Abstract
Background
Concentrating and washing apheresis platelets (APs) substantially reduce the number of allergic transfusion reactions likely due to removal of plasma. However, these processes may damage platelets. This study evaluated whether concentrating or washing APs decrease the Corrected Count Increment (CCI).
Study Design and Methods
This retrospective study evaluated individuals who initially received unmanipulated APs and subsequently received concentrated and/or washed APs at a large university hospital between 1998 and 2009. Concentrated units were prepared by reducing the plasma volume of APs by a goal of >67%. Washed units were prepared by washing the APs with 1L normal saline. The CCI (plt × m2/uL) for all transfusions was calculated. Hypothesis testing was performed with Student’s t-tests for continuous variables and chi-square tests for dichotomous variables.
Results
We evaluated 121 individuals; 46 patients who received unmanipulated, concentrated and then washed APs, 59 patients who received unmanipulated and then concentrated APs; and 16 patients who received unmanipulated and then washed APs. Patient demographics were similar among the three groups. The mean CCI for unmanipulated AP transfusions at 0–2 hours post transfusion were significantly higher than concentrated and washed platelet transfusions (p<0.001). However, when accounting for platelet loss due to manipulation, concentrating APs did not impact the CCI, but the CCI remained significantly lower for washed products at all time points post transfusion (40.7% mean reduction at 20–24 hours, p<0.001).
Conclusions
Washing APs significantly reduces platelet count recovery and survival, as demonstrated by a significantly reduced CCI.
Keywords: corrected count increment (CCI), allergic transfusion reaction (ATR), platelet, wash, concentrate, urticaria, hives, anaphylaxis, premedication
INTRODUCTION
Plasma reduction of red blood cells and platelets is a process common to blood banks and has multiple clinically significant uses. Plasma reduction through concentrating and washing apheresis platelets (APs) significantly reduces the number of chronic allergic transfusion reactions (ATRs).1 Washing APs can remove IgA from plasma, and can prevent anaphylaxis in sensitized IgA-deficient individuals.2 Reduction of plasma volume can also reduce the likelihood of fluid overload in patients with cardiac disease and individuals sensitive to small fluid increases.3 In addition, washing APs may reduce the risk of TRALI from anti-neutrophil or HLA donor antibodies, and hemolysis due to ABO incompatible plasma.2
Although concentrating and washing APs has previously been shown to be highly effective for mitigating certain clinical complications, a few studies indicate that washing or concentrating APs decreases the number of platelets or that the platelets may not be as functional. It has been demonstrated that approximately 15% of platelets are lost when AP units are concentrated.4 Up to 33% of the platelet yield may be lost during washing.2,5 It has been suggested that the corrected count increment (CCI) with washed APs drops more precipitously at subsequent time points, suggesting that the survival of the washed platelets is decreased.5 While this has not been definitively substantiated, the reduction in CCI may be due to platelet damage from the plasma reduction procedure. Consequently, while plasma reduction may reduce the risk of adverse transfusion reactions such as severe or recurrent allergic reactions, the platelet product itself may be rendered less beneficial to the patient due to the manipulation.
No large study to-date has directly addressed the effect that plasma reduction has on AP transfusion effectiveness as defined by the CCI. Consequently, we evaluated CCI pre- and post-transfusion from oncology patients who received concentrated or washed APs.
MATERIALS AND METHODS
This retrospective study evaluated all of the available CCI of platelet transfusions among oncology patients who received unmanipulated APs and subsequently plasma concentrated and/or washed APs between January 1, 1998 and December 31, 2009 due to refractory or severe ATRs. The study was approved by the Johns Hopkins Medical Institutions Institutional Review Board.
The diagnosis of ATRs was documented at the time of the reaction by transfusion medicine physicians. At The Johns Hopkins Hospital, it is a hospital requirement to report all suspected transfusion reactions. ATRs were clinically diagnosed by the definition provided in the concurrent AABB Technical Manuals.2 Transfusion reactions were evaluated by a transfusion medicine physician and recommendations for product manipulation on subsequent transfusions were communicated to clinicians by platelet transfusion coordinators.
Apheresis Platelets (APs)
APs were collected from our community blood center or collected onsite by standard procedures.2 All APs were irradiated and leukocyte reduced. One equivalent unit of platelets was defined as 5.5 × 1010 platelets, and the apheresis platelet units contained at least 3.0 × 1011 platelets.
