Abstract
Background
The identification and enlistment of suitable participants into clinical studies is often challenging, requiring a large commitment of time and staff resources. The recruitment and retention of populations typically underrepresented in research present additional challenges to enrollment of sufficient numbers of participants in clinical studies. Inadequate participation may undermine the pace and direction of new treatment discoveries.
Purpose
Registries of potential research participants are powerful tools to support research by providing a framework to streamline screening and recruitment and to maintain a communication history with potential research participants. The authors present a model for the development and implementation of a web-based database system to support recruitment, enrollment, and retention of potential study participants in close alignment with the goals of the Wisconsin Alzheimer’s Disease Research Center (ADRC).
Methods
The required data elements and major information domains for the registry were identified using a structured problem-solving and system design approach and the collaboration of a multidisciplinary team of stakeholders. The system performance, utility, and usability were assessed through multiple iterations with the users.
Results
The process-oriented approach culminated in a multifaceted tool that combined contact management and potential research participant registration to assist with the challenges of recruitment and retention in clinical research. A unique feature of the registry design model was its contact management capabilities for efficient tracking of all contacts with registrants.
Limitations
We have focused on the development and implementation of a system for the recruitment of older adults with specific cognitive and medical characteristics. However, our procedures for identifying data needs and database system utility and functionality can be transferred easily to other populations and settings. As with any multipurpose registry database system, careful management and training are essential to optimize efficiency.
Conclusion
Adding a contact management element to the registry design significantly improved the efficiency of communication between clinical study coordinators and potential research participants, as well as the communication among coordinators.
Introduction
Participant recruitment and retention are essential to the success of any clinical study and consequently to the evaluation of new treatments. However, identifying potential research participants who meet specific selection criteria and achieving enrollment goals are often challenging and require large commitments of time and staff resources. Some of the challenges associated with recruitment to clinical studies have been well documented in the literature [1–4], particularly recruitment and enrollment of underrepresented populations such as racial/ethnic minorities, older adults, those living in rural areas, and low-income individuals [2,5–11]. Moreover, targeting special populations very often requires expertise and a unique set of skills, as well as more time and a significant investment of resources to tailor recruitment strategies and retention plans to their particular needs [12–14].
In a review of more than 100 trials, McDonald et al. [4] showed that less than a third of the trials achieved their original recruitment target and half required an extension of the accrual period. A recent press release from the National Institutes of Health (NIH) indicated that only 4% of the US population had participated in clinical trials and about 85% of trials conducted nationwide did not finish on time due to low participation [15]. The risks of low participation and recruitment of less than the planned sample also threaten the statistical power of the study and consequently its clinical relevance and generalizability [16,17]. Targeting specific populations for research such as older adults can be uniquely challenging and thus requires more timely and efficient approaches to enroll adequate samples of individuals who meet inclusion and exclusion criteria for entry into the prospective studies and are representative of the majority of older people. Furthermore, the conduct of clinical studies that enroll participants with dementia typically requires the recruitment of participant dyads, that is, participant and a caregiver, and repeated evaluations during the course of the study, additional barriers to recruitment, retention, and adherence [18,19].
Integrated database management systems, such as patient registries, with a focus on collecting data for scientific and clinical purposes, may help to overcome some of the challenges associated with research study recruitment and retention. The identification of eligible potential (candidate) participants from a database with a sufficiently large pool of registrants from a wide range of backgrounds not only may facilitate the timely execution of clinical studies by increasing recruitment capabilities but also may reduce the risk of sample selection bias [20]. Carefully designed research registries can play a valuable role in successful planning and management of recruitment and retention of study participants. Registries can increase participation in and completion of clinical trials by providing an efficient mechanism to identify candidates and meet recruitment targets within specific time frames. For example, the ability to match registrants to the most appropriate current research protocols can be greatly enhanced by an efficient registry process. One of the goals of the Wisconsin Alzheimer’s Disease Research Center (ADRC) is to enhance the recruitment of individuals into ongoing clinical research with special attention to the inclusion of populations traditionally under-represented in research such as individuals from rural areas and racial/ethnic minority groups. Recruitment and outreach efforts target not only cognitively healthy adults but also individuals with memory impairments associated with underlying cognitive disorders, for example, mild cognitive impairment or Alzheimer’s disease. The new Alzheimer’s Association TrialMatch™ (www.alz.org/trialmatch) is a confidential and free interactive tool developed to minimize recruitment barriers and increase participation in Alzheimer disease research by providing an Internet-and-telephone–based system to match registrants to potential clinical studies based on personal profile and location [21]. The goal of crafting outreach initiatives to enhance the recruitment of participants from diverse backgrounds who meet cognitive status inclusion criteria was a key component in the design of the information elements and functions of the research registry. Well-designed and managed registries such as the one described herein can aid research staff to build and sustain a communication history with registrants that results in trust, commitment, and long-term research commitment and participation.
