Abstract
Background
Although slow and insufficient muscle activation is a hallmark of hemiparesis post-stroke, movement speed is rarely emphasized during upper extremity rehabilitation. Moving faster may increase intensity of task-specific training, but positive and/or negative effects on paretic-limb movement quality are unknown.
Objective
To determine whether moving quickly instead of at a preferred speed either enhances or impairs paretic limb task performance after stroke.
Methods
Sixteen people with post-stroke hemiparesis and 11 healthy controls performed reach-grasp-lift movements at their preferred speed and as fast as possible, using palmar and 3-finger grip types. We measured durations of the reach and grasp phases, straightness of the reach path, thumb-index finger separation (aperture), efficiency of finger movement, and grip force.
Results
As expected, reach and grasp phase durations decreased in the fast condition in both groups, showing that participants were able to move more quickly when asked. When moving fast, the hemiparetic group had reach durations equal to those of healthy controls moving at their preferred speed. Movement quality also improved. Reach paths were straighter and peak apertures were greater in both groups in the fast condition. The group with hemiparesis also showed improved efficiency of finger movement. Differences in peak grip force across speed conditions did not reach significance.
Conclusions
People with hemiparesis are able to move faster than they choose to, and when they do, movement quality is improved. Simple instructions to move faster could be a cost-free and effective means of increasing rehabilitation intensity after stroke.
Keywords: hemiparesis, speed, kinematics, upper extremity, motor control, reach-to-grasp
INTRODUCTION
The neurological system is known to be highly adaptable and capable of transforming functionally and structurally after injury, in a use-dependent manner1–4. Clinicians and researchers alike now seek to identify the parameters of physical training that maximize neural adaptation and allow individuals to approach their potential in terms of motor control and function. Repetitive task-specific training is a key stimulus to promote neural adaptation and recovery after stroke5–8. Implementation of task-specific training for the upper extremity remains challenging for clinicians, however, given the multitude of choices regarding what to practice and specifically how to practice9.
Stroke disrupts the sensorimotor neural network that governs movement, causing slow and often insufficient muscle activation10–14. Although this leads to slow, awkward movement15, speed of task performance during training is rarely emphasized in either clinical or experimental intervention protocols16–18. Reasons for this are unclear, but may stem from traditional therapeutic approaches where the focus was on minimizing hyperactive stretch reflexes and abnormal movements in order to promote better movement quality19. More currently, principles of neuroplasticity now suggest that kinematic, kinetic and temporal characteristics of movement should be optimized during practice, in order to produce appropriate task-specific sensory feedback that contributes to reshaping of the sensorimotor neural network20.
Intensity of upper extremity rehabilitation post-stroke is often insufficient, as revealed by the many clinical trials that show less improvement after standard care compared to experimental training protocols that increase intensity by adding training time21–24. We suggest that intensity can also be enhanced by increasing the speed of task performance during training. Instructions to move faster could increase intensity by encouraging more effortful practice, and by allowing more repetitions of task practice to be performed within the time allotted for each therapy session. A first step in evaluating whether speed-intensive practice may enhance rehabilitation outcomes is to test for effects of movement speed on task performance within a single session.
Only two studies have questioned the effects of movement speed on upper extremity task performance after stroke. In one, faster paretic limb movements were associated with increased opening of the hand, and a smaller percentage of the reaching movement was spent decelerating as the hand approached the object to be grasped25. In another study, non-paretic limb reaching trajectories were smoother (i.e. they included fewer acceleration/deceleration reversals), when speed was emphasized over accuracy26. Similar speed-related changes in motor performance have been demonstrated in healthy participants27–29 and people with other movement disorders30, but have not been adequately investigated in the paretic limb of people post-stroke.
The purpose of this study, therefore, was to determine whether moving quickly instead of at a preferred speed either enhances or impairs performance of a reach-grasp-lift task by the paretic limb of people with post-stroke hemiparesis. Based on previous findings25, we expected that participants would be able to increase their movement speed when asked. We hypothesized that faster movements would be associated with improved movement quality, as measured by reach path straightness, thumb-finger separation (aperture), smoothness of thumb-finger opening and closing, and grip force. If faster speeds benefit performance without negative consequences, simple instructions to move faster could be a cost-free and effective means of increasing intensity and maximizing the therapeutic dose of activity contained within each therapy session.
METHODS
Participants
Sixteen people with hemiparesis due to stroke were recruited from the St. Louis metropolitan area via the Brain Recovery Core Database and the Cognitive Rehabilitation Research Group Stroke Registry at Washington University, and from local support groups for people with stroke. Potential participants were included if they 1) had a recent diagnosis of ischemic or hemorrhagic stroke by a stroke neurologist, 2) had persistent hemiparesis, as evidenced by upper extremity Medical Research Council muscle test scores that were at least one muscle grade lower on the paretic side compared to the non-paretic side, 3) were able to reach, grasp and lift a vertical cylinder (3.4 cm diameter, 420 grams) using palmar and 3-finger grip types, as necessary to complete the study procedures, and 4) had the ability to follow 2-step commands. We excluded people who 1) had severe aphasia as indicated by a score of 2 or 3 on the Best Language item of the National Institutes of Health Stroke Scale (NIHSS), 2) had severe hemispatial neglect, as indicated by a score of 2 on the Extinction and Inattention item on the NIHSS, 3) had musculoskeletal or other medical conditions besides stroke that limited either upper extremity, or 4) were unable to give informed consent.
For comparison, eleven healthy adults were recruited from the Volunteer for Health Research Participant Registry at Washington University. Volunteers were included if they 1) were at least 30 years old, 2) had no known neurological disease, and 3) had no disability or injury affecting their upper extremity on either side. To evenly represent both genders across the age range, we recruited one male and one female within each decade between 30 and 79 years of age. One 81 year-old male also volunteered. This study was approved by the Washington University Human Research Protection Office, and all participants provided informed consent prior to beginning the study.