Concentrated AP units were prepared by reducing the plasma volume of APs by a goal of >67% while ensuring there was at least 100mL of plasma remaining for resuspension. Washed AP units were prepared by washing the APs with 1L normal saline. All washing was performed on the Cobe 2991 Cell Processor (CaridianBCT, Lakewood, CO).
CCI calculation
All CCIs were calculated in the following fashion:
The CCI was reported as plt × m2/uL. The CCI was determined from data collected at the time of transfusion and subsequent platelet counts; this study only evaluated individuals with previously documented CCIs. If multiple post-transfusion platelet counts within 24 hours were performed, then study subjects had multiple CCIs. These CCIs were then categorized into 0–2 hours, 2–8 hours, 8–14 hours, 14–20 hours, and 20–24 hours post-transfusion. The number of equivalent units was calculated from platelet yield at collection (not post manipulation).
We determined the loss of platelets post-manipulation over two years at our institution as part of quality control processes. The platelet count percent recovery was calculated by sending a sample of the platelet product pre- and post-manipulation to the hematology lab. The platelet counts were determined using the Sysmex ™ platform. Since we identified that approximately 20% of platelets are lost both by concentration and washing platelets, we accounted for this loss by increasing the calculated CCI for all washed and concentrated AP transfusions by 20%.
Statistical analysis
Statistical analyses were performed using STATA, v9.2 (StataCorp, College Station, TX) and Microsoft Excel (Redmond, WA). The mean and median, 25th and 75th percentile interquartile range (IQR) were calculated for demographic and transfusion data. Hypothesis testing was performed with Student’s t-tests for continuous variables and chi-square tests for dichotomous variables. The t-tests were two tailed and performed assuming unequal variance.
RESULTS
Subjects
There were 121 individuals at Johns Hopkins Hospital who received unmanipulated and subsequently concentrated and/or washed AP products who were evaluated for CCI. Of the 121 individuals, 46 patients received unmanipulated, concentrated and then washed APs, 59 patients received unmanipulated and then concentrated APs, and 16 patients received unmanipulated and then washed APs.
The mean age of the entire study population was 46.9 years (SD: 19.1; median: 49; range: 1–82) and 58.7% (71/121) were males (Table 1). Most of individuals in the study population were Caucasian (70.2%, 85/121). There were no significant differences in age, gender, or race between those who received concentrated or washed APs (Table 1). The majority of the study participants were oncology patients (93.4%, 113/121). None of the individuals had documented IgA deficiency.
Table 1.
Characteristics of study subjects who received unmanipulated and subsequently manipulated AP products.
| Concentrated only (n= 59) |
Concentrated and Washed (n=46) |
Washed only (n= 16) |
All individuals (n=121) |
|
|---|---|---|---|---|
| Males (%) | 37 (62.7) | 24 (52.2) | 10 (62.5) | 71 (58.7) |
| Age (years) | ||||
|
0–24 (%) 25–49 50–74 75+ |
11 (18.6) 17 (28.8) 25 (42.4) 6 (10.2) |
11 (23.9) 16 (34.9) 19 (41.3) 0 (0.0) |
1 (6.3) 7 (43.8) 7 (43.8) 1 (6.3) |
23 (19.0) 40 (33.1) 51 (42.1) 7 (5.8) |
| Race | ||||
|
Caucasian (%) African American Asian/Hispanic/Other |
44 (74.6) 6 (10.2) 9 (15.3) |
29 (63.0) 9 (19.6) 8 (17.4) |
12 (75.0) 3 (18.8) 1 (6.3) |
85 (70.2) 18 (14.9) 18 (14.9) |
|
Primary Oncologic diagnosis (%) |
53 (89.8) | 44 (95.7) | 16 (100.0) | 113 (93.4) |
|
Individuals who received APs prior to manipulation |
59 (100%) | 46 (100%) | 16 (100%) | 121 (100%) |
|
Months of transfusion observation (SD) |
14.8 (18.1) | 15.3 (19.2) | 13.5 (13.2) | 14.8 (17.8) |
|
Median number of AP units prior to manipulation (IQR) |
14 (6.0–35.0) | 10.5 (3.3–20.8) | 7 (4.5–11.5) | 11 (5.0–24.0) |
|
Median concentrated units (IQR) |
19 (10.0–38.0) | 5 (2.0–16.0) | NA | 13 (4.0–32.0) |
|
Median washed units (IQR) |
NA | 26.5 (12.3–61.8) | 21 (9.8–42.5) | 25.5 (11.3–57.0) |
Baseline transfusion history
In total, the previously determined CCI from 7,495 AP transfusions were evaluated. Of these, 4,641 transfusions had an associated 0–2 hr post transfusion CCI (61.9%). All study participants initially received unmanipulated APs (Table 1). These individuals received a median of 11 (IQR: 5–24) AP units prior to receiving either concentrated and/or washed APs. The average time each individual received AP transfusion support was 14.8 months (SD: 17.8); the mean overall duration of receiving unmanipulated and then concentrated and/or washed platelets was similar. Overall, the median number of concentrated APs received per person was 13 (IQR: 4–32), and washed APs was 25.5 (IQR: 11–57).
