Abstract
Objective
The mechanism by which anti-DNA antibodies mediate lupus nephritis has yet to be conclusively determined. Previously, we found that treatment of mesangial cells with anti-DNA antibodies induced high expression of Neutrophil Gelatinase Associated Lipocalin (NGAL), an iron-binding protein upregulated in response to kidney injury. However, whether NGAL is instrumental in pathogenesis, induced as part of repair, or irrelevant to damage/repair pathways, is not known.
Methods
To investigate the role of NGAL in antibody-mediated nephritis, we induced nephrotoxic nephritis by passive antibody transfer to B6 or 129 mice. To determine if NGAL upregulation is instrumental, we compared the severity of renal damage in NGAL wild-type and knock-out mice following induction of nephrotoxic nephritis.
Results
We found that kidney NGAL expression, as well as urinary NGAL levels, were significantly increased in nephrotoxic nephritis as compared to control injected mice. Tight correlations were observed between NGAL expression, renal histopathology, and urinary NGAL excretion. NGAL knock-out mice had attenuated proteinuria and improved renal histopathology as compared to wild-type mice. Similarly, following nephritis induction, NGAL injection significantly exacerbated nephritis and decreased survival. NGAL induces apoptosis via caspase-3 activation, and upregulates inflammatory gene expression in kidney cells in vitro and when injected in vivo.
Conclusion
We conclude that kidney binding of pathogenic antibodies stimulates local expression of NGAL, which plays a crucial role in the pathogenesis of nephritis via promotion of inflammation and apoptosis. NGAL blockade may be a novel therapeutic approach for the treatment of nephritis mediated by pathogenic antibodies, including anti-GBM disease and lupus nephritis.
INTRODUCTION
Experimentally, murine anti-glomerular basement membrane (GBM) disease can be induced by passive transfer of pre-formed heterologous anti-GBM antibodies, resulting in antibody deposition, recruitment of inflammatory cells, complement activation, and upregulation of proinflammatory mediators leading in concert to severe crescentic glomerulonephritis. The defined onset and reproducibility of the anti-GBM model has facilitated the exploration of mechanisms underlying kidney injury, and allowed investigators to reach conclusions that are often valid for other immune-mediated glomerulopathies as well, including lupus nephritis (LN) (1). Improved understanding of the pathways involved when nephritogenic autoantibodies deposit in kidney would have important diagnostic and therapeutic potential.
NGAL, a member of the lipocalin family of proteins, is widely expressed in a variety of cell types, including neutrophils, epithelial cells, and mesangial and tubular cells (2–5). Following early observations that NGAL is involved in kidney epithelial differentiation (6), studies in experimental renal ischemia suggested that NGAL is involved in epithelial repair, conferring a likely protective role for NGAL following kidney injury (7). This mechanism, however, has not been proven in the context of other animal models, and a pathway of action has not been fully elucidated.
NGAL is upregulated in resident kidney cells in vivo in response to renal injury, as demonstrated in patients with acute nephrotoxic damage or proliferative glomerulonephritis (7). The sensitivity of NGAL to acute kidney injury has been applied translationally, where serum and urine NGAL levels have been used successfully for the non-invasive assessment of renal damage in an increasing number of clinical conditions (8–14).
In previous studies directed at understanding the pathogenesis of LN, we had found that in vitro treatment of mesangial cells (MC) or systemic injection of pathogenic anti-DNA antibodies promoted significant NGAL overexpression by kidney cells and tissue, respectively (15). In addition, we and others have demonstrated that LN patients exhibit high levels of urinary NGAL that correlate with severity of renal involvement and may predict future activity (16–19). However, it is not known whether NGAL is actually instrumental in the downstream cascade leading from deposition of nephritogenic antibodies to renal damage in antibody-mediated nephritis such as SLE. Alternatively, renal NGAL can be induced as a protective response to ameliorate the local injury, or may simply reflect activation of other pathogenic pathways as an “innocent bystander”.
In the present study, we determined that NGAL is markedly upregulated in nephrotoxic serum nephritis (NTN), an experimental model for renal disease mediated by nephritogenic antibodies. We found that following induction of NTN, nephritis was significantly attenuated in NGAL knock-out (KO) mice while administration of exogenous NGAL to wild-type (WT) mice exacerbated the histological injury and worsened survival, thus conclusively establishing a central role for NGAL in the pathogenesis of antibody-induced nephritis.
MATERIALS and METHODS
Mice
Eight week old 129/SvJ (129) and C57Bl/6 (B6) mice were purchased from The Jackson Laboratory and housed 3–5 mice per cage in the animal facility of the Albert Einstein College of Medicine. All animal studies were approved by the Institutional Animal Care Committee.