Clinical Assessments
Clinical tests were used to describe the participants with post-stroke hemiparesis (Table 1). We assessed upper extremity function using the Action Research Arm Test (ARAT) on the paretic side31–33 and the Activities of Daily Living and Hand Function domains of the Stroke Impact Scale, version 3.034, 35 (SIS). Maximum grip strength was measured on each side using a Jamar grip dynamometer36, 37. Maximum pinch strength was measured on each side with a Jamar hydraulic pinch gauge positioned between the thumb and the lateral side of the index finger’s middle phalanx38, 39. Sensation on the palmar surface of the distal index finger was evaluated using Semmes-Weinstein monofilaments40. Spasticity of the elbow flexors was assessed on the paretic side using the Modified Ashworth Scale41.
Table 1.
Characteristics of participants with hemiparesisa
| Age, years | 59 ± 11 (39 – 88) |
| Gender, n | |
| Male | 9 |
| Female | 7 |
| Paretic side, n | |
| Dominant | 11 |
| Non-dominant | 5 |
| Right | 8 |
| Left | 8 |
| Type of stroke | |
| Ischemic | 14 |
| Hemorrhagic | 2 |
| Time since stroke (median, range) | 1.2 months (2 weeks – 9.4 years) |
| Grip strength | |
| paretic side in kg | 21.9 ± 8.8 (10.0 – 36.0) |
| paretic side as % of non-paretic | 69 ± 26 (37 – 112) |
| Pinch strength | |
| paretic side in kg | 5.5 ± 1.9 (2.0 – 8.0) |
| paretic side as % of non-paretic | 70 ± 21 (29 – 114) |
| Sensationb, n | |
| 2.83 | 6 |
| 3.61 | 6 |
| 4.31 | 2 |
| 6.25 | 2 |
| Spasticityc, n | |
| 0 | 8 |
| 1 | 6 |
| 2 | 1 |
| 4 | 1 |
| Action Research Arm Testd | 41 ± 10 (20 – 53) |
| Stroke Impact Scalee | |
| Activities in a Typical Day | 62 ± 15 (43 – 88) |
| Hand Function | 50 ± 21 (0 – 85) |
Data are mean ± standard deviation (range), except where otherwise noted.
Size of the smallest Semmes Weinstein monofilament sensed in 3 of 5 trials on the anterior distal index finger on the paretic side
Modified Ashworth Scale score for the elbow flexors on the paretic side
Paretic side. Range of possible scores is 0 to 57, 57 = normal
Range of possible scores is 0 to 100, 100 = normal
Experimental Procedures
For each participant, data collection was completed in a single session. Upper extremity movement and grip force were measured during reach-grasp-lift movements in preferred speed and fast conditions using palmar and 3-finger grip types. These grip types were chosen because they have been well characterized as two discrete patterns of prehension with different levels of accuracy and precision, and because they represent a range of actions observed in daily life42–45. The object that was grasped consisted of a custom-fabricated vertical cylinder (34 mm diameter, 113 mm height) attached to a rectangular base (135 by 60 mm) that was designed to hold a Tekscan I-scan electronic interface (Tekscan, Inc. South Boston, MA). The cylindrical portion of the object was covered with a Tekscan pressure sensor (112 by 112 mm, 1936 sensels, spatial resolution 155 sensels/mm2). Combined weight of the object and electronics was 420 grams. Pressure data were collected at 100 Hz. Measurement of grip force is a novel use of pressure sensor technology46. Although previous studies of grip force control in people with stroke have used custom-made devices equipped with strain gauges47, 48, we chose to use a pressure sensor because it does not require that participants place their hand or fingers on specific locations. Instead, each participant’s natural grasping performance was measured by the pressure sensor, which covered the entire surface of the vertical cylinder46. Using this method, we determined precisely when the hand contacted the object, and quantified grip force applied to the object. A disadvantage of the pressure sensor system is that it only measures grip forces (normal forces) and is unable to measure load forces (tangential or shear forces). For use in this study, we believed that the advantage of capturing natural movements outweighed the disadvantage of limiting our force analysis to grip (i.e. normal) forces.
We tested the contralesional, paretic upper extremity of participants with hemiparesis, and one randomly selected side for control participants. Three-dimensional movements of the tested upper extremity and the grasped object were recorded at 50 Hz using an electromagnetic tracking system (The MotionMonitor, Innovative Sports Training, Chicago, IL), capable of determining sensor positions to within 1.8 mm (root mean square accuracy, per manufacturer specifications). Nine sensors were attached to the trunk and upper extremity, as follows: 1) trunk: midline below the sternal notch, 2) upper arm: proximal to the lateral epicondyle, bisecting the upper arm mass, 3) forearm: midpoint between the radial and ulnar styloids on the dorsum of the forearm, 4) hand: midpoint of the third metacarpal on the dorsum of the hand, and 5 through 9) thumb and fingers: on the nail of each digit. The instrumentation produced minimal interference with reaching, grasping and lifting movements, since all sensors on the hand and forearm were placed dorsally, and since all leads were supported using wraps at the forearm and upper arm. In each session, after all sensors were applied, all upper extremity joints were moved through their available range of motion, to ensure that the leads did not restrict movement. One additional sensor was attached at the base of the cylindrical object, so that its vertical position could be used to determine precisely when the object was lifted.
As shown in Figure 1, participants were seated in a chair with back support for all data collection. A table was placed with its closest edge across the participant’s mid-thighs and the height was adjusted to be as low as possible without contacting the thighs, in order to allow clearance of the table edge while reaching. The object was placed on the table at a standardized horizontal distance from the participant’s shoulder (90% of the length of the arm from shoulder to wrist). In the frontal plane, the object was aligned with the mid-clavicle.
Figure 1.
Assessment of task performance. Illustration of the experimental set-up and a participant beginning (A) and finishing (B) the reach-grasp-lift task with a 3-finger grip.