The registry design model presented in this article was developed in multiple phases, ranging from identifying the essential data elements and system deliverables and establishing a common design vision from a multidisciplinary team of users and expert advisors to the design of a high-quality data information system in close alignment with the goals of the Wisconsin ADRC. The registry system was designed to provide an organizational structure; a set of procedures for screening assessment, updating, and recruitment; and a comprehensive data management framework to inform decision making and to support access to potential study participants who meet specific criteria readily available in the information system. A unique feature of the registry is its contact management capabilities tailored specifically for efficient tracking of all contacts with registrants.
In this report, we focus on the design of the registry and major steps followed to design and implement a web-based research registry system to optimize planning and management of participant recruitment efforts. Based on our experience, we provide design guidelines and procedural tips for enhancing the collaboration and teamwork needed for a successful development of a research registry of older adults. We also discuss issues that must be addressed to maintain overall registry functionality.
Methods
Defining data needs and identifying relevant content domains
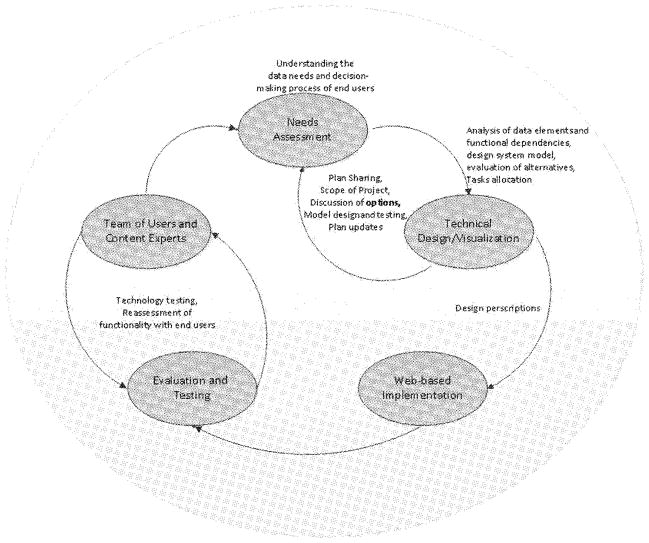
The initial step in the development of the design plan for the registry included a common definition of its major purposes and functions within the context of the Wisconsin ADRC as well as the projected information use and reporting activities to support research planning and decision making. The selection of the required data elements and the identification of data sources for the ADRC recruitment registry began with a thorough assessment of data needs and the identification of major information domains. To define the scope of data requirements and set the starting point for the system development, we used a structured problem-solving [22] and system design approach [23] that consisted of the following main nonlinear iterative phases: (1) describing the ADRC recruitment process; (2) streamlining and standardizing the recruitment process, which included the definition of minimal critical specifications for a systematic, valid, and consistent recruitment process; and (3) developing and testing appropriate system solutions to meet identified needs. Figure 1 depicts the overall process.
Figure 1.
Registry design process as dynamic inquiry and team operation approach.
Data needs based on the recruitment plan
The first phase involved developing workflow diagrams as graphic organizers to depict the recruitment processes and to identify and categorize information needs by different end users of the registry. To complete this phase, a multidisciplinary team of information users and expert advisors wrote a unified description and illustration of the recruitment processes as currently implemented. The multidisciplinary team consisted of clinicians, study coordinators, administrators, and information technologists with a clear investment in the registry process as a tool to support research. This first step not only led to the identification of additional information required to optimize recruitment efforts but also the discussion of strategies to gather valid, unbiased, and consistent information necessary to guide the registry design process and to specify relevant content.
