Abstract
A corticostriatal-dependent deficit in the release of ascorbate (AA), an antioxidant vitamin and neuromodulator, occurs concurrently in striatum with dysfunctional GLT1-dependent uptake of glutamate in the R6/2 mouse model of Huntington's disease (HD), an autosomal dominant condition characterized by overt corticostriatal dysfunction. To determine if deficient striatal AA release into extracellular fluid is related to altered GLT1 activity in HD, symptomatic R6/2 mice between 6 and 9 weeks of age and age-matched wild-type (WT) mice received single daily injections of 200 mg/kg ceftriaxone, a β-lactam antibiotic that elevates the functional expression of GLT1, or saline vehicle for 5 consecutive days. On the following day, in vivo voltammetry was coupled with corticostriatal afferent stimulation to monitor evoked release of AA into striatum. In saline-treated mice, we found a marked decrease in evoked extracellular AA in striatum of R6/2 relative to WT. Ceftriaxone, in contrast, restored striatal AA in R6/2 mice to WT levels. In addition, intra-striatal infusion of either the GLT1 inhibitor dihydrokainic acid (DHK) or DL-threo-beta-benzyloxyaspartate (TBOA) blocked evoked striatal AA release. Collectively, our results provide compelling evidence for a link between GLT1 activation and release of AA into the striatal extracellular fluid, and suggest that dysfunction of this system is a key component of HD pathophysiology.
Keywords: Huntington's disease, ceftriaxone, ascorbate, glutamate uptake, striatum
Introduction
The extracellular concentration of ascorbate (vitamin C; AA) in striatum is dynamic, serving both anti-oxidative and neuromodulatory roles (see Harrison and May, 2009, Rebec, 2007, and Rebec and Pierce, 1994). Corticostriatal glutamatergic afferent activity comprises the major driving force of AA release into striatal extracellular fluid (Grunewald, 1993; Rebec and Pierce, 1994; Rice, 2000). Interestingly, mice that model Huntington's disease (HD), an autosomal dominant condition caused by an expanded glutamine (CAG) repeat in exon 1 of the huntingtin gene (Huntington's Disease Collaborative Research Group, 1993), exhibit marked deficits in the level of extracellular striatal AA in response to both behavior (Rebec et al., 2002) and stimulation of corticostriatal afferents (Dorner et al., 2009; Rebec, 2009). Thus, it appears that dysfunction of the corticostriatal pathway, which is a major target of HD pathophysiology (see, Cepeda et al., 2007; Miller and Bezprozvanny, 2010; Miller et al., 2011), interferes with release of AA into striatal extracellular fluid.
Even more intriguing is evidence that striatal AA exerts a strong influence on behavioral output. For example, the level of behavioral activation in rodents is correlated with the level of striatal AA release (O'Neill and Fillenz, 1985). Conversely, depletion of extracellular AA in striatum impairs locomotion and other forms of motor sequences (Rebec and Wang, 2001). Related to these findings is evidence that AA treatment not only restores striatal AA extracellular concentrations in HD mice to wild-type (WT) levels, but also attenuates several motor-dependent behavioral signs of HD (Rebec et al., 2003).
Furthermore, blockade of glutamate uptake inhibits release of AA into the extracellular fluid of striatum (Grϋnewald and Fillenz, 1984; Miele et al., 1994), suggesting an AA-glutamate link that may be dysfunctional in HD. Indeed, HD mice exhibit diminished glutamate uptake in striatum, which is concomitant with down-regulation of GLT1 (Behrens et al., 2002, Estrada-Sanchez et al., 2009; Lievens et al., 2001, Miller et al., 2008; Faideau et al., 2010; Sari et al., 2010), the transporter primarily responsible for the clearance of extracellular glutamate (Robinson, 1998; Anderson and Swanson, 2000). We have previously shown that treatment with ceftriaxone, a β-lactam antibiotic that increases the functional expression of GLT1 (Lee et al., 2008; Rothstein et al., 2005), restores glutamate uptake in striatum of R6/2 mice to WT levels and attenuates several HD-related behavioral signs (Miller et al., 2008). Here, to determine if a relationship exists between diminished extracellular AA and GLT1-mediated glutamate uptake in HD striatum, we used slow-scan cyclic voltammetry, which provides a distinct and selective signal for in vivo levels of striatal AA (Gonon et al., 1981; Rebec, 2007), coupled with stimulation of corticostriatal afferents arising from primary motor cortex (M1). Evoked striatal AA was monitored in R6/2 mice, which express a rapidly progressive HD phenotype (Mangiarini et al., 1996; Carter et al., 1999), and in age-matched WT controls. Mice were treated with either ceftriaxone (200 mg/kg) or equivolume saline vehicle to investigate GLT1 involvement in striatal AA efflux. Moreover, dihydrokainic acid (DHK), a selective and non-transportable inhibitor of GLT1 (Arriza et al., 1994), or DL-threo-beta-Benzyloxyaspartate (TBOA), a broad-spectrum non-pump reversing GLT1 antagonist (Tzingounis and Wadiche, 2007) were infused into striatum to confirm the role of GLT1 in striatal AA efflux. Collectively, our results suggest that GLT1 activation drives AA release into the extracellular fluid of striatum and that dysfunction of this mechanism underlies diminished corticostriatal AA release in HD pathophysiology.
Materials and Methods
Animals
Male, transgenic R6/2 mice (B6CBA-TgN[HDexon1]62Gpb), which contain exon 1 of the human HD gene and are based on the C57BL/6 and CBA background strains (Mangiarini et al., 1996), and WT controls were obtained from The Jackson Laboratories (Bar Harbor, ME) at 5-6 weeks of age. Upon arrival, the mice were housed individually under standard laboratory conditions (12-hr light cycle from 7:00AM to 7:00PM) with ad libitum access to food and water. Mice were allowed several days of habituation and were used between 6-9 weeks of age, a range that corresponds to early symptom development extending to approximately the midpoint of symptom severity (Carter et al., 1999). Housing and animal use followed NIH guidelines and was approved by the Indiana University Institutional Animal Care and Use Committee. Groups were designated as WTsal and R6/2sal for mice that received saline or WTcef and R6/2cef for those that received ceftriaxone (see below for treatment protocol). Five WTsal, 5 WTcef, 4 R/62sal, and 6 R6/2cef mice ranging in age between 6 and 8 weeks with mean age of 7.4 ± 0.4, 7.2 ± 0.2, 7.2 ± 0.5, and 7.1 ± 0.2 weeks (mean ± S.E.M.), respectively, were used to determine the effects of ceftriaxone on AA dynamics in striatum. Eight WTcef and 6 R6/2cef mice ranging in age between 7 and 9 weeks with mean age of 7.6 ± 0.4 and 7.3 ± 0.3 (mean ± S.E.M.), respectively were used for DHK infusions. Three WTcef mice, all at 7 weeks of age, were used for TBOA infusions. Consistent with previous findings (Carter et al, 1999), there were no differences in body weights between the groups at all ages tested (p > 0.05).
