Abstract
Objective
To investigate the decisive role of preoperative serum thrombopoietin levels in the discrimination of benign and malignant ovarian pathologies and its value in the evaluation of treatment response.
Methods
Fifty patients with diagnoses of adnexal masses (25 benign, 25 malignant) were included in the study. Blood samples were collected from all cases preoperatively. Age, menopausal status, adnexal mass size, preoperative CA-125 level, platelet count, the stage of the disease (FIGO stage), tumor grade, histologic subgroup, the residual tumor mass, ascites cytology, surgical procedures, and postoperative treatments were recorded for the malignant group. Response to treatment was evaluated based on the revised RECIST guideline.
Results
The preoperative serum thrombopoietin levels of the malignant cases (median, 98; range, 7 to 768) were significantly higher when compared with those of benign cases (median, 27; range, 13 to 131; p=0.004). The positive predictive value of CA-125 was found to be 79%, when it was used as a single marker; however it had risen to 85% when both CA-125 and thrombopoietin levels were used. There was no significant relationship between preoperative serum thrombopoietin levels and tumor grade, ascites cytology, presence of residual mass, and response to treatment. The preoperative serum thrombopoietin levels were significantly higher in stage III-IV cases and cases with serous histology. The post-treatment serum thrombopoietin levels in the malignant group were significantly lower as compared with the preoperative thrombopoietin levels.
Conclusion
Thrombopoietin can play an additive role for prediction of ovarian cancer.
Keywords: Biological tumor markers, Ovarian neoplasms, Thrombopoietin
INTRODUCTION
Ovarian cancers have the highest fatality rate among gynecologic malignancies. The disease is identified in advanced stages in most patients and only one out of three cases has a chance of long-term survival [1]. The lack of specific symptoms in addition with the lack of reliable screening tests cause a delay in diagnosis, resulting in low survival rates. While the overall 5-year survival rate is 44%, it is 89% for localized disease, 36% for regional metastasis, and 17% for distant metastasis [2].
Transvaginal ultrasonography has a high false-positive rate and leads to unnecessary surgical procedures [3]. Frequent positivity of CA-125 in women with different benign diseases limits the efficacy of screening [4]. Although the sensitivity and specificity of CA-125 can be increased by combining it with ultrasonography, the predictive level remains still relatively low. The positive predictive value for invasive cancer is 3.7% for an abnormal CA-125, 1.0% for an abnormal transvaginal ultrasonography, and 23.5% if both tests are abnormal [5]. For this reason, determination of serum identifiers that can be used independently or in combination with CA-125 and/or transvaginal ultrasonography has a critical significance.
Gene expression analysis, proteomics, and new tumor markers are among the current alternatives of early tumor detection [6,7]. A novel candidate marker, thrombopoietin, which is known as a primary regulator protein, regulates platelet production as well as the megakaryopoiesis process. Thrombopoietin demonstrates its effect by binding to receptor c-Mpl, a cellular proto-oncogene product [8]. In the literature during the last decade, there are case reports associated with the following three types of malignant tumors that have been shown to secrete thrombopoietin: Ovarian cancer, hepatoblastoma, and hepatocellular carcinoma [9-11].
In the present study, the decisive role of preoperative serum thrombopoietin levels in the discrimination of benign and malignant ovarian pathologies and its value in the evaluation of treatment response have been investigated.
MATERIALS AND METHODS
Between January 2008 and April 2010, 50 patients with diagnoses of adnexal masses and hospitalized for surgery in the Department of Obstetrics and Gynecology, Ege University Hospital were included in the study. Blood samples were collected from all cases on the same day of surgery just after the induction of anesthesia. Cases with non-ovarian originating adnexal masses, and patients with inflammatory conditions, such as allergies or infections, were not included in the study. Informed consent of all cases were obtained and the study was approved by the Ege University Hospital Ethics Committee. The cases included in the study were divided into two groups (25 malignant and 25 benign cases) based on postoperative ovarian pathology results.