Four trial types were collected, each characterized by the preferred speed or fast movement condition and by the type of grip (i.e. palmar preferred speed, palmar fast, 3-finger preferred speed, and 3-finger fast). We collected preferred speed trials before fast trials in order to accurately capture natural preferred speed performance before introducing speed-related instructions that could bias all subsequent trials. Within each speed condition, we randomized the order in which palmar and 3-finger trials were collected. Although we considered the reach-grasp-lift task to be highly learned already, we recognized the potential for practice effects to confound the effects of speed condition, and therefore included three practice trials at the beginning of each data collection session, prior to recording any movements. Further, we tested for practice effects within each speed condition statistically.
Prior to each trial, the participant was instructed to rest both hands in their lap with thumb and fingers together, wait for the word ‘go’, then grasp and lift the object, hold it above the table for about 5 seconds until the examiner said ‘done’, then put it down and return to the starting position. No speed-related instructions were provided prior to preferred speed trials. Before each fast trial, the participant was instructed to wait for the word ‘go’, then complete the reach-grasp-lift movement as fast as possible while still being able to complete the task. Verbal instruction and demonstration was also provided regarding grip type. Three trials of each type were recorded consecutively, with approximately 10 seconds of rest between trials.
Analysis
Pressure data were converted to grams of force, using Tekscan software to multiply recorded pressure by the sensor’s spatial area. After low-pass filtering of kinematic data at 6 Hz using a second-order Butterworth filter, sensor position data were extracted using MotionMonitor software (Innovative Sports Training, Chicago, IL). Subsequent analysis was then completed using custom software written in MATLAB (The MathWorks, Inc., Natick, MA).
Durations of movement phases were determined based on hand velocity, force on the object, and object position (Figure 2). The reach phase began when velocity of the hand sensor first exceeded 5 mm/s, and ended when force on the object first exceeded 5 grams. Pre-lift delay began at the end of the reach, and ended when the vertical position of the object increased by 3 mm from its initial value (ie. object lift-off). Pre-lift delay represents the time needed to establish a stable grip and begin the lifting movement. Movement quality was evaluated using the following variables:
Figure 2.
Example data from a healthy control participant performing one trial of the reach-grasp-lift task at preferred speed, using a 3-finger grip. Vertical dashed lines and solid black circles demonstrate division of the task into movement phases. The reach phase began when speed of the hand sensor first exceeded 5 mm/sec and ended when grip force first exceeded 5 grams. Pre-lift delay began at the end of the reach and ended when the vertical position of the object increased by 3 mm from its initial value.
Reach path ratio quantified curvature of the reach path, based on the forearm sensor’s three-dimensional trajectory. Reach path ratio was calculated by dividing the length of the forearm sensor’s actual path during the reach phase, by the length of a straight line path from the forearm sensor’s position at the start of the reach phase to its position at the end of the reach phase49. In healthy people, reach path ratios are typically close to one, indicating a straight, direct reach50, achieved when the ratio of the shoulder and elbow angular velocities remains nearly constant throughout the reaching movement49, 51–53. Reach path ratios greater than one indicate curvature of the reach path, caused by reduced coordination of shoulder and elbow joint movements53, 54.
Peak aperture quantified how far the hand opened. Aperture was defined as the three-dimensional distance between sensors on the thumbnail and the index fingernail. Note that because the sensors were placed on the fingernails, this measurement includes the thickness of the thumb and index fingertips. Peak aperture was the maximum aperture occurring in the reach phase.
-
Aperture path ratio quantified the efficiency of thumb and index finger movement during the reach phase, and was calculated as follows46.
This equation is based on the idea that efficient finger movements during reach-to-grasp are characterized by a single, direct opening to the point of peak aperture, followed by a single direct closing onto the object. The denominator in the above equation thus represents the most efficient aperture path, and the numerator represents the participant’s actual aperture path. In healthy people, aperture path ratios are typically equal to one, indicating a single direct separation of the thumb and index finger to the maximum aperture value, followed by a single direct closing onto the object. Higher values indicate abnormal, inefficient opening and closing of the thumb and index fingers, typically seen when participants make multiple attempts to open their hand and then close it on the object.
Peak grip force was defined as the maximum force applied to the object, during an interval beginning when the hand first contacted the object and ending one second after the object was lifted off the table.
Reliability of kinematic reaching variables has been shown to be adequate in healthy individuals and people with post-stroke hemiparesis55–57. In a recent evaluation of a reach-to-grasp task similar to the task used in the current study, excellent reliability was reported for reach duration, reach path ratio, and peak aperture (r > 0.75) in a group of people with hemiparesis after stroke56. Although these reliability studies used video-based motion capture systems instead of the electromagnetic system we used, direct comparison supports similarity of results obtained with the two motion analysis methods58.
Variables were calculated separately for each trial. Each participant’s performance in each movement condition was represented by the mean of three trials. Kolmogorov-Smirnov tests were used to determine whether data were normally distributed. Since all data met the normality assumption (p > 0.05), parametric statistics were used. For each variable, we tested for effects of trial order (i.e. practice effects) in each group by comparing the six trials performed for each speed condition (three for each grip type) using repeated measures ANOVA. For each variable, 2 × 2 × 2 mixed effects analysis of variance was used to determine effects of movement condition (preferred speed vs. fast), grip type (3-finger vs. palmar), and group (control vs. hemiparesis). Statistica software was used for normality testing and analysis of variance (Version 6.1 Statsoft Inc., Tulsa, OK), and the criterion for significance was set at p < 0.05. Effect sizes were calculated for statistically significant differences between speed conditions using Hedges’ g, which is equal to the mean difference between conditions divided by the pooled unbiased standard deviation.
RESULTS
Characteristics of the 16 participants with hemiparesis are provided in Table 1. Time since stroke ranged from two weeks to nine years, and was less than four months in all except four participants. Severity of sensorimotor impairment and functional limitation ranged from mild to moderate, as shown by the strength measures and scores on the ARAT and SIS assessments. Eleven reported that they were right handed before their stroke, and five reported being left handed.