Genotype and CAG repeat length
Because the HD phenotype is related to CAG repeat length, R6/2 mice were genotyped, and PCR was used to determine CAG repeat length as reported elsewhere (Miller et al., 2008). The mean CAG repeat number for our R6/2 cohort was 125.9 ± 7.9 (S.E.M.) with a range of 116 to 140.
Ceftriaxone treatment protocol
Mice were treated with intraperitoneal (ip) injections of 200 mg/kg ceftriaxone (Sigma, St. Louis, MO), or equivolume saline vehicle once daily for 5 consecutive days. At this dose and treatment period, ceftriaxone increases GLT1 expression by enhancing its genetic transcription (Lee et al., 2008; Rothstein et al., 2005; Sari et al., 2010), and also augments glutamate uptake while attenuating several R6/2 behavioral signs (Miller et al., 2008).
Surgery for voltammetric recordings
All voltammetric testing was performed on the day after the last ceftriaxone injection (i.e., day 6). Mice were anesthetized with chloropent (chloral hydrate and sodium pentobarbital; 0.4 ml/100g, ip) and mounted in a stereotaxic frame. The skull was exposed via an anterior to posterior incision through the scalp and holes were drilled over striatum (anterior +0.5 mm and ±2.0 mm lateral from bregma) for the voltammetry recording electrode and infusion cannula and over cortex (anterior +1.0 mm and ±1.5 mm lateral from bregma) for stimulating electrodes (Franklin and Paxinos, 2008). The mice remained under anesthesia during the course of voltammetric testing and were killed at the end of the experiment. The depth of anesthesia was routinely monitored by ensuring that noxious pinch stimuli to the tail and subsequently the hind paw did not evoke reflexes. Booster doses of chloropent (~0.1 ml/100g, i.p.) were administered as needed.
Voltammetry coupled with cortical stimulation
Our goal was to stimulate corticostriatal afferents remotely and then measure the level of AA released into striatal extracellular fluid. For all experiments, a locally constructed, glass-insulated carbon fiber (10 μm in diameter) served as the working electrode. The active surface area of the carbon fiber extended ~150 μm beyond the glass tip. Electrochemical pretreatment was used to separate the oxidation signal for AA from other easily oxidized compounds such as 3,4-dihydroxyphenylacetic acid (DOPAC) (Gonon et al., 1981; Rebec 2007). The pretreatment protocol consisted of two applications of a 70-Hz triangular wave (versus a saturated calomel reference and stainless steel auxiliary in citrate phosphate buffer): first, for 140 s from zero to +2.1 V and then for 5 s from zero to +2.5 V. Each application, moreover, is followed by 20 s of a constant potential at +1.5 V (Rebec, 2007). Subsequent tests in citrate phosphate buffer containing 100 μM AA and 20 μM DOPAC, which are comparable to known physiological levels in striatum, were used to ensure adequate sensitivity and peak separation. Electrodes that showed poor AA/DOPAC peak separation were discarded. Storage of sampled current and generation of waveforms for slow-scan voltammetry were carried out by a computer interfaced to a locally constructed potentiostat operating in two-electrode mode.
For voltammetric recordings, the working electrode was attached to a custom stereotaxic arm and lowered into left or right striatum (3.2 - 3.5 mm below skull surface). A Ag/AgCl reference electrode was placed under the skin entering at the scalp incision and extending toward the back of the neck. A potential was applied in 6 mV steps from -350 to +450 mV to ensure oxidation of AA. The scan rate was set to100 mV/s to achieve the temporal resolution necessary to track stimulation-evoked changes in extracellular AA concentration. The AA oxidation peak occurs at ~100 mV, which is confirmed for each electrode in vitro. Intra-striatal infusion of AA oxidase, which depletes AA, blocks the AA oxidation signal without affecting DOPAC, confirming the AA peak in vivo (Rebec and Wang, 2001).
Cortical stimulations were evoked using a bipolar Polyamide ® coated stainless steel stimulating electrode (0.2 mm diameter; Plastics One, Inc., Roanoke, VA), which was lowered 0.5 mm using a custom stereotaxic arm into left or right M1 motor cortex. To ensure stable recordings of extracellular AA concentrations in striatum after the electrodes were lowered, preliminary voltammetric scans were obtained approximately every 5 min until the electrode and AA concentrations stabilized. Our voltammetric recording/stimulation protocol consisted of 3 steps: 1) a single baseline voltammetric scan occurred prior to cortical stimulation (pre-stim scan); 2) the pre-stim scan was followed immediately by 10 s of cortical stimulation (500 μA at 60 Hz); and 3) a final scan occurred 100 ms after the stimulation period (post-stim scan). Each animal underwent several (~4) consecutive protocol sessions in each hemisphere, totaling ~8 sessions per animal. A minimum of 5 min was allowed between sessions to ensure that AA concentrations stabilized. We based this inter-session-interval on evidence that the concentration of striatal glutamate decays back to baseline levels 40 s after cortical stimulation (Rebec et al., 2005).
Intra-striatal DHK and TBOA infusion
In a separate cohort of mice treated with only ceftriaxone, intra-striatal infusions of dihydrokainic acid (DHK; 1 mM; Sigma-Aldrich), or DL-threo-beta-Benzyloxyaspartate (TBOA; 0.5 mM; Tocris Bioscience), or equivolume saline vehicle were used to confirm GLT1 involvement in the release of AA into striatum. The DHK infusion concentration was based on evidence of selectivity for GLT1 over other glutamate transporters (e.g. GLAST, EAAC1) at concentrations up to 3 mM (Arriza et al., 1994; Hamann et al., 2002; Tanaka, 1994). Furthermore, we selected the concentration of TBOA because it was shown to be effective in blocking glutamate transport in vivo (Montiel et al., 2005). The infusion cannula was constructed by affixing the working electrode to a 5 μl/26-gauge Hamilton microsyringe. The needle diameter was tapered by inserting a 33-gauge stainless steel tube into the syringe opening. The tip of the infusion cannula extended ~0.2 mm beyond the tip of the working electrode and inter-tip distance was ~0.5 mm. The DHK sessions followed the same protocol as above; however, 4 pre-infusion protocol sessions were collected and mice then received either an intra-striatal infusion of DHK, TBOA, or equivolume saline (1 μl) followed by additional protocol sessions.