Induction of NTN
NTN was induced as described previously, with minor modifications (20). Briefly, nephrotoxic serum was generated by rabbit immunization with sonicated mouse glomeruli. Mice were primed intraperitoneally with 50 μg of rabbit IgG in CFA on day (d) 0. On d5, mice received an intravenous injection of either 1) rabbit nephrotoxic serum; 2) control serum from non-immunized rabbits (normal rabbit sera, or NRS); or 3) PBS. Blood and urine were obtained at baseline (d0) and subsequently every 3–7 days (usually d7, d14 and d21) for serological measurements. Levels of proteinuria were determined by Uristix (Bayer Corporation, Pittsburgh, PA), and urine creatinine measured by the Jaffe reaction.
To examine renal histopathology over time as a function of NGAL expression, 5 mice from each experimental group were sacrificed at d7, d14 and d21 in one dedicated cohort. Kidney histology was quantitated blindly by an experienced nephropathologist, as described (21).
Separately, induction of NTN was performed in NGAL-KO and WT mice, and those mice were sacrificed at d8–21. Kidneys from sacrificed mice were used for RNA extraction, and preparation of paraffin sections for histological and immunohistochemical studies.
RNA isolation, cDNA synthesis and real-time RT-PCR
Total RNA was extracted from whole kidney tissue using Trizol (Invitrogen, Carlsbad, CA). In other experiments, MC stimulated for 6 hrs with NGAL were harvested for RNA isolation. Reverse transcription and real-time PCR was performed in triplicate, as previously described (15).
Detection of NGAL in kidney sections by immunohistochemistry
Following antigen retrieval and blocking, slides were incubated with 15 μg/ml of anti-mouse NGAL (R&D Systems, Minneapolis, MN) in 2% BSA in a humid chamber for 1 hour at room temperature. Slides were developed with biotinylated anti-IgG followed by streptavidin-HRP, counterstained with Mayer’s hematoxylin, dehydrated and mounted. The score assigned by a blinded evaluator (including both glomerular and tubular staining) averaging multiple fields was based on intensity: 0, no staining; 1, trace; 2–4, increasing degrees of intensity.
Urine and serum NGAL detection by ELISA
Serum and urine NGAL were determined by ELISA, as previously described (16).
Generation of NGAL−/− (KO) and +/+ (WT) mice
NGAL deficient mice were originally generated in the laboratory of Dr. Tak W. Mak (The Ontario Cancer Institute) as described previously (22). Knockout mice were backcrossed into the B6 background for 10 generations.
NGAL expression, isolation and purification
NGAL expressing E. coli BL21(DE3) (23) was provided as a generous gift by Prof. Kiyoshi Takeda, Osaka University. Plasmid was purified and the correct sequence for mouse LCN2 was confirmed. E. coli was grown in LB broth containing ampicillin, and purified using a Glutathione-Sepharose-4B column (GE Life Sciences, Piscataway, NJ). The GST tag was cleaved by incubating the fusion protein with PreScission protease (GE Life Sciences), and removed by centrifugation. Purified NGAL was then filter-sterilised using a 0.2 μm filter. Size and immunoreactivity of the recombinant NGAL protein were confirmed by Western blot and ELISA.
Cell culture
Primary MC from MRL/lpr mice were isolated as described previously in detail (15), and used for the in vitro experiments.
Cell proliferation assay
MC proliferation was assessed using the CellTiter 96 aqueous one solution cell proliferation assay (Promega, Madison, WI), in which the OD is directly proportional to the degree of cellular proliferation.
For proliferation in the presence of a caspase-3-inhibitor (R & D systems) (100 μM), cells were treated with or without NGAL 2 μg/ml and the proliferation was measured as described above. For proliferation in the presence of 24p3R siRNA (Qiagen), cells were transfected with 24p3R siRNA or a negative control siRNA at a concentration of 40 nM when 30–40% confluent, and again after 24 hrs. Twenty-four hours later, transfected cells were treated with NGAL and the proliferation measured after 24 hrs.
TUNEL staining
TUNEL staining was performed using the fluorescein based in-situ cell death detection kit (Roche, Indianapolis, IN) using the manufacturer’s protocol. TUNEL positive (fluorescent) cells in 10–15 randomly selected kidney sections per mouse were counted by a blinded observer, and the images captured at 20× magnification.
Statistical analysis
Results are presented as mean±standard error of the mean (SEM). Comparisons between two groups were performed using the two tailed unpaired Student’s T test. Comparisons between three or more groups were performed by one way ANOVA followed by a Tukey’s Multiple Comparison post test. Correlations were analyzed by Pearson’s correlation coefficient (r). P values lower than 0.05 were considered statistically significant.