A specific aim of the Wisconsin ADRC recruitment process was to establish and provide support to an effective outreach program in order to gain access to and serve low-income and minority populations as well as those residing in rural communities of Wisconsin. To meet this aim, the registry was designed to capture the widest possible cross section of the target population. The systematic collection of anonymous, de-identified demographic (ethnic/racial background, education, age, zip code) data and sources of referral information from prospective registrants prior to any eligibility assessment or consent was thought to provide a way to gauge the effectiveness of recruitment efforts targeted at specific segments of the population. Additionally, an evaluation of the extent to which the actual registry population matched the characteristics of the target population could be used to inform, support, and evaluate efficacy of outreach and recruitment activities.
Another key requirement identified by the multi-disciplinary registry design team was to be able to record all contacts with a prospective registrant as well as all contacts and activities related to individuals who had consented to enroll in the Wisconsin ADRC registry. Those who had consented included all registrants from an existing registry database containing information collected over a 3-year span. Many of these registrants also had been recruited to participate in current or completed clinical studies. With over 2000 registrants, the existing registry database provided a large pool of individuals who already had consented to register and had agreed to participate in translational research in dementia. This existing database held a limited set of demographic and contact information about registrants yet did not provide much information about the status of their recruitment into individual clinical studies. Very often, required data fields had not been completed in an informative and consistent manner. Additionally, lists of registrant’s participation in clinical studies, information on current status, and registrant preferences for research participation were maintained by separate research staff and study coordinators. These limitations of the existing registry database made collaboration among study coordinators challenging since it prevented research staff from effectively tracking contacts and implementing recruitment efforts with potential study participants. Records of contacts with an individual registrant may have been recorded only in hard copy, for example, in case report files or staff member notes. Furthermore, a registrant may have been targeted by several study coordinators and may have been contacted multiple times and asked repeatedly for demographic and personal health and contact information. As a result of these limitations, the team recognized the need to develop a multipurpose registry system. One that (a) optimized recruitment into the registry, (b) offered a pool of potential candidates for clinical studies, (c) tracked and shared research participation by individual registrants, and (d) facilitated a collaborative infrastructure across research teams.
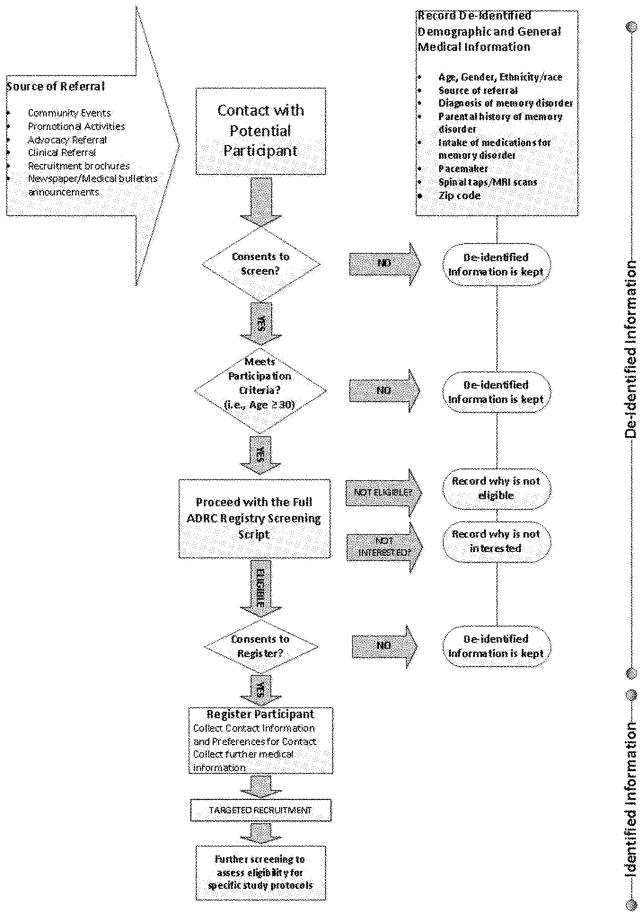
After a thorough assessment of the components of the registry recruitment process and experiences with the use of the existing registry database structure for recruiting individuals into studies, a list of critical information needs was compiled. A diagram illustrating main features of the recruitment process was drafted and used to establish “essential” requirements (see Figure 2). The new registry system would provide users with the ability to (a) record every contact and activity within the registry database; (2) identify and group registrants by health profiles, demographic characteristics, or risk factors for future recruitment into research studies; and (c) summarize and produce grouped and ungrouped reports on all recorded registrant characteristics. A key design goal was to create a database system that would support the staff to improve productivity while effectively encouraging research volunteers to participate according to their interests and characteristics.