The patients with ovarian cancer underwent cytoreductive surgery comprised of removal of adnexial mass, hysterectomy, total omentectomy and retroperitoneal debulking followed by adjuvant paclitaxel and carboplatin chemotherapy.
In this prospective case-control study, age, menopausal status, adnexal mass size, preoperative CA-125 level, platelet count, the stage of the disease, tumor grade, histologic subgroup, the residual tumor mass, ascites cytology, surgical procedures, and postoperative treatments were recorded for the malignant group. The FIGO stage was identified based on the surgery and pathology results. The pathologic analyses of all the cases included in the study were performed by an experienced gynecologic pathologist and tumors with a low potential of malignancy were not included in the study. Response to treatment was evaluated based on the revised RECIST guideline [12]. Blood was collected once more from the patients included in the malignant study group 6 weeks after treatment in order to re-analyze serum thrombopoietin levels. The serum samples were waited for 30 minutes and centrifuged at 2,000 g for 10 minutes and stored at -80℃ until the analysis.
Serum thrombopoietin levels were analyzed with a thrombopoietin-immunoassay kit (R&D Systems, Minneapolis, MN, USA) in accordance with the manufacturer's manual. The sensitivity of thrombopoietin ELISA was 15.7 pg/mL.
SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis of data in our study. The comparisons in line with the specifications of the variables were made using a t-test, chi-square test, Mann-Whitney U-test, and Spearman's rho test. The calculation sensitivity and specificity of serum thrombopoietin and CA-125 in the determination of malignancy was performed using logistic regression analysis and receiver operating characteristic (ROC) curve analysis. A value of p<0.05 was considered statistically significant.
RESULTS
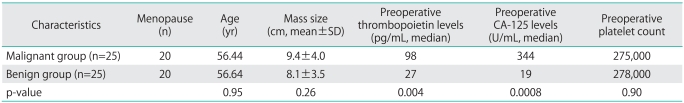
The clinical characteristics of patients and the number of patients with CA-125 and/or thrombopoietin positivity are summarized in Tables 1 and 2, respectively. The preoperative serum thrombopoietin levels of the malignant cases (median, 98 pg/mL; range, 7 to 768 pg/mL) were significantly higher when compared with those of benign cases (median, 27 pg/mL; range, 13 to 131 pg/mL; p=0.004). As expected, the preoperative serum CA-125 levels in the malignant group (median, 344 U/mL; range, 10 to 4,587 U/mL) were also significantly higher as compared with the benign group (median, 19 U/mL; range, 3 to 303 U/mL; p=0.0008).
Table 1.
Clinical characteristics of patients
p<0.05, statistically significant.
Table 2.
Number of patients with CA-125 and/or thrombopoietin positivity and/or negativity
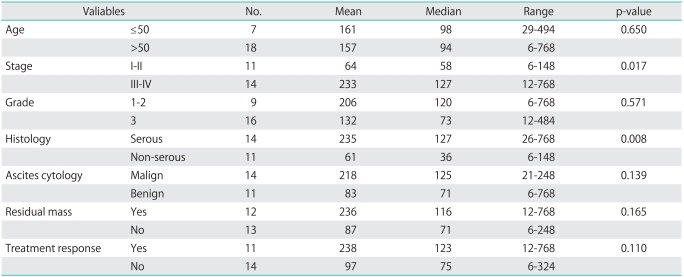
A comparison of preoperative serum thrombopoietin levels in patients with invasive ovarian cancer and certain characteristic features that are considered to have prognostic significance is provided in Table 3. The malignant cases were staged between 1 and 4 based on the severity of the disease according to the FIGO classification. According to surgical staging, 4 cases were stage I, 7 cases were stage II, 12 cases were stage III, and 2 cases were stage IV. It has not been possible to show a significant relationship between preoperative serum thrombopoietin levels and tumor grade, ascites cytology, presence of residual mass, and response to treatment. On the other hand, the relationship between the FIGO stage and histologic subgroup and preoperative serum thrombopoietin levels were significant. Out of 11 stage (I-II) patients in the malignant group, 4 patients had serous histology while 7 had non-serous. Out of 14 stage (III-IV) patients in the malignant group, 10 patients had serous histology while 4 patients had non-serous histology. The preoperative serum thrombopoietin levels were significantly higher in stage III-IV cases and cases with serous histology (Table 3).
