Abstract
Abnormalities in serotonin systems are presumably linked to various psychiatric disorders including schizophrenia and depression. Medications intended for these disorders aim to either block the reuptake or the degradation of this neurotransmitter. In an alternative approach, efforts have been made to enhance serotonin levels through dietary manipulation of precursor levels with modest clinical success. In the last 30 years, there has been little improvement in the pharmaceutical management of depression, and now is the time to revisit therapeutic strategies for the treatment of this disease. Tryptophan hydroxylase (TPH) catalyzes the first and rate-limiting step in the biosynthesis of serotonin. A recently discovered isoform, TPH2, is responsible for serotonin biosynthesis in the brain. Learning how to activate this enzyme (and its polymorphic versions) may lead to a new, more selective generation of antidepressants, able to regulate the levels of serotonin in the brain with fewer side effects.
Keywords: Antidepressants, depression, serotonin, tryptophan hydroxylase 2
Introduction
Depression is a serious medical disorder that presents with wide-ranging symptoms, including depressed mood, loss of interest or pleasure, feelings of guilt or low self-worth, low energy and poor concentration. Over 121 million people are affected by this disease around the world, and approximately one in six Americans will suffer from it during their lifetime (Krishnan and Nestler, 2008). Aside from the mortality associated with suicide, depressed individuals are more likely to develop coronary artery disease and diabetes (Knol et al., 2006; Krishnan and Nestler, 2008; Musselman et al., 2003), and when they occur, outcomes and mortality tend to be worse. Furthermore, depression worsens the prognosis of other concurrent chronic medical conditions (Evans et al., 2005; Gildengers et al., 2008; Krishnan and Nestler, 2008) and also has a deep impact on the families of those affected (van Wijngaarden et al., 2009). All in all, the economic burden of depression is estimated to be in the range of US$80–100 billion per year in the United States alone (Greenberg et al., 2003; Pincus and Pettit, 2001).
In the early 1970s, deficiencies in the neurotransmission of serotonin (5-hydroxytryptamine or 5-HT; Figure 1) were linked to depression (Meltzer, 1989). Based on this, a theory was postulated that depression results from a deficiency of monoamine function (5-HT or norepinephrine), commonly referred to as the monoamine hypothesis of depression (Wong and Licinio, 2004). Thus, chemicals that enhance 5-HT action by either blocking its uptake from the synaptic cleft or inhibiting its degradation have been widely used as antidepressants (Wong et al., 2005; Wong and Licinio, 2004). Also, alterations in the levels of the tryptophan in the brain, the main precursor to serotonin, were found to rapidly affect the rate at which serotonin was synthesized (Fernstrom and Wurtman, 1971; Wurtman, 1986). Thus, efforts to achieve increases in the serotonin levels through the dietary manipulation of tryptophan, in foods or supplements, were undertaken with modest success (Wurtman, 1988). Serotonergic dysfunctions have since been linked to many other neuropsychiatric illnesses including obsessive–compulsive disorder, schizophrenia and autism (Mockus and Vrana, 1998; Veenstra-VanderWeele and Cook, 2004; Zhang et al., 2006). However, it is important to note that serotonin is not the only story and that dysregulation of other neurotransmitters, such as norepinephine and dopamine, have also been implicated in the etiology of depression (Nutt, 2006).
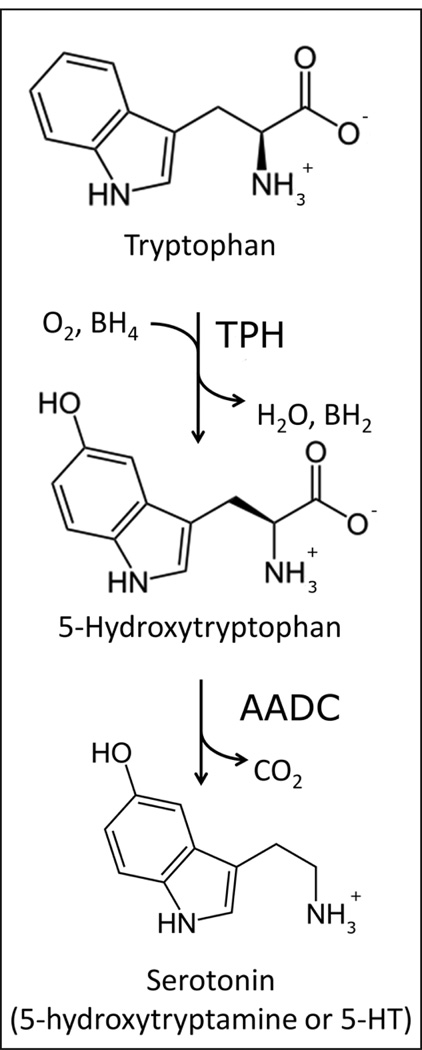
Figure 1.
The biosynthesis of serotonin. Tryptophan hydroxylase (TPH) catalyzes the first and rate-limiting step in the synthesis of serotonin (5-hydroxytryptamine) using tetrahydrobiopterin (BH4) and dioxygen as co-substrates and producing water and dihydrobiopterin (BH2) as byproducts. The second and final reaction in the biosynthesis of serotonin is catalyzed by the aromatic amino acid decarboxylase (AADC).
