Abstract
Decreased GABAergic synaptic strength (“disinhibition”) in the spinal dorsal horn is a crucial mechanism contributing to the development and maintenance of pathological pain. However, mechanisms leading to disinhibition in the spinal dorsal horn remain elusive. We investigated the role of glial glutamate transporters (GLT-1 and GLAST) and glutamine synthetase in maintaining GABAergic synaptic activity in the spinal dorsal horn. Electrically evoked GABAergic inhibitory postsynaptic currents (eIPSCs), spontaneous IPSCs (sIPSCs) and miniature IPSCs (mIPSCs) were recorded in superficial spinal dorsal horn neurons of spinal slices from young adult rats. We used (2S, 3S)-3-[3-[4-(trifluoromethyl)benzoylamino]benzyloxy]aspartate (TFB-TBOA), to block both GLT-1 and GLAST and dihydrokainic acid (DHK) to block only GLT-1. We found that blockade of both GLAST and GLT-1 and blockade of only GLT-1 in the spinal dorsal horn decreased the amplitude of GABAergic eIPSCs, as well as both the amplitude and frequency of GABAergic sIPSCs or mIPSCs. Pharmacological inhibition of glial glutamine synthetase had similar effects on both GABAergic eIPSCs and sIPSCs. We provided evidence demonstrating that the reduction in GABAergic strength induced by the inhibition of glial glutamate transporters is due to insufficient GABA synthesis through the glutamate-glutamine cycle between astrocytes and neurons. Thus, our results indicate that deficient glial glutamate transporters and glutamine synthetase significantly attenuate GABAergic synaptic strength in the spinal dorsal horn, which may be a crucial synaptic mechanism underlying glial-neuronal interactions caused by dysfunctional astrocytes in pathological pain conditions.
INTRODUCTION
γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system. GABA released from GABAergic interneurons exerts inhibition by acting on GABA receptors at presynaptic terminals and postsynaptic neurons to reduce presynaptic glutamate release and produce inhibitory postsynaptic currents (IPSCs), or membrane hyperpolarization, in postsynaptic neurons. Reduced inhibition, termed “disinhibition,” resulting from decreased GABAergic synaptic strength in the spinal dorsal horn is a crucial mechanism contributing to the development and maintenance of pathological pain (Coull et al. 2003; Moore et al. 2002; Yaksh 1989). However, mechanisms leading to disinhibition in the spinal dorsal horn remain unclear. One major mechanism underlying disinhibition is an alteration of the homeostasis between the consumption (i.e., exocytosis) and supply of GABA in presynaptic GABAergic neurons (Ortinski et al. 2011). Studies of forebrain areas and cultured cells have shown that the supply of GABA to presynaptic GABAergic vesicles comes from three different pathways: the GABA recycle pathway, the neuronal glutamate transporter pathway, and the glutamate-glutamine cycle (Bak et al. 2006; Liang et al. 2006; Mathews and Diamond 2003). In the GABA recycle pathway, released GABA is taken up by GABA transporters located in GABAergic presynaptic terminals and packed into the GABA vesicles to be reused. In the neuronal glutamate transporter pathway, extracellular glutamate is transported by neuronal glutamate transporters (such as EAAC1) into GABAergic neurons, where glutamate is transformed into GABA by glutamic acid decarboxylase (GAD). The glutamate-glutamine cycle takes place between neurons and astrocytes. Extracellular glutamate is taken up by glial glutamate transporters into astrocytes. Inside the astrocyte, glutamate is transformed to glutamine via glutamine synthetase. The astrocyte then exports glutamine outside the cell, where glutamine is taken up by neurons. Phosphate-activated glutaminase in neurons deaminates glutamine, producing glutamate. Glutamate in GABAergic neurons is then decarboxylated by GAD to become GABA. Finally, GABA is taken up into synaptic vesicles by vesicular GABA transporters, completing the cycle. This process suggests that astrocytes may regulate GABAergic synaptic activity by altering the function of glial glutamate transporters and glutamine synthetase. Dysfunction in spinal dorsal horn astrocytes has been widely recognized as a key mechanism underlying the pathogenesis of pain (Milligan and Watkins, 2010; Ren and Dubner, 2010). However, it remains unknown whether spinal GABAergic activity can be regulated by glial glutamate transporters and glutamine synthetase in astrocytes.
Two types of glial glutamate transporters (GLAST and GLT-1) exist in astrocytes of the spinal dorsal horn (Mao et al. 2002; Nie et al. 2010; Weng et al. 2005). Dysfunction of glial glutamate transporters is an important mechanism underlying the allodynia and hyperalgesia induced by nerve injury or morphine tolerance (Mao et al. 2002; Nie and Weng 2010; Nie et al. 2010; Sung et al. 2003). Deficient glutamate uptake by glia results in enhanced activation of both AMPA and NMDA receptors in the spinal dorsal horn neurons (Nie and Weng 2009; Weng et al. 2007). Glutamine synthetase is also expressed in astrocytes of the spinal dorsal horn. Recent studies have suggested that reduced activity of glutamine synthetase is associated with spinal central sensitization following intraplantar injection of carrageenan (Chen et al. 2010) and in cases of morphine tolerance (Muscoli et al. 2010). To study the impact of deficient glial glutamate uptake or glutamine synthetase on GABAergic synaptic activities in the spinal superficial dorsal horn, we examined the changes in GABAergic synaptic activity induced by pharmacological inhibition of glial glutamate transporters or glutamine synthetase. We found that both electrically evoked GABAergic IPSCs (eIPSCs) and spontaneous IPSCs (sIPSCs) in the spinal dorsal horn were reduced upon inhibition of glial glutamate transporters and glutamine synthetase. Our results indicate, to our knowledge for the first time, that GABAergic synaptic strength in the spinal dorsal horn is highly dependent on the intact function of glial glutamate transporters and the activity of glutamine synthetase in astrocytes.
MATERIALS AND METHODS
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Georgia and were fully compliant with the National Institutes of Health Guidelines for the Use and Care of Laboratory Animals.