Figure 2.
General recruitment scheme for the registry. MRI: magnetic resonance imaging; ADRC: Alzheimer’s Disease Research Center.
A significant component of the recruitment process also included revamping the Wisconsin ADRC outreach program to make sources of referral aware of the registry. As a parallel effort to aid the development of the registry and increase recruitment into the registry, the outreach program designed registry brochures; organized community events, conferences, and promotional events; and arranged meetings with key sources of referral. Other means to increase registry awareness included announcements in newspapers and medical bulletins.
Minimal critical specification approach to streamline and standardize recruitment
Another specific aim of the Wisconsin ADRC was to recruit a sufficiently large pool of potential research participants to support and stimulate translational research in dementia and to provide an infrastructure of resources and services to facilitate the recruitment into further studies in preclinical diagnosis, early diagnosis, and progression of Alzheimer’s disease. To ensure that the registry objectives would fulfill the recruitment needs for all studies, the registry design team reviewed and described the specific enrollment requirements for each ongoing study. This activity generated multiple lists of screening questions and specific study participation criteria linked to different information needs. For example, some protocols required collection of a complete listing of current medications from potential study participants, while others specified collection of information specifically about medications prescribed to treat memory and cognition problems; yet other study recruitment scripts had no specific medication requirements.
These variations in study screening questions and enrollment requirements provided an opportunity not only to discuss and outline strategies to streamline and standardize the overall registration process and enrollment into clinical studies but also to define required data fields and functions for the registry that would enhance the registry’s utility. The emphasis on standardization of procedures ensured the use of uniform and systematic methods for collecting registry data.
We used the principle of “minimal critical specification” [24] to develop system specifications for the design of the registry that were absolutely essential to accomplish tasks. As a result of the team discussions, it was suggested that all recruitment activities, both to the registry and to the individual clinical studies using registrants, would be managed within the registry database. Accordingly, the following eight preliminary requirement specifications and preferences served as guiding principles in the design of the new registry system: (1) a web-based, multiuser application that provided access to standardized registry data forms and processes; (2) a standard screening process for all initial contacts with potential registrants that met the minimum screening criteria for all Wisconsin ADRC studies; (3) the ability to group and assign registrants according to their research interests, eligibility, characteristics, and specific needs; (4) the ability to capture demographic information anonymously from all initial contacts whether or not the potential registrant met eligibility criteria for participation in a specific study or consented to participation; (5) search and query functions to generate data quality reports and to assess data completeness; (6) an audit plan to assess data integrity that focused on the most relevant data fields; (7) the ability to document verbal consent; and (8) an electronic contact record and activity workspace to record, track, and monitor all characteristics of registrants, as well as all staff activities concerning recruitment, screening, and communication with registrants.
This new multipronged design provided flexibility and a number of desirable advantages to users. Recognizing that recruitment of a candidate to an individual study may require multiple contacts with the potential participant, the Wisconsin ADRC registry system was designed to permit any staff member to see the contact history of the potential registrant or potential clinical study participant. Thus, any staff member would be able to address return calls or responses to mailings. Recruitment could be managed electronically by staff, decreasing the need for paper records and reducing the number of duplicative contacts. The registry made it possible for the Wisconsin ADRC staff to use information gathered during previous contacts with registrants and registration candidates, virtually eliminating the need to “cold call” potential registrants or study participants. Another significant advantage of the new registry recruitment process was the ability to record the registrant-identified preferred method and frequency of communications (telephone, letter, email, etc.). Moreover, by documenting the number and form of contacts, the registry provided data needed to evaluate the efficacy of recruitment strategies for both the registry and clinical studies using the pool of registrants. These features enhanced communication between staff and registrants and within research teams and optimized the rate of success in engaging study participants. In addition, the standardization of the initial contact screening process facilitated the quick identification of registrants who met specific criteria for participation in an individual study.
