used for continuous variables that followed a non-normal distribution.
A generalized estimating equations (GEE) approach for logistic regression for correlated outcomes was used to estimate the odds ratio of worsening in individual frailty characteristics at follow-up, where death at follow-up was considered as worsening in all five characteristics. GEE accounts for correlations among different frailty characteristics within an individual and uses all available data points to model the marginal probability of worsening of each characteristic. Three models were estimated, adding pertinent covariates in each subsequent model. Model 1 is an unadjusted model; Model 2 is adjusted for ethnic group, age, sex, and follow-up interval; Model 3 additionally adjusts for SES (income, education), comorbid disease, and diabetes. To further analyze the role of diabetes in frailty worsening, Model 4 includes the covariates from the previous model except diabetes with macrovascular complications and uncomplicated diabetes are entered as separate variables. Diabetes with and without macrovascular complications are each compared to no diabetes as the reference group. Analyses for Tables 1, 2, and 3 were completed using STATA version 10.1 (College Station, TX), and analyses for Table 4 were completed using SAS version 9.1 (Cary, NC).
Table 1.
Study Sample Characteristics: Individuals for whom Transitions Data, including Death, was Obtained at Follow-up 1
| Baseline Characteristics | Mexican Americans (n = 312) | European Americans (n = 285) | Total (n = 597)* | P-value for Ethnic Difference |
|---|---|---|---|---|
| n(%) or mean(SD)† | n(%) or mean(SD)† | n(%) or mean(SD)† | ||
| Age, years (range: 65–80) | 69.1 (3.2) | 70.2 (3.5) | 69.6 (3.4) | <0.001 |
| Female | 172 (55.1) | 157 (55.1) | 329 (55.1) | 0.992 |
| Hypertension | 148 (47.4) | 144 (50.5) | 292 (48.9) | 0.451 |
| Stroke | 34 (11.0) | 18 (6.4) | 52 (8.8) | 0.046 |
| Arthritis | 134 (43.1) | 135 (47.7) | 269 (45.3) | 0.259 |
| Ischemic heart disease (self-report) | 23 (7.4) | 20 (7.0) | 43 (7.3) | 0.851 |
| Ischemic heart disease (ECG-defined) | 45 (14.5) | 46 (16.1) | 91 (15.3) | 0.571 |
| Congestive heart failure | 0 (0.0) | 1 (0.4) | 1 (0.2) | 0.295 |
| Chronic obstructive pulmonary disease (n = 583) | 93 (30.7) | 104 (37.1) | 197 (33.8) | 0.100 |
| Diabetes (n = 532) | 99 (33.9) | 25 (10.4) | 124 (23.3) | <0.001 |
| Diabetes with complications ‡ (n = 449) | 34 (15.0) | 7 (3.2) | 41 (9.1) | <0.001 |
| Cognitive impairment¶ | 9 (2.9) | 1 (0.4) | 10 (1.7) | 0.016 |
| Comorbidity§ (n = 571) | 112 (36.8) | 120 (44.9) | 232 (40.6) | 0.049 |
| Comorbidity, score (range: 0–5) | 1.3 (1.0) | 1.4 (1.0) | 1.3 (1.0) | 0.122 |
| Income,# category (range: 1–15) | 10.7 (3.1) | 12.9 (2.3) | 11.8 (2.9) | <0.001 |
| Education, years (range: 0–23) | 9.4 (4.5) | 13.4 (2.6) | 11.3 (4.2) | <0.001 |
| Frailty Characteristics | ||||
| Weakness (n = 592) | 78 (25.2) | 39 (13.8) | 117 (19.8) | 0.001 |
| Slowness (n = 596) | 71 (22.8) | 44 (15.5) | 115 (19.3) | 0.025 |
| Exhaustion (n = 582) | 71 (23.4) | 100 (36.0) | 171 (29.4) | 0.001 |
| Weight Loss (n = 568) | 45 (15.5) | 35 (12.6) | 80 (13.4) | 0.333 |
| Low Physical Activity (n = 590) | 70 (22.7) | 43 (15.3) | 113 (19.2) | 0.021 |
| Frailty, Ordinal Trichotomous (n = 559) | 0.054 | |||
| Non-frail | 102 (35.3) | 107 (52.6) | 209 (37.4) | |
| Pre-frail | 152 (52.6) | 146 (54.1) | 298 (53.3) | |
| Frail | 35 (12.1) | 17 (6.3) | 52 (9.3) | |
Sample size of 597 includes individuals who were not missing information for all five frailty characteristics at baseline or follow-up, and whose transition could be classified in at least one frailty characteristic at baseline and follow-up. Sample size may be lower for individual baseline characteristics due to missing data; in this case, the sample size is listed next to the characteristic.