Post-calibration and histology
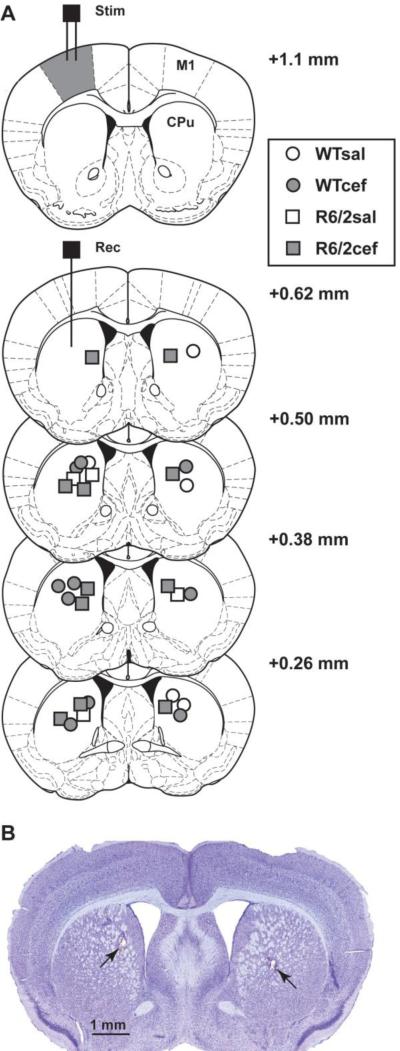
When all recording sessions were completed, the working electrode either was re-tested for sensitivity in buffer containing 100 μM AA and 20 μM DOPAC in order to provide a post-calibration estimation of AA concentration in vivo or was used to confirm striatal placement during subsequent histological analysis. Because lesion-verification is necessary to ensure proper electrode placement in striatum but is not possible when electrodes are re-tested ex vivo to obtain the estimated extracellular concentrations of AA (μM), not all placements could be verified. In all histological samples, however, striatum was consistently and accurately targeted. For histology, mice were anesthetized with chloropent (2 times the surgical dose), and current was passed through the working electrode to mark the recording site. The mice were transcardially perfused with saline followed by 10% formalin. Brains were removed and stored in 30% sucrose in 10% formalin, frozen, sectioned, stained with cresyl violet and examined under a light microscope (Franklin and Paxinos, 2008). Histological analysis confirmed placement of stimulating electrodes in M1 motor cortex and working voltammetry electrodes in striatum (Fig. 1).
Figure 1.
Histological verification of electrode placements. (A) Diagram of coronal sections showing locations of bipolar stimulation electrodes in M1 cortex (stim, filled gray) and voltammetry electrodes in striatum (rec). Symbols in striatum represent electrode placements and numbers indicate distance (mm) anterior to bregma. (B) Photomicrograph of coronal section from an R6/2 mouse showing voltammetry electrode placement in striatum of both hemispheres (arrows).
Data Analysis
Peak heights (measured from the apex of the waveform; see Fig. 3) of the voltammograms (Basse-Tomusk and Rebec, 1990, Rebec et al., 2002, 2003, 2006) were calculated to determine the level of extracellular striatal AA. To compare extracellular striatal AA levels before and after stimulation, the percent change between peak heights of scans taken before stimulation (prestim scans) to scans taken 100 ms after stimulation (post-stim scans) was calculated. Protocol sessions were averaged for each mouse to provide a mean percent change in AA for each animal. Two-way analysis of variance (ANOVA) was used to test differences in striatal AA between the genotypes, the treatment groups and the DHK infusion groups. Tukey tests were used for multiple comparisons. Student's t-test was used to compare the difference in the mean percent change in AA between the pre- and post-saline infusion condition and the TBOA infusion condition. Data represent mean ± standard error of the mean (S.E.M.). The alpha level of significance was p < 0.05. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA) and SigmaPlot 11 (Systat Software Inc, Chicago, IL).
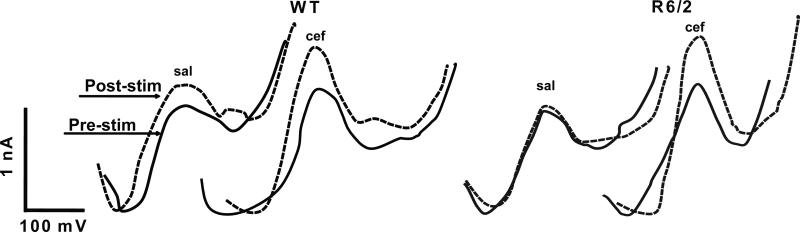
Figure 3.
Representative voltammograms obtained from WT and R6/2 mice treated with saline or ceftriaxone. The pre-stim AA peak (solid line) depicts a single scan taken before cortical stimulation. The post-stim peak (dashed line) depicts a single scan taken 100 ms following stimulation. Note that the peak amplitude of the AA signal is relative to the sensitivity and variability of each electrode; thus, comparison of peak amplitudes across condition or genotype is irrelevant (hence the use of % change to normalize data). Consequently, the difference in amplitude for each condition is the key focus.
Results
Ceftriaxone enhances the level of evoked extracellular striatal AA in R6/2 mice
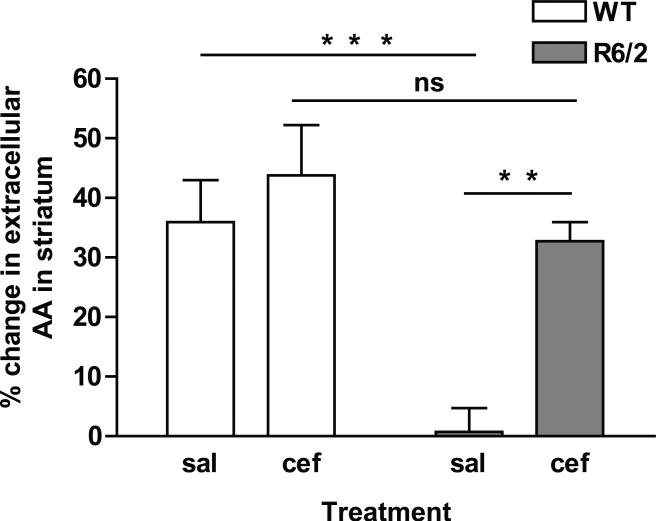
Stimulation of M1 motor cortex resulted in a main effect between both genotype [WT and R6/2; F(1,16) = 14.4, p < 0.01] and treatment [saline and ceftriaxone; F(1,16) = 10.7, p < 0.01]. Cortical M1 stimulation elevated AA in striatal extracellular fluid of WTsal mice, while stimulated release of AA was markedly diminished in R6/2sal mice (Fig. 2; p < 0.001). To investigate whether increasing the level of glutamate uptake would restore striatal AA in R6/2 mice to WT levels, we treated a separate cohort of mice with ceftriaxone. Indeed, ceftriaxone treatment significantly increased the level of evoked striatal AA in R6/2cef relative to R6/2sal mice (Fig. 2; p < 0.01). In fact, the increase in extracellular striatal AA in R6/2cef mice matched WTcef levels (p > 0.05). Ceftriaxone treatment, however, had no effect on striatal AA in WTcef compared to WTsal mice (Fig. 2; p > 0.05). Table I shows the estimated extracellular concentration of striatal AA both before and after cortical stimulation. For representative AA voltammograms for each group, see Fig. 3.
Figure 2.
Ceftriaxone elevates evoked levels of extracellular AA in striatum of R6/2 mice. Data represent the percent change in AA release into striatal extracellular fluid in WT and R6/2 mice treated with either saline (sal) or ceftriaxone (cef). The change in AA was measured by comparing the pre-stimulation voltammetric AA peak height to the post-stimulation peak height. There was a main effect of genotype [F(1,16) = 14.4, p < 0.01] and treatment [F(1,16) = 10.7, p < 0.01]. R6/2sal had little to no increase in extracellular levels of striatal AA following cortical stimulation relative to WT mice. R6/2cef mice, however, had a significantly greater magnitude of striatal AA release than R6/2sal and did not differ from WTcef mice (asterisks represent Tukey test, **p < 0.01; ***p < 0.001; ns = not significant; n = 5 for WTsal, n = 5 for WTcef, n = 4 for R6/2sal, n = 6 for R6/2cef).