Impact of concentrating and washing APs on platelet count
To determine the impact of washing (n=83) and concentrating (n=27) APs on the platelet loss, quality control data for two years was evaluated (Table 2). There was a 79.0% platelet count recovery for concentrated products and an 80.1% platelet count recovery for washed products This was a significant reduction in platelet count for both washing and concentrating platelets (p<0.001).
Table 2.
The impact of product manipulation on platelet count
|
Manipulation |
Pre-manipulation volume (ml) [mean, (SD)] |
Post-manipulation volume (ml) [mean, (SD)] |
P value |
Pre-manipulation plt count [mean, (SD)] |
Post-manipulation plt count [mean, (SD)] |
P value |
Average Percent recovery [mean, (SD)] |
|---|---|---|---|---|---|---|---|
| Concentrated Platelets (n=27) |
303.8 (67.1) | 107.6 (15.9) | < 0.001 | 354,421.9 (70,305.4) |
276,866.5 (54,135.5) |
< 0.001 | 79.0 (10.5) |
| Washed Platelets (n=83) |
281.8 (72.5) | 173.1 (11.5) | < 0.001 | 349,098.9 (87,937.7) |
278,825.9 (71,554.9) |
< 0.001 | 80.1 (7.8) |
In Vivo Impact of Washing and Concentrating APs
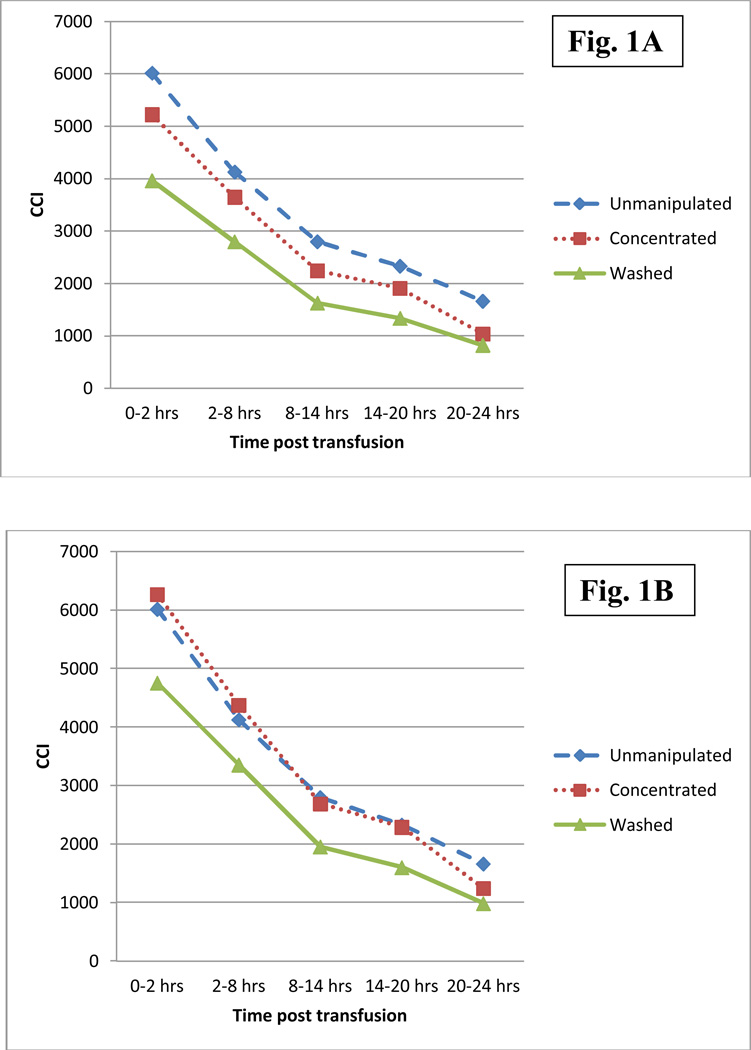
There was a significant decrease in mean CCI during the 24 hour period post-transfusion for all platelet product types (p<0.001; 72.3% mean reduction for unmanipulated products, 80.2% for concentrated, and 79.2% for washed) (Figure 1a). Table 3 shows that the mean CCI 0–2 hours post transfusion for unmanipulated APs was 6017.7 ± 4086.4, 5224.7 ± 3208.3 for concentrated APs, and 3963.6 ± 2874.7 for washed APs. The CCI for concentrated and washed APs was significantly lower at all time-points post transfusion in comparison to unmanipulated products (p=0.04). The percent difference between the mean CCI of unmanipulated APs and plasma reduced APs ranged from 11.6% – 37.8% for concentrated products, and 32.2% – 50.6% for washed products (Table 3). The greatest difference in CCI between unmanipulated, concentrated, and washed APs was noted at 20–24 hours after the AP transfusion (p<0.001).