The ability to capture anonymous demographic information during the initial contact with potential registrants while providing the necessary identity protection until eligibility was determined and consent gained met a number of program and institutional needs concerning the effectiveness of overall recruitment and outreach activities to enhance registry population diversity. The main goal of the registry was to enroll the broadest possible cross section of the population in Wisconsin. The process was structured as follows: Upon initial contact, a potential registrant was informed of the anonymous screening process and asked to give oral consent to provide nonidentifiable personal demographic information that included an eligibility screening questionnaire. Individuals were also asked to provide information on medical history, for example, age, parental history of memory disorders, usage of medications for memory problems, and diagnosis of a memory disorder. Whenever an individual did not meet registry eligibility criteria, nonidentifiable personal information was collected and the registry recorded an anonymous set of demographic responses that could be used to survey the characteristics of all contacts. When individuals met registry eligibility criteria, they were to be informed of registry eligibility and asked to provide oral consent to enroll in the research registry. A critical component of the registration process was recruitment while complying with the Health Insurance Portability and Accountability Act (HIPPA) privacy regulations.
Documenting a formal functional specification for the registry development
Concurrent with the identification of data needs and the standardization of the recruitment process, the team created a written registry plan that incorporated all stakeholder and user functionality requirements identified as relevant to meet the main registry goals. This document became the basis for developing the functional specifications for the registry design. The document consisted of a collection of (a) task-related requirements, (b) program interface requirements, (c) system features to minimize potential user interaction barriers, (d) online documentation and dialogues to assist users through the recruitment process, and (e) specifications for tracking, monitoring, and managing recruitment tasks and activities. Table 1 outlines the complete list of requirements and functionalities that guided the technical design and development of the registry.
Table 1.
Summary of recruitment registry requirements
| Web-based application to simplify and automate the process for collecting, storing, monitoring, and reporting registry data |
|---|
| Secure/manage access |
| Secure login features and role-based access |
| Manage staff access to information |
| Screening/storing/documenting |
| Ability to store anonymous initial contact demographic information |
| Ability to document consent |
| Ability to screen a potential participant |
| Ability to store contact and sensitive information and previously anonymous demographic information about registered participants |
| Ability to store mailing information and generate mailing labels, mailing logs, and form letters |
| Searching/filtering/tracking |
| Ability to track participant’s progress in processes, status, contacts, and events/activities |
| Ability to track time to progress from first contact to every other step |
| Ability to track in which studies/programs a participant is screened, eligible for, enrolled in, ineligible for (and reasons), interested in, not interested in |
| Search and query functions on all entered data. |
| Report generation and auditing capabilities |
| Ability to profile characteristics of participants recruited, for example, demographics and referral source |
| Ability to audit the quality of the data |
| Multisite management capabilities |
| Program and study management |
| Manage program and study status |
| Manage multiple type of contacts and workflow |
| Activity management |
| Recent activity list for potential participants/participants |
| Recruitment task and activity management |
| Dashboard to facilitate data use and management |
All the key components contained in the final registry functional specification documentation were generated in an iterative fashion through weekly meetings with users, administrators, and subject matter experts. Directly observed and reported difficulties with the existing registry tools also were taken into account in developing system requirements.
Assessing options for meeting functional specification needs
Once the functional specifications for the database registry had been drafted and confirmed, the system development team conducted an in-depth analysis of viable options for the “best fitting” platform and features to meet the ADRC operational objectives and to optimize data management. The main purposes of the viability study were (a) to gather data on the various platform options and approaches, (b) to compare and analyze the strengths and weaknesses of each option for meeting the functional specifications, (c) to enumerate the opportunities and threats presented by each possible option, and (d) to evaluate the technical, practical, and economic feasibility that each platform and approach offered. This study provided a thorough analysis of the range of possible options and a basis to evaluate, as a group, how well each possible option would meet data requirement needs. Table 2 shows the platform options and approaches considered in the viability analysis.
Table 2.
Platform options
| Options |
|---|
| Update and optimize existing database and convert to Oracle |
| Develop the required registry functions as part of an existing research database (OnCore)a developed for the University of Wisconsin-Madison ICTR, funded since 2007 by the NIH CTSA |
| Develop and implement a stand-alone clinical trials management software (e.g., REDCap) |
| Develop a new web-based registry application |
| Modify and implement an off-the-shelf web-based CRM-type software |
ICTR: Institute for Clinical and Translational Research; NIH: National Institute of Health; CTSA: Clinical and Translational Science Award; CRM: customer relationship management.