Table 3.
The relationship of prognostic factors with thrombopoietin (pg/mL) levels in cases of ovarian cancer
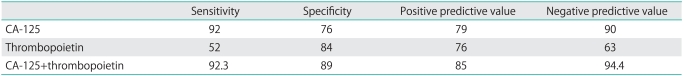
The utility of preoperative serum thrombopoietin levels for predicting malignancy, sensitivity and specificity calculations were performed (Table 4). In all the cases, when the preoperative serum thrombopoietin cut-off level was taken as 90 pg/mL to identify malignancy, the sensitivity was 52%, the specificity was 84%, the positive predictive value (PPV) was 76%, and the negative predictive value (NPV) was 63%. In all the cases, when the cut-off level for CA-125 was taken as 30 U/mL, the sensitivity was 92%, specificity was 76%, and NPV was 84%. The PPV of CA-125 was found to be 79%, when it was used as a single marker; however it had risen to 85% when both CA-125 and thrombopoietin levels were used. There was only 1 patient with normal CA-125 and elevated thrombopoietin levels (Table 4).
Table 4.
Additive role of thrombopoietin for ovarian cancer prediction
Values are presented as percentage (%).
The post-treatment serum thrombopoietin levels in the malignant group were significantly lower as compared with the preoperative thrombopoietin levels (p=0.002). However, there was no significant relationship between responders and non-responders to treatment in terms of post-treatment serum thrombopoietin levels (p=0.907).
DISCUSSION
The reason for increased thrombopoietin levels in cases with invasive ovarian cancer could be secondary to direct production from the tumoral tissue or certain other growth factors that trigger thrombopoietin production from the target organs or to inflammatory cytokines. Studies that support both situations exist in the literature. Furuhashi et al. [9] showed increased immunohistochemical expression of thrombopoietin in a case of ovarian cancer. Torres et al. [13] and Chambers [14] found evidence in association with the fact that cytokines, such as IL-6, IL-10, CSF-1, TGF-b, and TNF contribute to tumor development in the pathogenesis of ovarian cancer. In a study that supports these data, Nowak et al. [15] compared the levels of IL-6, IL-8, and IL-10 in benign cases, and in early and advanced stage ovarian cancer cases, and concluded that these cytokines could be useful in the identification of malignancy.
The only study that has evaluated the relation of thrombopoietin with benign ovarian cysts and ovarian cancers includes 51 invasive ovarian cancer cases and 25 cases of benign adnexal masses [16]. This study is in agreement with our study with respect to serum preoperative thrombopoietin levels, which are reported to be significantly higher in malignant cases, and with respect to thrombocyte count and thrombopoietin levels, as no relationship has been found between the two groups. Also in agreement to this study, it has not been possible to show a significant relationship between preoperative serum thrombopoietin levels and tumor grade, ascites cytology, presence of residual mass, and response to treatment. However, an important difference from Tsukishiro et al. [16] is that serum thrombopoietin levels are found significantly higher in stage III-IV cases and cases with serous histology in our study. Because thrombopoietin is not only a protein synthesized from ovarian cancer cells and can be influenced by many physiologic and pathologic situations, it does not seem possible to conclude this high level in advanced stage serous histology cases can be totally attributed to malignancy.
We have also attempted to assess whether any relation exists between response to treatment and thrombopoietin by evaluating serum levels following surgery and chemotherapy. Although thrombopoietin levels fell significantly following treatment, there was no relationship between the patients that responded and did not respond to treatment. This postoperative/post-chemotherapy decline is an expected result and this finding limits the relevance of thrombopoietin in the evaluation of response to treatment in cases with ovarian cancer.