Figure 1.
The mean CCI as a function of time post transfusion (1A) and corrected for estimated platelet loss during the manipulation in the laboratory (1B).
Table 3.
The mean CCI as a function of time post transfusion.
| 0–2 hrs | 2–8 hrs | 8–14 hrs | 14–20 hrs | 20–24 hrs | |
|---|---|---|---|---|---|
|
Unmanipulated APs (n=3139) Mean (SD) |
6016.7 (4086.4) | 4126.9 (3115.9) | 2798.2 (2886.3) | 2331.7 (2795.7) | 1665.6 (2028.6) |
|
Concentrated APs (n=3260) Mean (SD) |
5224.7 (3208.3)*** | 3648.7 (3008.5)*** | 2242.2 (2757.0)* | 1908.7 (2056.3)* | 1036.6 (1411.9)*** |
|
Washed APs (n=3611) Mean (SD) |
3963.6 (2874.7)*** | 2796.4 (2537.7)*** | 1628.8 (1970.2)*** | 1336.1 (1946.3)*** | 823.4 (1387.0)*** |
P < 0.05
P < 0.001
Since approximately 20% of platelets were lost both by concentration and washing APs at our institution and the CCI calculation is dependent on the number of platelets transfused (Table 2), we corrected the mean CCI for concentrated and washed AP products by increasing each CCI by 20%. After correction, as shown in Figure 1b and Table 4, there were, overall, no substantial differences between mean CCI of unmanipulated APs and CCI of corrected concentrated APs. The only difference that remained significant was the difference in the CCI at 20–24hrs (P=0.03).
Table 4.
The CCI of unmanipulated and corrected concentrated APs (+) as a function of time post transfusion.
| 0–2 hrs | 2–8 hrs | 8–14 hrs | 14–20 hrs | 20–24 hrs | |
|---|---|---|---|---|---|
| Unmanipulated APs (n=3139) Mean (SD) |
6016.7 (4086.4) | 4126.9 (3115.9) | 2798.2 (2886.3) | 2331.7 (2795.7) | 1665.6 (2028.6) |
| Concentrated APs (n=3260) Mean (SD) |
6269.6 (3850.0) | 4378.4 (3610.2) | 2690.6 (3308.4) | 2290.4 (2467.5) | 1243.9 (1694.2) |
| Percent change in CCI (unmanipulated vs. concentrated) Mean (%) |
4 | 5.7 | −3.8 | −1.8 | −25.3* |
P < 0.05
The corrected mean was calculated by adding 20% to the concentrated calculated CCI to account for the platelet loss due to the manipulation process.
In contrast to concentrated APs, the corrected CCI for washed APs remained significantly lower at all time points post-transfusion (P<0.001), and the percent difference between the mean CCI of unmanipulated APs and CCI of corrected washed APs ranged from 18.7% – 40.7%. As with the uncorrected CCIs, the greatest change in CCI between unmanipulated, concentrated, and washed APs was noted at 20–24 hours after the AP transfusion.
Due to possible changes in clinical status and platelet refractory conditions, we also evaluated CCI transfusion data in a more limited window of only 2 weeks before and 2 weeks after a change in AP transfusion protocol. We found that there was no significant difference in the overall trend of corrected CCIs between the primary analysis and the 2 week analysis for unmanipulated, concentrated and washed APs.