Until the late 1980s, depression was primarily treated with electroconvulsive therapy and drugs such as tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) (Licinio and Wong, 2005). The majority of the TCAs act as serotonin–norepinephrine reuptake inhibitors (SNRIs) by blocking the serotonin and norepinephrine transporters and producing a rise in the extracellular concentrations of these neurotransmitters and therefore an enhancement of neurotransmission (Wong et al., 2005). In contrast, MAOIs increase the concentrations of monoamines by preventing the degradation of the neurotransmitters by the enzyme monoamine oxidase. While TCAs and MAOIs are effective at treating the symptoms of depression, they also can cause severe adverse reactions and toxicity in overdose (Wong et al., 2005).
In the early 1980s, selective serotonin reuptake inhibitors (SSRIs) were introduced. These drugs (see Table 1) also increase the availability of monoamines in the synapse between neurons, but they do so by specifically and selectively inhibiting the uptake of serotonin. Although these treatments have provided valuable relief to millions of patients around the world, they take weeks to exert full efficacy, 60–70% of patients fail to reach remission on initial treatment, and many fail to take the medicine as directed due to side effects (de Bodinat et al., 2010). While there are a number of alternate treatments available, next-step options when an SSRI fails to produce remission are ambiguous, and most antidepressants employ similar pharmacologic mechanisms. With the exception of the melatonin agonist/serotonin (5-HT)2C antagonist agomelatine, there have been no major breakthroughs in the pharmaceutical management of depression despite massive advancements in the field of neuroscience over the past 30 years (de Bodinat et al., 2010; Kennedy, 2009; Spedding et al., 2005).
Table 1.
Selective serotonin reuptake inhibitors (SSRIs) available in the US market.
| Trade Name in the US | Generic Name | Year Introduced |
|---|---|---|
| Prozac® | Fluoxetine | 1987 |
| Zoloft® | Sertraline | 1991 |
| Paxil® | Paroxetine | 1992 |
| Luvox® | Fluvoxamine | 1994 |
| Celexa® | Citalopram | 1998 |
| Lexapro® | Escitalopram | 2002 |
There is an obvious and compelling public-health need for conceptually novel antidepressants capable of rapidly and safely treating patients. A better understanding of serotonin biosynthesis may provide opportunities for developing such novel approaches. Rather than block the reuptake or the degradation of serotonin, we suggest exploring the direct activation of brain serotonin synthesis as a novel approach to treating depression. It is our hope that this strategy will open the door to a new, more selective and effective generation of antidepressants with fewer side effects.
Brain serotonin biosynthesis
The biosynthesis of serotonin involves the hydroxylation and decarboxylation of the essential amino acid tryptophan (Figure 1). Tryptophan hydroxylase (TPH) catalyzes the first and rate-limiting step in this process. Subsequent decarboxylation of 5-hydroxytryptophan by the aromatic amino acid decarboxylase (AADC) produces serotonin (Carkaci-Salli et al., 2006).
TPH, together with tyrosine hydroxylase (TH) and phenylalanine hydroxylase (PAH), belongs to the aromatic amino acid hydroxylase family, a small group of monooxygenases that utilize tetrahydrobiopterin (BH4) and molecular oxygen as substrates (Figure 1), in the presence of iron as a cofactor (Fitzpatrick, 1999). In eukaryotes, these enzymes are homotetramers composed of subunits comprising a catalytic domain in between an N-terminal regulatory domain and a C-terminal tetramerization domain (Figure 2) (Fitzpatrick, 1999). The catalytic domains are highly homologous and thus their catalytic mechanisms are thought to be similar; however, sequence identities between the regulatory domains are low, suggesting that they may be subject to distinct regulatory mechanisms (Fitzpatrick, 1999).
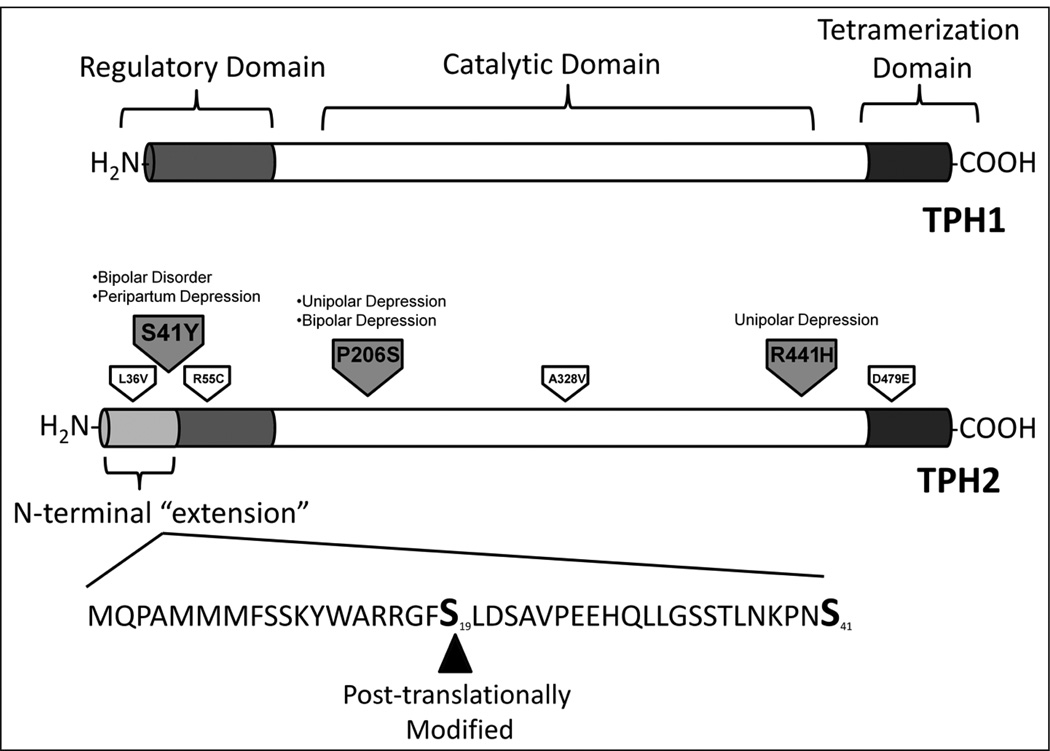
Figure 2.