Spinal slice preparation
Young adult male Sprague-Dawley rats (150–180 g) were deeply anesthetized using inhaled isoflurane, and a laminectomy was then performed to remove the lumbar spinal cord. The Lumbar spinal cord section was placed in ice-cold sucrose-based artificial cerebrospinal fluid (aCSF) pre-saturated with 95% O2 and 5% CO2. The sucrose-based aCSF contained 234 mM sucrose, 3.6 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.2 mM NaH2PO4, 12.0 mM glucose, and 25.0 mM NaHCO3. The pia-arachnoid membrane was removed from each section. The L4–5 spinal segment, identified by the lumbar enlargement and large dorsal roots, was attached with cyanoacrylate glue to a cutting support, which was then glued onto the stage of a vibratome (Series 1000, Technical Products International, St. Louis, MO). Transverse spinal cord slices (400 μm) were cut in the ice-cold sucrose aCSF and then pre-incubated for at least 2 hours in Krebs solution oxygenated with 95% O2 and 5% CO2 at 35°C before they were transferred to the recording chamber. The Krebs solution contained 117.0 mM NaCl, 3.6 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.2 mM NaH2PO4, 11.0 mM glucose, and 25.0 mM NaHCO3.
Whole-cell voltage-clamp recordings
Following pre-incubation, a single slice was placed in the recording chamber (1.5 ml in volume), perfused with Krebs solution at 35°C, and saturated with 95% O2 and 5% CO2. Borosilicate glass recording electrodes (resistance, 3–5 MΩ) were made and filled with an internal solution containing 110 mM Cs2SO4, 5 mM KCl, 2.0 mM MgCl2, 0.5 mM CaCl2, 5.0 mM HEPES, 5.0 mM EGTA, 5.0 mM ATP-Mg, 0.5 mM Na-GTP, and 10 mM lidocaine N-ethyl bromide (QX314), adjusted to pH7.2–7.4 with 1 M CsOH (290–300 mOsm). The recording electrodes were directed to the superficial spinal dorsal horn (laminae I and II areas). Live dorsal horn neurons were visualized with an infrared Nomarski microscope system (Leads Instruments, Inc., Texas, USA) and approached with a 3-dimensional motorized manipulator (Sutter Instrument Company, California, USA). Then, whole-cell configurations were established by applying moderate negative pressure after electrode contact (Weng et al. 2006, 2007). A seal resistance of ≥2 GΩ and an access resistance of 20–35 MΩ were considered acceptable. Series resistance was optimally compensated by ≥70% and constantly monitored throughout the experiments. A series resistance of more than 35 MΩ was considered loss of voltage control and the data were discarded (Calcagnotto et al, 2002; Lipp et al, 2002). Signals were amplified with an amplifier (Axopatch 700B, Molecular Devices, California, USA) and displayed and stored in a computer.
GABAergic IPSCs were recorded in the presence of 0.5 μM strychnine, a glycine receptor antagonist, at a holding potential of 0 mV (Hunt el al. 2011; Moore et al. 2002). The currents recorded under such condition were completely abolished by the classic antagonist of GABAA receptors, bicuculline (10 μM) (tested in 8 neurons). To evoked GABAergic IPSCs, neurons in the spinal lamina II area were stimulated by a rectangular electrical pulse (0.1 ms, 0.5 mA, repeated every 40 s) delivered through a concentric bipolar stainless electrode (50 μm diameter, isolated except for the tip) placed within 150 μm of the recorded neurons in the dorsal horn (Yang et al. 2004). Only monosynaptic GABAergic IPSCs were recorded. Identification of IPSCs as monosynaptic was based on a constant latency with graded intensity and high-frequency repetitive stimulation (20 Hz) (Yoshimura and Jessell 1989). Miniature IPSCs (mIPSCs) were recorded in the presence of tetrodotoxin (TTX, 1 μM). When sIPSCs were recorded, DNQX (10 μM) and D-aminophosphonovaleric acid (D-AP5; 25 μM) were used to block non-NMDA receptors and NMDA receptors during recordings.
Materials
(2S, 3S)-3-[3-[4-(trifluoromethyl)benzoylamino]benzyloxy]aspartate (TFB-TBOA), dihydrokainic acid (DHK), tetrahydropyridin-4-yl methylphosphinic acid (TPMPA), strychnine, bicuculline, DNQX and D-AP5 were purchased from Tocris Bioscience (Park Ellisville, MO, USA). TTX and methionine sulfoximine (MSO) were obtained from Sigma (St. Louis, MO, USA). All pharmacological agents were applied by perfusion into the recording chamber.
Data analysis
Data were recorded with Axopatch 700B amplifiers, digitized at 10 kHz and analyzed offline. Three to four eIPSCs evoked at baseline and in the presence of tested drugs, were averaged. Clampfit 10.2 software (Molecular Devices) was used to measure the amplitude of eIPSCs. The frequencies and amplitudes of sIPSCs and mIPSCs were analyzed with Mini Analysis software (synaptosoft, NJ, USA). Data are presented as means ± standard errors (SEs). The paired Student’s t test was used to determine statistical differences between measurements before and after the addition of tested drugs. A P value <0.05 was considered statistically significant.
RESULTS
GABAergic eIPSCs were reduced by TFB-TBOA
To study the impact of glial glutamate uptake deficiency on GABAergic synaptic functions, we recorded eIPSCs in spinal laminae I and II before and during bath perfusion of TFB-TBOA (concentration in the bath, 200 nM). TFB-TBOA is a high-affinity, selective blocker for both GLT-1 and GLAST. The IC50 values of TFB-TBOA for GLT-1 and GLAST are about 1/15 that for the neuronal glutamate transporter EAAC1 (Shimamoto et al. 2004). TFB-TBOA significantly and reversibly reduced the peak amplitude of GABAergic eIPSCs in all neurons by a mean (± SE) of 17.98 ± 2.00% (n = 11, P < 0.001, Fig. 1A). The reduction in GABAergic eIPSCs may be attributed to any or a combination of the following: (a) reduced release probability of GABA at presynaptic terminals; (b) decreased function of GABA receptors at postsynaptic neurons; and (c) decreased synthesis of GABA. We conducted the following experiments to determine which of these mechanisms occurred.
Figure 1. TFB-TBOA significantly reduces electrically evoked GABAergic IPSC amplitudes without changing P2/P1 ratios or GABA currents evoked by exogenous GABA.