RESULTS
NTN induces overexpression of kidney, urine, and serum NGAL
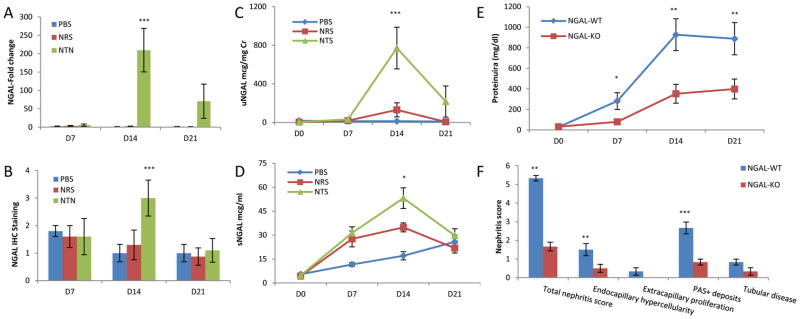
To establish whether NGAL is induced in murine nephritis mediated by pathogenic antibodies, we studied experimental anti-GBM disease in B6 mice. By real-time PCR, we found that kidney mRNA expression of NGAL was significantly upregulated at d14 in NTN injected mice when compared to baseline (p<0.001), and to the NRS (p<0.001) or PBS (p<0.001) injected groups at d14 (Figure 1A). Similar results were found by kidney immunohistochemical staining (Figure 1B), with NTN injected mice showing significant NGAL staining at d14, as compared to NRS and PBS injected mice (p<0.001 for each comparison). NGAL staining in the NTN group was attenuated by d21, and at that time point was not significantly different from the control groups (Figure 1B).
Figure 1.
NGAL deficiency is protective in kidney injury. Mice injected with nephrotoxic serum, as opposed to those who received NRS or PBS, showed significant increases in kidney NGAL mRNA expression (A), intensity of NGAL immunohistochemical staining (B), uNGAL (C), and sNGAL levels (D). In each group, urine/serum NGAL were measured in 15 mice on d0 and d7, 10 mice on d14, and 4–5 mice on d21; NGAL mRNA expression and immunohistochemistry were analyzed in 5 randomly selected mice from each group at each time point. The p values indicated refer to the comparison between the NTN and NRS groups. (E) NGAL-KO and WT mice were injected with nephrotoxic serum and proteinuria was followed to assess the degree of nephritis. Results shown are the average proteinuria levels (in mg/dl) from two independent experiments with similar results, for a total of 23 NGAL-KO and 21 WT mice. (F) Comparison of renal histopathology at d9 between NGAL-WT and KO mice with induced NTN, n=6 in each group. For E and F, the p values indicated refer to the comparison between NGAL-WT and KO mice with NTN. For all panels in this figure, *: p<0.05; **: p<0.01; ***: p<0.001.
The main distribution of NGAL staining observed in NTN challenged mice was in the tubules, in the form of a homogeneous brown coloration of the tubular epithelial cells or a droplet pattern in the apical part of the tubular epithelial cells. Some glomerular staining was present as well, but was relatively weak. As tubular disease became severe at d14, intense staining of damaged tubuli together with staining of intraluminal hyaline material became apparent (not shown).
Urine NGAL (uNGAL) levels closely tracked the temporal pattern of kidney NGAL mRNA expression. A peak of uNGAL was detected at d14 (p<0.001), with a subsequent decrease by d21 (p<0.001) (Figure 1C). Serum NGAL (sNGAL) levels in the NTN treated mice reached their peak at d14, being significantly higher than the levels at d7 (p<0.001) (Figure 1D). Although sNGAL did increase by d14 in the NRS group as well, the mean values were significantly lower than in the NTN group (p<0.05). PBS injected mice also showed a modest increment in sNGAL levels over time; nevertheless, the average values were significantly lower than those in the NTN group at d7 (p<0.001) and d14 (p<0.001). By d21, levels of sNGAL in the NTN, NRS and PBS groups were similar, but had not yet returned to baseline (Figure 1D).
NGAL overexpression correlates with the severity of renal damage
To ascertain if there is a linear relationship between the severity of renal injury and NGAL expression, correlation analyses were performed. Renal damage was closely associated with upregulation of kidney NGAL, as indicated by a tight correlation between the histopathological score and NGAL mRNA expression in kidney tissue (kNGAL) (r=0.93, p<0.0001). Moreover, uNGAL concentrations significantly correlate with kNGAL (r=0.95, p<0.0001), suggesting that the kidney itself is the main source of uNGAL in this model. Similarly, the less robust (albeit still significant) correlation between uNGAL and proteinuria (r=0.77, p<0.0001) suggests that uNGAL is not a mere consequence of increased protein filtration, but rather a reflection of its production in the kidney.
While urine and serum levels of NGAL were associated (r=0.62, p<0.0001) the correlation however is not excellent, perhaps due to different kinetics of NGAL absorption into the blood and excretion into the urine. The imperfect correlation between serum and urine NGAL together with the fact that uNGAL was more tightly associated with kNGAL than sNGAL may also imply that while sNGAL in NTN does mostly originate in the kidney, a non-kidney source may be contributing as well.
The degree of nephritis in the NTN model can be assessed functionally (proteinuria) or by analysis of histopathology. These parameters were concordant in this study (r=0.88, p<0.0001), as was the relationship between renal pathology and uNGAL (r=0.86, p<0.0001), indicating that both proteinuria and uNGAL perform similarly, and with high accuracy, as non-invasive biomarkers of structural renal disease in this model. When the different score components were analyzed separately, the main histopathological feature responsible for the association of the total nephritis score with uNGAL was tubular disease (r=0.92, p<0.0001).