The structures of tryptophan hydroxylase 1 (TPH1) and tryptophan hydroxylase 2 (TPH2). The two isoforms of this enzyme contain a C-terminal tetramerization domain (shown in dark gray), a catalytic domain (in white) and an N-terminal regulatory domain (in gray). In TPH2, the regulatory domain contains 41 ‘extra’ amino acids that TPH1 lacks. Within this N-terminal ‘extension’, TPH2 is phosphorylated at Serine 19. Non-synonymous polymorphisms found on human TPH2 are shown. Psychiatric diseases associated with particular mutations are noted; polymorphisms for which no diseased phenotype has been found are also shown.
For many years, a single gene encoding TPH was believed to be responsible for all serotonin synthesis in vertebrates. However, a second gene encoding a different isoform of TPH (TPH2) was discovered in 2003 (Walther et al., 2003). While TPH1 was found to be expressed in the periphery and the pineal gland (McKinney et al., 2005; Zhang et al., 2004), the newly discovered variant, TPH2, was found to be preferentially expressed in the brain (almost exclusively in the raphe nuclei) (Patel et al., 2004). Thus, TPH2 is thought to be responsible for the majority of serotonin synthesis in the brain (Alenina et al., 2009; Gutknecht et al., 2008; Savelieva et al., 2008). TPH1 and TPH2 share considerable sequence identity, particularly within their catalytic domains; nevertheless, their regulatory domains differ (Figure 2) (Murphy et al., 2008).
Structure and regulation of TPH2
The characterization of both TPH1 and TPH2 has been hindered by the fact that TPH is a particularly unstable enzyme; in addition, formation of insoluble inclusion bodies in bacteria has limited its purification in large quantities (Carkaci-Salli et al., 2006). Despite this, expression of recombinant TPH2 has been achieved in Escherichia coli and some mammalian cell systems such as PC12 and HEK cells (Carkaci-Salli et al., 2006; McKinney et al., 2005; Winge et al., 2007; Zhang et al., 2004, 2005). From protein produced through these expression systems, a few structural properties of TPH2 have been revealed. TPH2 shares a 71% sequence identity with TPH1(McKinney et al., 2005); however, TPH2 contains an ‘extended’ regulatory domain with an additional 41 amino acids absent in TPH1 (Figure 2). This region of the protein is thought to be involved in the regulation of enzyme expression (Murphy et al., 2008) and it has been found to increase TPH2’s instability (Carkaci-Salli et al., 2006).
To date, no crystal structure of TPH2 has been elucidated; however, information can be extrapolated from available crystal structures for TPH1 (Wang et al., 2002; Windahl et al., 2008). A crystal structure of the catalytic domain of human TPH1 with bound dihydrobiopterin (BH2) and iron shows the catalytic domain of TPH1 to be similar to the catalytic domain of PAH with either BH4 or BH2 bound (Wang et al., 2002). The crystal structure of the catalytic domain of chicken TPH1 bound to tryptophan and iron shows the binding of tryptophan causes structural changes in the catalytic domain compared with the structure of the human TPH1 without tryptophan (Windahl et al., 2008). Similar structural changes have been observed to occur in the catalytic domain of iron-bound PAH upon binding of substrate analogues (Andersen et al., 2003).
While little is known about the mechanisms that regulate TPH2’s function, a fair amount of information has been collected on the regulation of TH and PAH activity. TH is subject to feedback inhibition by catecholamine products and is also subject to allosteric regulation. In the case of TH, heparin, phospholipids, and polyanions have all been shown to reversibly interact with the enzyme to produce an increase in enzymatic activity. These effectors are thought to bind to the regulatory domain of TH protein and produce a conformational change that activates the enzyme (Kumer and Vrana, 1996). Moreover, TH is known to be regulated presynaptically in vivo by a number of autoreceptor feedback-modulated events (reviewed in Dunkley et al., 2004). In the case of PAH, its substrate, phenylalanine, has been found to act as an allosteric activator (Kobe et al., 1999). Recent findings support a model in which phenylalanine binding causes a conformational change in the regulatory domain that alters the interaction between it and the catalytic domain of the protein (Li et al., 2010). As a member of the aromatic amino acid hydroxylase family, it is conceivable that TPH2 will be subject to some form of feedback or allosteric regulation yet to be discovered. No direct evidence of such regulation has been observed to date; however, knowledge of TPH2’s control mechanisms may open the doors to new ways of increasing brain serotonin levels.