DISCUSSION
This study evaluated the impact of concentrating or washing APs on platelet recovery, as documented by CCI. This study evaluated a large number of oncology patients receiving concentrated and washed APs to account for the biologic variability of platelet products and transfusion recipients. Over 24 hours, the mean CCI decreased by 72.3% in unmanipulated APs and by 79.2% in washed APs. When correcting for platelet loss, only washed platelets had CCIs which were significantly reduced in comparison to unmanipulated APs.
Our patient population required plasma reduction due to refractory or severe allergic transfusion reactions (ATRs). ATRs have often created difficulties with the administration of blood and are the most common complication of blood transfusion. Estimates of ATRs range from 1 to 3 percent of all transfusions2,6–7 but may be as high as 10% in prospective, active surveillance research protocols.8 The pathophysiology of ATRs are not well characterized, and both clinical trials and observational studies have demonstrated that premedications administered to prevent ATRs are ineffective.9 Recipient, donor, and product characteristics are all associated with ATRs.10–12 Additionally, a plasma component is necessary to cause an ATR.1,10,13 We have recently demonstrated that plasma reduction (concentrating APs (73% reduction) and washing APs (95% reduction)) is likely the most effective method to reduce urticarial reactions, especially for platelet recipients who experience recurrent ATRs due to an underlying predisposition.1 Thus, it is important to understand the adverse consequences of plasma reduction methods.
This study demonstrates that plasma reduction decreases platelet in vivo, as defined by a lower CCI. It is widely accepted that a 1 hour post-transfusion CCI < 5000 on 2 consecutive transfusions defines platelet refractoriness.2 When the CCI is corrected for initial platelet loss, both unmanipulated and concentrated platelet products, but not washed platelets, yielded adequate CCIs at 0–2 hours. Consequently, patients who are receiving washed units are receiving suboptimal transfusions, as defined by CCI, and more transfusions would logically be needed to maintain their optimal target platelet count. In this study, the median number of washed AP transfusions per patient was higher than the median number of unmanipulated or concentrated transfusions combined (Table 1).
Because the CCI of the unmanipulated units closely mimics the CCI of the concentrated products after the 20% correction factor (Figure 1b), these data suggest that the platelets, once concentrated will have initial loss and some failure to survive, but those that do survive do so in a normal manner. These data further suggest that if you could correct for the expected platelet loss by increasing the platelet dose to be concentrated, the decay will be generally normal, so that the transfusion interval and CCI would not be affected. Clinical trial data recently suggested that transfusions of low doses of platelets (defined as 1.1× 1011 platelets per square meter of body surface area in this study) used for the prophylactic prevention of bleeding does not affect the incidence of bleeding.8 Consequently, the lower CCI found in washed platelets, even when accounting for platelet loss, may not be clinically significant.
This study confirms the previously reported observation that significant numbers of platelets are lost in the plasma reduction process.2,4 We found that 20% of platelets are lost to both the concentration and washing process. It has been demonstrated previously that approximately 15% of platelets are lost when AP units are concentrated, and up to 33% of the platelet yield may be lost during washing.2,5 The similar recovery between washing and concentrating APs at our institution is unclear. However, the differences observed are likely due to methodology. The current washing technique at our institution is more automated, and perhaps preserves more platelets than presented in previous studies.
As this study is retrospective, there are a number of limitations. First, while previous reports have shown that plasma reduced platelets have hemostatic effectiveness,13–14 we could not evaluate whether there were changes in bleeding event frequency over the study period. This study does not differentiate patients in terms of those who had or developed HLA alloantibodies or splenomegaly during the study period, two factors that could significantly alter the CCI. These factors likely explain the high variability in CCI. In addition, the post-transfusion platelet count assessment could not be standardized, as only 62% of our evaluated transfusions actually had a 0–2 hr post-transfusion CCI. The study aim was to determine the difference in CCI due to only product manipulation; consequently, the effect of patient-specific variables, such as fever, the presence of disseminated intravascular coagulation, or infection, were not evaluated as independent variables.