Table I.
Estimated extracellular concentration of AA (μM)
| Group | N | Pre-stim | Post-stim |
|---|---|---|---|
| WTsal | 22(5) | 86 ± 15 | 107 ± 18 |
| WTcef | 20(5) | 110 ± 32 | 153 ± 47 |
| R6/2sal | 12(3) | 61 ± 13 | 64 ± 13 |
| R6/2cef | 26(6) | 102 ± 15 | 130 ± 21 |
n = number of sessions (number of mice)
Pre-stim = before cortical stimulation
Post-stim = after cortical stimulation
Data are presented as mean ± S.E.M.
Estimates are based on post-calibration ex vivo
Evoked striatal AA is blocked by DHK and TBOA
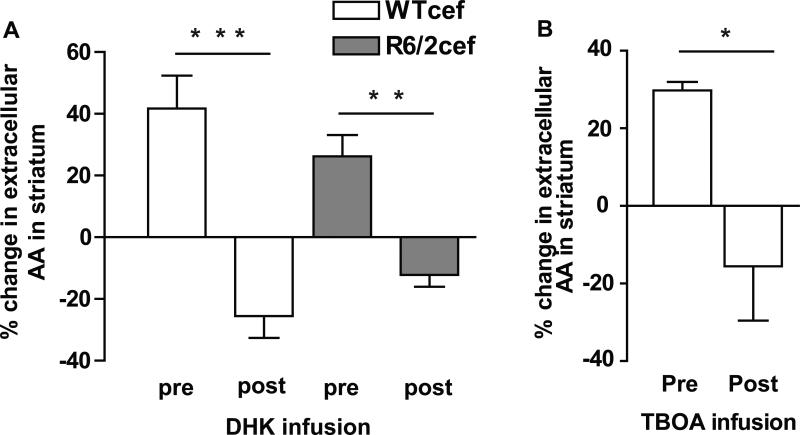
To investigate the role of GLT1 and evoked striatal AA, DHK or TBOA, inhibitors of GLT1, were locally infused into striatum near the site of the voltammetric recording electrode. Our first goal was to ensure that DHK and TBOA did not alter the characteristic voltammetric AA oxidation signal. Ex vivo experiments revealed that DHK (1 mM) and TBOA (0.5 mM) had no effect on the voltammetric detection of AA (100 μM) or DOPAC (20 μM) (data not shown). Because the level of evoked striatal AA was negligible in R6/2sal mice and to circumvent a null effect of DHK and TBOA, infusions were only performed in mice treated with ceftriaxone.
To ensure that infusion alone did not disrupt evoked changes in striatal AA, saline vehicle was infused in a subset of mice. Saline infusion had no effect on the magnitude of evoked AA into extracellular striatum as the percent change in AA was the same both before (40% ± 12) and after (45% ± 17) saline infusion [t-test; p > 0.05; data are from WTcef (n= 4) and R6/2cef (n =1), and because no difference emerged between WTcef and R6/2cef (see above), the mice were pooled for statistical analysis; each mouse underwent 4 protocol sessions (see methods)]. Note that the magnitude of this response is consistent with the magnitude that was found in the WTcef and R6/2cef groups in Fig. 2. Intra-striatal infusion of DHK, however, produced a main effect for infusion [F(1,16) = 49.6, p < 0.001], with no effect on genotype [F(1,16) = 0.02, p > 0.05] in both the WTcef and R6/2cef mice. Indeed, DHK completely blocked evoked AA in WTcef (p < 0.001) and R6/2cef mice (p < 0.01; Fig. 4A). In fact, in the presence of DHK, the level of striatal AA after cortical stimulation was lower than pre-stimulation levels (i.e., mean differences in the % change of AA between pre- vs. post-infusion of DHK was negative). To confirm the role of GLT1 and AA efflux, we infused TBOA into striatum, and like DHK, it lowered evoked AA in WTcef mice in response to cortical stimulation (p < 0.05; Fig. 4B).
Figure 4.
Intrastriatal infusion of either DHK or TBOA blocked evoked levels of AA in striatum. X-axis labels for A and B represent data collected with the cannula placed in striatum but before infusion (pre), and data collected following infusion (post) in the same animals. (A)There was a main effect of infusion [F(1,16) = 49.6, p < 0.001]. No effect of genotype emerged [F(1,16) = 0.02, p > 0.05]. Note the similarity between the WTcef and R6/2cef data here compared to Fig. 2. A Tukey test indicated that DHK infusion diminished striatal AA in WTcef (***p < 0.001) and in R6/2cef mice (**p < 0.01) compared to the pre-DHK condition. (B) TBOA also reduced evoked release of AA in WTcef striatum (t-test, *p< 0.05). n = 8 for WTcef and n = 6 for R6/2cef in DHK experiments, and n = 3 for WTcef TBOA experiments.
Discussion
Our results not only support previous evidence of diminished extracellular AA in striatum of HD mice (Dorner et al., 2007, 2009; Rebec et al., 2002, 2006), but implicate GLT1 dysfunction as a probable mechanism. A ceftriaxone-mediated enhancement of glutamate uptake through elevated GLT1 expression (see Miller et al., 2008; Sari et al., 2010) resulted in complete restoration of evoked striatal AA in R6/2 mice that matched WT levels. Conversely, blockade of glutamate uptake by intra-striatal infusion of either DHK or TBOA led to a marked decrease in the level of evoked AA into the striatum. Collectively, our results show that GLT1 activation is linked to the release of AA into striatal extracellular fluid and lend further support to the view that dysfunction of this mechanism plays a critical role in HD pathophysiology.
Diminished striatal AA in R6/2 mice
In line with previous findings (Rebec et al., 2002, 2003, 2006), evoked striatal AA release was significantly diminished in R6/2sal relative to WTsal mice. In fact, our data recapitulate the findings of Dorner et al., 2009, in which striatal AA increased ~40% in WT, but below ~10% in R6/2 mice in response to cortical stimulation. That the AA response depends on glutamate release from corticostriatal fibers is supported by ample evidence. For example, transient episodes of glutamate release in response to corticostriatal stimulation in rats are tetrodotoxin-sensitive, calcium-dependent, and regulated by metabotropic glutamate receptors, all of which indicate a neuronal origin of glutamate (Lada et al., 1998). Moreover, cortical stimulation elevates striatal extracellular glutamate for up to 40 s (Rebec et al., 2005), well within the time frame of our AA measurements.