Post-translational modification of TPH2
Post-translational modifications (PTMs) have been shown to regulate protein function and interactions (Young et al., 2010). Given the central importance of TPH2, it is likely that this enzyme will be regulated through several types of PTMs at multiple sites throughout the protein. Within the regulatory domain ‘extension’ unique to TPH2, the enzyme is known to be phosphorylated on Ser19 by both protein kinase A and Ca2+/calmodulin dependant protein kinase II in vitro (Kuhn et al., 2007; Murphy et al., 2008; Winge et al., 2008) (Figure 2). This modification results in increased stability and activity (Kuhn et al., 2007; Murphy et al., 2008). Moreover, 14-3-3 proteins have been shown to bind this modification, further stabilizing TPH2 (Winge et al., 2008). The only other established TPH2 phosphorylation site is Ser104. It is likely that many other PTM sites and binding partners for TPH2 remain undiscovered.
We hypothesize that the PTM of TPH2 could play an important role in the regulation of this key enzyme. For instance, increased nocturnal serotonin synthesis in the pineal gland has been shown to be mediated by the phosphorylation of TPH1 at serine 58 (Huang et al., 2008); however, in the 8 years since its discovery, in vivo PTMs of TPH2 and their consequences on enzyme activity remain poorly characterized. Furthermore, it is possible for a phosphorylation site to affect phosphorylation (or other modifications) on other sites of the protein. Such hierarchical phosphorylation has been shown to occur for TH both in vitro and in situ: phosphorylation of TH at Ser19 increases the rate of Ser40 phosphorylation leading to an increase in the enzyme’s activity (Dunkley et al., 2004). Thus far under-utilized in this context, newly developed techniques, including high-end proteomic methods, have the potential to uncover key information regarding the in vivo regulation of TPH2.
TPH2 defects are linked to depression
To date, over 300 single nucleotide polymorphisms have been identified in the human TPH2 gene (Zhang et al., 2006). Some of these have been associated with mental disorders, including several types of depression. For instance, R441H (the arginine residue at position 441 converted to histidine) and P206S (proline-206 converted to serine) have been associated with unipolar and unipolar and bipolar depression, respectively (Cichon et al., 2008; Zhang et al., 2005). Both mutations result in decreased thermal stability and an increased rate of aggregation of TPH2 in vitro, supporting a role for these mutations in the clinical phenotype (Cichon et al., 2008; Haavik et al., 2008; Zhang et al., 2005).
Aside from structural effects, it is possible for mutations to hinder TPH2’s function in diseased individuals by creating or eliminating PTM sites. For example, a recently discovered mutation in the regulatory domain of TPH2 (S41Y; serine-41 converted to tyrosine) has been identified to be associated with bipolar disorder and peripartum depression in a Chinese population (Lin et al., 2007, 2009). S41Y significantly reduces the capacity of the enzyme to produce serotonin (Lin et al., 2007). S41 is a highly conserved residue in mammalian TPH2 and thus it is likely that this residue will be important in the regulation of TPH2 (Lin et al., 2007). Furthermore, as S41 is in a potential protein kinase site (Gnad et al., 2007), it is possible for its importance to stem from its PTM. Moreover, the substitution of a tyrosine may create an alternative kinase substrate. Other non-synonymous TPH2 mutations have been discovered, but have yet to be associated with specific behavioral phenotypes (Figure 2) (Haavik et al., 2008).