(A) Samples of original recordings recorded from the same cell before, during and after washout of TFB-TBOA are displayed (left). Bar graphs (right) show mean (±SE) amplitudes before, during and after washout of TFB-TBOA (200 nM). (B) The raw data (left) show two GABAergic IPSCs evoked by paired pulse stimuli (100 ms apart) before, during and after washout of TFB-TBOA (200 nM). Bar graphs (right) show mean (±SE) P2/P1 ratios at baseline, during and after washout of TFB-TBOA (200 nM). (C) Samples of GABA currents evoked by perfusing exogenous GABA (1 mM, 20 s) at baseline and in the presence of TFB-TBOA (200 nM) are shown (left). Mean (±SE) percentages of baseline peak amplitudes of GABA currents in the presence of TFB-TBOA (200 nM) are presented in bar graphs (right). * P < 0.05; *** P < 0.001; n.s., no statistical significance.
TFB-TBOA did not alter the P2:P1 ratio
To determine whether the changes induced by TFB-TBOA in GABAergic eIPSCs was due to altered release probability of GABA transmitter at the presynaptic site, we first recorded two GABAergic eIPSCs elicited by paired pulses separated by 100 ms and analyzed the paired pulse ratio (i.e., P2:P1 ratio). P1 was the amplitude of the first evoked current and P2 was the second response amplitude measured after subtraction of the remaining P1 “tail” current” (Balakrishnan et al, 2009; Marty et al, 2011). An increase or decrease in the P2:P1 ratio is conventionally used to detect a decrease or increase in neurotransmitter release probability at the presynaptic site (Foster and McNaughton 1991; Manabe et al. 1993; Weng et al. 2007; Xu et al. 2008; Zucker 1989), including GABAergic synapses in the spinal dorsal horn (Hugel and Schlichter, 2000; Ingram et al, 2008). As shown in Fig. 1B, although both P2 and P1 values of the GABAergic eIPSCs were reduced after bath perfusion of TFB-TBOA, the P2:P1 ratios remained unchanged (0.66 ± 0.08 at baseline and 0.67 ± 0.08 during TFB-TBOA perfusion, n = 11, P = 0.24). These data indicate that the TFB-TBOA–induced reduction in GABAergic eIPSCs was not due to the reduced release probability of GABA from presynaptic terminals.
TFB-TBOA did not alter GABA currents induced by exogenous GABA
To determine whether functions of postsynaptic GABA receptors changed during perfusion of TFB-TBOA (200 nM), we recorded GABA currents evoked by exogenous GABA perfusion (1 mM, 20 s) into the recording bath before and during perfusion of TFB-TBOA. As shown in Fig. 1C, TFB-TBOA did not alter GABA currents evoked by exogenous GABA perfusion (n = 6, P = 0.11), indicating that the function of postsynaptic GABA receptors remained unchanged during perfusion of TFB-TBOA under our experimental conditions.
TFB-TBOA reduced GABA concentrations at the synaptic cleft
If the TFB-TBOA–induced reduction in GABAergic eIPSCs were due to a reduction of GABA synthesis, one would expect that the amount of GABA released from presynaptic terminals would be reduced—that is, the concentrations in the GABAergic synaptic cleft would be reduced per se (Liang et al. 2006). To test this notion, we examined the inhibition induced by TPMPA on GABAergic eIPSCs in the absence and presence of TFB-TBOA. Because TPMPA is a low-affinity competitive antagonist of GABAA receptors, TPMPA binding to GABAA receptors is rapidly replaced by synaptic GABA transmitter. When GABAA receptors are exposed to TPMPA, the degree of inhibition caused by TPMPA on GABAergic IPSCs is inversely related to the concentration of GABA transients (the amount of GABA release) in the synaptic cleft (Foster et al. 2005; Liang 2006; Liu et al. 1999; Shen et al. 2002; Wadiche and Jahr 2001). As shown in Fig. 2 the percentage of inhibition by TPMPA (60 μM) on GABAergic IPSCs in the presence of TFB-TBOA (45.22 ± 2.11%, n = 10) was significantly larger (P < 0.001) than that in the absence of TFB-TBOA (20.90 ± 2.16%, n = 10). These data indicate that inhibition induced by TFB-TBOA on GABAergic IPSCs was indeed due to a decrease in the amount of GABA release from presynaptic neurons.
Figure 2. GABA concentrations at the synaptic cleft is reduced by TFB-TBOA.
(A) Shown are electrically evoked GABAergic IPSCs (eIPSCs) at baseline (a1), in the presence of TPMPA (60 μM) (a2), after TPMPA washout (a3), in the presence of TFB-TBOA (200 nM) (a4), in the presence of TFB-TBOA (200 nM) plus TPMPA (60 μM) (a5), and after washout of both TFB-TBOA and TPMPA (a6). In the presence of TFB-TBOA, the percentage of inhibition induced by TPMPA on eIPSCs (a4 and a5) was significantly stronger than that induced by TPMPA in the absence of TFB-TBOA (a1 and a2). (B) Mean (±SE) percentages of eIPSC amplitudes during perfusion of TPMPA in the absence of TFB-TBOA (second bar to the left), and in the presence of TFB-TBOA (the far right bar). Amplitudes prior to TPMPA perfusion in the absence (the far left bar) or presence of TFB-TBOA (the second bar to the right) were used as the baseline for calculating the percentages. *** P <0.001.
Exogenous glutamine prevented the reduction induced by TFB-TBOA in GABAergic eIPSCs
Although TFB-TBOA has an IC50 value for EAAC1 15 times higher than that for GLT-1 and GLAST, it was not clear that the TFB-TBOA induced reduction of GABAergic activity in the preceding experiment occurred through its inhibition on GLT-1 and GLAST in the astrocyte or on EAAC1 in the neuron. Based on the glutamate-glutamine cycle theory (Bak et al. 2006), we reasoned that if the effects induced by TFB-TBOA occur through its inhibition on GLT-1 and GLAST, then glutamine concentrations in the extracellular space in the presence of TFB-TBOA must be reduced, resulting in a deficient glutamine supply to GABAergic neurons and a reduction in GABA synthesis. If this is the case, supplementing exogenous glutamine in the recording bath would prevent the reduction induced by TFB-TBOA in GABAergic eIPSCs. As expected, in the presence of 1 mM glutamine, TFB-TBOA no longer altered the amplitudes of GABAergic eIPSCs (baseline: 712.88 ± 78.90 pA; TFB-TBOA: 699.00 ± 79.64 pA, n = 8, P = 0.24, Fig. 3).