Eleven healthy adults also participated, including five males and six females between 34 and 81 years of age (mean 55, SD 15 years). Nine were right handed and two were left handed, by self report. Random selection of the side to be tested resulted in six rights and five lefts. The dominant side was tested in six participants (five right-handed, one left-handed).
Group differences were not significant with respect to age (t-test, p = 0.45), gender (Mann-Whitney U, p = 0.64), hand dominance (Mann-Whitney U, p = 0.57), or dominant/non-dominant status of the tested side (Mann-Whitney U, p = 0.54).
Effects of trial order
In each group, comparison of the six trials within each speed condition (3 for each grip type) revealed no significant effects of trial order on reach duration, pre-lift delay, reach path ratio, peak aperture, aperture path ratio, or peak grip force (repeated measures ANOVA, p > 0.05).
Effects of movement speed
As expected, participants in both groups were able to move faster than their preferred speed when asked (Figure 3). Means, standard errors, and differences between speed conditions are presented in Table 2, with ANOVA results and effect sizes. Compared to the preferred speed condition, reach duration (Figure 3A) was 40% shorter in the fast condition in the hemiparetic group (post-hoc p < 0.05), and 47% shorter in the fast condition in the control group (post-hoc p < 0.05). Within each speed condition, reach durations were longer in the hemiparetic group compared to controls (post hoc p < 0.05). When moving fast, however, the hemiparetic group showed reach durations equal to those of healthy controls moving at their preferred speed (post-hoc p = 0.93). Compared to the preferred speed condition, pre-lift delay (Figure 3B) was 37% shorter in the fast condition in the hemiparetic group (post-hoc p < 0.05), and 52% shorter in the fast condition in the control group (post-hoc p < 0.05).
Figure 3.
Comparison of phase durations in the preferred speed versus fast conditions. Values are means ± 1 standard error. For healthy controls in the fast condition, some of the standard errors do not extend past the edges of the symbols (circles or triangles), and error bars therefore do not appear. Reach duration (A) and pre-lift delay (B) were shorter in the fast condition, in both groups. Reach duration was longer in the hemiparetic group compared to controls. When moving fast, however, the hemiparetic group had reach durations equal to that of the control group moving at preferred speed.
Table 2.
Effects of movement speed on task performancea
| Group | Palmar Grip
|
3-Finger Grip
|
Preferred vs. Fast Effect Size (g) | |||||
|---|---|---|---|---|---|---|---|---|
| Preferred Speed | Fast | Difference | Preferred Speed | Fast | Difference | |||
| Reach Duration (msec) bcdef | Hemiparesis | 1279 ± 86 | 803 ± 60 | −476 ± 79 | 1590 ± 139 | 913 ± 94 | −677 ± 104 | 1.26 |
| Control | 788 ± 80 | 423 ± 19 | −365 ± 81 | 792 ± 50 | 417 ± 40 | −375 ± 40 | 1.54 | |
| Pre-Lift Delay (msec) bcdf | Hemiparesis | 659 ± 61 | 371 ± 23 | −288 ± 64 | 790 ± 60 | 551 ± 58 | −239 ± 80 | 1.24 |
| Control | 588 ± 90 | 237 ± 15 | −351 ± 86 | 562 ± 31 | 309 ± 29 | −253 ± 49 | 1.51 | |
| Reach Path Ratio bc | Hemiparesis | 1.37 ± 0.04 | 1.32 ± 0.04 | −0.05 ± 0.03 | 1.35 ± 0.04 | 1.29 ± 0.04 | −0.06 ± 0.03 | 0.37 |
| Control | 1.23 ± 0.05 | 1.14 ± 0.03 | −0.09 ± 0.03 | 1.18 ± 0.04 | 1.14 ± 0.05 | −0.03 ± 0.03 | 0.48 | |
| Peak Aperture (mm) cd | Hemiparesis | 118 ± 7 | 123 ± 7 | 6 ± 3 | 101 ± 7 | 108 ± 6 | 7 ± 4 | 0.24 |
| Control | 120 ± 4 | 125 ± 3 | 5 ± 4 | 97 ± 3 | 106 ± 6 | 9 ± 6 | 0.62 | |
| Aperture Path Ratio ce | Hemiparesis | 1.24 ± 0.07 | 1.14 ± 0.04 | −0.10 ± 0.05 | 1.38 ± 0.07 | 1.15 ± 0.04 | −0.22 ± 0.06 | 0.75 |
| Control | 1.13 ± 0.04 | 1.14 ± 0.05 | 0.01 ± 0.03 | 1.12 ± 0.03 | 1.16 ± 0.07 | 0.04 ± 0.08 | ns | |
| Peak Grip Force (grams) d | Hemiparesis | 3590 ± 436 | 4018 ± 491 | 428 ± 295 | 1942 ± 285 | 2013 ± 260 | 72 ± 68 | ns |
| Control | 4062 ± 787 | 5566 ± 1781 | 1504 ± 1286 | 1162 ± 76 | 1644 ± 209 | 481 ± 217 | ns | |
Data are mean ± standard error. Negative numbers indicate lower values in the fast condition. Positive numbers indicate higher values in the fast condition.
Main effect of group, p < 0.05
Main effect of preferred vs. fast speed, p < 0.05
Main effect of grip, p < 0.05
Speed × group interaction effect, p < 0.05
Grip × group interaction effect, p < 0.05
Effect sizes are reported for significant effects of speed condition in each group, collapsed across grip types. ns indicates no significant difference across speed conditions.