Abbreviations: SD = standard deviation
Diabetes with complications was defined as the presence of diabetes (defined by American Diabetes Association criteria) as well as the presence of stroke (defined by self-report of physician-diagnosed disease) or ischemic heart disease, either self-reported (assessed by Rose questionnaire) or ECG-defined.
Comorbidity defined as presence of two or more of seven chronic conditions, including angina, hypertension, myocardial infarction, stroke, arthritis, and cancer (non-skin). Comorbidity score is a sum of the number of these chronic diseases present.
Cognitive impairment was defined as Mini Mental State Examination score of less than 18.
Monthly household income categories: 1=$0–49, 2=$50–99, 3=$100–149, 4=$150–199, 5=$200–299, 6=$300–399, 7=$400–499, 8=$500–749, 9=$750–999, 10=$1000–1249, 11=$1250–1499, 12=$1500–1999, 13=$2000–2499, 14=$2500–2999, 15=$3000+. Dollar equivalents of annual household incomes are: 10=$13,500, 11=$16,500, 12=$21,000, 13=$27,000.
Table 2.
Follow-up Frailty Score and Death Status, by Baseline Frailty Score for Total Sample
| Total sample (n = 597)* | Baseline Frailty Score | |||||
|---|---|---|---|---|---|---|
| Non-frail | Pre-frail | Frail | ||||
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Follow-up Frailty | (n = 228) | (n = 212) | (n = 105) | (n = 37) | (n = 12) | (n = 3) |
| Score | n (%)† | n (%) | n (%) | n (%) | n (%) | n (%) |
| 0 (n = 145) | 94 (41.2) | 38 (17.9) | 11 (10.5) | 2 (5.4) | 0 (0.0) | 0 (0.0) |
| 1 (n = 159) | 68 (29.8) | 67 (31.6) | 20 (19.1) | 3 (8.1) | 1 (8.3) | 0 (0.0) |
| 2 (n = 93) | 26 (11.4) | 34 (16.0) | 24 (22.9) | 7 (18.9) | 1 (8.3) | 1 (33.3) |
| 3 (n = 59) | 9 (4.0) | 27 (12.7) | 16 (15.2) | 6 (16.2) | 1 (8.3) | 0 (0.0) |
| 4 (n = 14) | 1 (0.4) | 2 (0.9) | 9 (8.6) | 2 (5.4) | 0 (0.0) | 0 (0.0) |
| 5 (n = 3) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 0 (0.0) |
| Deceased (n = 124) | 30 (13.2) | 43 (20.3) | 24 (22.9) | 17 (46.0) | 8 (66.7) | 2 (66.7) |
| Total sample (n = 597)* | Baseline Frailty Category | |||
|---|---|---|---|---|
| Follow-up Frailty | Non-frail | Pre-frail | Frail | Cannot classify |
| Category | (n = 209) | (n = 298) | (n = 52) | (n = 38) |
| Non-frail (n = 121) | 79 (37.8) | 37 (12.4) | 1 (1.9) | 4 (10.5) |
| Pre-frail (n = 201) | 70 (33.5) | 110 (36.9) | 6 (11.5) | 15 (39.5) |
| Frail (n = 76) | 6 (2.9) | 49 (16.4) | 10 (19.2) | 11 (29.0) |
| Deceased (n = 124) | 30 (14.4) | 67 (22.5) | 27 (51.9) | 0 (0.0) |
| Cannot classify (n = 75) | 24 (11.5) | 35 (11.7) | 8 (15.4) | 8 (21.1) |
Analysis includes individuals who were not missing information for all five frailty characteristics, and whose transition could be classified in at least one frailty characteristic at baseline and follow-up.
Column percentages are shown.
Table 3.
