Abstract
Background
There are limited data assessing bronchodilator responsiveness (BDR) in infants and toddlers with recurrent wheezing, and factors associated with a positive response.
Objectives
In a multicenter study of children ≤ 36 months old we assessed the prevalence of and factors associated with BDR among infants/toddlers with recurrent episodes of wheezing.
Methods
Forced expiratory flows and volumes using the raised-volume rapid thoracic compression method were measured in 76 infants/toddlers (mean (sd) age 16.8 (7.6) mos.) with recurrent wheezing before and after administration of albuterol. Prior history of hospitalization or emergency department treatment for wheezing, use of inhaled or systemic corticosteroids, physician treatment of eczema, environmental tobacco smoke exposure, and family history of asthma or allergic rhinitis were ascertained.
Results
Using the published upper limit of normal for post bronchodilator change (FEV0.5 ≥ 13% and/or FEF25–75 ≥ 24%) in healthy infants, 24% (n=18) of children in our study exhibited BDR. The BDR response was not associated with any clinical factor other than body size. Dichotomizing subjects into responders (defined by published limits of normal)or by quartile to identify children with the greatest change from baseline (4th quartile vs. other) did not identify any other factor associated with BDR.
Conclusions
Approximately one quarter of infants/toddlers with recurrent wheezing exhibited BDR at their clinical baseline. However, BDR in wheezy infants/toddlers was not associated with established clinical asthma risk factors.
Keywords: recurrent wheezing, infants, pulmonary function, raised-volume rapid thoracoabdominal compression, bronchodilator responsiveness, asthma
Introduction
Recurrent wheezing affects 20–30% of infants and toddlers, yet resolves in at least 50% of these children by school age.1 Infants and toddlers with recurrent wheezing are a heterogeneous group. The majority of wheezy infants and toddlers do not have asthma, but rather may wheeze for a variety of anatomic and/or pathophysiologic reasons, including small airway caliber, tracheomalacia, dysphagia, post-viral airway inflammation and injury, or alternations in the maturation of the immune system such as reduced IFN-γ production. 2,3 Clinicians generally cannot distinguish young children with transient wheezing from those with early persistent asthma. Therefore, predictors of asthma in wheezy infants and toddlers could lead to major improvements in the management of these children, potentially reducing preventable healthcare utilization for thousands of young children and preventing under- or over-treatment with corticosteroids. In school age children, airflow obstruction that is at least partially reversible is a hallmark of asthma.4 International expert panel asthma treatment guidelines 4,5 recommend assessment of reversibility of airflow obstruction following administration of a bronchodilator during spirometry as a key method to assist with the diagnosis of asthma in children over age 5 years. However, among infants and toddlers it is not known whether short-term reversibility of airflow obstruction in response to treatment with a bronchodilator is correlated with or predictive of asthma.
Although infants and toddlers are not capable of independently performing spirometry, the raised-volume rapid thoracoabdominal compression (RVRTC) technique can be used to measure forced expiratory flows and volumes in children ≤ 3 years of age under sedation with chloral hydrate. When comparing different infant lung function parameters, RVRTC parameters were found to be the least variable and thus most likely to identify a change following administration of a bronchodilator. 6. During the RVRTC maneuver an infant’s lungs are inflated to near total lung capacity followed by a forced expiration.7–9 The RVRTC technique allows generation of flow-volume curves that are similar to adult-type flow-volume curves. RVRTC forced expiratory flows and volumes have been used to assess infants with bronchiolitis and recurrent wheezing,10,11 and reference values from healthy infants are published.12 Although published data describing forced expiratory volumes and flows following administration of albuterol in healthy children exist,13 there is a paucity of data regarding the prevalence of bronchodilator responsiveness (BDR) among infants and toddlers with recurrent wheezing.14 Furthermore, studies in this population exploring factors associated with BDR, as well as the predictive value of BDR for asthma later in childhood, are lacking.
The objectives of this study were to assess the prevalence of BDR and factors associated with BDR among infants and toddlers with recurrent episodes of wheezing. To accomplish these aims we analyzed forced expiratory flows and volumes obtained at three study sites in the United States and Canada using the RVRTC technique in a convenience sample of children less than 36 months of age with a history of recurrent episodes of physician-diagnosed and treated wheezing. We then investigated associations between clinical factors and changes in lung function following administration of albuterol. We hypothesized that BDR would be associated with well established epidemiologic risk factors of asthma, specifically personal history of eczema and family history of asthma or atopy.
Materials and Methods
Subjects
Data collected from children 4–36 months of age with ≥ 3 prior episodes of physician-diagnosed wheezing treated with bronchodilators or corticosteroids who were studied at three academic children’s hospitals were included in this study (Seattle Children’s Hospital, Seattle, Washington., USA.; Hospital for Sick Children, University of Toronto, Toronto, Ontario Canada; and C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, Michigan, USA). Subjects born < 36 weeks gestation or with congenital heart disease, dysphagia, severe gastroesophageal reflux, oxygen saturation ≤ 90% on room air, an active seizure disorder, or upper airway obstruction were excluded. Subjects did not receive systemic steroids for at least 3 weeks prior to lung function measurements. Subjects at the Seattle and Michigan sites did not receive inhaled corticosteroids within 3 weeks of lung function testing, whereas at the Toronto site all subjects were receiving inhaled corticosteroids at the time of testing. Written consent was obtained from parents of subjects. The study was approved by the Institutional Review Boards of each institution.