TPH2 as a novel drug target
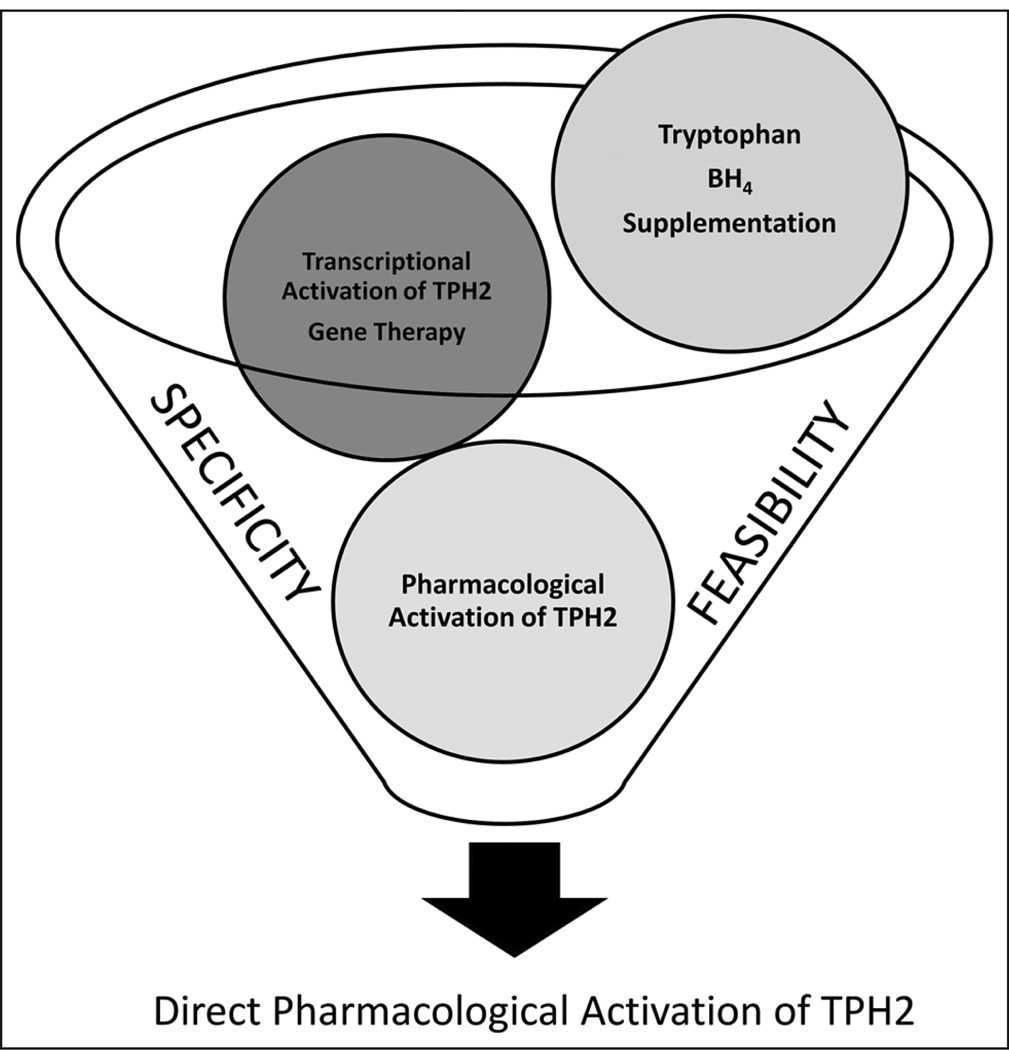
TPH2 offers a brain-specific, novel pharmacological target for antidepressant discovery, potentially bypassing the side effects produced by the stimulation of the peripheral serotonergic system (Matthes et al., 2010). Moreover, given the growing number of coding region polymorphisms in TPH2, it is clear that some individuals may be uniquely suited to increasing the activity of the enzyme. The biggest obstacle in achieving pharmacological activation of TPH2 is how little we know about this enzyme; a great deal of research is needed to overcome this hurdle. In order to increase serotonin levels, a first approach might be to increase the levels of TPH2 enzyme (Figure 3). Theoretically, this could be achieved through transcriptional activation of the TPH2 gene; however, little is known about the promoter region of the gene and this strategy is unlikely to be specific only to the intended target gene, thus the probability of extraneous gene activation would be high. Furthermore, the interference of epigenetic mechanisms, such as histone and DNA modification, is likely to compromise such approach. Epigenetic factors have been extensively implicated in the etiology of numerous neuropsychiatric disorders (Plazas-Mayorca and Vrana, 2010). For instance, inhibitors of histone deacetylases have been recently found to have antidepressant actions (Covington et al., 2009).
Figure 3.
A novel approach for the treatment of depression based on the activation of serotonin biosynthesis must be both selective and feasible. While gene therapy and transcriptional activation aimed at the TPH2 gene may be selective, these approaches are not currently feasible. On the other hand, dietary manipulation of precursor and co-substrate levels is very feasible, but not selective, and activation of TPH1 in the periphery is liable to produce unwanted side effects and decreased utility. Thus, the selective pharmacological activation of TPH2 may be the best compromise between feasibility and selectivity to yield an improved generation of antidepressants with fewer side effects.
Another possibility to increase the expression of TPH2 in the brain is the use of gene therapy. Viral vectors have been shown to be an efficient way to transduce cells in the central nervous system (Eckman and Eckman, 2005; Marr et al., 2003). However, this approach poses significant challenges, such as issues of delivery and safety, which are unlikely to be addressed in the near future.
A more feasible approach to repopulate the antidepressant drug pipeline would entail directly boosting serotonin synthesis in the brain. Figure 3 displays some possible mechanisms by which TPH2 could be stimulated to directly increase the production of 5-HT. A first approach would involve the administration of tryptophan and/or BH4. Lack of tryptophan in the diet has been linked to decreases in tissue tryptophan and in brain serotonin (Young and Leyton, 2002). In fact, due to the low concentrations of precursors in the brain, TPH is generally assumed to be only half-saturated (Markus, 2008). As a consequence, changes in tryptophan availability have a direct impact on the rate of 5-HT synthesis. Such a direct association is not the case for other neurotransmitters. For example, TH is usually more than 75% saturated with its substrate (Markus, 2008). While this approach is certainly intriguing, increasing precursor levels to stimulate TPH2 would most likely activate TPH1 as well, resulting in undesirable side effects. Indeed, supplementation with tryptophan and 5-hydroxytryptophan (Figure 1) has been proven to increase brain serotonin concentrations and have modest antidepressant effects (Birdsall, 1998; Wurtman, 1986); in addition, as this approach increases serotonin in the periphery, it also results in gastrointestinal side effects (Turner et al., 2006). Supplementation with BH4 has been used to enhance PAH in the treatment of phenylketonuria (Moens and Kass, 2006), however, and so it is incompatible with the exclusive activation of TPH2.