OnCore or “Online Collaborative Research Environment” is a research informatics platform developed by Forte Research Systems that provides a web-based data management system.
After completing the viability study, the development team met with the major stakeholders in the ADRC to review the analysis of options and to present alternative solutions. This meeting gave the stakeholders the opportunity to participate actively in the selection of an optimal registry development strategy and ensured that both the development team and stakeholders held a common view of the purpose and scope of the project. In addition, the meeting served to establish role assignments and a clear commitment to the registry objectives and development direction. The off-the-shelf web-based customer relationship management (CRM) software was chosen as the best solution according to the following criteria: financial advantage and value, efficient and innovative components for contact management and querying, accreditations and qualifications, and general capabilities and support of all core activities and processes.
Results
From functional specification needs to project development
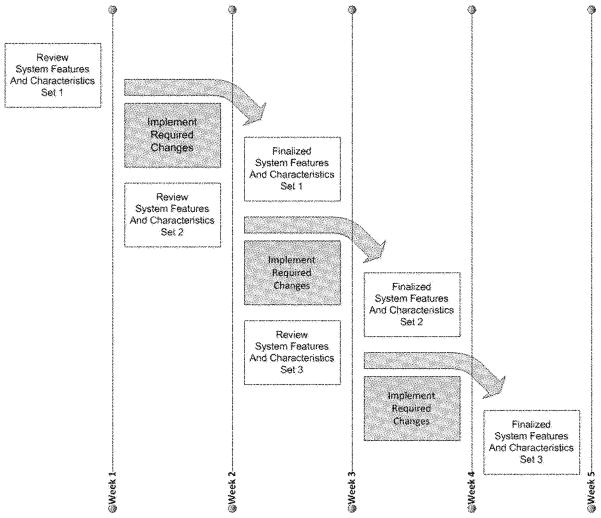
The project development process occurred in an iterative fashion tailored primarily to meet the ADRC recruitment process needs. As depicted in Figure 3, the development project plan included three registry “feature set” releases at 1-week intervals over the course of 5 weeks. Each “feature set” or “system functions set” was completed in a 3-week cycle: release in the first week, implementation of required changes in the second week, and release of the final feature set in the third week. This iterative development strategy allowed the team to manage the specification requirements by feature set, to monitor and verify the quality of the overall registry functioning, and to provide the ADRC staff with functioning registry features with real-time applications and full testing facilities.
Figure 3.
Iterative application development process: review–realignment–approval cascade.
During this development stage, a working prototype of the registry database with a limited set of simulated data based on new specifications was made available to a select set of ADRC users. Availability of the prototype ensured that all of the required data fields, functions, and system-integrated data quality assessment capabilities were working properly. Furthermore, it served as an opportunity to educate and train the ADRC staff on the new registry by allowing them to see how the new system would meet the general recruitment and study enrollment process needs. A natural result of this opportunity was the identification of additional ways to streamline and enhance the screening, recruitment, study enrollment, and retention procedures. Documentation, including a data and coding dictionary to ensure data consistency, was also developed at this point. In addition, the system development phase set the stage for the identification of training, support, and maintenance needs as well as the discussion of a plan for transitioning the ongoing operational support and maintenance responsibilities to the ADRC staff.
To ensure data consistency, all users of the registry were required to participate in standard, documented, hands-on training on how to use the registry. Training included reporting initial contacts, screening, entering data in a consistent manner, and scheduling and recording registrant activities. Additionally, all study coordinators met at least quarterly to review the use of the registry and standard methods of documenting interactions with registrants who were also participants in a particular clinical study. The study coordinators also identified recurring problems and introduced corrections to the system. Within 6 months after the introduction of the registry, all reporting on recruitment and retention of registrants into studies was done using the registry system.