Follow-up Status, including Death, for Individual Frailty Characteristics by Their Corresponding Baseline Status
| Individual Frailty Characteristic | ||||
|---|---|---|---|---|
| Grip Strength | Weakness at Baseline (n = 84) | No Weakness at Baseline (n = 382) | ||
| Weakness at F/U* | 26 (30.9) | Weakness at F/U | 27 (7.1) | |
| No Weakness at F/U | 24 (28.6) | No Weakness at F/U | 271 (70.9) | |
| Died at F/U | 34 (40.5) | Died at F/U | 84 (22.0) | |
| Slowness at Baseline (n = 82) | No Slowness at Baseline (n = 384) | |||
| Walking Speed | Slowness at F/U | 28 (34.2) | Slowness at F/U | 37 (9.6) |
| No Slowness at F/U | 21 (25.6) | No Slowness at F/U | 262 (68.2) | |
| Died at F/U | 33 (40.2) | Died at F/U | 85 (22.1) | |
| Exhaustion | Exhaustion at Baseline (n = 138) | No Exhaustion at Baseline (n = 328) | ||
| Exhaustion at F/U | 71 (51.5) | Exhaustion at F/U | 50 (15.2) | |
| No Exhaustion at F/U | 25 (18.1) | No Exhaustion at F/U | 202 (61.6) | |
| Died at F/U | 42 (30.4) | Died at F/U | 76 (23.2) | |
| Weight Loss | Weight Loss at Baseline (n = 65) | No Weight Loss at Baseline (n = 401) | ||
| Weight Loss at F/U | 21 (32.3) | Weight Loss at F/U | 91 (22.7) | |
| No Weight Loss at F/U | 24 (36.9) | No Weight Loss at F/U | 212 (52.9) | |
| Died at F/U | 20 (30.8) | Died at F/U | 98 (24.4) | |
| Physical Activity | Low Physical Activity at Baseline (n = 84) | No Low Physical Activity at Baseline (n = 382 | ||
| Low Physical Activity at F/U | 22 (26.2) | Low Physical Activity at F/U | 24 (6.3) | |
| No Low Physical Activity at F/U | 27 (32.1) | No Low Physical Activity at F/U | 275 (72.0) | |
| Died at F/U | 35 (41.7) | Died at F/U | 83 (21.7) | |
Abbreviations: F/U = follow-up
Table 4.
Odds ratios of frailty worsening (vs. remaining unchanged or improving) in multivariate models using generalized estimating equations.*
| N = 597 | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Dependent Variables | OR† (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Grip Strength | 0.56 (0.47–0.66) | <0.001 | 0.3 (0.21–0.43) | <0.001 | 0.28 (0.19–0.41) | <0.001 | 0.28 (0.19–0.41) | <0.001 |
| Walking Speed | 0.65 (0.55–0.77) | <0.001 | 0.39 (0.27–0.56) | <0.001 | 0.40 (0.27–0.58) | <0.001 | 0.4 (0.27–0.58) | <0.001 |
| Exhaustion | 0.73 (0.61–0.88) | 0.001 | 0.57 (0.41–0.81) | 0.001 | 0.54 (0.37–0.78) | 0.001 | 0.54 (0.37–0.78) | 0.001 |
| Physical Activity | 0.59 (0.49–0.7) | <0.001 | 0.36 (0.25–0.51) | <0.001 | 0.36 (0.25–0.52) | <0.001 | 0.36 (0.25–0.52) | <0.001 |
| Weight Loss | 1 | 1 | 1 | 1 | ||||
| Predictor Variables | ||||||||
| Ethnicity (MA vs. EA) | 1.05 (0.82–1.36) | 0.68 | 0.78 (0.56–1.07) | 0.09 | 0.76 (0.56–1.05) | 0.09 | ||
| Age (years) | 1.04 (1–1.08) | 0.05 | 1.04 (1.0–1.08) | 0.06 | 1.04 (1.0–1.08) | 0.06 | ||
| Sex (male vs. female) | 1.11 (0.86–1.42) | 0.42 | 1.21 (0.92–1.59) | 0.23 | 1.20 (0.91–1.58) | 0.19 | ||
| Follow-up interval (years) | 1.39 (1.26–1.54) | <0.001 | 1.30 (1.17–1.46) | <0.001 | 1.30 (1.17–1.46) | <0.001 | ||
| Income‡ (1-category increment) | 0.95 (0.9–1.01) | 0.09 | 0.96 (0.91–1.01) | 0.11 | ||||
| Education (1-year increment) | 0.96 (0.93–1.0) | 0.04 | 0.96 (0.93–1.0) | 0.04 | ||||
| Comorbidity (not including diabetes) | 1.30 (0.99–1.71) | 0.06 | 1.26 (0.95–1.67) | 0.11 | ||||
| Diabetes (yes vs. no) | 1.38 (1.0–1.91) | 0.05 | ||||||
| Diabetes, no complications (vs. no diabetes) | 1.25 (0.88–1.79) | 0.21 | ||||||
| Diabetes, with complications§ (vs. no diabetes) | 1.84 (1.02–3.33) | 0.04 | ||||||
The GEE analysis includes all subjects whose change in at least one of the five frailty characteristics can be determined.