Our pre-stimulation AA concentration for WTsal animals (see Table I) is lower than the basal values previously reported for behaving mice (Rebec et al., 2002) and rats (Miele and Fillenz, 1996; Pierce and Rebec, 1990) most likely because our stimulation paradigm required general anesthesia. In fact, chloral hydrate can block electrically stimulated release of AA in rats (O'Neill et al., 1984). Although the concentration of chloral hydrate (170 mg/kg) in our anesthetic cocktail (chloral hydrate and pentobarbital) did not completely block evoked AA release, the level of extracellular AA in striatum is lower when animals are anesthetized than when they are awake (Rebec et al., 2002). Regardless of the pre-stimulation AA concentration, however, R6/2sal mice exhibited a clear decrease in the evoked level of extracellular striatal AA relative to the WTsal group. It is also relevant that barbiturates, including pentobarbital (0.4 ml/100g), can increase AA in the liver (Hahn et al., 1978), which could lead to a subsequent increase in brain AA. Elevated AA in the liver, however, is not likely to be a confounding variable in our study since all data were collected from mice anesthetized with the same cocktail. In fact, relative to waking, anesthesia lowers rather than increases extracellular AA in striatum (Rebec et al., 2002), arguing against an anesthetic effect on striatal AA release.
Interestingly, only when mice become behaviorally active does an extracellular AA difference appear between WT and R6/2s (Rebec et al., 2002). Because activation of corticostriatal fibers is necessary to drive striatal AA release (Basse-Tomusk and Rebec, 1990; O'Neill et al., 1983), our results support growing evidence for corticostriatal dysfunction in HD pathophysiology (Cepeda et al., 2007; Miller and Bezprozvanny, 2010; Miller et al., 2011; Milnerwood and Raymond, 2010b; Raymond et al., 2011).
Mechanism of corticostriatal-dependent release of AA and its dysfunction in HD
Although release of glutamate from corticostriatal terminals triggers the rise in striatal extracellular AA, it is not clear if the source of AA is the corticostriatal projection itself or adjacent astrocytes or both. For example, neurons accumulate AA via the sodium-dependent vitamin C transporter (SVCT2; Harrison and May, 2009; Tsukaguchi et al., 1999), which is highly expressed in cortical pyramidal neurons, but not in striatum (Mun et al., 2006; Berger and Hediger, 2000). In fact, the intracellular concentration of AA is at least 10-times higher in neurons than glia (Rice, 1999) and corticostriatal lesions lower striatal extracellular AA by >70% (Basse-Tomusk and Rebec, 1990). A role for astrocytes cannot be ruled out, however, because they also have the ability to store AA and release it in a glutamate-dependent manner (Wilson et al., 2000). Another consideration is that neuropathology can increase the expression of SVCT2 (Berger et al., 2003), suggesting that HD could change how AA is regulated and stored in striatal neurons. HD also could change how cells use AA. For example, although the level of AA in R6/2 striatal cells is not different from that of WT at 8 weeks of age (Tkac et al., 2007), less AA may be available for release in HD because of a need to combat oxidative stress caused by protein misfolding and mitochondrial dysfunction in striatal neurons (Browne and Beal, 2006). Thus, further research is required to examine the role of AA and its regulation in HD models.
Striatal AA release is thought to result from either activation of glutamate receptors and/or glutamate uptake. Evidence for a receptor-mediated mechanism comes from evidence that striatal AA release is diminished by blocking NMDA receptors with either the non-selective antagonist amantadine, or the non-competitive antagonist MK-801 (Liu et al., 1999; Pierce and Rebec, 1992). Release of AA in retina, moreover, is mediated by activation of AMPA receptors (Portugal et al., 2009). Although activation of postsynaptic glutamate receptors may modulate striatal AA release, it is unlikely that they are the driving force since a loss of striatal cells fails to alter the extracellular level of AA (Pierce et al., 1992).
It appears, instead, that glutamate uptake is the main contributor to striatal AA release. For example, infusion of L-glutamate, the naturally occurring isomer, promotes the rapid release of AA in rat striatal tissue (Ghasemzedah et al., 1991) and in synaptosomal fractions (Grunewald and Fillenz, 1984), whereas D-glutamate, which has less affinity for transport, fails to alter AA release. Similarly, global blockade of glutamate uptake using non-selective uptake inhibitors diminishes extracellular AA in striatum (Grϋnewald and Fillenz, 1984; Miele et al., 1994). Thus, the level of striatal AA in extracellular fluid appears to depend on the level of glutamate uptake. Additional insight into the link between glutamate uptake and striatal AA release comes from studies of HD mice. For example, glutamate release into striatum from corticostriatal fibers is decreased (Joshi et al., 2009; Traficante et al., 2007), but basal extracellular glutamate concentrations are not different (Behrens et al., 2002; Miller et al., 2008) in HD compared to WT animals, suggesting deficient removal of synaptic glutamate. In fact, GLT1 mRNA and protein expression are down-regulated in mouse models of HD (Behrens et al., 2002; Estrada-Sanchez et al., 2009; Lievens et al., 2001), and in postmortem brain tissue taken from HD patients (Arzberger et al., 1997). Deficient glutamate uptake is also prevalent while R6/2 mice are behaving (Miller et al., 2008). In HD, therefore, impaired glutamate uptake occurs in conjunction with diminished levels of AA in striatal extracellular fluid. The relationship between striatal AA release and glutamate uptake by GLT1 is intriguing because GLT1 is present in both astrocytes and at presynaptic densities, albeit expression is ~10% lower in neurons than astrocytes (Furness et al., 2008; de Vivo et al., 2010). It seems likely, therefore, that the efflux of AA from intracellular stores into striatal extracellular fluid is driven by a heteroexchange process, in which AA release from astrocytes occurs in response to glutamate uptake via astrocytic GLT1 activation. This view supports earlier work confirming AA release concomitant with glutamate uptake (Cammack et al., 1991; Miele et al., 1994; O'Neill and Fillenz, 1984). The precise molecular mechanism underlying this exchange, even whether GLT1 operates alone or in combination with other proteins, remains to be determined. It is interesting to note, however, that extracellular AA has a modulatory effect on striatal neuronal activity (Kiyatkin and Rebec, 1998). In R6/2 mice, which have low extracellular AA and a high rate of striatal neuronal firing, treatments that increase extracellular AA also lower striatal activity (Rebec et al., 2006).
Ceftriaxone restores AA in striatum of R6/2 mice
Treatment with ceftriaxone restored the evoked release of striatal AA in R6/2 mice to WT levels. Interestingly, there was a subtle but non-significant increase in striatal AA in WTcef compared to WTsal mice. The lack of a significant ceftriaxone effect in WT mice may be due to compensatory mechanisms that regulate synaptic levels of AA, which is a process necessary for normal function of the corticostriatal synapse (Sandstrom and Rebec, 2007). It is also conceivable that our cortical stimulation protocol evoked a near-maximal response in the release of AA. Thus, AA release into striatal extracellular fluid likely reached a plateau, thereby limiting the effectiveness of ceftriaxone in WT mice, whereas R6/2sal mice. Our stimulation protocol, moreover, promotes neuronal glutamate release (Lada et al., 1998), which in turn drives the efflux of AA into striatal extracellular fluid on a time scale that likely represents release from intracellular stores (Dorner et al., 2009; Rebec et al., 2005). Interestingly, apart from upregulating GLT1, ceftriaxone may promote glutamate release by increasing the cystine-glutamate exchanger (Knackstedt et al., 2009; Lewerenz et al., 2009). This would make more glutamate available for uptake and thus promote a further increase in AA release. Although this mechanism cannot be ruled out, it seems unlikely since glutamate released by activation of the cysteine-glutamate exchanger also acts on presynaptic metabotropic glutamate receptors to inhibit further release (Baker et al., 2003). Collectively, the data suggest that GLT1plays a key role in driving AA into striatal extracellular fluid.