Cost effectiveness is also calculated for thrombopoietin, as it is an important factor for all screening tests. The cost of thrombopoietin amounts to approximately 9.75$ per patient and this is a well price when compared to other tools such as CA-125 and transvaginal ultrasonography. While CA-125 has a cost of 6.5$ per patient, transvaginal ultrasonography has a cost of 15$.
In conclusion, although thrombopoietin can play an additive role for prediction of ovarian cancer, it has no superiority to CA-125, which is in widespread use. It has also no relationship with the majority of prognostic factors and its significance in the evaluation of 'response to treatment' is limited.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 2.Ries LA, Harkins D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al. SEER cancer statistics review, 1975-2003. Bethesda, MD: National Cancer Institute; 2005. [Google Scholar]
- 3.Bell R, Petticrew M, Sheldon T. The performance of screening tests for ovarian cancer: results of a systematic review. Br J Obstet Gynaecol. 1998;105:1136–1147. doi: 10.1111/j.1471-0528.1998.tb09966.x. [DOI] [PubMed] [Google Scholar]
- 4.Markman M. The role of CA-125 in the management of ovarian cancer. Oncologist. 1997;2:6–9. [PubMed] [Google Scholar]
- 5.Buys SS, Partridge E, Greene MH, Prorok PC, Reding D, Riley TL, et al. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol. 2005;193:1630–1639. doi: 10.1016/j.ajog.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Rapkiewicz AV, Espina V, Petricoin EF, 3rd, Liotta LA. Biomarkers of ovarian tumours. Eur J Cancer. 2004;40:2604–2612. doi: 10.1016/j.ejca.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Dupont J, Tanwar MK, Thaler HT, Fleisher M, Kauff N, Hensley ML, et al. Early detection and prognosis of ovarian cancer using serum YKL-40. J Clin Oncol. 2004;22:3330–3339. doi: 10.1200/JCO.2004.09.112. [DOI] [PubMed] [Google Scholar]
- 8.de Sauvage FJ, Hass PE, Spencer SD, Malloy BE, Gurney AL, Spencer SA, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369:533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 9.Furuhashi M, Miyabe Y, Oda H. A case of thrombopoietin-producing ovarian carcinoma confirmed by immunohistochemistry. Gynecol Oncol. 1999;74:278–281. doi: 10.1006/gyno.1999.5428. [DOI] [PubMed] [Google Scholar]
- 10.Komura E, Matsumura T, Kato T, Tahara T, Tsunoda Y, Sawada T. Thrombopoietin in patients with hepatoblastoma. Stem Cells. 1998;16:329–333. doi: 10.1002/stem.160329. [DOI] [PubMed] [Google Scholar]
- 11.Ryu T, Nishimura S, Miura H, Yamada H, Morita H, Miyazaki H, et al. Thrombopoietin-producing hepatocellular carcinoma. Intern Med. 2003;42:730–734. doi: 10.2169/internalmedicine.42.730. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Torres MP, Ponnusamy MP, Lakshmanan I, Batra SK. Immunopathogenesis of ovarian cancer. Minerva Med. 2009;100:385–400. [PubMed] [Google Scholar]
- 14.Chambers SK. Role of CSF-1 in progression of epithelial ovarian cancer. Future Oncol. 2009;5:1429–1440. doi: 10.2217/fon.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowak M, Glowacka E, Szpakowski M, Szyllo K, Malinowski A, Kulig A, et al. Proinflammatory and immunosuppressive serum, ascites and cyst fluid cytokines in patients with early and advanced ovarian cancer and benign ovarian tumors. Neuro Endocrinol Lett. 2010;31:375–383. [PubMed] [Google Scholar]
- 16.Tsukishiro S, Suzumori N, Nishikawa H, Arakawa A, Suzumori K. Preoperative serum thrombopoietin levels are higher in patients with ovarian cancer than with benign cysts. Eur J Obstet Gynecol Reprod Biol. 2008;140:67–70. doi: 10.1016/j.ejogrb.2005.10.037. [DOI] [PubMed] [Google Scholar]