Abbreviations: OR = odds ratio, CI = confidence interval, vs. = versus.
Monthly household income categories: 1=$0–49, 2=$50–99, 3=$100–149, 4=$150–199, 5=$200–299, 6=$300–399, 7=$400–499,
8=$500–749, 9=$750–999, 10=$1000–1249, 11=$1250–1499, 12=$1500–1999, 13=$2000–2499, 14=$2500–2999, 15=$3000+.
Diabetes with complications was defined as the presence of diabetes (defined by American Diabetes Association criteria) as well as the presence of stroke (defined by self-report of physician-diagnosed disease) or ischemic heart disease, either self-reported (assessed by Rose questionnaire) or ECG-defined.
RESULTS
Figure 1 provides a flowchart of the sample included in the analysis. 597 individuals had information to determine change in at least one frailty characteristic from baseline to follow-up. Individuals who were deceased at follow-up were considered as progressed in all frailty characteristics. Baseline sample characteristics are shown in Table 1. MAs were older, and had lower SES compared to EAs. While there was significant ethnic difference in comorbidity, MAs had higher prevalence of diabetes, as well as diabetes with complications. There was no significant ethnic difference in the presence of hypertension, ischemic heart disease, chronic obstructive pulmonary disease, stroke, arthritis, or comorbidity. MAs were more likely to have cognitive impairment; however, the overall prevalence of cognitive impairment in the cohort was low, at approximately 2%. More EAs than MAs had comorbid disease (44.9% vs. 36.8%, p = 0.049), and the overall prevalence of comorbidity in the cohort was 40.6%. More MAs than EAs were frail (12.1% vs. 6.3%), but approximately equal proportions of both ethnic groups were pre-frail or non-frail. Ethnic differences in overall frailty state were not statistically significant.
Figure 1.
Sample Size
Frailty score and frailty state at follow-up (including death) are shown in Table 2 by baseline frailty score and frailty state. Among those who were pre-frail at baseline, 36.9% remained pre-frail. Individuals with two characteristics were almost twice as likely to progress to frail compared to those with only one (24.3% vs. 13.6%, respectively). Conversely, those with only one characteristic were almost twice as likely to regress compared to those with two (17.9% vs. 10.5%, respectively). The death rate was similar for frailty scores of 1 and 2, and was approximately 10% higher than the rate among non-frail. Among those who were frail at baseline, whether they had a frailty score of 3, 4, or 5, the dominant transition was to death. The transition to death more than doubled for those who were frail compared to pre-frail. Nonetheless, among those with only 3 frailty characteristics, 32.4% regressed, while few individuals with 4 or 5 characteristics regressed.
Table 3 shows presence or absence of each frailty characteristic (or death) at follow-up, stratified by presence or absence of that characteristic at baseline. Transition to death was almost twice as high for individuals with characteristics at baseline that were classified using performance-based measures and low physical activity compared to those classified using self-report measures.