In contrast to our findings, data obtained from cultured avian retinal cells argues against a link between glutamate transport and AA release (Portugal et al., 2009). This report is difficult to interpret, however, because expression of GLT1 in striatum is thought to be localized primarily to astrocytes (Lehre and Danbolt, 1998; Rothstein et al., 1994), whereas GLT1 is localized to amacrine and photoreceptor cells in the avian retina (Reye et al., 2002). In fact, the avian retina is completely devoid of astrocytes (Won et al., 2000). Thus, regulation of AA dynamics in the avian retina and mammalian striatum is likely to be very different. Within striatum, however, recent evidence for differential expression of three GLT1 splice variants (Holmseth et al., 2009) suggests a further complication in understanding GLT1 involvement in AA release.
DHK and TBOA prevent evoked extracellular AA in striatum
To establish a role for GLT1 in mediating the efflux of AA into striatal extracellular fluid, we infused DHK or TBOA into striatum. This infusion completely blocked evoked AA efflux into striatum. In fact, in the presence of either DHK or TBOA, the percent change in the level of evoked striatal AA was negative relative to the pre-infusion level most likely because the GLT1 inhibitors prevented the release of AA. Ample evidence, moreover, indicates that DHK is a selective and non-transportable blocker of GTL1 with low affinity for other glutamate transporters (e.g., GLAST and EAAC1). For example, the Ki of DHK for GLT1 is 23 μM, but ~3,000 μM for GLAST and EAAC1 (Arriza et al., 1994, Tanaka, 1994). It also is relevant that ceftriaxone selectively enhances GLT1 expression (Rothstein et al., 2005), further arguing against a role for other glutamate transporters in the ability of DHK to reverse the ceftriaxone-induced increase in AA. Although our dose of DHK has affinity for kainate receptors (Shimamoto et al., 1998; Shigeri et al., 2004), which are expressed on striatal neurons (Nansen et al., 2000), DHK-activation of kainate receptors cannot explain our results because striatal neurons are not the main source of striatal AA release (Pierce et al., 1992). To confirm the relationship of GLT1 activation and AA release, we infused the broad-spectrum glutamate uptake antagonist TBOA (Montiel et al., 2005) and found similar results to our DHK experiments. Thus, cortical activation appears to trigger AA release via GLT1.
Conclusion
Our results indicate that glutamate uptake through GLT1 activation is necessary for evoked release of AA into striatal extracellular fluid and that this process is deficient in vivo in the R6/2 mouse model of HD. Although the mechanism for the interaction between AA release into striatum and GLT1 activation has not been identified, increasing the functional expression of GLT1 with ceftriaxone (Miller et al., 2008; Sari et al., 2010) directly elevates extracellular AA in striatum of R6/2 mice. Conversely, inhibition of GLT1 leads to a marked decrease in evoked striatal AA. Importantly, release of AA into striatum, in conjunction with corticostriatal activation, likely protects against glutamate-induced excitotoxicity and oxidation (for reviews see, Qiu et al., 2007; Rice, 2000), both of which are major problems in the HD corticostriatal system (Browne & Beal, 2006; Rice, 2000; Estrada-Sanchez et al., 2008; Fan and Raymond, 2007; Milnerwood and Raymond, 2010b; Raymond et al., 2011; Stack et al., 2008). In fact, in mouse models of HD, augmented glutamate signaling, which activates extrasynaptic N-methyl-D-aspartate receptors (Milnerwood et al., 2010a), is suppressed by TBOA-mediated GLT1 blockade. It is likely, therefore, that the temporal coupling of AA with increased glutamate activity ensures some level of protection against excitotoxic mechanisms. Restoring this coupling in HD could point the way toward a novel but effective therapeutic strategy.
Acknowledgments
This work was supported by grants from the National Institute of Neurological Disorders and Stroke: R01 NS-35663 (GVR), F31 NS-064791 (BRM) and F31 NS-060218 (JLD); and from the Neuroscience Node of the Indiana University Metabolomics and Cytomics (METACyt) Initiative funded, in part, by a grant from the Lilly Endowment. Faye Caylor and Paul Langley provided administrative and technical support, respectively. We thank Youssef Sari for assistance with the photomicrograph and Dr. Estrada-Sanchez for helpful discussion.
References
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzberger T, Krampfl K, Leimgruber S, Weindl A. Changes of NMDA receptor subunit (NR1, NR2B) and glutamate transporter (GLT1) mRNA expression in Huntington's disease--an in situ hybridization study. J. Neuropathol. Exp. Neurol. 1997;56:440–454. doi: 10.1097/00005072-199704000-00013. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat. Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Basse-Tomusk A, Rebec GV. Corticostriatal and thalamic regulation of amphetamine-induced ascorbate release in the neostriatum. Pharmacol. Biochem. Behav. 1990;35:55–60. doi: 10.1016/0091-3057(90)90204-u. [DOI] [PubMed] [Google Scholar]
- Behrens PF, Franz P, Woodman B, Lindenberg KS, Landwehrmeyer GB. Impaired glutamate transport and glutamate-glutamine cycling: downstream effects of the Huntington mutation. Brain. 2002;125:1908–1922. doi: 10.1093/brain/awf180. [DOI] [PubMed] [Google Scholar]
- Berger UV, Hediger MA. The vitamin C transporter SVCT2 is expressed by astrocytes in culture but not in situ. Neuroreport. 2000;11:1395–1399. doi: 10.1097/00001756-200005150-00009. [DOI] [PubMed] [Google Scholar]
- Berger UV, Lu XC, Liu W, Tang Z, Slusher BS, Hediger MA. Effect of middle cerebral artery occlusion on mRNA expression for the sodium-coupled vitamin C transporter SVCT2 in rat brain. J Neurochem. 2003;4:896–906. doi: 10.1046/j.1471-4159.2003.01891.x. [DOI] [PubMed] [Google Scholar]
- Bridges RJ, Kavanaugh MP, Chamberlin AR. A pharmacological review of competitive inhibitors and substrates of high-affinity, sodium-dependent glutamate transport in the central nervous system. Curr. Pharm. Des. 1999;5:363–379. [PubMed] [Google Scholar]
- Browne SE, Beal MF. Oxidative damage in Huntington's disease pathogenesis. Antioxid. Redox. Signal. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J. Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casassus G, Mulle C. Functional characterization of kainite receptors in the mouse nucleus accumbens. Neuropharmacology. 2002;42:603–611. doi: 10.1016/s0028-3908(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Wu N, Andre VM, Cummings DM, Levine MS. The corticostriatal pathway in Huntington's disease. Prog. Neurobiol. 2007;81:253–271. doi: 10.1016/j.pneurobio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder TL, Weiner JL. Functional characterization of kainite receptors in the rat nucleus accumbens core region. J. Neurophysiol. 2002;88:41–48. doi: 10.1152/jn.2002.88.1.41. [DOI] [PubMed] [Google Scholar]