Improvements in movement quality were also evident during faster task performance. Representative data from individual participants with hemiparesis and healthy controls are shown in Figure 4. In the participant with hemiparesis, reach trajectories in the fast condition were straighter than they were in the preferred speed condition (Figure 4A, left panel vs. middle panel). Aperture path ratio was decreased (improved) in the fast condition compared to the preferred speed condition, shown by smoother aperture traces (Figure 4B, left panel vs. middle panel). When moving at preferred speed, the hemiparetic participants’ performance differed from that of the healthy controls moving at preferred speed (Figure 4, left panels vs. right panels). When moving fast, however, hemiparetic participants’ performance was similar to that of the healthy controls moving at preferred speed (Figure 4, middle panels vs. right panels).
Figure 4.
Examples of improved movement quality in the fast condition compared to the preferred speed condition in individual participants with hemiparesis. Trajectories of the forearm sensor in the sagittal plane (A), plotted from the start of movement (open circle), until the hand contacted the object. In one representative participant with hemiparesis, reach paths were straighter during three fast trials (middle panel) compared to three preferred speed trials (left panel). Aperture traces (B) plotted from the start of movement (0 msec) until the hand contacted the object. In a different representative participant with hemiparesis, aperture traces were smoother during three fast trials (middle panel) compared to three preferred speed trials (left panel). In each example, performance of the participant with hemiparesis during three fast trials (middle panels) approached that of a representative healthy control participant performing three trials at preferred speed (right panels).
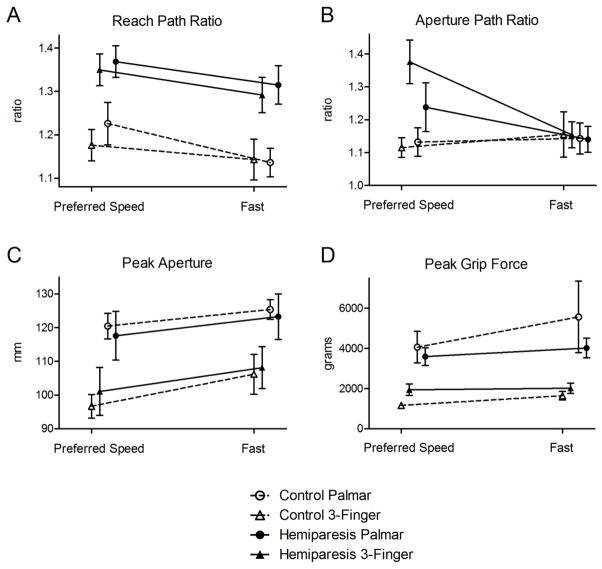
Group data for the measures of movement quality are shown in Figure 5. Means, standard errors, and differences between speed conditions are presented in Table 2, with ANOVA results and effect sizes. Both groups showed lower (better) reach path ratios and greater peak apertures in the fast condition compared to the preferred speed condition (main effect of speed, p < 0.05). In the hemiparetic group only, aperture path ratios were lower (better) in the fast condition compared to the preferred speed condition (speed condition by group interaction effect, p < 0.05, post hoc p < 0.05 for hemiparetic group only). Although the main effect of movement speed on peak grip force approached significance (p = 0.06), post hoc testing failed to find a difference between speed conditions in either group (hemiparetic group p = 0.94, control group p = 0.20).
Figure 5.
Comparison of movement quality in the preferred speed versus fast conditions. Both groups showed lower reach path ratios (A) and greater peak apertures (C) in the fast condition compared to the preferred speed condition. In the hemiparetic group only, aperture path ratios were lower in the fast condition compared to the preferred speed condition (B). The standard error for peak grip force in healthy controls in the preferred speed condition did not extend past the edges of the symbol (triangle), and error bars therefore do not appear.
Effects of group and grip type
Some additional significant effects of group and grip type were also found (Table 2). Pre-lift delays and reach path ratios were greater in the hemiparetic group than in controls (main effects of group, p < 0.05, Figures 3B and 5A, respectively), regardless of speed condition (no group x condition interactions). Differences between groups did not reach significance for aperture path ratio, peak aperture, or grip force (Figures 5B, 5C, and 5D).
In the hemiparetic group only, reach duration and pre-lift delay were longer during 3-finger grip trials compared to palmar grip trials (post-hoc p < 0.05). In both groups, peak aperture and peak grip force were greater during palmar grip trials compared to 3-finger grip trials (main effect of grip type p < 0.05). Grip type did not affect reach path ratios or aperture path ratios significantly in either group.
DISCUSSION
Faster is possible
This study demonstrated that people with mild to moderate post-stroke hemiparesis are able to increase their movement speed upon request, and when they do, movement quality is improved. Reach paths are straighter, finger movements are more efficient, and the fingers open wider. These measures of task performance are known to be altered after stroke and have the potential for recovery59, 60. As most of our participants were within a few months post-stroke, our findings suggest that incorporating the fast movement condition into rehabilitation may improve movement quality during training and thus potentially may contribute to improved outcomes.
Shorter reach durations and pre-lift delays in the fast condition demonstrate that people with hemiparesis can voluntarily move faster than their preferred speed and can approach the movement speeds of healthy individuals. This is likely accomplished through earlier activation of upper extremity muscles (quicker muscle onsets) and better modulation of muscle activity (higher levels of activation), as has been shown to occur during recovery of reaching15. Slow and insufficient muscle activation is a fundamental movement problem after stroke10–14, and people with hemiparesis often choose to use their opposite limb instead when the paretic limb is too slow or unreliable61. Aside from any potential improvements in movement quality, aiming to increase paretic limb movement speed is itself a reasonable goal of upper extremity rehabilitation.
Faster is better
This is the first study to demonstrate improved paretic-limb coordination during faster movement, as shown by the reach path and aperture path ratios. These variables reflect the efficiency of reach and grasp movements and have been shown to be related to upper extremity function at various time points post stroke31, 59, 60. For peak aperture, our results echo those of van Vliet and Sheridan25, who also showed wider finger opening during fast movements in healthy controls and people post-stroke, and suggested that excessive aperture may be a compensation for increased spatial variability during faster movement25. Since larger peak apertures were found at faster speeds in both groups (main effect of speed, figure 5C), this is likely a normal compensation during fast movement, and not one that is due to movement impairment post-stroke. It should be noted that even after accounting for the thickness of the fingertips, peak apertures in both groups were substantially larger than the 34 mm diameter of the target object. If larger apertures result in more reliable task completion, then this would be functionally beneficial for people with stroke.