Adjusted and unadjusted odds of worsening in individual frailty characteristics are shown in Table 4. Weight loss is used as the reference category in all models as it had the highest rate of both progression and regression among the five frailty characteristics. As indicated in the table, the dependent variable for the GEE analysis is worsening in any of the five frailty characteristics. The predictor variables are age, sex, ethnic group, household income, education, diabetes without complications, diabetes with complications, and comorbid diseases excluding diabetes. In the unadjusted model for worsening, the odds of worsening relative to weight loss were lowest for grip strength, followed by physical activity, walking speed, and exhaustion. In Model 2, age and follow-up interval were significant predictors of worsening in any frailty characteristic, with a 4% increased risk of worsening for each year of age, and a 39% increased risk of worsening in frailty for each year of follow-up. In Model 3 diabetes and fewer years of education were significant predictors of worsening. Diabetes was associated with an approximately 40% increased risk of worsening (OR = 1.38, 95% CI: 1.0–1.91), while each year of education was associated with a 4% decreased risk of worsening (OR = 0.96, 95% CI: 0.92–1.0). In Model 4, diabetes with macrovascular complications – but not uncomplicated diabetes – was a significant predictor of worsening (OR = 1.84, 95% CI: 1.02–3.33). The magnitude of the effect was higher for diabetes with macrovascular complications than for undifferentiated diabetes in model 3 (OR = 1.38).
DISCUSSION
In this study of frailty transitions over an average 6.4 years among community-dwelling older MA and EA participants in SALSA, we found that pre-frail individuals with two baseline characteristics were more likely than those with only one to worsen in frailty state. Similarly, those with only one baseline characteristic were more likely to improve than those with two. Follow-up death rates increased by baseline frailty state, and the rate was higher for frailty characteristics classified based on performance-based measures and low physical activity compared to those classified based on self-reported frailty measures. In GEE analyses of frailty worsening, significant predictors were diabetes with macrovascular complications, fewer years of education, and follow-up interval. Individuals with undifferentiated diabetes were approximately 40% more likely to progress in any frailty characteristic. In Model 4, those who had diabetes with macrovascular complications were 84% more likely to worsen in any frailty characteristic compared to non-diabetics. These findings suggest that diabetes plays a pervasive role in frailty worsening, affecting all five frailty characteristics.
Previous studies have shown that diabetes is associated with prevalent2 and incident frailty;3, 5 and, fasting hyperglycemia is associated with frailty in individuals without diabetes.17 Several studies support associations between frailty and insulin resistance and diabetes.18 In particular, insulin resistance has been shown to be predictive of incident frailty, and increasing hemoglobin A1c is also associated with frailty.19 It should be noted that frailty has also been linked with other diseases, such as cardiovascular disease, congestive heart failure, peripheral vascular disease, and stroke.20, 21 Although more research is needed to develop agreed upon clinical criteria for identifying frailty in the clinical setting, in the future performing a clinical frailty assessment at the onset of diabetes in older adults may help identify those at risk of frailty and lead to early initiation of preventive interventions.
To our knowledge, this is the first study to examine diabetes as a predictor of worsening in individual frailty characteristics. Interestingly, in spite of the fact that diabetes is two to three times more prevalent in MAs than in EAs, we found no ethnic difference in worsening in any frailty characteristic. We have previously reported that MAs were 60% less likely than EAs to develop incident frailty (OR = 0.40, 95%CI: 0.23–0.72) after covariate adjustment for relevant covariates.22 The present finding suggests, however, that MAs and EAs may be equally likely to transition in individual frailty characteristics, and that factors predicting worsening of any frailty characteristic, including diabetes, may operate similarly in both ethnic groups.
This study also showed that fewer years of education, a key indicator of SES, is also a significant predictor of worsening in any individual frailty characteristic (OR = 0.96, 95% CI: 0.93–1.0). Previous studies have shown that low SES is associated with prevalent and incident frailty.2, 3, 23 A potential mechanism explaining this low SES-frailty association is increased inflammation, which is thought to be a major physiologic alteration operant in frailty,24 and which may result from poorer nutritional status, less access to medical care, and higher prevalence of chronic disease among lower SES individuals.25 Our study extends these findings by showing that education is a predictor of worsening in any frailty characteristic. In combination with our findings relative to diabetes, there may be an increased burden of frailty in older diabetics with lower education. Certainly more research is needed; however, it is possible that education interventions in the area of diabetes management for older adults could indirectly reduce frailty progression by lowering the incidence of diabetes with complications.
We found that mortality rates were higher for individual frailty characteristics classified using objective or quasi-objective measures compared to self-reported. One possibility for this finding is that more objective measures of frailty may be less affected by individual perceptions or self-report bias, and may be more reflective of underlying physiologic deficits compared to self-report measures. Prior studies have shown that individual frailty measures are predictive of mortality.26, 27 However, previous reports of which individual frailty characteristics predict mortality vary across study populations and have included both objective and self-reported characteristics as the strongest mortality predictor.26–28 Because pre-frail individuals may have only one frailty characteristic but still at significant risk of death and incident frailty,2, 6 the potential ability of individual frailty characteristics to predict these outcomes in diverse populations should continue to be examined to identify appropriate targets for intervention in different population subgroups.