Amelioration of proteinuria and kidney structural damage in NGAL deficient mice
To determine if NGAL overexpression following deposition of nephritogenic antibodies is instrumental in renal damage, we induced NTN in NGAL-KO mice and in their littermate WT controls. NGAL sufficient mice (n=21) showed a quick rise in proteinuria which starts to be detectable at d7 (2 days after NTN injection), peaks at d14, and begins to decrease by d21 (Figure 1E). However, the average proteinuria found in the NGAL-KO mice (n=23) was significantly lower than their matched WT littermates at d7 (p=0.0149), d14 (p=0.0022) and d21 (p=0.0098) (Figure 1E). There were, however, no significant differences in the titers of the mouse anti-rabbit IgG antibody response, or kidney immunoglobulin deposition, between NGAL-WT and KO mice (not shown).
To determine if NGAL deficiency would also improve renal structural damage, we analyzed kidney histology in NGAL-WT and KO mice with induced NTN. Since the peak of disease appears between d7 and d14 followed by spontaneous improvement, we induced NTN in NGAL-WT and KO mice (n=6 in each group) and sacrificed them on d9. NGAL-WT mice injected with nephrotoxic sera had significantly worse renal disease than NGAL-KO mice (a total renal histopathology score of 5.33±0.15 in NGAL-WT vs. 1.67±0.23 in NGAL-KO, p<0.006)(Figure 1F, Figure 2A–D). When the score components were analyzed separately, NGAL-WT mice had significantly increased endocapillary hypercellularity (1.5±0.33 vs. 0.5±0.21, p<0.0035) and glomerular PAS+ deposits (2.67±0.32 vs. 0.83±0.16, p<0.001) than NGAL-KO mice, while tubular disease approached significance (0.83±0.16 vs. 0.33±0.20, p=0.09)(Figure 1F, Figure 2A–D).
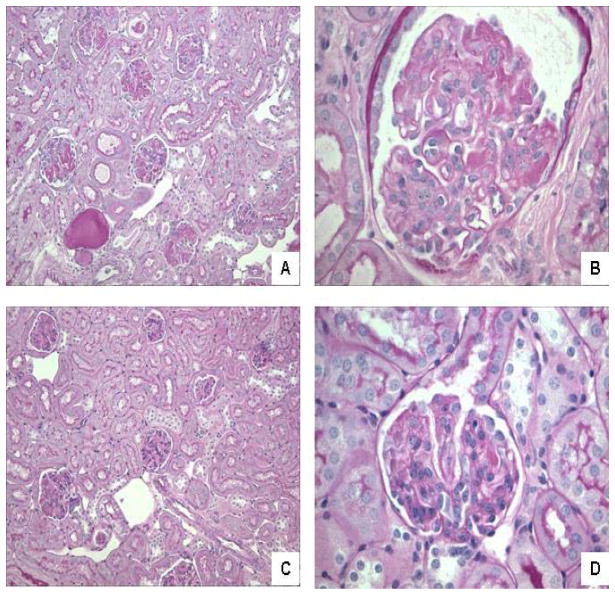
Figure 2.
NGAL-KO mice have decreased kidney damage following induction of NTN. (A) A representative kidney section from a B6 NGAL-WT mouse with NTN (PAS; 20× magnification) showing glomeruli that appear mildly enlarged with patchy, segmental to global involvement by PAS+ deposits. Also seen is a dilated tubule containing a proteinaceous cast. (B) A representative kidney section from a B6 NGAL-WT mouse with NTN (PAS; 60× magnification) showing an enlarged glomerulus with PAS+ deposition accompanied by prominent segmental endocapillary proliferation. (C) A representative kidney section from a B6 NGAL-KO mouse with NTN (PAS; 20× magnification) showing glomeruli that appear predominantly intact, with focal segmental PAS+ deposits and no evidence of tubular disease. (D) A representative kidney section from a B6 NGAL-KO mouse with NTN (PAS; 60× magnification) showing a glomerulus that is normal in size and cellularity with a small focus of a PAS+ deposit (2 o’clock).