Study Visits
Study visits occurred at each child’s baseline state of health, and a minimum of 2 weeks following resolution of an upper or lower respiratory infection, or acute exacerbation of wheezing. Bronchodilators were held for at least 8 hours prior to lung function measurements. A detailed medical history was obtained by a study investigator, including ascertainment of prior history of hospitalization or emergency department treatment for wheezing, use of inhaled or systemic corticosteroids, physician treatment of eczema, environmental tobacco smoke exposure, first-degree relative history of asthma or allergic rhinitis, and ethnicity. Length and weight were measured with a calibrated stadiometer and digital scale at the time of infant lung function testing.
Lung Function Measurements
Forced expiratory volumes and flows (FVC, FEV0.5, FEF25–75, and FEF75) were measured using the RVRTC technique according to ATS/ERS guidelines for raised volume forced expirations in infants15 via the nSpire® Infant Pulmonary Lab (IPL). Measurement of forced expiratory flows and volumes were repeated 10 minutes after administration of albuterol 13. Two puffs of albuterol were administered using a metered dose inhaler with a spacer. Each puff of albuterol was followed by an inflation of the lungs to 25 cm H2O. Heart rate was continuously monitored throughout the study. Adequate systemic delivery was assumed when a 10% increase in heart rate was achieved. Repeat doses of 2 puffs were given if a 10% rise in heart rate was not achieved by 2 minutes after the previous dose, up to a maximum of 8 puffs. The mean dose of albuterol administered to subjects was 4 puffs. Lung function outcomes are reported as the single best pre- and post albuterol maneuvers with the highest sum of FVC and FEF25–75. All curves used for analysis had FVC measurements within 10% of the highest FVC. Lung function parameters were analyzed both as raw values and as z-scores calculated from published normative data. 12
Statistical Analysis
Data were pooled from the three centers; outliers were investigated and excluded if values were not biologically plausible (e.g. change in FEV0.5 >200%). Summary statistics were performed by center, and for the whole group. Baseline comparisons between groups were done using an ANOVA and Chi squared test. Generalized estimating equations (GEE), adjusting for clustering by centers, were used to analyze the association between changes in lung function post bronchodilator and potential predictors. Logistic regression was applied for the following dichotomous outcome variables: 1) bronchodilator response defined as FEV0.5 ≥13% and/or FEF25–75 ≥24%13 and 2) highest quartile for relative change in FEV0.5, FEF25–75, or FEF75.. The predictor variables considered were sex, age, height-for-age z-score, weight-for-age z-score, hospital admissions, emergency room visits, environmental tobacco exposure, prior treatment for eczema, family history of asthma and family history of allergy. Height-for-age and weight-forage z-scores were derived using the 2006 WHO Growth Charts. 16 All statistical analysis was done using STATA (StataCorp. 2001. Statistical Software: Release 7.0. College Station, TX: Stata Corporation)
Results
Seventy-six infants from the three centers had acceptable pre and post bronchodilator results. At the Seattle center 44/47 enrolled subjects completed acceptable infant lung function testing, of which 36 subjects completed acceptable post-bronchodilator lung function testing. At the Toronto site 32/40 subjects completed lung function testing, and 25 completed acceptable post-bronchodilator results. At the Michigan site 15/15 enrolled subjects completed lung function testing with acceptable post-bronchodilator results. Table 1 summarizes the study population. There were a greater proportion of male infants in all three sites, and 75% of the study population was Caucasian. The majority of infants were less than 18 months at the time of testing (median 16 months, range 4.5–36). On average the children included in the study were shorter-for-age and heavier-for-age than the international reference population 16 and had reduced lung function outcomes compared to a North American reference population 12. Prior to enrollment most children in the study had been treated with bronchodilators and/or inhaled steroids and more than half had been treated with at least one course of systemic corticosteroids. The rate of previous hospitalizations and emergency room visits was greater at the Toronto site than at the Seattle and Michigan sites. The combined prevalence of a positive family history of asthma or allergy was high and differed between the centers. Other than FEF25–75 z-score, which was highest in Michigan with the youngest infants, baseline lung function did not differ between the centers.
Table 1.