For the comparison across speeds in the hemiparetic group, effect sizes were medium for aperture path ratio (0.5 ≤ g ≤ 0.8), small for (0.3 ≤ g ≤ 0.5), and minimal for peak aperture (g ≤ 0.3). No detrimental effects of the fast movement condition were observed. These results imply that fast training has the potential to increase rates of muscle activation and improve the spatiotemporal pattern of activation that controls proximal and distal multi-joint movements. In addition, fast training may allow for more task repetitions during the same allotted therapy time, as has been explored recently in a proof-of-concept trial62.
Although outcomes of fast training have not been examined in the upper extremity, outcomes from fast gait training studies provide valuable insight. Simply informing people with stroke of their fast walking speed each day during inpatient rehabilitation resulted in dramatic improvements in walking speed63. Likewise, when instructed to walk faster, people with stroke show improved symmetry, increases in joint excursions and muscle activations, and less compensatory paretic-limb circumduction64. Cardiovascular fitness, walking endurance and functional mobility have also been shown to increase much more after speed-intensive gait training compared to a less intense protocol65. The benefit of fast training may not be restricted to one type of movement, but may extend to training of many functional movements.
Limitations
Recruitment into the hemiparetic group in this study was limited to a convenience sample of people who were able to perform reach-grasp-lift movements with both palmar and 3-finger grip types. Participants were, therefore, mildly to moderately impaired, and the results may not generalize to people with more severe hemiparesis. People with mild to moderate impairments post-stroke are most likely to benefit from task-specific training and are most likely to be enrolled in stroke rehabilitation trials9. Our investigation was also limited to the within-session effects of movement speed on kinematic and kinetic characteristics of task performance, and did not explore training effects across multiple sessions. Further investigation into the feasibility, safety, and potential benefits of incorporating the fast movement condition into task-specific training appears warranted. It is possible that muscular, neurological, and/or cardiorespiratory fatigue may limit implementation of fast task-specific training, particularly in certain individuals with comorbidities. Lastly, the number of trials each participant performed in each condition was limited to three, and the preferred speed trials were always performed first. While this order of conditions was necessary so that instructions to move fast would not bias the preferred speed trials, it leaves open the possibility that the results are due to practice effects. We consider this possibility unlikely however, because the reach-grasp-lift task is an often used, highly learned movement, because all participants had practice trials before the recorded ones, and because our within-condition comparison of trial order failed to show any practice effects.
Conclusions
In summary, this study showed that people with mild to moderate hemiparesis post-stroke are able to perform upper extremity reach-grasp-lift tasks substantially faster than their preferred movement speed and, further, that movement quality is enhanced during faster movements. No detrimental effects were observed in the fast condition. Simple instructions to move faster could be a cost-free and effective means of increasing the intensity of task specific training after stroke. Further studies of feasibility, safety and therapeutic effects are needed.
Acknowledgments
This work was supported in part by NIH R01HD055964 (CEL), NIH T32HD007434 (SLD), and the Foundation for Physical Therapy (SLD). The authors acknowledge Dustin Hardwick, DPT, PhD for assistance with data collection.
References
- 1.Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101:1776–1782. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- 2.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller MJ, Maluf KS. Tissue adaptation to physical stress: A proposed “Physical stress theory” To guide physical therapist practice, education, and research. Phys Ther. 2002;82:383–403. [PubMed] [Google Scholar]
- 4.Jones TA, Allred RP, Adkins DL, Hsu JE, O’Bryant A, Maldonado MA. Remodeling the brain with behavioral experience after stroke. Stroke. 2009;40:S136–138. doi: 10.1161/STROKEAHA.108.533653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Askim T, Indredavik B, Vangberg T, Haberg A. Motor network changes associated with successful motor skill relearning after acute ischemic stroke: A longitudinal functional magnetic resonance imaging study. Neurorehabil Neural Repair. 2009;23:295–304. doi: 10.1177/1545968308322840. [DOI] [PubMed] [Google Scholar]
- 6.Jang SH, Kim YH, Cho SH, Lee JH, Park JW, Kwon YH. Cortical reorganization induced by task-oriented training in chronic hemiplegic stroke patients. Neuroreport. 2003;14:137–141. doi: 10.1097/00001756-200301200-00025. [DOI] [PubMed] [Google Scholar]
- 7.Liepert J, Graef S, Uhde I, Leidner O, Weiller C. Training-induced changes of motor cortex representations in stroke patients. Acta Neurol Scand. 2000;101:321–326. doi: 10.1034/j.1600-0404.2000.90337a.x. [DOI] [PubMed] [Google Scholar]
- 8.Schaechter JD, Kraft E, Hilliard TS, Dijkhuizen RM, Benner T, Finklestein SP, Rosen BR, Cramer SC. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: A preliminary study. Neurorehabil Neural Repair. 2002;16:326–338. doi: 10.1177/154596830201600403. [DOI] [PubMed] [Google Scholar]