Exogenous NGAL accelerates renal disease in NTN
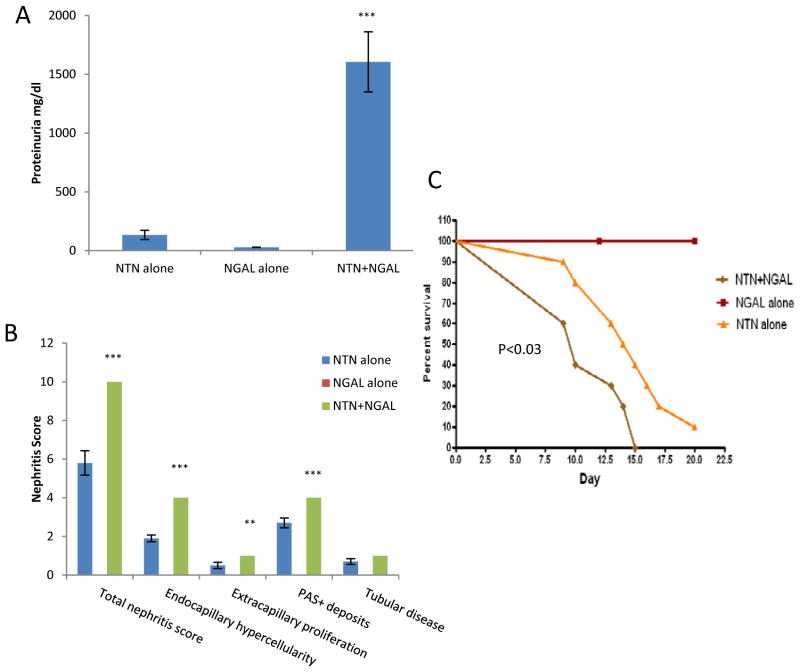
To confirm that indeed NGAL is instrumental in the pathogenesis of antibody-mediated nephritis, we induced NTN in NGAL-WT mice as above and studied the effect of a single injection of 250 μg/mouse of NGAL intravenously on d6. Since 129 mice are more susceptible to induction of NTN than B6 (24), the subsequent experiments were performed in the former strain. Within 2 days of injection, NGAL leads to a dramatic increase in proteinuria (“NTN+NGAL”), as compared to mice with induced NTN injected with PBS (“NTN alone”), or mice injected with NGAL but without prior induction of NTN (“NGAL alone”) (Figure 3A; n=10 in each group). Moreover, mice with NTN injected with NGAL had significantly higher BUN at d8 (81.2±5.5) as compared to NGAL alone (30.4±1; p<0.00001) or NTN alone (40.9±3.3; p<0.00001) treated mice. Two days after injection of nephrotoxic sera (d7), there were no significant differences between NTN+NGAL, NGAL alone, and NTN alone groups in serum titers of mouse IgG anti-rabbit antibodies (not shown).
Figure 3.
Exogenous NGAL exacerbates NTN. 129/SvJ mice received nephrotoxic serum, and in addition received a single injection of 250 μg of NGAL (“NTN+NGAL”; n=10) or PBS (“NTN alone”; n=10) intravenously on d6. An additional control group of mice was immunized with rabbit IgG as above but received an injection of PBS on d5, and an injection of 250 μg NGAL on d6 (“NGAL alone”; n=10). (A) Proteinuria on d7. (B) Mice were sacrificed on d8, and the kidney paraffin sections were stained for analysis of histopathology. Mice receiving NGAL without concurrent induction of NTN all scored 0 for each of the kidney sub-scores, and therefore these are not evident in this graph. Since all mice in the NTN+NGAL group had identical scores for each subcategory, the error bar equals 0 for this group. (C) NTN was induced in an independent cohort as above, with mice treated with NTN+NGAL (n=10), NTN alone (n=10), and NGAL alone (n=10). Starting from d5, survival was monitored daily. Mice appearing moribund were sacrificed without knowledge of the treatment assignment. The p values indicated refer to the comparison between NTN+NGAL and NTN alone groups, **:p<0.01; ***: p<0.001.
Next, we compared renal histopathology between the NTN+NGAL, NGAL alone, and NTN alone groups. We found that NGAL significantly worsened kidney damage in mice with NTN, with a total nephritis score of 10±0 in the NTN+NGAL group vs. 5.8±0.65 in mice with NTN that were not treated with exogenous NGAL (Figure 3B, n=10 in each group, d8, p<0.001). Mice receiving NGAL together with nephrotoxic sera uniformly had the worst possible degree of endocapillary hypercellularity and glomerular PAS+ deposits, with some crescent formation and tubular disease (Figures 3B, 4E–F). In contrast, renal histopathology was less severe in mice with NTN who did not receive NGAL (Figures 3B, 4C–D). The differences in scores between the NTN+NGAL vs. NTN alone were highly significant (p≤0.008) for endocapillary hypercellularity, extracapillary proliferation, and PAS+ deposits, while the difference in tubular disease was of borderline significance (p=0.065) (Figure 3B). No renal abnormalities were observed in mice who received NGAL but without prior induction of NTN (Figure 3B, Figure 4A–B).
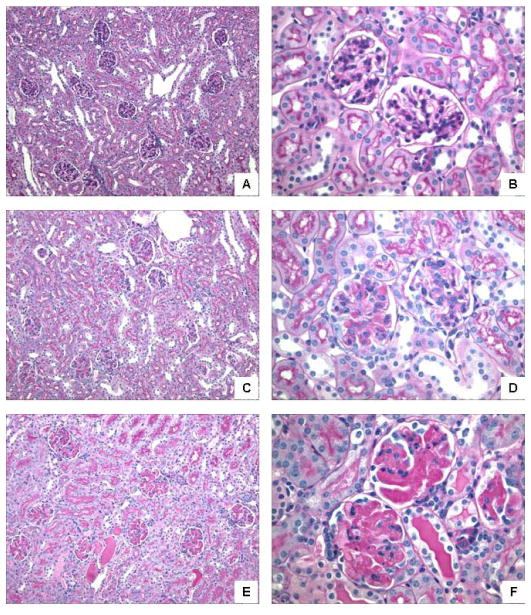
Figure 4.