Figure 3. Exogenous glutamine prevents the reduction induced by TFB-TBOA in electrically evoked GABAergic IPSCs (eIPSCs).

(A) Samples of original recordings show that in the presence of glutamine (1 mM), amplitudes of eIPSCs were not changed by bath-application of TFB-TBOA (200 nM). (B) Bar graphs show that mean (±SE) amplitudes of eIPSCs in the presence of glutamine (1 mM) were not significantly altered by TFB-TBOA. n.s., no statistical significance.
Contents of spontaneously released GABAergic vesicles were reduced by TFB-TBOA
The data described above clearly demonstrate that TFB-TBOA reduced the contents of GABAergic vesicles in the vesicle pool used by action potential–evoked release. It has been suggested that the presynaptic vesicle pool used by spontaneous neurotransmitter release in the absence of action potentials is separate from the presynaptic vesicle pool used by action potential–evoked neurotransmitter release (Fredj and Burrone 2009; Mathew et al. 2008; Schikorski and Stevens 2001). To determine whether TFB-TBOA also affects the contents of GABAergic vesicles in the vesicle pool used by spontaneous release, GABAergic mIPSCs were recorded in the presence of 1 μM TTX (Fredj and Burrone 2009; Mathew et al. 2008; Murthy and Stevens 1999) (Fig. 4). Perfusion of TFB-TBOA immediately (in <3 min) and significantly reduced the mean mIPSC amplitudes by 13.32 ± 3.09% (n = 9, P < 0.01) and frequencies by 27.37 ± 7.33% (n = 9, P < 0.01). Because TFB-TBOA did not alter the function of postsynaptic GABA receptors or the release probability at the presynaptic site (Fig. 1), we reasoned that the reduction in GABAergic mIPSC frequencies was ascribed to a failure to detect some of the GABAergic mIPSCs with low amplitude during perfusion of TFB-TBOA. It is conceivable that the missing of events with low amplitudes during analysis also led to an underestimation of the percentage reduction of the mean mIPSC amplitudes induced by TFB-TBOA. Our data are in line with previous reports that decreasing the amount of GABA and glutamate in synaptic vesicles not only reduces amplitudes of mIPSCs and miniature excitatory postsynaptic currents (mEPSCs), but also reduces frequencies of mIPSCs and mEPSCs (Juge et al. 2010; Zhou et al. 2000). Together, these findings suggest that the contents of GABAergic vesicles in the vesicle pool used by spontaneous neurotransmitter release were reduced upon blockage of glial glutamate transporters.
Figure 4. Contents of spontaneously released GABAergic vesicles are reduced by TFB-TBOA.
(A) A sample of GABAergic miniature IPSC (mIPSC) recording before, during, and after washout of TFB-TBOA (200 nM). a1 and a2 show enlargements of the recording before and during perfusion of TFB-TBOA, respectively. (B) Bar graphs show that mean (±SE) frequencies (top) and amplitudes (bottom) of GABAergic mIPSCs were reduced by bath perfusion of TFB-TBOA. * P < 0.05; ** P < 0.01.
Selective inhibition of GLT-1 reduced GABAergic eIPSCs and sIPSCs
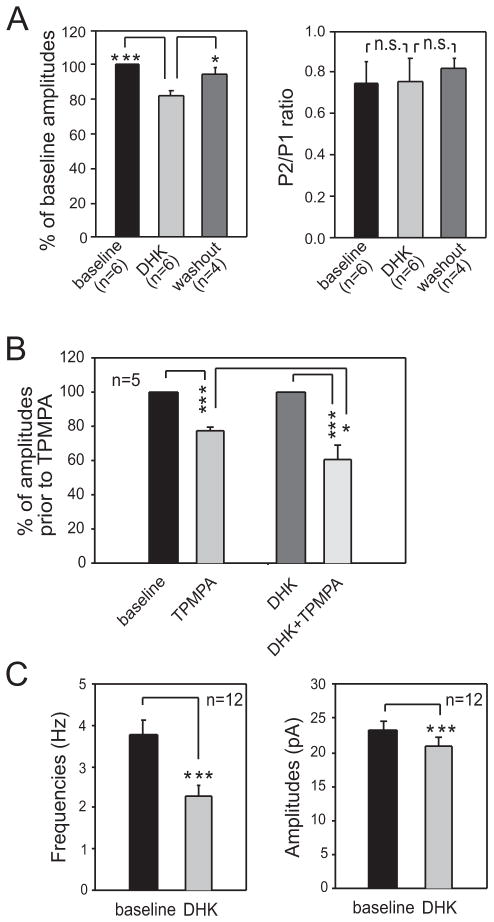
Two types of glial glutamate transporters, GLAST and GLT-1, are blocked by TFB-TBOA (Shimamoto et al. 2004). It is generally believed that GLT-1 plays a major role in taking up glutamate into glial cells. To specifically determine the role of GLT-1 in the GABAergic activity in the spinal dorsal horn, we first examined the effects induced by DHK, which selectively blocks GLT-1 (Arriza et al. 1994; Shimamoto et al. 2004), on GABAergic eIPSCs. Similar to the effects induced by TFB-TBOA, bath perfusion of DHK (concentration in the bath, 300 μM) significantly and reversibly reduced the amplitude of GABAergic IPSCs by 17.81 ± 2.69% (n = 6, p<0.001). The P2:P1 ratios remained unchanged (baseline: 0.74 ± 0.10; DHK: 0.75 ± 0.11) (Fig. 5A). Next, TPMPA was used to determine the changes in GABA concentrations induced by DHK in the synaptic cleft (Fig. 5B). We found that the mean percentage of inhibition induced by TPMPA (60 μM) on GABAergic eIPSCs in the presence of DHK (39.56 ± 7.28%, n = 5) was significantly (P < 0.05) stronger than that in the absence of DHK (22.28 ± 2.46%, n = 5), indicating that GABA concentrations in the synaptic cleft were reduced in the presence of DHK. We then determined the effects of DHK on GABAergic sIPSCs, which reflect vesicles released from both the vesicle pool used by action potentials and that used by spontaneous release in the absence of action potentials. To eliminate effects induced by changes in excitatory synaptic activities during the blockade of glutamate transporters, we used DNQX (10 μM) and D-AP5 (25 μM) to block non-NMDA receptors and NMDA receptors during recordings. Bath perfusion of DHK reduced the amplitude of GABAergic sIPSCs by 10.40 ± 1.55% (n = 12, P < 0.001) and the mean frequency by 37.60 ± 5.64% (n = 12, P < 0.001) (Fig. 5C). Furthermore, in the presence of 1 mM glutamine, bath perfusion of DHK no longer altered the amplitude (baseline: 22.62 ± 1.40 pA; DHK: 22.32 ± 1.48 pA; n=6) and the frequency (baseline: 5.00 ± 1.24 Hz; DHK: 5.11 ± 1.34Hz; n=6) of GABAergic sIPSCs. These data indicate that GLT-1 is a key glial glutamate transporter for maintaining the homeostasis of GABA activity in the spinal dorsal horn.