The SALSA cohort was 69.6 ±3.4 years at baseline, an average of 8.8 years younger than the cohort studied by Gill et al. (78.4±5.3 years), and 12.9 years younger than the H-EPESE cohort (82.5±4.5 years).5 Despite these age differences, all studies found that frail individuals were unlikely to regress. Both the SALSA and H-EPESE studies found that frail individuals were more likely to die than remain frail, while Gill et al., who followed participants for a shorter time interval, found that frail individuals were more likely to remain frail than die. The advanced age of the H-EPESE cohort and slightly longer follow-up interval likely account for the greater proportion of frail individuals who died in that study compared with SALSA (84% vs. 52%).
The present study has several limitations. There were minor modifications of the CHS criteria. Results obtained for MAs living in a single major urban area in south Texas may not be generalizable to MAs living in other urban areas in the U.S. or those living in rural areas. The sample size was relatively small, and only about 7% of the analytic cohort regressed in overall frailty category. Bias could have been introduced if the individuals lost to follow-up differed systematically from those who completed the follow-up exam. To address this concern, we compared baseline frailty information for those included in the analytic cohort non-completers (data not shown); no differences were found in either individual frailty characteristics or frailty state between the two groups. Disease ascertainment is also a potential limitation. Although diabetes, hypertension, and ischemic heart disease were measured by clinical criteria or validated measures, many were ascertained by self-report of physician-diagnosed disease and were not adjudicated. Finally, the varying length of follow-up among SALSA participants was by design. Interval lengths were varied across individuals by reversing the order of enrollment at baseline in order to maximize information from the assessment. Interval length was included as a covariate in the GEE analyses.
The findings of an increased mortality among pre-frail relative to non-frail, approximately equivalent death rates among pre-frail with either 1 or 2 frailty characteristics, and a substantially lower death rate among pre-frail relative to frail provides further validation of the trichotomous frailty classification proposed by Fried in the CHS,2 highlighting the significance of pre-frailty as a separate risk state. Further, the finding that pre-frail individuals were more likely than frail to improve in frailty state suggests that this group of individuals is capable of significant improvement over time and may be responsive to clinical and/or behavioral interventions to slow or reverse worsening toward frailty.
Although there is no established clinical intervention for frailty per se, characteristics of the frailty phenotype include the domains of physical activity, muscle strength, and nutrition. One study has shown that physical activity, in the form of strength training, is more effective than a nutritional intervention in improving muscle strength and gait speed in older adults, and that it is safe, even in nursing home residents.29 Other studies have shown that exercise interventions can ameliorate frailty and prevent disability in frail older adults,30 but there are conflicting findings regarding the effectiveness of therapeutic exercise, as well as the specific type of exercise that should be recommended to older adults.31 Future research should focus on translating exercise and strength training interventions into clinical prescriptions for therapeutic exercise in older adults as well as testing and translating into clinical practice various approaches to frailty screening in the clinical setting. Given the increasing rates of diabetes in the U.S. population, including older adults, the role of diabetes in the development of frailty is an important issue and should be considered when developing methods and interventions for improving the health of older adults in the future.
Acknowledgments
This research was supported by National Institute on Aging (NIA) R01-AG10444, R01-AG16518, and National Center for Research Resources (NCRR) M01-RR01345. This work was also supported by The Harold Amos Medical Faculty Development Program with the Robert Wood Johnson Foundation and Clinical Translational Science Award KL2 RR025766 from NCRR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR of the National Institutes of Health. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U. S. Government. This material is the result of work supported with resources and the use of facilities at the Audie Murphy VA Medical Center, San Antonio, TX. The authors gratefully acknowledge Adrienne Boulton for her assistance in writing the computer programs used to construct the criteria for classifying frailty in the SALSA study.
Sponsor’s Role: The funding institutes had no role in the collection, analysis, or interpretation of the data or in the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Sara Espinoza: study concept and design, statistical analysis and interpretation of data, and manuscript preparation. Inkyung Jung: study design, statistical analysis and interpretation of data, and preparation of manuscript preparation. Helen Hazuda: study concept and design, study concept and design for cohort, interpretation of data, and manuscript preparation.