NGAL worsens kidney injury in NTN. 129/SvJ mice received nephrotoxic serum, and in addition received 250 μg of NGAL (“NTN+NGAL”; n=10) or PBS (“NTN alone”; n=10) intravenously on d6. An additional control group of mice was immunized with rabbit IgG as above but received injections of PBS on d5, and 250 μg NGAL on d6 (“NGAL alone”; n=10). (A) Kidney from a NGAL alone mouse (PAS; 20×) showing preserved glomeruli and tubules. (B) Kidney from a NGAL alone mouse (PAS; 60×) showing 2 normocellular glomeruli without PAS+ deposits. (C) Kidney from a NTN alone mouse (PAS; 20×) showing mildly enlarged glomeruli with patchy segmental PAS+ deposits. (D) Kidney from a NTN alone mouse (PAS; 60×) showing 2 glomeruli, one of which shows moderate PAS+ deposition and endocapillary proliferation. (E) Kidney from a NTN+NGAL mouse (PAS; 20×) showing enlarged glomeruli with prominent PAS+ deposits and patchy dilatation of tubules with proteinaceous casts. (F) Kidney from a NTN+NGAL mouse (PAS; 60×) showing 3 glomeruli globally expanded by abundant PAS+ deposits, accompanied by moderate segmental endocapillary proliferation. Also seen are mildly dilated distal tubules with proteinaceous casts. Selected kidney sections are representative of the histology in each group.
Finally, we examined whether the more severe renal disease observed in mice with NTN receiving NGAL would also translate into accelerated mortality. In this experiment, cohorts of NTN+NGAL, NGAL alone, and NTN alone (n=10 mice in each group) were monitored for survival. As seen in Figure 3C, there was no mortality in the NGAL alone group. However, mice in the NTN+NGAL group had significantly accelerated mortality, with 60% of the mice dead by d10 as compared to d15 in the NTN group. Moreover, 100% of the mice in the NTN+NGAL group were dead by d15, at which time 40% of the mice in the NTN group were still alive (p<0.03) (Figure 3C).
NGAL induces apoptosis of resident kidney cells via 24p3R
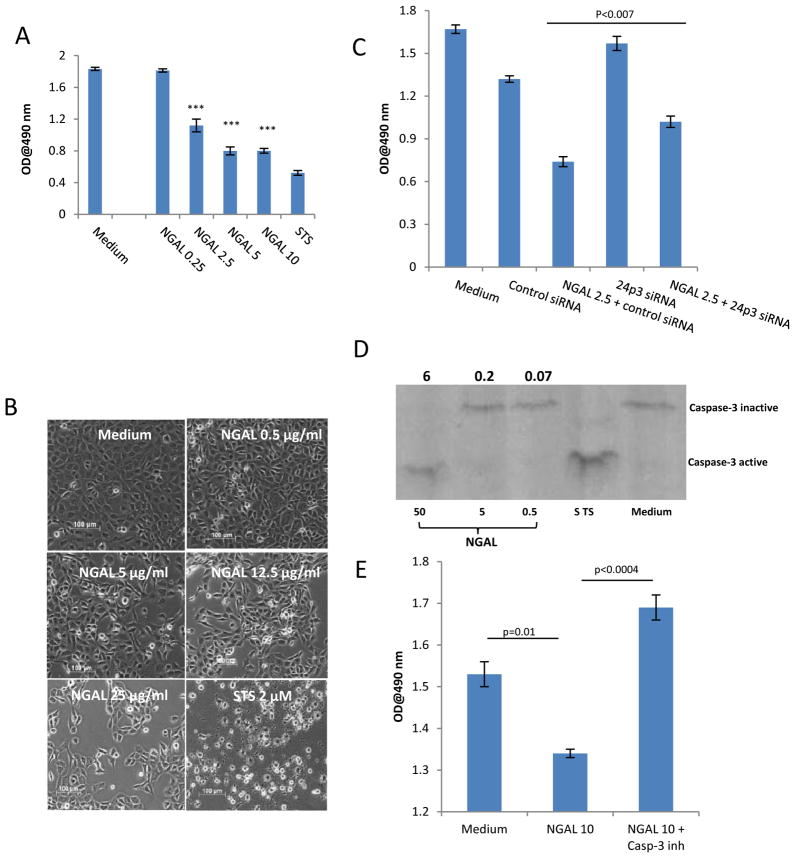
To understand the detrimental effect of NGAL in immune-mediated nephritis, we studied the effect of NGAL on survival of resident kidney cells. We found that NGAL led to a dose-dependent decrease in proliferation of primary MRL/lpr MC (Figure 5A). This effect was confirmed by direct visualization, with NGAL at a concentration of 0.5, 5, and 25 μg/ml decreasing the actual cell count by 8, 46, and 61%, respectively (Figure 5B). NGAL had a similar anti-proliferative effect in murine glomerular endothelial and tubular cell lines, and human HK-2 cells (not shown). LPS in a wide dose range (0.1–200 μg/ml) did not show any significant effect on MC proliferation (not shown), confirming that the observed effects were due to NGAL alone, rather than a bacterial contaminant. Flow cytometry using Annexin-V and 7-AAD staining indicated that NGAL is actually promoting apoptotic cell death, with MC treated with NGAL for 24 hours demonstrating significantly increased numbers of cells undergoing early and late apoptosis (Supplemental Figure 1). Finally, induction of apoptosis in MC by NGAL was confirmed by an ELISA-based cellular DNA fragmentation assay (Supplemental Figure 2).