The documentation of the diminished recovery and survival of washed platelets demonstrates the need for the investigation and implementation of new methods to provide better platelet support for patients who cannot tolerate unmanipulated platelet products. The technology for washing platelets has changed little over several decades, and research into alternative methods of washing are needed. The use of platelet additive solutions (PAS) in this context may have value. It has been recently shown that platelets stored in platelet additive solution are effective and reduce adverse events associated with platelet transfusions.15–17 The use of platelet additive solutions has recently been FDA approved, however it remains unclear whether the CCI in PAS platelets are diminished similarly to washed APs. Current studies in Europe do suggest, however, that PAS platelets have an adverse effect on CCI. In one study, compared to unmanipulated platelets, the mean difference for the 1-h CCI was −31% for plasma reduced PAS platelets and −9% for PAS platelets, respectively.17 Future clinical trials comparing the CCI of PAS platelets to those that have been concentrated or washed, are clearly needed.
This study also demonstrates the need to improve our ability to monitor platelet efficacy in-vivo. As noted, the CCI is an imperfect and artificial method for evaluating whether the platelets in a platelet transfusion are effective.18–19 The CCI is not able to determine specifically determine platelet function. However, washing may also impact platelet function. One recent study evaluated 11 platelet units and found that washed platelets are physically altered such that they had reduced ability to aggregate and increased activation in comparison to both unmanipulated and concentrated platelets.20
In conclusion, while washing APs clearly has multiple important uses, washing APs appears to shorten the survival time of platelets in vivo. These data support the widely adopted practices of 1) concentrating or washing APs be undertaken only for clearly defined indications that cannot otherwise be prevented and only after the careful assessment of a transfusion medicine specialist and 2) monitoring chronic platelet recipients receiving washed platelets with serial platelet counts. Additional prospective, comparative studies are needed to evaluate the effects of AP function and survival in vivo, as are new approaches for preparing and monitoring plasma reduced platelet products.
Table 5.
The CCI of unmanipulated and corrected washed APs (+) as a function of time post transfusion
| 0–2 hrs | 2–8 hrs | 8–14 hrs | 14–20 hrs | 20–24 hrs | |
|---|---|---|---|---|---|
| Unmanipulated APs (n=3139) Mean (SD) |
6016.7 (4086.4) | 4126.9 (3115.9) | 2798.2 (2886.3) | 2331.7 (2795.7) | 1665.6 (2028.6) |
| Washed APs (n=3611) Mean (SD) |
4756.3 (3449.7) | 3355.7 (3045.2) | 1954.6 (2364.3) | 1603.3 (2335.6) | 988.1 (1664.4) |
| Percent change in CCI (unmanipulated vs. washed) Mean (%) |
−20.9*** | −18.7*** | −30.1*** | −31.2*** | −40.7*** |
P < 0.001
The corrected mean was calculated by adding 20% to the washed calculated CCI to account for the platelet loss due to the manipulation process.
Acknowledgements
We gratefully the nurses and laboratory medical technologists who performed the transfusion reaction workups. AART is supported by the Doris Duke Charitable Foundation (grant 2011036), NIH 1K23AI093152-01A1, and Johns Hopkins University Clinician Scientist Development Award.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011 doi: 10.1111/j.1537-2995.2010.03008.x. [DOI] [PubMed] [Google Scholar]
- 2.Technical Manual. 16 ed. Bethesda: AABB; 2008. [Google Scholar]
- 3.Schoenfeld H, Spies C, Jakob C. Volume-reduced platelet concentrates. Curr Hematol Rep. 2006;5:82–88. [PubMed] [Google Scholar]
- 4.Moroff G, Friedman A, Robkin-Kline L, Gautier G, Luban NL. Reduction of the volume of stored platelet concentrates for use in neonatal patients. Transfusion. 1984;24:144–146. doi: 10.1046/j.1537-2995.1984.24284173346.x. [DOI] [PubMed] [Google Scholar]
- 5.Tanz WS, Ansari-Lari M, King KE, Ness PM. Efficacy of washed platelets for the treatment of severe allergic transfusion reactions. Blood. 2001;98:827a. [Google Scholar]