Summary of participant characteristics.
| Seattle (n=36) | Michigan (n=15) | Toronto (n=25) | Combined (n=76) | P value§ | |
|---|---|---|---|---|---|
| Sex; n (%) male | 25 (69.4) | 10 (66.7) | 19 (76.0) | 54 (71.1) | 0.836 |
| Age (months); mean (sd) | 17.8 (7.2) | 10.8 (3.7) | 18.9 (8.3) | 16.8 (7.6) | 0.0024 |
| Ethnicity; n (%) Caucasian | 27 (75.0) | 13 (86.7) | 17 (68.0) | 57 (75.0) | 0.520 |
| z-score height; mean (sd) | −0.12 (1.1) | −0.67 (1.4) | −0.40 (1.0) | −0.32 (1.1) | 0.2758 |
| z-score weight; mean (sd) | 0.61 (1.1) | 0.20 (1.2) | 0.90 (4.7) | 0.62 (2.8) | 0.7347 |
| z-score FVC; mean (sd) | −0.22 (1.3) | −0.41 (1.7) | 0.13 (1.4) | −0.15 (1.4) | 0.5253 |
| z-score FEV0.5; mean (sd) | −0.77 (1.2) | −0.23 (1.6) | −0.48 (1.6) | −0.57 (1.4) | 0.3734 |
| z-score FEF25–75; mean (sd) | −0.91 (1.4) | −0.33 (1.4) | −0.76 (1.8) | −0.75 (1.6) | 0.0140 |
| z-score FEF75; mean (sd) | −1.0 (1.4) | −0.24 (1.4) | −0.97 (1.6) | −0.84 (1.5) | 0.2246 |
| Previous hospitalization; n (%) | 14 (38.9) | 5 (33.3) | 18 (72.0) | 37 (48.7) | 0.007 |
| Previous ED visit; n (%) | 27 (75.0) | 11 (73.3) | 25 (100) | 63 (82.9) | 0.029 |
| Current ICS use | 0 (0) | 0 (0) | 25 (100) | 25 (32.8) | <0.001 |
| History of ICS use; n (%) | 6 (17.0) | 5(33.3) | 25 (100) | 29 (38.2) | <0.001 |
| History of systemic steroid use; n (%) | 28 (78.0) | 6 (40.0) | 19 (76.0) | 53(69.7) | <0.001 |
| Household exposure to smoke n (%) | 8 (22.2) | 5 (33.3) | 12 (48.0) | 25 (32.9) | 0.082 |
| Eczema treatment; n (%) | 12 (33.3) | 3 (20.0) | 2 (8.0) | 17 (23.3) | 0.042 |
| Family history of asthma; n (%) | 19 (52.8) | 3 (20.0) | 12 (48.0)* | 45 (59.2) | 0.000 |
| Family history of allergy; n (%) | 28 (77.8) | 11 (73.3) | 14 (56.0)* | 53 (69.7) | 0.141 |
In Toronto, history of asthma and allergy are for parental history only, whereas in Michigan and Seattle all first-degree relatives are included.
p values obtained from ANOVA analysis for continuous outcomes and chi squared analysis for categorical outcomes
Definition of Abbreviations: ED = emergency department; ICS = inhaled corticosteroid
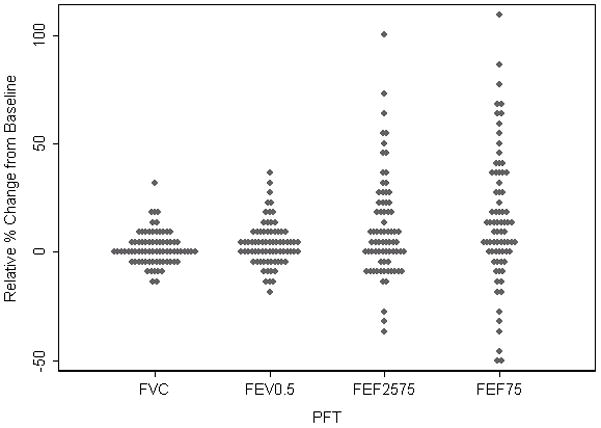
Figure 1 presents the average relative change from baseline for each of the lung function outcomes. For FVC the median relative change from baseline following administration of albuterol was 1.4% (range −12.0; 30.2); For FEV0.5 the median relative change after albuterol was 2.4% (range −19.8; 36.8); whereas the observed change was much greater and more variable for the two flow outcomes, 10.9% (range −49.6; 110.0) for FEF75, and 6.1% (range −36.3; 100.9) for FEF25–75. Using the published upper limit of normal (ULN) for post bronchodilator change (FEV0.5 ≥ 13% and/or FEF25–75 ≥ 24%)13, 24% (n=18) of infants had a post-bronchodilator change greater than that observed in healthy subjects. Using the same cut-off for FEF75 instead of FEF25–75, the percentage of children who were classified as responders increased to 34% (n=26). Only 16% (n=12) of infants had a post-BDR increase in FEV0.5 of >12%. The median relative change from baseline in the ‘responders’ was 14.9% (IQR 6.7–22.4) for FEV0.5, 0.34% (IQR −3.0; 6.1) for FVC, 35.6% (IQR 28.3; 52.8) for FEF25–75 and 50.5% (IQR 34.3; 63.3) for FEF75. Stratifying the results by center, using the published ULN for post bronchodilator, 17% of children were classified as ‘responders’ in Seattle, 40% in Michigan and 21% in Toronto.