References
- 1.Fried LP, Walston J. Frailty and Failure to Thrive. In: Hazzard WR, Blass JP, Ettinger WH Jr, Halter JB, Ouslander J, editors. Principles of Geriatric Medicine and Gerontology. 4. New York: McGraw Hill Publisher; 1998. pp. 1387–1402. [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: Emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 4.Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 5.Ottenbacher KJ, Graham JE, Al Snih S, et al. Mexican americans and frailty: Findings from the Hispanic Established Populations Epidemiologic Studies of the Elderly. Am J Public Health. 2009;99:673–679. doi: 10.2105/AJPH.2008.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinoza SE, Hazuda HP. Frailty in older Mexican American and European American adults: Is there an ethnic disparity? J Am Geriatr Soc. 2008;56:1744–1749. doi: 10.1111/j.1532-5415.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 7.Stern MP, Pugh JA, Gaskill SP, et al. Knowledge, attitudes, and behavior related to obesity and dieting in Mexican Americans and Anglos: The San Antonio Heart Study. Am J Epidemiol. 1982;115:917–928. doi: 10.1093/oxfordjournals.aje.a113379. [DOI] [PubMed] [Google Scholar]
- 8.Hazuda HP, Comeaux PJ, Stern MP, et al. A comparison of three indicators for identifying Mexican Americans in epidemiologic research. Methodological findings from the San Antonio Heart Study. Am J Epidemiol. 1986;123:96–112. doi: 10.1093/oxfordjournals.aje.a114228. [DOI] [PubMed] [Google Scholar]
- 9.Taylor HL, Jacobs DR, Jr, Schucker B, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 10.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 11.Anonymous. Clinical practice recommendations--American Diabetes Association, 1991–1992. Diabetes Care. 1992;15(Suppl 2):1–80. [PubMed] [Google Scholar]
- 12.Anonymous. The sixth report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Erratum appears in arch intern med 1998 Mar 23;158:573. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 13.Rose GA, Blackburn H. Cardiovascular Survey Methods. World Health Organization; 1968. [PubMed] [Google Scholar]
- 14.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 15.Pauwels R, Buist A, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO global initiative for chronic obstructive lung disease (GOLD) workshop summary. Am J Respir Crit Care Med. 2001;163:1256. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard RE, Lang IA, Llewellyn DJ, et al. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. 2010;65:377. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 19.Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch Intern Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 20.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–66. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 21.Blaum CS, Xue QL, Michelon E, et al. The association between obesity and the frailty syndrome in older women: The Women’s Health and Aging Studies. J Am Geriatr Soc. 2005;53:927–934. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- 22.Espinoza SE, Jung I, Hazuda HP. Lower frailty incidence in older Mexican Americans than in older European Americans: The San Antonio Longitudinal Study of Aging. J Am Geriatr Soc. 2010;58:2142–2148. doi: 10.1111/j.1532-5415.2010.03153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szanton SL, Seplaki CL, Thorpe RJ, et al. Socioeconomic status is associated with frailty: The Women’s Health and Aging Studies. J Epidemiol Community Health. 2010;64:63–67. doi: 10.1136/jech.2008.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging research conference on frailty in older adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 25.Taylor SE, Repetti RL, Seeman T. Health psychology: What is an unhealthy environment and how does it get under the skin? Annu Rev Psychol. 1997;48:411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- 26.Al Snih S, Markides KS, Ray L, et al. Handgrip strength and mortality in older Mexican Americans. J Am Geriatr Soc. 2002;50:1250–1256. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- 27.Hardy SE, Studenski SA. Fatigue predicts mortality in older adults. J Am Geriatr Soc. 2008;56:1910–1914. doi: 10.1111/j.1532-5415.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham JE, Snih SA, Berges IM, et al. Frailty and 10-year mortality in community-living Mexican American older adults. Gerontology. 2009;55:644–651. doi: 10.1159/000235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 30.Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: Results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50:1921–1928. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 31.Singh MF. Exercise comes of age. J Gerontol A Biol Sci Med Sci. 2002;57:M262. doi: 10.1093/gerona/57.5.m262. [DOI] [PubMed] [Google Scholar]