Figure 5. Selective inhibition of GLT-1 reduces GABAergic eIPSCs and sIPSCs.
(A) Bar graphs show that selective inhibition of GLT-1 with DHK (300 μM) significantly and reversibly reduced the amplitude of electrically evoked GABAergic IPSCs (eIPSCs) (left) without altering the P2/P1 ratio (right). (B) In the presence of DHK (300 μM), the percentage of inhibition induced by TPMPA (60 μM) on eIPSCs was significantly stronger than that induced by TPMPA in the absence of DHK. Mean (±SE) percentages of eIPSC amplitudes during perfusion of TPMPA in the absence of DHK (the second bar to the left) and in the presence of DHK (the far right bar). Amplitudes prior to TPMPA perfusion in the absence (the far left bar) or presence of DHK (the second bar to the right) were used as the baseline for calculating the percentages. (C) Selective inhibition of GLT-1 reduces amplitudes and frequencies of spontaneous IPSCs (sIPSCs). Bar graphs show that mean (±SE) frequencies (left) and amplitudes (right) of GABAergic sIPSCs were reduced by bath perfusion of DHK. * P < 0.05; *** P < 0.01; n.s., no statistical significance.
Selective inhibition of glutamine synthetase reduced the amplitude of GABAergic eIPSCs, as well as both the amplitude and frequency of GABAergic sIPSCs
One key step in the glutamate-glutamine cycle is the transformation of glutamate to glutamine by glutamine synthetase in astrocytes. Thus, we used MSO to selectively block glutamine synthetase (Fricke et al. 2007; Liang et al. 2006; Sellinger 1967) to determine the role of glutamine synthetase in GABAergic synaptic activity. Bath application of MSO (concentration in the recording chamber, 5 mM) significantly and reversibly reduced the peak amplitude of GABAergic eIPSCs by a mean of 29.36 ± 3.90% (n = 8, p<0.001) without changing the P2:P1 ratio (Fig. 6). GABAergic sIPSCs were then recorded in the presence of DNQX (10 μM) and D-AP5 (25 μM) before and after bath perfusion of MSO (concentration in the recording chamber, 5 mM). In the presence of MSO, the amplitude of GABAergic sIPSCs was reduced by 12 ± 3.15% (n = 12, P < 0.01) and the frequency by 35.50 ± 5.23% (n = 12, P < 0.01) (Fig. 7). These data indicate that spinal GABAergic activities are dependent on the tonic activity of glial glutamine synthetase, which also further supports the idea that GABA synthesis through the glutamate-glutamine cycle is crucial to maintaining normal GABAergic synaptic activities in the spinal dorsal horn.
Figure 6. Selective inhibition of glutamine synthetase reduces the amplitude of electrically evoked GABAergic IPSCs (ePISCs) without altering P2/P1 ratios.

(A) The raw data show that bath perfusion of the glutamine synthetase inhibitor (MSO, 5 mM) reversibly reduced amplitudes of two GABAergic IPSCs evoked by paired pulse stimuli (100 ms apart) without altering the P2/P1 ratio. (B) Bar graphs show that MSO reduced mean amplitudes of electrical evoked GABAergic IPSCs, but did not alter the ratio. * P < 0.05; *** P < 0.01; n.s., no statistical significance.
Figure 7. Selective inhibition of GLT-1 reduces amplitudes and frequencies of spontaneous IPSCs.
(A) A sample of GABAergic sIPSC recording before, during, and after washout of bath perfusion of the glutamine synthetase inhibitor (MSO, 5 mM). a1 and a2 show enlargements of the recording before and during perfusion of MSO, respectively. (B) Bar graphs show that mean (±SE) frequencies (top) and amplitudes (bottom) of GABAergic sIPSCs were reduced by bath perfusion of MSO. ** P < 0.01.
DISCUSSION
In this study, we reveal that astrocytes regulate spontaneous and action-potential–dependent GABAergic activities in the spinal dorsal horn via glial glutamate transporters and glutamine synthetase. Specifically, we found that blockade of both GLAST and GLT-1 and blockade of GLT-1 only in the spinal dorsal horn suppressed the peak amplitude of eIPSCs, sIPSCs or mIPSCs. Frequency of GABAergic sIPSCs and mIPSCs was also decreased upon blockade of glial glutamate transporters. Inhibition of glial glutamine synthetase by MOS produced similar effects in GABAergic eIPSCs and sIPSCs. We provide evidence to demonstrate that the reduction of GABAergic activity results from insufficient GABA synthesis through the glutamate-glutamine cycle between astrocytes and GABAergic neurons.