The overall project development effort required a total of 580 person-hours. Of this total amount of effort, approximately 145 h of time of a data analyst and of a project manager were spent identifying data requirement needs in collaboration with the multidisciplinary team of information users. The development of the registry plan required an additional 115 h of effort from a senior programmer and a data analyst, and the actual registry development and testing phases involved a total of 320 h of time from a programmer, a test engineer, and a project manager. The ongoing support and maintenance of the registry system, which included usage analysis, training, quality control, and coding of required tasks, shared by three staff members, required approximately 300 person-hours yearly.
Conclusion
Elements key to the successful design of the Wisconsin ADRC registry
The Wisconsin ADRC research registry was developed with two clearly defined goals: (1) to facilitate the communication with potential research participants and among program personnel and (2) to record the minimal information needed to identify potential participants for clinical studies efficiently. A fundamental step in the successful development of the registry was the implementation of a collaborative governance model involving all stakeholders across disciplines in the definition of required data elements and functions.
Several factors were essential to the successful design and implementation of the Wisconsin ADRC registry. The process involved a multidisciplinary team and an iterative design process (Figure 1). Staff members who directed this process were experienced in leading process-oriented projects and were able to recognize and facilitate the discrete steps involved in developing a new programmatic tool. At every step, there was careful documentation of group discussion; this resulted in a document outlining the findings of the systematic needs assessment. Only by following these procedures were we able to recognize the programmatic need for more than a searchable database to identify potential research participants. Adding the contact management element to the registry design improved the efficiency of communication between research coordinators and potential participants, as well as the communication among coordinators. Moreover, communications were recorded in the registry, eliminating the need for email and written correspondence among staff. The advantages of a combined contact management and participant registry were readily apparent to staff and easily implemented, especially given the staff involvement during the design phases. Overall, the process-oriented approach culminated in a multifaceted tool to assist with the challenges of recruitment and retention in clinical research.
Although the model presented focuses on the development and implementation of a system for the recruitment of older adults with specific cognitive and medical characteristics, the procedures for identifying data needs and database system utility and functionality can be easily transferred to other populations. As with any multipurpose registry database system, careful management and training are essential to maximize its functionality and utility.
A key challenge for the optimal use of information technologies to enhance recruitment and retention is sustainability, which requires investment in infrastructure and appropriate personnel. Managing a registry calls for a commitment of resources to support the efficient use of information systems, integrity of the data, and long-term service demands. The primary financial requirements of registries are for human resources and salary rather than equipment and are best served by continued administrative and institutional support. There can be a substantial gain in efficiency and feasibility of study recruitment through an existing registry compared to the use of traditional ad hoc methods [25]. The initial institutional costs for registry development, however, may be offset later by grants and other research support arising from the availability of the registry as a major research resource.
Acknowledgments
The authors would like to thank the editor, Dr Barbara S Hawkins, and two anonymous reviewers for their valuable comments and suggestions that helped to improve the article. The authors would also like to thank the Biomedical Computing Group at the University of Wisconsin-Madison for the design of the Participant Recruitment Registry and the Administrative and Clinical Cores of the Wisconsin ADRC for their contributions to the discussions that preceded the outline of the content specifications for the development of the registry, whose model is presented in this report.
Funding
This work was partially supported by NIH grant P50 AG033514.