An effective alternative to enhance serotonin levels would be to directly and selectively activate TPH2. In this way, side effects arising from peripheral serotonin could be avoided (Figure 3). Targeting TPH2 specifically would increase the efficiency of potential antidepressant medications (Matthes et al., 2010). Furthermore, given the correlation between TPH2 polymorphisms and depression, TPH2 genotyping could be used to identify those patients that are at unique risk because of reduced serotonin biosynthesis and so would respond particularly well to a treatment exploiting TPH2 activation.
It is important to note that we are proposing activating or ‘turning on’ TPH2. While pharmaceutical discovery efforts generally focus on enzyme inhibitors, the tide is turning and perhaps a dozen non-natural, small molecules that activate enzyme catalysis have been identified within the past decade (Zorn and Wells, 2010). In an effort to discriminate between TPH1 and TPH2, pharmacological agents should target the first 41 residues on the N-terminal regulatory domain of TPH2 (Figure 2) as these are unique to this isoform. Low-molecular weight compounds or short peptides could exploit potential allosteric mechanisms and/or PTM-dependent activation of the enzyme. In fact, small molecules have already shown some degree of potential as pharmacological chaperones capable of stabilizing TH in vitro (Calvo et al., 2010).
Although the selective pharmacological activation of TPH2 we propose might be able to bypass many side effects brought by the effects of serotonin increase in the periphery, such therapy would still affect the serotonergic system; therefore, this tactic might still encompass some of the issues inherent in current medications, including the several-week delay in treatment response. While treatments modulating serotonergic activity are likely to continue to be important in the fight against depression, there are other promising therapeutic approaches affecting other mechanisms, including potassium channel TREK1 agonists (reviewed in Honore, 2007), substance P (NK-1) and corticotrophin-releasing factor (CRF)-1 receptor antagonists (reviewed in Rakofsky et al., 2009), among several others.
Summary and outlook
Deficiencies in the levels of the neurotransmitter serotonin in the synapse have been hypothetically linked to various psychiatric disorders, including depression. The discovery of TPH2, specifically responsible for serotonin synthesis in the brain, has provided a new target for antidepressant discovery. Several diminished function, coding-region polymorphisms in human TPH2 have been associated with various types of depression, highlighting the importance of this enzyme in the etiology of the depression.
Understanding the physiological regulation of TPH2 is a key issue in the development of better medications with the potential to help millions of patients suffering from diseases resulting from serotonergic dysfunctions. Increasing the production of serotonin through pharmacological activation of TPH2 represents a novel therapeutic strategy. This new approach might lead to a more selective generation of antidepressants, able to regulate the levels of serotonin in the brain with fewer side effects. Elevating brain, but not peripheral tryptophan hydroxylase activity might ameliorate the incidence of gastric side effects, as well as bleeding problems and weight changes. On balance, therefore, it may be a propitious time to explore a novel approach to antidepressant therapy development. Specifically, targeting the brain-specific TPH2 will boost the serotonin stores and evoked release of the neurotransmitter. Coupled with genotyping for TPH2 polymorphisms, this may open the door to individualized treatments for depression.
Acknowledgments
Funding
This work was supported by grants from the U.S. National Institutes of Health (grant numbers GM38931-18 and AA016613-03) to KEV. MPT also gratefully acknowledges funding from a National Institutes of Health F32 NRSA postdoctoral fellowship (grant number 5F32MH094071).
Footnotes
Conflict of interest
The authors declare no conflicts of interest in preparing this paper.