Degree of dependence of GABAergic activity on the glutamate-glutamine cycle differs in synapses from different areas of the central nervous system
In this study, we found that maintenance of normal GABAergic activity in the spinal dorsal horn is highly dependent on the intact function of glial glutamate transporters and the activity of glutamine synthetase, as demonstrated by the our findings that amplitudes of both GABAergic sIPSCs and eIPSCs decreased immediately upon pharmacological blockade of glial glutamate transporters or glutamine synthetase. This high degree of dependency is strikingly different from that of synapses in the hippocampal CA1 area. Studies of that area in rats showed that blocking the glutamate-glutamine cycle by inhibiting GLT-1 with DHK or inhibiting glutamine synthetase with MOS did not alter GABAergic sIPSCs or eIPSCs (Fricke et al. 2007; Liang et al. 2006). Only after repetitive burst stimulation (four pulses of 50-Hz bursts repeated at 20-s intervals, 0.05 Hz, for 15 min) did application of DHK significantly reduce eIPSC amplitudes (Liang et al. 2006). These differences between previous studies and ours may be ascribed to several possibilities: (a) spinal dorsal horn GABAergic neurons have lower GABA storage and/or a higher consumption rate of GABA vesicles than hippocampal GABAergic neurons; (b) among the three pathways to supply GABA to GABA vesicles, the glutamate-glutamine cycle plays a more dominant role in the spinal dorsal horn than in the hippocampal area; or (c) other pathways, such as the GABA recycle pathway and/or the neuronal glutamate transporter pathway, may compensate more efficiently in GABA supply in hippocampal areas than in the spinal dorsal horn. Consistent with this view, studies of the hippocampal area have shown that blockade of glial glutamate transporters can reduce the amplitude of mIPSCs when neuronal glutamate transporters are blocked (Fricke et al. 2007). These data reiterate the long-held notion that the role of glutamate transporters in synaptic functions is region and synapse specific (Danbolt 2001).
Glial glutamate transporters are key molecules used by astrocytes to regulate both glutamatergic and GABAergic synaptic strength in the spinal dorsal horn
Abnormal activation of spinal dorsal horn neurons and dysfunction in glial cells are two fundamental phenomena in pathological pain conditions (Milligan and Watkins, 2010; Ren and Dubner, 2010). Imbalance between excitatory and inhibitory synaptic strength results in abnormal neuronal activation. Glutamate concentrations in the spinal dorsal horn are elevated in pathological pain conditions (Yang et al. 2011). Mechanisms by which glial cells increase excitatory synaptic strength have been accumulated. For example, cytokines and chemokines released from activated glial cells can enhance responses of AMPA receptors and NMDA receptors and spontaneous activities in dorsal horn neurons (Kawasaki et al. 2008). Glial cells also directly regulate the activation of glutamate receptors by glial glutamate transporters. Recent studies by us and others have shown that pharmacological blockade of glial glutamate transporters induces mechanical and thermal hypersensitivity in normal rats (Liaw et al. 2005; Weng et al. 2006). Down-regulation of glial glutamate transporter expression is has been associated with allodynia and hyperalgesia in neuropathic rats with neuropathy induced by nerve injury (Nie et al. 2010; Sung et al. 2003; Weng et al. 2005). In our previous studies, when we mimicked the glial deficiency of glial glutamate uptake by pharmacologically blocking glial glutamate transporters, excitatory postsynaptic currents induced by activation of both AMPA receptors and NMDA receptors increased (Nie and Weng 2009; Weng et al. 2007). Mechanisms by which glial cells regulate GABAergic synaptic strength in the spinal dorsal horn are still not fully understood. The findings of the current study indicate that the disinhibition induced by impairment of glial glutamate uptake and glutamine synthetase may be a crucial synaptic mechanism underlying glial-neuronal interactions caused by dysfunctional astrocytes in pathological pain conditions.
It is generally believed that glutamate in neurons is maintained by the glutamate-glutamine cycle (Kandel et al. 2000). Because glutamate is both an excitatory neurotransmitter and a precursor for the synthesis of GABA transmitter, one would expect that blockade of glial glutamate transporters (which impairs the glutamate-glutamine cycle) should, in addition to causing a reduction of GABAergic synaptic activities, reduce glutamatergic synaptic activities. However, previous studies by us and others have consistently shown that glial glutamate transporter inhibitors enhance the activation of glutamate receptors in both the spinal dorsal horn (Nie and Weng 2009; Weng et al. 2007) and forebrain areas (Overstreet et al. 1999; Tong and Jahr 1994). Furthermore, low expression of glial glutamate transporters has been associated with neuronal hyperactivation in neuropathic pain (Nie et al. 2010; Sung et al. 2003; Weng et al. 2005; Xin et al. 2009), and gene knockout of glial glutamate transporters has been shown to result in neuronal excitotoxicity (Rothstein et al. 1996) and epilepsy (Tanaka et al. 1997). The mechanisms by which glutamatergic synapses resist the impairment of the glutamate-glutamine cycle induced by reduced glial glutamate uptake remain unknown. It is possible that the glutamate resource, the compensatory pathways for the glutamate supply that are activated upon impairment of glial glutamate uptake, the size of the glutamate pool, and the efficacy of glutamate use in glutamatergic neurons differ from those in GABAergic neurons. Indeed, the central role of the glutamate-glutamine cycle in maintaining the vesicular glutamate pool in glutamatergic neurons has been challenged in recent years. Studies have shown that direct uptake of released glutamate accounted for a significant contribution to vesicular glutamate in photoreceptors in the retina (Hasegawa et al. 2006; Winkler et al. 1999) and in bipolar cells (Palmer et al. 2003). Glutamatergic neurons in hippocampus have the capacity to store or produce glutamate for long periods of time, independent of glia and the glutamate-glutamine cycle (Kam and Nicoll 2007). It has been suggested that glutamatergic neurons are capable of de novo synthesis of glutamate from tricarboxylic acid cycle intermediates, which can then be replenished from glutamine, glucose, or any other source of carbons that can be transported into the cell (Kam and Nicoll 2007). Although these previous studies suggest that the glutamate-glutamine cycle may not be crucial to supplying glutamate to excitatory neurons, our current study indicate that GABA synthesis in spinal dorsal horn GABAergic neurons is highly dependent on the glutamate-glutamine cycle. Consistent with our data, a recent study shows that exogenous glutamine reversed the hippocampal circuit hyperexcitability induced by GABAergic IPSC erosion resulting from failure of the astrocytic glutamate-glutamine cycle (Ortinski et al, 2010).