Figure 1.
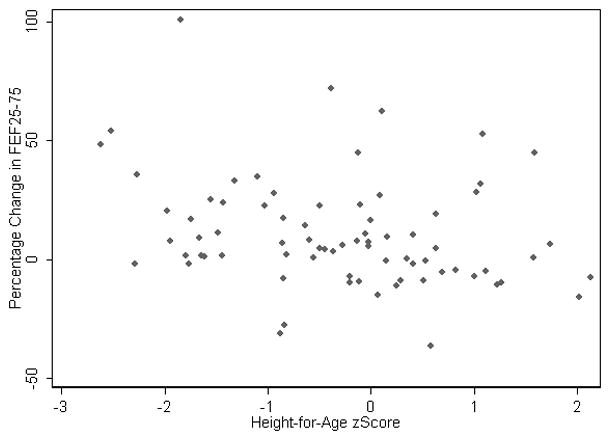
Subsequently we assessed which factors were associated with the greatest change in lung function parameters following administration of a bronchodilator. Table 2 summarizes the results from a multi-variable regression model, taking into account the differences between centers. The magnitude of change was less for females, however, this gender difference was not statistically significant. The change in FEF25–75 was lower in older children and in infants who were taller-for-age (higher height-for-age z-scores) (Figure 2). However, the relationship with age was no longer evident after taking into account the non-linear relationship in age with lung function. There was no association between baseline lung function and height-for-age, thus these results cannot be explained by regression to the mean. Furthermore, a sensitivity analysis excluding a potential outlier confirmed these findings. Similarly, dichotomizing the outcomes into responders as defined by published limits of normal 13 and by quartile to identify those children with the greatest change from baseline (4th quartile vs. other) did not identify any factors that were statistically predictive of greater BDR. For all of the multi-variable models, the variability between centers was greater than that variability within centers.
Table 2.
Summary of multi-variable regression, adjusted for clustering by center.
| Relative change following albuterol; (Post-Pre)/Pre | FVC slope (95% CI) |
FEV 0.5 slope (95% CI) |
FEF 75 slope (95% CI) |
FEF 25–75 slope (95% CI) |
|---|---|---|---|---|
| Sex (Females) | −2.68 (−6.36; 1.00) | −4.67 (−9.67; 0.34) | −14.07 (−28.83; 0.69) | −1.77 (−13.13; 9.59) |
| Age (months) | 0.12 (−0.11; 0.34) | −0.08 (−0.38; 0.23) | −0.29 (−1.19; 0.62) | −0.73 (−1.40; −0.05) |
| z-score height | −0.93 (−2.43; 0.57) | −1.84 (−3.88; 0.19) | −6.31(−12.28; −0.33) | −5.47(−9.99; −0.95) |
| z-score weight | −0.25 (−0.84; 0.34) | 0.68 (−0.13; 1.49) | −0.33 (−2.76; 2.10) | 0.54 (−1.31; 2.39) |
| More than 1 hospital admission | 1.30 (−2.13; 4.73) | 1.66 (−3.04; 6.36) | 0.95 (−13.34; 15.25) | 8.50 (−2.31; 19.30) |
| More than 2 ED visit | −0.21 (−2.45; 2.02) | 1.44 (−1.62; 4.50) | 0.39 (−8.82; 9.60) | 2.65 (−4.40; 9.71) |
| Household smoke exposure | −1.85 (−5.43; 1.73) | 1.25 (−3.68; 6.19) | 3.59 (−11.22; 18.41) | 1.41 (−9.78; 12.60) |
| Previous treatment for eczema | −0.12 (−4.37; 4.13) | −2.04 (−7.83; 3.76) | −1.06 (−18.67; 16.55) | −7.62 (−20.83; 5.60) |
| Family history of asthma | 3.14 (−0.20; 6.47) | 1.09 (−3.58; 5.77) | 2.67 (−11.16; 16.51) | 4.74 (−5.79; 15.29) |
| Family history of allergy | −1.06 (−4.73; 2.61) | −3.71 (−8.69; 1.27) | −11.25 (−26.11; 3.62) | −7.06 (−18.31; 4.20) |
Bold indicates p values <0.05.
Definition of Abbreviations: ED = emergency department; FEV0.5 = forced expiratory volume in 0.5 seconds; FEF25–75 = forced expiratory flow 25–75% of expiration; FEF75 = forced expiratory flow at 75% of expiration
Figure 2.
Discussion
In a multi-center study of infants and toddlers with recurrent physician-diagnosed episodes of wheezing we found that 24% of children exhibited a response to albuterol more than 2 standard deviations greater than that observed in previous published data assessing BDR in healthy infants.13 For forced expiratory flows, the only factor associated with increased BDR amongst wheezy infants and toddlers was lower z-scores for height. Aside from body size we did not find any other factors associated with BDR among young children with recurrent wheezing, including the well established asthma risk factors of family history of asthma, family history of allergy, and personal history of eczema. Furthermore, prior hospitalization or emergency department visits for wheezing, were not associated with BDR in children with known wheezing disorders.