- 9.Pomeroy V, Aglioti SM, Mark VW, McFarland D, Stinear C, Wolf SL, Corbetta M, Fitzpatrick SM. Neurological principles and rehabilitation of action disorders: Rehabilitation interventions. Neurorehabil Neural Repair. 2011;25(S):33–42. doi: 10.1177/1545968311410942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frontera WR, Grimby L, Larsson L. Firing rate of the lower motoneuron and contractile properties of its muscle fibers after upper motoneuron lesion in man. Muscle Nerve. 1997;20:938–947. doi: 10.1002/(sici)1097-4598(199708)20:8<938::aid-mus2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995;18:1101–1114. doi: 10.1002/mus.880181006. [DOI] [PubMed] [Google Scholar]
- 12.Jakobsson F, Grimby L, Edstrom L. Motoneuron activity and muscle fibre type composition in hemiparesis. Scand J Rehabil Med. 1992;24:115–119. [PubMed] [Google Scholar]
- 13.Rosenfalck A, Andreassen S. Impaired regulation of force and firing pattern of single motor units in patients with spasticity. J Neurol Neurosurg Psychiatry. 1980;43:907–916. doi: 10.1136/jnnp.43.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young JL, Mayer RF. Physiological alterations of motor units in hemiplegia. J Neurol Sci. 1982;54:401–412. doi: 10.1016/0022-510x(82)90203-9. [DOI] [PubMed] [Google Scholar]
- 15.Wagner JM, Dromerick AW, Sahrmann SA, Lang CE. Upper extremity muscle activation during recovery of reaching in subjects with post-stroke hemiparesis. Clin Neurophysiol. 2007;118:164–176. doi: 10.1016/j.clinph.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan PW, Zorowitz R, Bates B, Choi JY, Glasberg JJ, Graham GD, Katz RC, Lamberty K, Reker D. Management of adult stroke rehabilitation care: A clinical practice guideline. Stroke. 2005;36:e100–143. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- 17.Winstein CJ, Rose DK, Tan SM, Lewthwaite R, Chui HC, Azen SP. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: A pilot study of immediate and long-term outcomes. Arch Phys Med Rehabil. 2004;85:620–628. doi: 10.1016/j.apmr.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 19.Bobath B. Adult hemiplegia: Evaluation and treatment. London: William Heinmann Medical Books Ltd; 1970. [Google Scholar]
- 20.Dobkin BH. Strategies for stroke rehabilitation. Lancet Neurol. 2004;3:528–536. doi: 10.1016/S1474-4422(04)00851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duncan PW, Studenski S, Richards L, Gollub S, Lai SM, Reker D, Perera S, Yates J, Koch V, Rigler S, Johnson D. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173–2180. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 22.Harris JE, Eng JJ, Miller WC, Dawson AS. A self-administered graded repetitive arm supplementary program (GRASP) improves arm function during inpatient stroke rehabilitation: A multi-site randomized controlled trial. Stroke. 2009;40:2123–2128. doi: 10.1161/STROKEAHA.108.544585. [DOI] [PubMed] [Google Scholar]
- 23.Kuys SS, Brauer SG, Ada L. Higher-intensity treadmill walking during rehabilitation after stroke is feasible and not detrimental to walking pattern or quality: A pilot randomized trial. Clin Rehabil. 2011;25:316–326. doi: 10.1177/0269215510382928. [DOI] [PubMed] [Google Scholar]
- 24.Sunderland A, Tinson DJ, Bradley EL, Fletcher D, Langton Hewer R, Wade DT. Enhanced physical therapy improves recovery of arm function after stroke. A randomised controlled trial. J Neurol Neurosurg Psychiatry. 1992;55:530–535. doi: 10.1136/jnnp.55.7.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Vliet PM, Sheridan MR. Coordination between reaching and grasping in patients with hemiparesis and healthy subjects. Arch Phys Med Rehabil. 2007;88:1325–1331. doi: 10.1016/j.apmr.2007.06.769. [DOI] [PubMed] [Google Scholar]
- 26.Lin KC, Wu CY, Lin KH, Chang CW. Effects of task instructions and target location on reaching kinematics in people with and without cerebrovascular accident: A study of the less-affected limb. Am J Occup Ther. 2008;62:456–465. doi: 10.5014/ajot.62.4.456. [DOI] [PubMed] [Google Scholar]
- 27.Adam JJ. The effects of objectives and constraints on motor control strategy in reciprocal aiming movements. J Mot Behav. 1992;24:173–185. doi: 10.1080/00222895.1992.9941613. [DOI] [PubMed] [Google Scholar]
- 28.Fisk JD, Goodale MA. Differences in the organization of visually guided reaching to ipsilateral and contralateral targets. Behavioral Brain Research. 1984;12:189–190. [Google Scholar]
- 29.Rival C, Olivier I, Ceyte H. Effects of temporal and/or spatial instructions on the speed-accuracy trade-off of pointing movements in children. Neurosci Lett. 2003;336:65–69. doi: 10.1016/s0304-3940(02)01246-6. [DOI] [PubMed] [Google Scholar]
- 30.Rand MK, Stelmach GE, Bloedel JR. Movement accuracy constraints in Parkinson’s disease patients. Neuropsychologia. 2000;38:203–212. doi: 10.1016/s0028-3932(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 31.Lang CE, Wagner JM, Dromerick AW, Edwards DF. Measurement of upper-extremity function early after stroke: Properties of the Action Research Arm Test. Arch Phys Med Rehabil. 2006;87:1605–1610. doi: 10.1016/j.apmr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the Action Research Arm Test. Neurorehabil Neural Repair. 2008;22:78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]
- 34.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The Stroke Impact Scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 35.Lai SM, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke. 2002;33:1840–1844. doi: 10.1161/01.str.0000019289.15440.f2. [DOI] [PubMed] [Google Scholar]
- 36.Fess EE. Grip strength. In: Casanova JS, editor. Clinical assessment recommendations. Chicago: American Society of Hand Therapists; 1992. pp. 41–45. [Google Scholar]
- 37.Schmidt RT, Toews JV. Grip strength as measured by the Jamar dynamometer. Arch Phys Med Rehabil. 1970;51:321–327. [PubMed] [Google Scholar]