Functional implications
The spinal superficial dorsal horn is a first station for processing nociceptive inputs from peripheral nociceptive Aδ and C fibers. Reduced GABAergic synaptic strength in this area is thought to be an important mechanism leading to allodynia in neuropathic pain conditions. Extracellular GABA levels in the lumbar dorsal horn have been shown to be decreased by nerve injury (Stiller et al. 1996). Amplitudes and frequencies of GABAA receptor–mediated IPSCs in spinal dorsal horn neurons in neuropathic rats have also been found to be decreased (Moore et al. 2002). Mechanical allodynia and thermal hyperalgesia induced by nerve injury are known to be reversed by spinal application of GABA agonists (Malan et al. 2002). In addition, apoptosis of GABAergic neurons (Scholz et al. 2005), reduction of the chloride gradient across the neuronal membrane (Coull et al. 2003), and low expression of GAD (an enzyme that converts glutamate to GABA in GABAergic neurons) (Moore et al. 2002) have all been suggested to contribute to disinhibition in the neuropathic spinal dorsal horn induced by nerve injury. Our findings that GABAergic synaptic strength in the spinal superficial dorsal horn is highly dependent on the continuous supply of glutamine from glial cells through the glutamate-glutamine cycle highlight a novel pathway by which glial cells can regulate inhibitory synaptic activities in the spinal superficial dorsal horn. Physiological and pathological regulation of glial glutamine synthetase (which converts glutamate to glutamine) and glial glutamate transporters could be a crucial mechanism to alter the balance between excitatory and inhibitory inputs to spinal dorsal horn neurons. Indeed, down-regulation of glial glutamate transporters has been found in neuropathic rats and those with morphine tolerance (Mao et al. 2002; Nie et al. 2010; Sung et al. 2003; Weng et al. 2005). Recent studies have also shown nitration of glutamine synthetase, which reduces the function of glutamine synthetase, in spinal central sensitization following intraplantar injection of carrageenan (Chen et al. 2010) and in cases of morphine tolerance (Muscoli et al. 2010). Further experiments are needed to confirm that down-regulation of glial glutamate transporters and nitration of glutamine synthetase cause the decreased GABAergic synaptic activity and increased excitability in the spinal dorsal horn in these animal models.
In conclusion, we have demonstrated that glial cells consistently regulate GABAergic synaptic activities in the spinal superficial dorsal horn by taking up glutamate into glial cells and converting glutamate to glutamine through glutamine synthetase. Our findings suggest that normalization of glial glutamate transporters and glutamine synthetase may be a potential approach to counter the imbalance between excitatory and inhibitory synaptic strength in the spinal dorsal horn in pathological pain conditions.
Acknowledgments
This project was supported by NIH R01 grant NS064289 to H.R.W.
References
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Balakrishnan V, Kuo SP, Roberts PD, Trussell LO. Slow glycinergic transmission mediated by transmitter pooling. Nat Neurosci. 2009;12:286–94. doi: 10.1038/nn.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnotto ME, Paredes MF, Baraban SC. Heterotopic neurons with altered inhibitory synaptic function in an animal model of malformation-associated epilepsy. J Neurosci. 2002;22:7596–7605. doi: 10.1523/JNEUROSCI.22-17-07596.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Muscoli C, Doyle T, Bryant L, Cuzzocrea S, Mollace V, Mastroianni R, Masini E, Salvemini D. NMDA-receptor activation and nitroxidative regulation of the glutamatergic pathway during nociceptive processing. Pain. 2010;149:100–106. doi: 10.1016/j.pain.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JAM, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, DeKoninck P, DeKoninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Foster TC, McNaughton BL. Long-term enhancement of CA1 synaptic transmission is due to increased quantal size, not quantal content. Hippocampus. 1991;1:79–91. doi: 10.1002/hipo.450010108. [DOI] [PubMed] [Google Scholar]
- Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci. 2009;12:751–758. doi: 10.1038/nn.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke MN, Jones-Davis DM, Mathews GC. Glutamine uptake by System A transporters maintains neurotransmitter GABA synthesis and inhibitory synaptic transmission. Journal of Neurochemistry. 2007;102:1895–1904. doi: 10.1111/j.1471-4159.2007.04649.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa J, Obara T, Tanaka K, Tachibana M. High-density presynaptic transporters are required for glutamate removal from the first visual synapse. Neuron. 2006;50:63–74. doi: 10.1016/j.neuron.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Hugel S, Schlichter R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neurosci. 2000;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Scheff SW, Smith BN. Synaptic reorganization of inhibitory hilar interneuron circuitry after traumatic brain injury in mice. J Neurosci. 2011;31:6880–6890. doi: 10.1523/JNEUROSCI.0032-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RA, Fitzgerald M, Baccei ML. Developmental changes in the fidelity and short-term plasticity of GABAergic synapses in the neonatal rat dorsal horn. J Neurophysiol. 2008;99:3144–3150. doi: 10.1152/jn.01342.2007. [DOI] [PubMed] [Google Scholar]
- Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam K, Nicoll R. Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle. J Neurosci. 2007;27:9192–9200. doi: 10.1523/JNEUROSCI.1198-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E, Schwartz J, Jessell T. Principles of neural science. 4. McGraw-Hill; New York: 2000. [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. Journal of Neuroscience. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SL, Carlson GC, Coulter DA. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. Journal of Neuroscience. 2006;26:8537–8548. doi: 10.1523/JNEUROSCI.0329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw WJ, Stephens RL, Jr, Binns BC, Chu Y, Sepkuty JP, Johns RA, Rothstein JD, Tao YX. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain. 2005;115:60–70. doi: 10.1016/j.pain.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lipp P, Egger M, Niggli E. Spatial characteristics of sarcoplasmic reticulum Ca2+ release events triggered by L-type Ca2+ current and Na+ current in guinea-pig cardiac myocytes. J Physiol. 