References
- Alenina N, Kikic D, Todiras M, et al. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen OA, Stokka AJ, Flatmark T, et al. 2.0A resolution crystal structures of the ternary complexes of human phenylalanine hydroxylase catalytic domain with tetrahydrobiopterin and 3-(2-thienyl)-L-alanine or L-norleucine: substrate specificity and molecular motions related to substrate binding. J Mol Biol. 2003;333:747–757. doi: 10.1016/j.jmb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Birdsall TC. 5-Hydroxytryptophan: a clinically-effective serotonin precursor. Altern Med Rev. 1998;3:271–280. [PubMed] [Google Scholar]
- Calvo AC, Scherer T, Pey AL, et al. Effect of pharmacological chaperones on brain tyrosine hydroxylase and tryptophan hydroxylase 2. J Neurochem. 2010;114:853–863. doi: 10.1111/j.1471-4159.2010.06821.x. [DOI] [PubMed] [Google Scholar]
- Carkaci-Salli N, Flanagan JM, Martz MK, et al. Functional domains of human tryptophan hydroxylase 2 (hTPH2) J Biol Chem. 2006;281:28105–28112. doi: 10.1074/jbc.M602817200. [DOI] [PubMed] [Google Scholar]
- Cichon S, Winge I, Mattheisen M, et al. Brain-specific tryptophan hydroxylase 2 (TPH2): a functional Pro206Ser substitution and variation in the 5′-region are associated with bipolar affective disorder. Hum Mol Genet. 2008;17:87–97. doi: 10.1093/hmg/ddm286. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Maze I, LaPlant QC, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bodinat C, Guardiola-Lemaitre B, Mocaer E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010a;9:628–642. doi: 10.1038/nrd3140. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Bobrovskaya L, Graham ME, et al. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem. 2004;91:1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- Eckman EA, Eckman CB. A beta-degrading enzymes: modulators of Alzheimer’s disease pathogenesis and targets for therapeutic intervention. Biochem Soc Trans. 2005;33:1101–1105. doi: 10.1042/BST20051101. [DOI] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science. 1971;173:149–152. doi: 10.1126/science.173.3992.149. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Annu Rev Biochem. 1999;68:355–381. doi: 10.1146/annurev.biochem.68.1.355. [DOI] [PubMed] [Google Scholar]
- Gildengers AG, Whyte EM, Drayer RA, et al. Medical burden in late-life bipolar and major depressive disorders. Am J Geriatr Psychiatry. 2008;16:194–200. doi: 10.1097/JGP.0b013e318157c5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnad F, Ren S, Cox J, et al. PHOSIDA (phosphorylation site database): management, structural and evolutionary investigation, and prediction of phosphosites. Genome Biol. 2007;8:R250. doi: 10.1186/gb-2007-8-11-r250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Gutknecht L, Waider J, Kraft S, et al. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J Neural Transm. 2008;115:1127–1132. doi: 10.1007/s00702-008-0096-6. [DOI] [PubMed] [Google Scholar]
- Haavik J, Blau N, Thony B. Mutations in human monoamine-related neurotransmitter pathway genes. Hum Mutat. 2008;29:891–902. doi: 10.1002/humu.20700. [DOI] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Huang Z, Liu T, Chattoraj A, et al. Posttranslational regulation of TPH1 is responsible for the nightly surge of 5-HT output in the rat pineal gland. J Pineal Res. 2008;45:506–514. doi: 10.1111/j.1600-079X.2008.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH. Agomelatine: efficacy at each phase of antidepressant treatment. CNS Drugs. 2009;23(Suppl. 2):41–47. doi: 10.2165/11318660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Knol MJ, Twisk JW, Beekman AT, et al. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- Kobe B, Jennings IG, House CM, et al. Structural basis of auto-regulation of phenylalanine hydroxylase. Nat Struct Biol. 1999;6:442–448. doi: 10.1038/8247. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Sakowski SA, Geddes TJ, et al. Phosphorylation and activation of tryptophan hydroxylase 2: identification of serine-19 as the substrate site for calcium, calmodulin-dependent protein kinase II. J Neurochem. 2007;103:1567–1573. doi: 10.1111/j.1471-4159.2007.04855.x. [DOI] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Li J, Dangott LJ, Fitzpatrick PF. Regulation of phenylalanine hydroxylase: conformational changes upon phenylalanine binding detected by hydrogen/deuterium exchange and mass spectrometry. Biochemistry. 2010;49:3327–3335. doi: 10.1021/bi1001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, Wong ML. Depression, antidepressants and suicidality: a critical appraisal. Nat Rev Drug Discov. 2005;4:165–171. doi: 10.1038/nrd1634. [DOI] [PubMed] [Google Scholar]
- Lin YM, Chao SC, Chen TM, et al. Association of functional polymorphisms of the human tryptophan hydroxylase 2 gene with risk for bipolar disorder in Han Chinese. Arch Gen Psychiatry. 2007;64:1015–1024. doi: 10.1001/archpsyc.64.9.1015. [DOI] [PubMed] [Google Scholar]
- Lin YM, Ko HC, Chang FM, et al. Population-specific functional variant of the TPH2 gene 2755C>A polymorphism contributes risk association to major depression and anxiety in Chinese peripartum women. Arch Womens Ment Health. 2009;12:401–408. doi: 10.1007/s00737-009-0088-z. [DOI] [PubMed] [Google Scholar]
- Markus CR. Dietary amino acids and brain serotonin function; implications for stress-related affective changes. Neuromolecular Med. 2008;10:247–258. doi: 10.1007/s12017-008-8039-9. [DOI] [PubMed] [Google Scholar]
- Marr RA, Rockenstein E, Mukherjee A, et al. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci. 2003;23:1992–1996. doi: 10.1523/JNEUROSCI.23-06-01992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes S, Mosienko V, Bashammakh S, et al. Tryptophan hydroxylase as novel target for the treatment of depressive disorders. Pharmacology. 2010;85:95–109. doi: 10.1159/000279322. [DOI] [PubMed] [Google Scholar]