- de Vivo L, Melone M, Rothstein JD, Conti F. GLT-1 promoter activity in astrocytes and neurons of mouse hippocampus and somatic sensory cortex. Frontiers in Neuroanatomy. 2010;3:3. doi: 10.3389/neuro.05.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner JL, Miller BR, Barton SJ, Brock TJ, Rebec GV. Sex differences in behavior and striatal ascorbate release in the 140 CAG knock-in mouse model of Huntington's disease. Behav. Brain Res. 2007;178:90–97. doi: 10.1016/j.bbr.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner JL, Miller BR, Klein EL, Murphy-Nakhnikian A, Andrews RL, Barton SJ, Rebec GV. Corticostriatal dysfunction underlies diminished striatal ascorbate release in the R6/2 mouse model of Huntington's disease. Brain Res. 2009;1290:111–120. doi: 10.1016/j.brainres.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Sanchez AM, Montiel T, Segovia J, Massieu L. Glutamate toxicity in the striatum of the R6/2 Huntington's disease transgenic mice is age-dependent and correlates with decreased levels of glutamate transporters. Neurobiol. Dis. 2009;34:78–86. doi: 10.1016/j.nbd.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Estrada Sanchez AM, Mejia-Toiber J, Massieu L. Excitotoxic neuronal death and the pathogenesis of Huntington's disease. Arch. Med. Res. 2008;39:265–276. doi: 10.1016/j.arcmed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Fan MM, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington's disease. Prog. Neurobiol. 2007;81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd ed. Academic Press; New York: 2008. with CD-ROM. [Google Scholar]
- Furness DN, Dehnes Y, Akhtar AQ, Rossi DJ, Hamann M, Grutle NJ, Gundersen V, Holmseth S, Lehre KP, Ullensvang K, Wojewodzic M, Zhou Y, Attwell D, Danbolt NC. A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: new insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2). Neuroscience. 2008;157:80–94. doi: 10.1016/j.neuroscience.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh B, Cammack J, Adams RN. Dynamic changes in extracellular fluid ascorbic acid monitored by in vivo electrochemistry. Brain Res. 1991;547:162–166. [PubMed] [Google Scholar]
- Gonon F, Buda M, Cespuglio R, Jouvet M, Pujol JF. Voltammetry in the striatum of chronic freely moving rats: detection of catechols and ascorbic acid. Brain Res. 1981;223:69–80. doi: 10.1016/0006-8993(81)90807-6. [DOI] [PubMed] [Google Scholar]
- Grunewald RA, Fillenz M. Release of ascorbate from synaptosomal fraction of rat brain. Neurochem. Int. 1984;6:491–500. doi: 10.1016/0197-0186(84)90120-7. [DOI] [PubMed] [Google Scholar]
- Grunewald RA. Ascorbic acid in the brain. Brain Res. Brain Res. Rev. 1993;18:123–133. doi: 10.1016/0165-0173(93)90010-w. [DOI] [PubMed] [Google Scholar]
- Hahn HK, Barak AJ, Tuma DJ, Sorrell MF. Effects of Phenobarbital administration on levels of physiological antioxidants in rat liver. Pharmacology. 1978;17:341–344. doi: 10.1159/000136875. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Marie H, Attwell D. Knocking out the glial glutamate transporter GLT-1 reduces glutamate uptake but does not affect hippocampal glutamate dynamics in early simulated ischaemia. Eur. J. Neurosci. 2002;2:308–314. doi: 10.1046/j.0953-816x.2001.01861.x. [DOI] [PubMed] [Google Scholar]
- Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic. Bio.l and Med. 2009;46:719–730. doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmseth S, Scott HA, Real K, Leher KP, Leergaard TB, Bjaalie JG, Danbolt NC. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience. 2009;162:1055–1071. doi: 10.1016/j.neuroscience.2009.03.048. [DOI] [PubMed] [Google Scholar]
- Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Joshi PR, Wu NP, Andre VM, Cummings DM, Cepeda C, Joyce JA, Carroll JB, Leavitt BR, Hayden MR, Levine MS, Bamford NS. Age-dependent alterations of corticostriatal activity in the YAC128 mouse model of Huntington disease. J. Neurosci. 2009;29:2414–2427. doi: 10.1523/JNEUROSCI.5687-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Ascorbate modulates glutamate-induced excitations of striatal neurons. Brain Res. 1998;812:14–22. doi: 10.1016/s0006-8993(98)00814-2. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol. Psychiatry. 2009;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lada MW, Vickroy TW, Kennedy RT. Evidence for neuronal origin and metabotropic receptor-mediated regulation of extracellular glutamate and aspartate in rat striatum in vivo following electrical stimulation of the prefrontal cortex. J. Neurochem. 1998;70:617–625. doi: 10.1046/j.1471-4159.1998.70020617.x. [DOI] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J. Biol. Chem. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Albrecht P, Tien ML, Henke N, Karumbayaram S, Kornblum HI, Wiedau-Pazos M, Schubert D, Maher P, Methner A. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J. Neurochem. 2009;111:332–343. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J. Neurosci. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievens JC, Woodman B, Mahal A, Spasic-Boscovic O, Samuel D, Kerkerian-Le Goff L, Bates GP. Impaired glutamate uptake in the R6 Huntington's disease transgenic mice. Neurobiol. Dis. 2001;8:807–821. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- Liu J, Wu C, Liu W, Zhang H, Li CL. Involvement of the corticostriatal glutamatergic pathway in ethanol-induced ascorbic acid release in rat striatum. Addict. Biol. 1999;4:273–281. doi: 10.1080/13556219971489. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Miele M, Boutelle MG, Fillenz M. The physiologically induced release of ascorbate in rat brain is dependent on impulse traffic, calcium influx and glutamate uptake. Neuroscience. 1994;62:87–91. doi: 10.1016/0306-4522(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Miele M, Fillenz M. In vivo determination of extracellular brain ascorbate. J. Neurosci. Methods. 1996;70:15–19. doi: 10.1016/S0165-0270(96)00094-5. [DOI] [PubMed] [Google Scholar]
- Miller BR, Bezprozvanny I. Corticostriatal circuit dysfunction in Huntington's disease: intersection of glutamate, dopamine, and calcium. Future Neurol. 2010;5:735–756. doi: 10.2217/fnl.10.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington's disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Walker AG, Barton SJ, Rebec GV. Dysregulated Neuronal Activity Patterns Implicate Corticostriatal Circuit Dysfunction in Multiple Rodent Models of Huntington's Disease. Front. Syst. Neurosci. 2011;5:26. doi: 10.3389/fnsys.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD, Ko RW, Vasuta OC, Graham RK, Hayden MR, Murphy TH, Raymond LA. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington's disease mice. Neuron. 2010;65(2):178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Milnerwood AJ, Raymond LA. Early synaptic pathophysiology in neurodegeneration: insights from Huntington's disease. Trends Neurosci. 2010;33:513–523. doi: 10.1016/j.tins.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Montiel T, Camacho A, Estrada-Sanchez AM, Massieu L. Differential effects of the substrate inhibitor L-transpyrrolidine-2,4-Dicarboxylate (PDC) and the non-substrate inhibitor DL-threo-β-Benzyloxyaspartate (DL-TBOA) of glutamate transporters on neuronal damage and extracellular amino acid levels in rat brain in vivo. Neuroscience. 2005;133:667–678. doi: 10.1016/j.neuroscience.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Mun GH, Kim MJ, Lee JH, et al. Immunohistochemical study of the distribution of sodium-dependent vitamin C transporters in adult rat brain. J. Neurosci. Res. 2006;83:919–928. doi: 10.1002/jnr.20751. [DOI] [PubMed] [Google Scholar]