2002;542:383–393. doi: 10.1113/jphysiol.2001.013382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TP, Mata HP, Porreca F. Spinal GABA A and GABAB receptor pharmacology in a model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. Journal of Neurophysiology. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. Journal of Neuroscience. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty V, Kuzmiski JB, Baimoukhametova DV, Bains JS. Short-term plasticity impacts information transfer at glutamate synapses onto parvocellular neuroendocrine cells in the paraventricular nucleus of the hypothalamus. J Physiol. 2011;589:4259–4270. doi: 10.1113/jphysiol.2011.208082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SS, Pozzo-Miller L, Hablitz JJ. Kainate modulates presynaptic GABA release from two vesicle pools. J Neurosci. 2008;28:725–731. doi: 10.1523/JNEUROSCI.3625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews GC, Diamond JS. Neuronal glutamate uptake Contributes to GABA synthesis and inhibitory synaptic strength. J Neurosci. 2003;23:2040–2048. doi: 10.1523/JNEUROSCI.23-06-02040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. Journal of Neuroscience. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Stevens CF. Reversal of synaptic vesicle docking at central synapses. Nat Neurosci. 1999;2:503–507. doi: 10.1038/9149. [DOI] [PubMed] [Google Scholar]
- Muscoli C, Doyle T, Dagostino C, Bryant L, Chen Z, Watkins LR, Ryerse J, Bieberich E, Neumman W, Salvemini D. Counter-regulation of opioid analgesia by glial-derived bioactive sphingolipids. J Neurosci. 2010;30:15400–15408. doi: 10.1523/JNEUROSCI.2391-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Weng HR. Glutamate Transporters Prevent Excessive Activation of NMDA Receptors and Extrasynaptic Glutamate Spillover in the Spinal Dorsal Horn. Journal of Neurophysiology. 2009;101:2041–2051. doi: 10.1152/jn.91138.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Weng HR. Impaired glial glutamate uptake induces extrasynaptic glutamate spillover in the spinal sensory synapses of neuropathic rats. J Neurophysiol. 2010;103:2570–2580. doi: 10.1152/jn.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Zhang H, Weng HR. Minocycline prevents impaired glial glutamate uptake in the spinal sensory synapses of neuropathic rats. Neuroscience. 2010;170:901–912. doi: 10.1016/j.neuroscience.2010.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2011;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet LS, Kinney GA, Liu YB, Billups D, Slater NT. Glutamate transporters contribute to the time course of synaptic transmission in cerebellar granule cells. Journal of Neuroscience. 1999;19:9663–9673. doi: 10.1523/JNEUROSCI.19-21-09663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MJ, Taschenberger H, Hull C, Tremere L, von Gersdorff H. Synaptic activation of presynaptic glutamate transporter currents in nerve terminals. J Neurosci. 2003;23:4831–4841. doi: 10.1523/JNEUROSCI.23-12-04831.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellinger OZ. Inactivation of cerebral glutamine synthetase by DL-methionine-DL-sulfoximine. Biochim Biophys Acta. 1967;132:514–516. doi: 10.1016/0005-2744(67)90172-6. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Sakai R, Takaoka K, Yumoto N, Nakajima T, Amara SG, Shigeri Y. Characterization of novel L-threo-beta-benzyloxyaspartate derivatives, potent blockers of the glutamate transporters. Mol Pharmacol. 2004;65:1008–1015. doi: 10.1124/mol.65.4.1008. [DOI] [PubMed] [Google Scholar]
- Stiller CO, Cui JG, O’Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of y-aminobutyric acid in the dorsal horn and suppression of tactile alloydynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery. 1996;39 doi: 10.1097/00006123-199608000-00026. [DOI] [PubMed] [Google Scholar]
- Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. Journal of Neuroscience. 2003;23:2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tong G, Jahr CE. Block of glutamate transporters potentiates postsynaptic excitation. Neuron. 1994;13:1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Weng HR, Aravindan N, Cata JP, Chen JH, Shaw AD, Dougherty PM. Spinal glial glutamate transporters downregulate in rats with taxol-induced hyperalgesia. Neurosci Lett. 2005;386:18–22. doi: 10.1016/j.neulet.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Weng HR, Chen JH, Cata JP. Inhibition of glutamate uptake in the spinal cord induces hyperalgesia and increased responses of spinal dorsal horn neurons to peripheral afferent stimulation. Neuroscience. 2006;138:1351–1360. doi: 10.1016/j.neuroscience.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Weng HR, Chen JH, Pan ZZ, Nie H. Glial glutamate transporter 1 regulates the spatial and temporal coding of glutamatergic synaptic transmission in spinal lamina II neurons. Neuroscience. 2007;149:898–907. doi: 10.1016/j.neuroscience.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Winkler BS, Kapousta-Bruneau N, Arnold MJ, Green DG. Effects of inhibiting glutamine synthetase and blocking glutamate uptake on b-wave generation in the isolated rat retina. Vis Neurosci. 1999;16:345–353. doi: 10.1017/s095252389916214x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin WJ, Weng HR, Dougherty PM. Plasticity in expression of the glutamate transporters GLT-1 and GLAST in spinal dorsal horn glial cells following partial sciatic nerve ligation. Molecular Pain. 2009;5 doi: 10.1186/1744-8069-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci. 2008;28:7445–7453. doi: 10.1523/JNEUROSCI.1812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by a spinal glycine inhibition:effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- Yang CP, Cherng CH, Wu CT, Huang HY, Tao PL, Wong CS. Intrathecal ultra-low dose naloxone enhances the antinociceptive effect of morphine by enhancing the reuptake of excitatory amino acids from the synaptic cleft in the spinal cord of partial sciatic nerve-transected rats. Anesth Analg. 2011;113:1490–500. doi: 10.1213/ANE.0b013e31822d39c1. [DOI] [PubMed] [Google Scholar]
- Yang K, Fujita T, Kumamoto E. Adenosine inhibits GABAergic and glycinergic transmission in adult rat substantia gelatinosa neurons. J Neurophysiol. 2004;92:2867–2877. doi: 10.1152/jn.00291.2004. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62:96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Petersen CC, Nicoll RA. Effects of reduced vesicular filling on synaptic transmission in rat hippocampal neurones. J Physiol. 2000;525:195–206. doi: 10.1111/j.1469-7793.2000.t01-1-00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]