- McKinney J, Knappskog PM, Haavik J. Different properties of the central and peripheral forms of human tryptophan hydroxylase. J Neurochem. 2005;92:311–320. doi: 10.1111/j.1471-4159.2004.02850.x. [DOI] [PubMed] [Google Scholar]
- Meltzer H. Serotonergic dysfunction in depression. Br J Psychiatry Suppl. 1989;8:25–31. [PubMed] [Google Scholar]
- Mockus SM, Vrana KE. Advances in the molecular characterization of tryptophan hydroxylase. J Mol Neurosci. 1998;10:163–179. doi: 10.1007/BF02761772. [DOI] [PubMed] [Google Scholar]
- Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2006;26:2439–2444. doi: 10.1161/01.ATV.0000243924.00970.cb. [DOI] [PubMed] [Google Scholar]
- Murphy KL, Zhang X, Gainetdinov RR, et al. A regulatory domain in the N terminus of tryptophan hydroxylase 2 controls enzyme expression. J Biol Chem. 2008;283:13216–13224. doi: 10.1074/jbc.M706749200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, Betan E, Larsen H, et al. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54:317–329. doi: 10.1016/s0006-3223(03)00569-9. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry. 2006;67(Suppl. 6):3–8. [PubMed] [Google Scholar]
- Patel PD, Pontrello C, Burke S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol Psychiatry. 2004;55:428–433. doi: 10.1016/j.biopsych.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Pincus HA, Pettit AR. The societal costs of chronic major depression. J Clin Psychiatry. 2001;62(Suppl. 6):5–9. [PubMed] [Google Scholar]
- Plazas-Mayorca MD, Vrana KE. Proteomic investigation of epigenetics in neuropsychiatric disorders: a missing link between genetics and behavior? J Proteome Res. 2010;10:58–65. doi: 10.1021/pr100463y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakofsky JJ, Holtzheimer PE, Nemeroff CB. Emerging targets for antidepressant therapies. Curr Opin Chem Biol. 2009;13:291–302. doi: 10.1016/j.cbpa.2009.04.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelieva KV, Zhao S, Pogorelov VM, et al. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS One. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spedding M, Jay T, Costa e Silva J, et al. A pathophysiological paradigm for the therapy of psychiatric disease. Nat Rev Drug Discov. 2005;4:467–476. doi: 10.1038/nrd1753. [DOI] [PubMed] [Google Scholar]
- Turner EH, Loftis JM, Blackwell AD. Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Ther. 2006;109:325–338. doi: 10.1016/j.pharmthera.2005.06.004. [DOI] [PubMed] [Google Scholar]
- van Wijngaarden B, Koeter M, Knapp M, et al. Caring for people with depression or with schizophrenia: are the consequences different? Psychiatry Res. 2009;169:62–69. doi: 10.1016/j.psychres.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Cook EH., Jr Molecular genetics of autism spectrum disorder. Mol Psychiatry. 2004;9:819–832. doi: 10.1038/sj.mp.4001505. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Wang L, Erlandsen H, Haavik J, et al. Three-dimensional structure of human tryptophan hydroxylase and its implications for the biosynthesis of the neurotransmitters serotonin and melatonin. Biochemistry. 2002;41:12569–12574. doi: 10.1021/bi026561f. [DOI] [PubMed] [Google Scholar]
- Windahl MS, Petersen CR, Christensen HE, et al. Crystal structure of tryptophan hydroxylase with bound amino acid substrate. Biochemistry. 2008;47:12087–12094. doi: 10.1021/bi8015263. [DOI] [PubMed] [Google Scholar]
- Winge I, McKinney JA, Knappskog PM, et al. Characterization of wild-type and mutant forms of human tryptophan hydroxylase 2. J Neurochem. 2007;100:1648–1657. doi: 10.1111/j.1471-4159.2006.04290.x. [DOI] [PubMed] [Google Scholar]
- Winge I, McKinney JA, Ying M, et al. Activation and stabilization of human tryptophan hydroxylase 2 by phosphorylation and 14-3-3 binding. Biochem J. 2008;410:195–204. doi: 10.1042/BJ20071033. [DOI] [PubMed] [Google Scholar]
- Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac) Nat Rev Drug Discov. 2005;4:764–774. doi: 10.1038/nrd1821. [DOI] [PubMed] [Google Scholar]
- Wong ML, Licinio J. From monoamines to genomic targets: a paradigm shift for drug discovery in depression. Nat Rev Drug Discov. 2004;3:136–151. doi: 10.1038/nrd1303. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ. Ways that foods can affect the brain. Nutr Rev. 1986;44(Suppl.):2–6. doi: 10.1111/j.1753-4887.1986.tb07671.x. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ. Presynaptic control of Release of Amine Neurotransmitters by Precursor Levels. News Physiol Sci. 1988;3:158–163. [Google Scholar]
- Young NL, Plazas-Mayorca MD, Garcia BA. Systems-wide proteomic characterization of combinatorial post-translational modification patterns. Expert Rev Proteomics. 2010;7:79–92. doi: 10.1586/epr.09.100. [DOI] [PubMed] [Google Scholar]
- Young SN, Leyton M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol Biochem Behav. 2002;71:857–865. doi: 10.1016/s0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- Zhang X, Beaulieu JM, Gainetdinov RR, et al. Functional polymorphisms of the brain serotonin synthesizing enzyme tryptophan hydroxylase-2. Cell Mol Life Sci. 2006;63:6–11. doi: 10.1007/s00018-005-5417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Beaulieu JM, Sotnikova TD, et al. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov RR, Beaulieu JM, et al. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zorn JA, Wells JA. Turning enzymes ON with small molecules. Nat Chem Biol. 2010;6:179–188. doi: 10.1038/nchembio.318. [DOI] [PubMed] [Google Scholar]