- 38.Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: Normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74. [PubMed] [Google Scholar]
- 39.Werle S, Goldhahn J, Drerup S, Simmen BR, Sprott H, Herren DB. Age- and gender-specific normative data of grip and pinch strength in a healthy adult Swiss population. J Hand Surg Eur Vol. 2009;34:76–84. doi: 10.1177/1753193408096763. [DOI] [PubMed] [Google Scholar]
- 40.Bell-Krotoski J. Advances in sensibility evaluation. Hand Clin. 1991;7:527–546. [PubMed] [Google Scholar]
- 41.Bohannon RW, Smith MB. Interrater reliability of a Modified Ashworth Scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 42.Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision- versus power-grip tasks: An fMRI study. J Neurophysiol. 2000;83:528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- 43.Landsmeer JM. Power grip and precision handling. Ann Rheum Dis. 1962;21:164–170. doi: 10.1136/ard.21.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Napier JR. The prehensile movements of the human hand. J Bone Joint Surg Br. 1956;38-B:902–913. doi: 10.1302/0301-620X.38B4.902. [DOI] [PubMed] [Google Scholar]
- 45.Pouydebat E, Laurin M, Gorce P, Bels V. Evolution of grasping among anthropoids. J Evol Biol. 2008;21:1732–1743. doi: 10.1111/j.1420-9101.2008.01582.x. [DOI] [PubMed] [Google Scholar]
- 46.DeJong SL, Birkenmeier R, Lang CE. Person-specific changes in motor performance accompany upper extremity functional gains after stroke. J Appl Biomech. 2011 doi: 10.1123/jab.28.3.304. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blennerhassett JM, Carey LM, Matyas TA. Grip force regulation during pinch grip lifts under somatosensory guidance: Comparison between people with stroke and healthy controls. Arch Phys Med Rehabil. 2006;87:418–429. doi: 10.1016/j.apmr.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 48.Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: Brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23:641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- 49.Bastian AJ, Martin TA, Keating JG, Thatch WT. Cerebellar ataxia: Abnormal control of interaction torques across multiple joints. J Neurophysiol. 1996;76:492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- 50.Morasso P. Spatial control of arm movements. Exp Brain Res. 1981;42:223–227. doi: 10.1007/BF00236911. [DOI] [PubMed] [Google Scholar]
- 51.Flanagan RJ, Ostry DJ, Feldman AG. Control of trajectory modifications in target-directed reaching. J Mot Behav. 1993;25:175–192. doi: 10.1080/00222895.1993.9942045. [DOI] [PubMed] [Google Scholar]
- 52.Lacquaniti F. Automatic control of limb movement and posture. Curr Opin Neurobiol. 1992;2:807–814. doi: 10.1016/0959-4388(92)90138-b. [DOI] [PubMed] [Google Scholar]
- 53.McCrea PH, Eng JJ, Hodgson AJ. Biomechanics of reaching: Clinical implications for individuals with acquired brain injury. Disabil Rehabil. 2002;24:534–541. doi: 10.1080/09638280110115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cirstea MC, Mitnitski AB, Feldman AG, Levin MF. Interjoint coordination dynamics during reaching in stroke. Exp Brain Res. 2003;151:289–300. doi: 10.1007/s00221-003-1438-0. [DOI] [PubMed] [Google Scholar]
- 55.Caimmi M, Carda S, Giovanzana C, Maini ES, Sabatini AM, Smania N, Molteni F. Using kinematic analysis to evaluate constraint-induced movement therapy in chronic stroke patients. Neurorehabil Neural Repair. 2008;22:31–39. doi: 10.1177/1545968307302923. [DOI] [PubMed] [Google Scholar]
- 56.Patterson TS, Bishop MD, McGuirk TE, Sethi A, Richards LG. Reliability of upper extremity kinematics while performing different tasks in individuals with stroke. J Mot Behav. 2011;43:121–130. doi: 10.1080/00222895.2010.548422. [DOI] [PubMed] [Google Scholar]
- 57.Wagner JM, Rhodes JA, Patten C. Reproducibility and minimal detectable change of three-dimensional kinematic analysis of reaching tasks in people with hemiparesis after stroke. Phys Ther. 2008;88:652–663. doi: 10.2522/ptj.20070255. [DOI] [PubMed] [Google Scholar]
- 58.Hassan EA, Jenkyn TR, Dunning CE. Direct comparison of kinematic data collected using an electromagnetic tracking system versus a digital optical system. J Biomech. 2007;40:930–935. doi: 10.1016/j.jbiomech.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 59.Lang CE, Wagner JM, Bastian AJ, Hu Q, Edwards DF, Sahrmann SA, Dromerick AW. Deficits in grasp versus reach during acute hemiparesis. Exp Brain Res. 2005;166:126–136. doi: 10.1007/s00221-005-2350-6. [DOI] [PubMed] [Google Scholar]
- 60.Lang CE, Wagner JM, Edwards DF, Sahrmann SA, Dromerick AW. Recovery of grasp versus reach in people with hemiparesis poststroke. Neurorehabil Neural Repair. 2006;20:444–454. doi: 10.1177/1545968306289299. [DOI] [PubMed] [Google Scholar]
- 61.Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: Implications for rehabilitation. Eura Medicophys. 2006;42:241–256. [PubMed] [Google Scholar]
- 62.Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: A proof-of-concept study. Neurorehabil Neural Repair. 2010;24:620–635. doi: 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dobkin BH, Plummer-D’Amato P, Elashoff R, Lee J. International randomized clinical trial, stroke inpatient rehabilitation with reinforcement of walking speed (SIRROWS), improves outcomes. Neurorehabil Neural Repair. 2010;24:235–242. doi: 10.1177/1545968309357558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lamontagne A, Fung J. Faster is better: Implications for speed-intensive gait training after stroke. Stroke. 2004;35:2543–2548. doi: 10.1161/01.STR.0000144685.88760.d7. [DOI] [PubMed] [Google Scholar]
- 65.Macko RF, Ivey FM, Forrester LW, Hanley D, Sorkin JD, Katzel LI, Silver KH, Goldberg AP. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: A randomized, controlled trial. Stroke. 2005;36:2206–2211. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]