References
- 1.Guadagnolo BA, Petereit DG, Helbig P, et al. Involving American Indians and medically underserved rural populations in cancer clinical trials. Clin Trials. 2009;6:610–17. doi: 10.1177/1740774509348526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawranik P, Pangman V. Recruitment of community-dwelling older adults for nursing research: A challenging process. Can J Nurs Res. 2002;33:171–84. [PubMed] [Google Scholar]
- 3.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. New Engl J Med. 1999;341:2061–67. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 4.McDonald AM, Knight RC, Campbell MK, et al. What influences recruitment to randomized controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:1–8. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross S, Grant A, Counsell C, et al. Barriers to participation in randomized controlled trials: A systematic review. J Clin Epidemiol. 1999;52:1143–56. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 6.Boles M, Getchell WS, Feldman G, McBride R, Hart RG. Primary prevention studies and the healthy elderly: Evaluating barriers to recruitment. J Community Health. 2000;25:279–92. doi: 10.1023/a:1005153909429. [DOI] [PubMed] [Google Scholar]
- 7.Jackson J, Mandel D, Blanchard J, et al. Confronting challenges in intervention research with ethnically diverse older adults: The USC Well Elderly II Trial. Clin Trials. 2009;6:90–101. doi: 10.1177/1740774508101191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai GY, Gary TL, Tilburt J, et al. Effectiveness of strategies to recruit underrepresented populations into cancer clinical trials. Clin Trials. 2006;3(2):133–41. doi: 10.1191/1740774506cn143oa. [DOI] [PubMed] [Google Scholar]
- 9.Ridda I, MacIntyre CR, Lindley RI, Tan TC. Difficulties in recruiting older people in clinical trials: An examination of barriers and solutions. Vaccine. 2010;28:901–6. doi: 10.1016/j.vaccine.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 10.Rochon PA, Berger P, Gordon M. The evolution of clinical trials: Inclusion and representation. Can Med Assoc J. 1998;159:1373–74. [PMC free article] [PubMed] [Google Scholar]
- 11.Wujcik D, Wolff SN. Recruitment of African Americans to national oncology clinical trials through a clinical trial shared resource. J Health Care Poor Underserved. 2010;21:38–50. doi: 10.1353/hpu.0.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballard E, Nash F, Raiford K, Harrell L. Recruitment of Black elderly for clinical research studies of dementia: The CERAD experience. Gerontologist. 1993;33:561–65. doi: 10.1093/geront/33.4.561. [DOI] [PubMed] [Google Scholar]
- 13.Ejiogu N, Norbeck JH, Mason MA, et al. Recruitment and retention strategies for minority or poor clinical research participants: Lessons from the healthy aging in neighborhoods of diversity across the life span study. Gerontologist. 2011;51:33–45. doi: 10.1093/geront/gnr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarlow BA, Mahoney DF. The cost of recruiting Alzheimer’s disease caregivers for research. J Aging Health. 2000;12:490–510. doi: 10.1177/089826430001200403. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health-NIH News. [accessed November 2010];NIH Announces First National Research Study Recruitment Registry. [press release, 10 November 2009]. Available at http://www.nih.gov/news/health/nov2009/ncrr-10.htm.
- 16.Ganguli M, Lytle ME, Reynolds MD, Dodge HH. Random versus volunteer selection for a community-based study. J Gerontol A Biol Sci Med Sci. 1998;53:M39–M46. doi: 10.1093/gerona/53a.1.m39. [DOI] [PubMed] [Google Scholar]
- 17.Treves TA, Verchovsky R, Klimovitsky S, Korczyn A. Recruitment rate to drug trials for dementia of the Alzheimer’s type. Alzheimer Dis Assoc Disord. 2000;14:209–11. doi: 10.1097/00002093-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Connell CM, Shaw BA, Holmes SB, Foster NL. Caregivers’ attitudes toward their family members’ participation in Alzheimer disease research: Implications for recruitment and retention. Alzheimer Dis Assoc Disord. 2001;15:137–45. doi: 10.1097/00002093-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Shatenstein B, Kergoat M-J, Reid I. Issues in recruitment, retention and data collection in a longitudinal nutrition study of community dwelling older adults with early-stage Alzheimer dementia. J Appl Gerontol. 2008;27:267–85. [Google Scholar]
- 20.Dugas M, Lange M, Berdel WE, Müller-Tidow C. Workflow to improve patient recruitment for clinical trials within hospital information systems: A case-study. Trials. 2008;9:2–7. doi: 10.1186/1745-6215-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alzheimer’s Association - Research Center. [accessed November, 2010];About Alzheimer’s Association TrialMatch. http://www.alz.org/research/clinical_trials/find_clinical_trials_trialmatch.asp.
- 22.Parker G. Structured Problem Solving. Gower; Brookfield, VT: 1995. [Google Scholar]
- 23.Ramo S, St Clair RK. The Systems Approach: Fresh Solutions to Complex Problems Through Combining Science and Practical Common Sense. KNI, Inc; Anaheim, CA: 1998. [Google Scholar]
- 24.Cherns A. The principles of socio-technical design revisited. Hum Relat. 1987;40:153–61. [Google Scholar]
- 25.Fellows LK, Stark M, Berg A, Chatterjee A. Patient registries in cognitive neuroscience research: Advantages, challenges, and practical advice. J Cognit Neurosci. 2008;20:1107–13. doi: 10.1162/jocn.2008.20065. [DOI] [PubMed] [Google Scholar]