One of our aims was to estimate the prevalence of BDR in wheezy infants and toddlers utilizing infant lung function testing. This is the largest study published to date assessing bronchodilator responsiveness in infants and toddlers with recurrent wheezing. Hayden et al.11 studied 27 wheezy infants and 5 controls, of which 20 infants received a bronchodilator and 7 received placebo, and found that none of the infants had a bronchodilator response despite a 10% rise in heart-rate following administration of albuterol indicating adequate delivery. This population was similar to ours in that infants were tested when asymptomatic. A second study by Saito et al studied 19 children under age 3 years with recurrent wheezing who had failed empiric anti-asthma and/or anti-reflux therapy and were scheduled to undergo bronchoscopy, 17 of which completed pre and post-albuterol infant lung function testing.14 They reported BDR in 18% of their subjects using a definition of a >13% change in FEV0.5 and/or >24% change in FEF25–75 following albuterol. The prevalence of BDR reported by Saito et al. was slightly lower than what we observed. However, 95% of the children studied by Saito et al. had failed anti-asthma therapies prior to enrollment, potentially limiting the generalizability of their finding to the larger population of infants and toddlers with recurrent wheezing. In contrast, the characteristics and severity of the subjects in our study are more representative of infants and toddlers with recurrent wheezing managed by most pediatric caregivers.
BDR in school age children and adults is considered a key feature of asthma, and international asthma management guidelines recommend assessment of lung function after administration of a bronchodilator as an important evaluation to assist in the diagnosis of asthma. 4,5 Among school age children with asthma, the prevalence of BDR has been reported between 30 and 60% 17,18,19. BDR has been found to be associated with poor asthma control and worse clinical outcomes in school age children with asthma. 19,20 The presence of BDR in older patients with asthma has also been reported to be a good predictor of responsiveness to treatment with inhaled corticosteroids. 21–23 Given the association between BDR and asthma in older children and adults, BDR in infants and toddlers with recurrent wheezing might help separate transient wheezers from children who will go on to develop chronic asthma as well as assist in decision making regarding long term use of inhaled corticosteroids in infants and toddlers with recurrent wheezing. Parental history of asthma and personal history of eczema are two well established risk factors for asthma in young children that serve as the major criteria in the Asthma Predictive Index proposed by Castro-Rodriquez 24 and recommended by asthma management guidelines to guide decision making regarding the initiation of inhaled corticosteroids in young children with recurrent wheezing.4 We hypothesized that these asthma risk factors would be associated with BDR. However, not only did we find no relationship between BDR and known asthma risk factors, we also found that despite a high prevalence of inhaled steroid use (100%) in Toronto at the time of testing, there was no difference in the prevalence of BDR or in baseline lung function when compared to Seattle where none of the infants were on inhaled steroids at the time of testing, a finding in contrast to studies in older children and adults demonstrating that inhaled steroids attenuate bronchial hyperresponsiveness. 25 This is consistent with the observations of Saglani et al who reported that bronchial biopsy specimens from infants with recurrent wheeze and evidence of BDR lacked evidence of reticular basement membrane thickening or eosinophilic airway inflammation characteristic of asthma in older children and adults, suggesting that BDR in wheezy infants is not pathognomonic of future asthma 26 and thus may not predict responsiveness to inhaled steroids.
Interestingly, the only factor that was related to BDR was a lower height for age z-score. These results are seemingly in contrast to work in infants with acute bronchiolitis where age did not seem to predict the magnitude of the bronchodilator response. 27 However, in our study, we also found that the response with age was non-linear and the effect was not seen with age but with height z-score. Of note, Modl et al 27 also found that BDR had no relationship with known asthma risk factors. Our findings raise several concerns regarding the clinical significance of BDR among infants and toddlers with recurrent wheezing. First, if BDR in this population is not associated other established asthma risk factors, namely eczema and family history of asthma and allergy, it calls into question the utility of BDR assessment during infant pulmonary function testing in predicting future asthma. This is consistent with recently reported longitudinal data from one of our centers (Seattle) demonstrating that BDR in wheezing infants and toddlers is not a strong predictor of subsequent respiratory exacerbations or changes in lung function through 3 years of age; and in fact, tests of airway inflammation such as single breath exhaled nitric oxide (SB-FENO) seem to have greater predictive value. 28,29 Second, the lack of an association between BDR and asthma risk factors suggests that the presence of BDR alone in a wheezy infant or toddler is insufficient evidence to guide clinical decision making regarding initiation of an inhaled corticosteroid medication as a long term asthma controller.