Figure 5.
NGAL inhibits proliferation and induces apoptosis of mesangial cells. (A) MRL/lpr MC were cultured with increasing concentrations of NGAL (in μg/ml) for 48 hours, and the proliferation was assessed by the MTS assay. The p values indicated refer to the comparison between NGAL and media alone treated cells, ***: p<0.001. STS, staurosporine (4 μM). (B) Following 24 hours of serum starvation, mesangial cells were stimulated with different doses of NGAL, STS 2 μM, or medium for 24 hrs (20× magnification). (C) Mesangial cells transfected with 24p3R ("24p3 siRNA") or control siRNA were treated with NGAL at 2.5 μg/ml for 48 hours, and the proliferation measured as above. (D) Lysates from MC treated with NGAL, STS (4 μM), or medium alone for 24 hours were stained with antibodies against total and active caspase 3 by Western blot. The number above each of the NGAL treated lanes indicates the band density ratio of activated versus total caspase 3. (E) MC were treated with 10 μg/ml of NGAL for 24 hours, with or without previous treatment with a specific inhibitor of caspase 3, and the proliferation measured as above. Data in this Figure is representative of 2–3 independent experiments.
By cell surface ELISA and confocal microscopy, we found that 24p3R, the membrane-associated receptor for NGAL believed to be important in transducing its pro-apoptotic effects (25–26), was expressed on the surface of MC (not shown). To demonstrate that the effects of NGAL on MC are mediated via this receptor, MC were exposed to NGAL following pre-treatment with 24p3R siRNA (which decreased receptor expression by 30–50%; not shown). MC transfected with the 24p3R siRNA as compared to control siRNA and treated with NGAL had significantly less inhibition of proliferation (p<0.007) (Figure 5C).
NGAL induces apoptosis of erythroid progenitor cells through induction of caspase 3/7, thus playing an important role in the regulation of erythropoesis (27). To study if NGAL induced apoptosis in MC is mediated by caspase 3, lysates from NGAL treated cells were stained with antibodies to total and activated (cleaved) caspase 3. Resting cells, or those treated with low doses of NGAL, did not show evidence for caspase 3 cleavage. However, with a higher NGAL dose, activated caspase 3 was generated (together with a decrease in total caspase 3 levels) (Figure 5D), while pretreatment with a specific inhibitor of caspase 3 blocked the effects of NGAL on cell numbers (Figure 5E).
NGAL upregulates mediators of inflammation and apoptosis
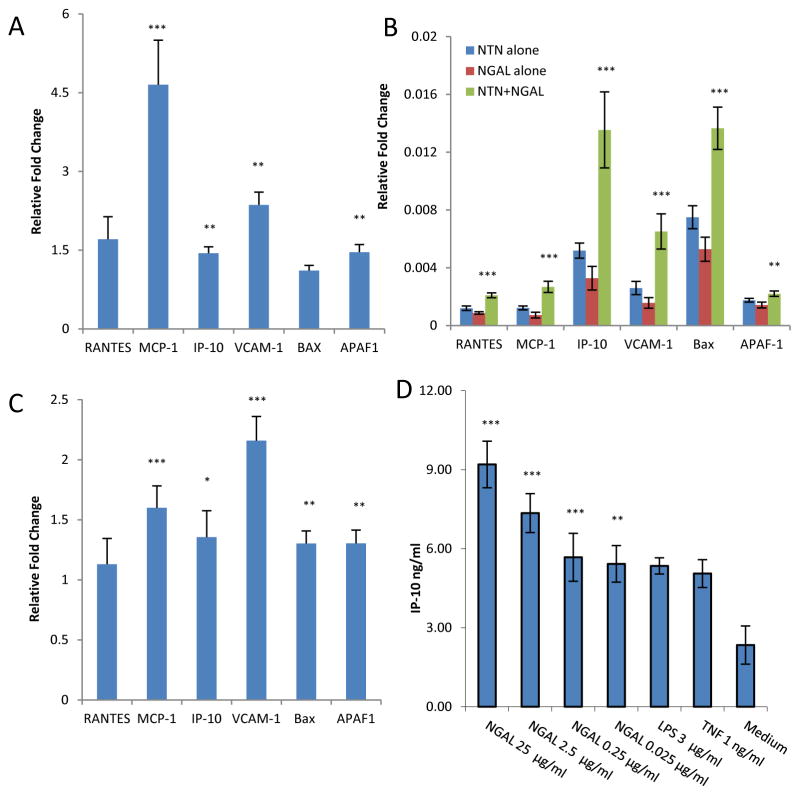
To establish whether NGAL also promotes kidney damage in NTN via enhancement of local inflammation, we studied the effects of NGAL on gene expression in kidney in vivo and in MC in vitro. We injected NGAL (250 μg) or PBS intraperitoneally to groups of 4 B6 mice for 3 days, and sacrificed the mice 12 hours after the last injection. We found that NGAL led to renal activation of the NF-κB signaling pathway (not shown), with upregulation of proinflammatory mediators including MCP-1, IP-10, and VCAM-1 (Figure 6A). These effects of NGAL on kidney gene expression were physiologically significant, as injection of NGAL to B6 mice significantly increased serum MCP-1 levels (not shown). Additional support for a direct stimulatory effect of NGAL on MCP-1, IP-10, and VCAM-1 expression by renal cells in vivo was found in analyzing gene expression in response to exogenous NGAL following induction of NTN (Figure 6B), and when comparing NGAL-WT and KO mice (Figure 6C).