- Nansen EA, Jokel ES, Lobo MK, Micevych PE, Ariano MA, Levine MS. Striatal ionotropic glutamate receptor ontogeny in the rat. Dev. Neurosci. 2000;22:329–340. doi: 10.1159/000017457. [DOI] [PubMed] [Google Scholar]
- O'Neill RD, Grunewald RA, Fillenz M, Albery WJ. The effect of unilateral cortical lesions on the circadian changes in rat striatal ascorbate and homovanillic acid levels measured in vivo using voltammetry. Neurosci. Lett. 1983;42:105–110. doi: 10.1016/0304-3940(83)90430-5. [DOI] [PubMed] [Google Scholar]
- O'Neill RD, Fillenz M, Sundstrom L, Rawlins JN. Voltammetrically monitored brain ascorbate as an index of excitatory amino acid release in the unrestrained rat. Neurosci. Lett. 1984;52:227–233. doi: 10.1016/0304-3940(84)90166-6. [DOI] [PubMed] [Google Scholar]
- O'Neill RD, Fillenz M. Circadian changes in extracellular ascorbate in rat cortex, accumbens, striatum and hippocampus: correlations with motor activity. Neurosci. Lett. 1985;60:331–336. doi: 10.1016/0304-3940(85)90599-3. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Miller DW, Reising DB, Rebec GV. Unilateral neostriatal kainate, but not 6-OHDA, lesions block dopamine agonist-induced ascorbate release in the neostriatum of freely moving rats. Brain Res. 1992;597:138–143. doi: 10.1016/0006-8993(92)91515-g. [DOI] [PubMed] [Google Scholar]
- Portugal CC, Miya VS, Calaza Kda C, Santos RA, Paes-de-Carvalho R. Glutamate receptors modulate sodium-dependent and calcium-independent vitamin C bidirectional transport in cultured avian retinal cells. J. Neurochem. 2009;108:507–520. doi: 10.1111/j.1471-4159.2008.05786.x. [DOI] [PubMed] [Google Scholar]
- Qiu S, Li L, Weeber EJ, May JM. Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J. Neurosci. Res. 2007;85:1046–1056. doi: 10.1002/jnr.21204. [DOI] [PubMed] [Google Scholar]
- Raymond LA, André VM, Cepeda C, Gladding CM, Milnerwood AJ, Levine MS. Pathophysiology of Huntington's disease: time-dependent alterations in synaptic and receptor function. Neuroscience. 2011;198:252–273. doi: 10.1016/j.neuroscience.2011.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Pierce RC. A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog. Neurobiol. 1994;43:537–565. doi: 10.1016/0301-0082(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Wang Z. Behavioral activation in rats requires endogenous ascorbate release in striatum. J. Neurosci. 2001;21:668–675. doi: 10.1523/JNEUROSCI.21-02-00668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Barton SJ, Ennis MD. Dysregulation of ascorbate release in the striatum of behaving mice expressing the Huntington's disease gene. J. Neurosci. 2002;22:RC202. doi: 10.1523/JNEUROSCI.22-02-j0006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Barton SJ, Marseilles AM, Collins K. Ascorbate treatment attenuates the Huntington behavioral phenotype in mice. Neuroreport. 2003;14:1263–1265. doi: 10.1097/00001756-200307010-00015. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Witowski SR, Sandstrom MI, Rostand RD, Kennedy RT. Extracellular ascorbate modulates cortically evoked glutamate dynamics in rat striatum. Neurosci. Lett. 2005;378:166–170. doi: 10.1016/j.neulet.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Conroy SK, Barton SJ. Hyperactive striatal neurons in symptomatic Huntington R6/2 mice: variations with behavioral state and repeated ascorbate treatment. Neuroscience. 2006;137:327–336. doi: 10.1016/j.neuroscience.2005.08.062. [DOI] [PubMed] [Google Scholar]
- Rebec GV. From interferant anion to neuromodulator: ascorbate oxidizes its way to respectability. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. CRC Press; Boca Raton, FL: 2007. pp. 149–165. [PubMed] [Google Scholar]
- Reye P, Sullivan R, Fletcher EL, Pow DV. Distribution of two splice variants of the glutamate transporter GLT1 in the retinas of humans, monkeys, rabbits, rats, cats, and chickens. J. Comp. Neurol. 2002;445:1–12. doi: 10.1002/cne.10095. [DOI] [PubMed] [Google Scholar]
- Rice ME. Ascorbate compartmentalization in the CNS. Neurotox. Res. 1999;2:81–90. doi: 10.1007/BF03033272. [DOI] [PubMed] [Google Scholar]
- Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- Robinson MB. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem. Int. 1998;33:479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sandstrom MI, Rebec GV. Extracellular ascorbate modulates glutamate dynamics: role of behavioral activation. BMC Neurosci. 2007;168:32. doi: 10.1186/1471-2202-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Prieto AL, Barton SJ, Miller BR, Rebec GV. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington's disease. J. Biomed. Sci. 2010;17:62. doi: 10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Brain Res Rev. 2004;45:250–265. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-thre-beta-benzyloxyaspartate, a potent bloker of excitatory amino acid transmission. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Stack EC, Matson WR, Ferrante RJ. Evidence of oxidant damage in Huntington's disease: translational strategies using antioxidants. Ann. N. Y. Acad. Sci. 2008;1147:79–92. doi: 10.1196/annals.1427.008. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Pharmacological characterization of a cloned glutamate transporter (GluT-1). Mol. Brain. Res. 1994;21:167–170. doi: 10.1016/0169-328x(94)90390-5. [DOI] [PubMed] [Google Scholar]
- Tkac I, Dubinsky JM, Keene CD, Gruetter R, Low WC. Neurochemical changes in Huntington R6/2 mouse striatum detected by in vivo 1H NMR spectroscopy. J. Neurochem. 2007;100:1397–1406. doi: 10.1111/j.1471-4159.2006.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traficante A, Riozzi B, Cannella M, Rampello L, Squitieri F, Battaglia G. Reduced activity of cortico-striatal fibres in the R6/2 mouse model of Huntington's disease. NeuroReport. 2007;18:1997–2000. doi: 10.1097/WNR.0b013e3282f262ca. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat. Rev. Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Wilson JX, Peters CE, Sitar SM, Daoust P, Gelb AW. Glutamate stimulates ascorbate transport by astrocytes. Brain Res. 2000;858:61–66. doi: 10.1016/s0006-8993(99)02433-6. [DOI] [PubMed] [Google Scholar]
- Won MH, Kang TC, Cho SS. Glial cells in the bird retina: immunochemical detection. Microsc. Res. Tech. 2000;50:151–160. doi: 10.1002/1097-0029(20000715)50:2<151::AID-JEMT7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]