At the present time the clinical utility of BDR in the assessment of wheezing infants is unclear. This is in part due to the lack of sufficient longitudinal data to demonstrate the outcome of these infants by school age. It is known that a significant proportion of infants and toddlers with recurrent wheezing will be asymptomatic with normal lung function at school age. 30. Ongoing new birth cohorts such as the Canadian Healthy Infant Longitudinal Development study (CHILD) will study the pattern of bronchodilator responses in infant lung function and correlate them with outcomes that are clinical and physiologic in later life as well as epidemiologic risk factors 24 and other promising biomarkers (eg, FENO). These types of longitudinal studies are needed to fully understand the role of BDR in infancy.
Airway reactivity in response to a challenge with methacholine or histamine is not synonymous with BDR, and we did not assess airway reactivity in our study population. In a cohort of infants with atopic dermatitis (mean enrollment age of 10.7 months) Tepper et al. recently reported that airway reactivity to methacholine was not a risk factor for subsequent wheezing over a 1 year period, although they did find that wheezing during follow-up was associated with airway reactivity measured at the 1 year follow-up visit. 31 In an Australian birth cohort followed from early infancy through school age Palmer et al. reported that reactivity to histamine at 1 month of age, as measured by a 40% decrease in the maximal flow at functional residual capacity, was associated with a physician diagnosis of asthma and decreased lung function at age 6 years. 32 However, the relationship between airway reactivity and BDR among infants and toddlers with recurrent wheezing has not been well elucidated.
There are several limitations inherent in our study design. First, our study did not include a healthy control group, but rather compared bronchodilator responses in wheezy infants to those reported in a previously published study conducted in healthy infants wherein BDR cut-offs were assessed among 28 infants treated with albuterol and 13 subjects that received an inhaled placebo. 13 However, given that our aims were to assess the prevalence of BDR among wheezy infants, and associations between BDR and asthma risk factors, this limitation had little impact on our analyses. Second, prenatal maternal smoking history, a factor associated with decreased lung function among children 33,34,35,36, was not assessed in all of our study centers. Third, because our study population was a convenience sample with common inclusion and exclusion criteria, potential for selection bias was a limitation. For example, subjects enrolled in Seattle and Michigan completed lung function testing a minimum of 3 weeks after discontinuation of any inhaled or systemic corticosteroids, whereas all subjects enrolled in Toronto were studied while receiving maintenance inhaled steroids, albeit during an asymptomatic period while not receiving systemic steroids. In addition to corticosteroid use, between-center differences existed with regards to age, eczema, emergency department treatment, hospitalization, and family history of asthma. However, we adjusted for center in our GEE model when analyzing the association between lung function changes post bronchodilator and potential predictors, and included all of the variables that differed between centers in our logistic regression models. Finally, the asthma risk factors we assessed in this cross-sectional analysis do not confirm or exclude future asthma. Longitudinal analyses of this and other cohorts of infants and toddlers with recurrent wheezing followed into the school age years and beyond will be required to definitively determine whether BDR in children less than age 3 years is associated with or predictive of clinical asthma later in childhood.
In summary, in the first multicenter study of infants and toddlers with recurrent wheezing to rigorously assess BDR we estimate that approximately one quarter of infants exhibit an improvement in forced expiratory flows in response to albuterol. Infant lung function testing utilizing the RVRTC method is technically challenging as well as time and labor intensive, limiting the feasibility of conducting such a study with a sufficient number of subjects at a single center. Our multicenter approach utilizing the same lung function testing equipment and standardized operating procedures is a significant strength of this study. Interestingly, only height z-score was associated with BDR among infants and toddlers with recurrent wheezing. In contrast, well established asthma risk factors of family history of asthma, family history of allergy, and personal history of eczema had no relationship with BDR. Although a longitudinal study will be required to definitively answer the question of whether BDR can predict asthma among wheezy infants, it is likely that identification of other biomarkers will be required to accurately diagnose asthma and guide the use of long term asthma controller medications in this important pediatric population.
Acknowledgments
Supported by: NHLBI: K23HL077626 (JD, Seattle); CTSA Grant Number I ULI RR025014-01 (Seattle); Seattle Children’s Hospital; AllerGen NCE (PS, Toronto); Don and Debbie Morrison (PS, Toronto); K23HL067881 (AGF, Michigan).
Abbreviations
- FEV0.5
forced expiratory volume in 0.5 seconds
- FEF25–75
forced expiratory flow 25–75% of expiration
- FEF75
forced expiratory flow at 75% of expiration
- RVRTC
raised volume rapid thoracoabdominal compression
- NHLBI
National Heart Lung Blood Institute
- ATS
American Thoracic Society
- ERS
European Respiratory Society
- FENO
fraction of exhaled nitric oxide
References
- 1.Martinez FD. Development of wheezing disorders and asthma in preschool children. Pediatrics. 2002;109(2 Suppl):362–367. [PubMed] [Google Scholar]
- 2.Morton RL, Sheikh S, Corbett ML, Eid NS. Evaluation of the wheezy infant. Ann Allergy Asthma Immunol. 2001;86(3):251–256. doi: 10.1016/S1081-1206(10)63293-0. [DOI] [PubMed] [Google Scholar]
- 3.Guerra S, Lohman IC, Halonen M, Martinez FD, Wright AL. Reduced interferon gamma production and soluble CD14 levels in early life predict recurrent wheezing by 1 year of age. Am J Respir Crit Care Med. 2004;169(1):70–76. doi: 10.1164/rccm.200304-499OC. [DOI] [PubMed] [Google Scholar]
- 4.NIH/NHLBI. National Asthma Education and Prevention Program Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma. [Google Scholar]
- 5.Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2010 Available from: http://www.ginasthma.org/Guidelines/guidelines-resources.html.