- 6.Domen RE, Hoeltge GA. Allergic transfusion reactions: an evaluation of 273 consecutive reactions. Arch Pathol Lab Med. 2003;127:316–320. doi: 10.5858/2003-127-0316-ATR. [DOI] [PubMed] [Google Scholar]
- 7.Enright H, Davis K, Gernsheimer T, McCullough JJ, Woodson R, Slichter SJ. Factors influencing moderate to severe reactions to PLT transfusions: experience of the TRAP multicenter clinical trial. Transfusion. 2003;43:1545–1552. doi: 10.1046/j.1537-2995.2003.00529.x. [DOI] [PubMed] [Google Scholar]
- 8.Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, Gernsheimer TB, Ness PM, Brecher ME, Josephson CD, Konkle BA, Woodson RD, Ortel TL, Hillyer CD, Skerrett DL, McCrae KR, Sloan SR, Uhl L, George JN, Aquino VM, Manno CS, McFarland JG, Hess JR, Leissinger C, Granger S. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362:600–613. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobian AA, King KE, Ness PM. Transfusion premedications: a growing practice not based on evidence. Transfusion. 2007;47:1089–1096. doi: 10.1111/j.1537-2995.2007.01242.x. [DOI] [PubMed] [Google Scholar]
- 10.Savage WJ, Savage JH, Tobian AAR, Thoburn C, Hamilton RG, Schroeder JT, Ness P. Allergic agonists in apheresis platelet products are associated with allergic transfusion reactions. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03310.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savage WJ, Tobian AA, Fuller AK, Wood RA, King KE, Ness PM. Allergic transfusion reactions to platelets are associated more with recipient and donor factors than with product attributes. Transfusion. 2011 doi: 10.1111/j.1537-2995.2010.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savage WJ, Tobian AA, Savage JH, Hamilton RG, Ness PM. Atopic predisposition of recipients in allergic transfusion reactions to apheresis platelets. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck SA, Kickler TS, McGuire M, Braine HG, Ness PM. The utility of platelet washing using an automated procedure for severe platelet allergic reactions. Transfusion. 1987;27:391–393. doi: 10.1046/j.1537-2995.1987.27587320530.x. [DOI] [PubMed] [Google Scholar]
- 14.Silvergleid AJ, Hafleigh EB, Harabin MA, Wolf RM, Grumet FC. Clinical value of washed-platelet concentrates in patients with non-hemolytic transfusion reactions. Transfusion. 1977;17:33–37. doi: 10.1046/j.1537-2995.1977.17177128881.x. [DOI] [PubMed] [Google Scholar]
- 15.de Wildt-Eggen J, Nauta S, Schrijver JG, van Marwijk Kooy M, Bins M, van Prooijen HC. Reactions and platelet increments after transfusion of platelet concentrates in plasma or an additive solution: a prospective, randomized study. Transfusion. 2000;40:398–403. doi: 10.1046/j.1537-2995.2000.40040398.x. [DOI] [PubMed] [Google Scholar]
- 16.Azuma H, Hirayama J, Akino M, Miura R, Kiyama Y, Imai K, Kasai M, Koizumi K, Kakinoki Y, Makiguchi Y, Kubo K, Atsuta Y, Fujihara M, Homma C, Yamamoto S, Kato T, Ikeda H. Reduction in adverse reactions to platelets by the removal of plasma supernatant and resuspension in a new additive solution (M-sol) Transfusion. 2009;49:214–218. doi: 10.1111/j.1537-2995.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- 17.Kerkhoffs JL, Eikenboom JC, Schipperus MS, van Wordragen-Vlaswinkel RJ, Brand R, Harvey MS, de Vries RR, Barge R, van Rhenen DJ, Brand A. A multicenter randomized study of the efficacy of transfusions with platelets stored in platelet additive solution II versus plasma. Blood. 2006;108:3210–3215. doi: 10.1182/blood-2006-04-020131. [DOI] [PubMed] [Google Scholar]
- 18.Davis KB, Slichter SJ, Corash L. Corrected count increment and percent platelet recovery as measures of posttransfusion platelet response: problems and a solution. Transfusion. 1999;39:586–592. doi: 10.1046/j.1537-2995.1999.39060586.x. [DOI] [PubMed] [Google Scholar]
- 19.Salama ME, Raman S, Drew MJ, Abdel-Raheem M, Mahmood MN. Platelet function testing to assess effectiveness of platelet transfusion therapy. Transfus Apher Sci. 2004;30:93–100. doi: 10.1016/j.transci.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Veeraputhiran M, Ware J, Dent J, Bornhorst J, Post G, Cottler-Fox M, Pesek G, Theus J, Nakagawa M. A comparison of washed and volume-reduced platelets with respect to platelet activation, aggregation, and plasma protein removal. Transfusion. 2011;51:1030–1036. doi: 10.1111/j.1537-2995.2010.02897.x. [DOI] [PubMed] [Google Scholar]