- 6.Beardsmore CS, Page C, Silverman M. The response to beta-agonists in wheezy infants: three methods compared. Respir Med. 2004;98(11):1138–1145. doi: 10.1016/j.rmed.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Feher A, Castile R, Kisling J, Angelicchio C, Filbrun D, Flucke R, Tepper R. Flow limitation in normal infants: a new method for forced expiratory maneuvers from raised lung volumes. J Appl Physiol. 1996;80(6):2019–2025. doi: 10.1152/jappl.1996.80.6.2019. [DOI] [PubMed] [Google Scholar]
- 8.Turner DJ, Lanteri CJ, LeSouef PN, Sly PD. Improved detection of abnormal respiratory function using forced expiration from raised lung volume in infants with cystic fibrosis. Eur Respir J. 1994;7(11):1995–1999. [PubMed] [Google Scholar]
- 9.Turner DJ, Stick SM, Lesouef KL, Sly PD, Lesouef PN. A new technique to generate and assess forced expiration from raised lung volume in infants. Am J Respir Crit Care Med. 1995;151(5):1441–1450. doi: 10.1164/ajrccm.151.5.7735598. [DOI] [PubMed] [Google Scholar]
- 10.Modl M, Eber E, Weinhandl E, Gruber W, Zach MS. Assessment of bronchodilator responsiveness in infants with bronchiolitis. A comparison of the tidal and the raised volume rapid thoracoabdominal compression technique. Am J Respir Crit Care Med. 2000;161(3 Pt 1):763–768. doi: 10.1164/ajrccm.161.3.9812063. [DOI] [PubMed] [Google Scholar]
- 11.Hayden MJ, Wildhaber JH, LeSouef PN. Bronchodilator responsiveness testing using raised volume forced expiration in recurrently wheezing infants. Pediatr Pulmonol. 1998;26(1):35–41. doi: 10.1002/(sici)1099-0496(199807)26:1<35::aid-ppul7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Jones M, Castile R, Davis S, Kisling J, Filbrun D, Flucke R, Goldstein A, Emsley C, Ambrosius W, Tepper RS. Forced expiratory flows and volumes in infants. Normative data and lung growth. Am J Respir Crit Care Med. 2000;161(2 Pt 1):353–359. doi: 10.1164/ajrccm.161.2.9903026. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein AB, Castile RG, Davis SD, Filbrun DA, Flucke RL, McCoy KS, Tepper RS. Bronchodilator responsiveness in normal infants and young children. Am J Respir Crit Care Med. 2001;164(3):447–454. doi: 10.1164/ajrccm.164.3.2005080. [DOI] [PubMed] [Google Scholar]
- 14.Saito J, Harris WT, Gelfond J, Noah TL, Leigh MW, Johnson R, Davis SD. Physiologic, bronchoscopic, and bronchoalveolar lavage fluid findings in young children with recurrent wheeze and cough. Pediatr Pulmonol. 2006;41(8):709–719. doi: 10.1002/ppul.20387. [DOI] [PubMed] [Google Scholar]
- 15.ATS/ERS statement: raised volume forced expirations in infants: guidelines for current practice. Am J Respir Crit Care Med. 2005;172(11):1463–1471. doi: 10.1164/rccm.200408-1141ST. [DOI] [PubMed] [Google Scholar]
- 16.de Onis M, Onyango AW, Borghi E, Garza C, Yang H. Comparison of the World Health Organization (WHO) Child Growth Standards and the National Center for Health Statistics/WHO international growth reference: implications for child health programmes. Public Health Nutr. 2006;9(7):942–947. doi: 10.1017/phn20062005. [DOI] [PubMed] [Google Scholar]
- 17.Bussamra MH, Cukier A, Stelmach R, Rodrigues JC. Evaluation of the magnitude of the bronchodilator response in children and adolescents with asthma. Chest. 2005;127(2):530–535. doi: 10.1378/chest.127.2.530. [DOI] [PubMed] [Google Scholar]
- 18.Galant SP, Morphew T, Amaro S, Liao O. Value of the bronchodilator response in assessing controller naive asthmatic children. J Pediatr. 2007;151(5):457–462. 462, e451. doi: 10.1016/j.jpeds.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Galant SP, Morphew T, Newcomb RL, Hioe K, Guijon O, Liao O. The Relationship of the Bronchodilator Response Phenotype to Poor Asthma Control in Children with Normal Spirometry. J Pediatr. 2011 doi: 10.1016/j.jpeds.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma S, Litonjua AA, Tantisira KG, Fuhlbrigge AL, Szefler SJ, Strunk RC, Zeiger RS, Murphy AJ, Weiss ST. Clinical predictors and outcomes of consistent bronchodilator response in the childhood asthma management program. J Allergy Clin Immunol. 2008;122(5):921–928. e924. doi: 10.1016/j.jaci.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerstjens HA, Overbeek SE, Schouten JP, Brand PL, Postma DS. Airways hyperresponsiveness, bronchodilator response, allergy and smoking predict improvement in FEV1 during long-term inhaled corticosteroid treatment. Dutch CNSLD Study Group. Eur Respir J. 1993;6(6):868–876. [PubMed] [Google Scholar]
- 22.Martin RJ, Szefler SJ, King TS, Kraft M, Boushey HA, Chinchilli VM, Craig TJ, Dimango EA, Deykin A, Fahy JV, Israel E, Lazarus SC, Lemanske RF, Jr, Leone FT, Pesola GR, Peters SP, Sorkness CA, Szwejbka LA, Wechsler ME. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J Allergy Clin Immunol. 2007;119(1):73–80. doi: 10.1016/j.jaci.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zielen S, Christmann M, Kloska M, Dogan-Yildiz G, Lieb A, Rosewich M, Schubert R, Rose MA, Schulze J. Predicting short term response to anti-inflammatory therapy in young children with asthma. Curr Med Res Opin. 2010;26(2):483–492. doi: 10.1185/03007990903485148. [DOI] [PubMed] [Google Scholar]
- 24.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 25.van Grunsven PM, van Schayck CP, Molema J, Akkermans RP, van Weel C. Effect of inhaled corticosteroids on bronchial responsiveness in patients with “corticosteroid naive” mild asthma: a meta-analysis. Thorax. 1999;54(4):316–322. doi: 10.1136/thx.54.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saglani S, Malmstrom K, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, Turpeinen M, Rogers AV, Payne DN, Bush A, Haahtela T, Makela MJ, Jeffery PK. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171(7):722–727. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 27.Modl M, Eber E, Malle-Scheid D, Weinhandl E, Zach MS. Does bronchodilator responsiveness in infants with bronchiolitis depend on age? J Pediatr. 2005;147(5):617–621. doi: 10.1016/j.jpeds.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Debley JS, Stamey DC, Cochrane ES, Gama KL, Redding GJ. Exhaled nitric oxide, lung function, and exacerbations in wheezy infants and toddlers. J Allergy Clin Immunol. 2010;125(6):1228–1234. e1213. doi: 10.1016/j.jaci.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debley JSS, Cochrane E, Elliot M, Redding G. Exhaled Nitric Oxide Predicts Persistence Of Wheezing, Exacerbations, And Decline In Lung Function In Wheezy Infants And Toddlers. Am J Respir Crit Care Med. 2011;183:A1033. doi: 10.1111/cea.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 31.Yao W, Barbe-Tuana FM, Llapur CJ, Jones MH, Tiller C, Kimmel R, Kisling J, Nguyen ET, Nguyen J, Yu Z, Kaplan MH, Tepper RS. Evaluation of airway reactivity and immune characteristics as risk factors for wheezing early in life. J Allergy Clin Immunol. 2010;126(3):483–488. e481. doi: 10.1016/j.jaci.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer LJ, Rye PJ, Gibson NA, Burton PR, Landau LI, Lesouef PN. Airway responsiveness in early infancy predicts asthma, lung function, and respiratory symptoms by school age. Am J Respir Crit Care Med. 2001;163(1):37–42. doi: 10.1164/ajrccm.163.1.2005013. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham J, Dockery DW, Speizer FE. Maternal smoking during pregnancy as a predictor of lung function in children. Am J Epidemiol. 1994;139(12):1139–1152. doi: 10.1093/oxfordjournals.aje.a116961. [DOI] [PubMed] [Google Scholar]
- 34.Li YF, Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Rappaport EB, Peters JM. Effects of in utero and environmental tobacco smoke exposure on lung function in boys and girls with and without asthma. Am J Respir Crit Care Med. 2000;162(6):2097–2104. doi: 10.1164/ajrccm.162.6.2004178. [DOI] [PubMed] [Google Scholar]
- 35.Gilliland FD, Berhane K, Li YF, Rappaport EB, Peters JM. Effects of early onset asthma and in utero exposure to maternal smoking on childhood lung function. Am J Respir Crit Care Med. 2003;167(6):917–924. doi: 10.1164/rccm.200206-616OC. [DOI] [PubMed] [Google Scholar]
- 36.Moshammer H, Hoek G, Luttmann-Gibson H, Neuberger MA, Antova T, Gehring U, Hruba F, Pattenden S, Rudnai P, Slachtova H, Zlotkowska R, Fletcher T. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173(11):1255–1263. doi: 10.1164/rccm.200510-1552OC. [DOI] [PubMed] [Google Scholar]