Figure 6.
NGAL promotes inflammation and apoptosis. (A) B6 mice received 250 μg NGAL intraperitoneally for 3d (“NGAL”; n=4) or PBS (“PBS”; n=4). Kidneys were obtained 12 hours after the last injection for real-time PCR. The y-axis and the p values refer to the fold change in gene expression in NGAL versus PBS treated mice. (B) NTN was induced as described in Figure 3 (n=10 in each group), and real-time PCR performed on d8 kidney mRNA. The p values indicated reflect the comparison between the NTN+NGAL and NTN alone groups. (C) NTN was induced in NGAL-WT (n=6) and KO (n=6) mice, and real-time PCR for the indicated genes performed on d9 kidney mRNA. The y-axis and the p values refer to the fold change in gene expression in NGAL-WT versus KO mice. (D) MC were cultured in medium alone, medium with added TNF or NGAL for 24 hours, and IP-10 concentrations measured in supernatants by ELISA. The p values indicated refer to the comparison between NGAL treated cells at each concentration and cells grown in media alone. For all panels in this figure, *: p<0.05; **: p<0.01; ***: p<0.001. Data in this Figure is representative of 2–3 independent experiments.
We could demonstrate a direct proinflammatory effect of NGAL in kidney cells at the protein level as well, where treatment of MC for 48 hours with recombinant NGAL led to a dose dependent increase in IP-10 secretion (Figure 6D). Moreover, NGAL treatment activated NF-κB signaling intracellularly (Supplemental Figure 3), while an inhibitor of NF-κB signaling blocked cytokine secretion in response to NGAL stimulation (not shown). Preincubation with polymyxin-B did not inhibit the proinflammatory effects of NGAL on MC. Furthermore, in MC, cytokine secretion (not shown) and the induction of apoptosis (Supplemental Figure 2) in response to NGAL were inhibited with an anti-NGAL mAb. In addition, we found that a similarly prepared control protein, glutathione-S-transferase, did not display the same effect as NGAL in vitro (not shown). Taken together, these latter experiments indicate a specific effect of NGAL on renal MC, rather than any spurious effect induced by endotoxin and/or other possible contaminants in the recombinant protein preparation.
Similarly, we examined the effect of systemic administration of NGAL on induction of apoptosis-related genes. We found that following 3 days of NGAL injection to B6 mice, significant upregulation of APAF-1 kidney mRNA was present (Figure 6A). Significantly increased Bax and APAF-1 mRNA expression was found in kidneys of NTN mice injected with NGAL as compared to mice with NTN alone (Figure 6B), and in NGAL-WT mice with NTN as compared to NGAL-KO mice (Figure 6C). Finally, to confirm NGAL’s apoptotic effects in vivo, we quantitated the actual number of kidney cells undergoing apoptosis following NGAL injection. Injection of 250 μg of NGAL per day for 3 days to B6 mice resulted in a significant increase in the number of glomerular and tubular cells undergoing apoptosis (8.4±1 TUNEL positive cells/field in NGAL injected mice as compared to 4.6±0.5 in control injected mice, p<0.003). Similarly, an increased number of apoptotic glomerular cells following induction of NTN was found in NGAL-WT versus KO mice (not shown).
DISCUSSION
In this study we demonstrated that NGAL plays a central role in the pathogenesis of glomerulonephritis mediated by pathogenic antibodies. Mice treated with nephrotoxic antibodies demonstrated high levels of kidney, urine, and serum NGAL. NGAL expression was clearly detrimental, as NGAL-KO mice had significantly less kidney dysfunction and structural damage following injection of nephrotoxic sera. While it is important to consider whether NGAL-KO mice had less severe nephritis due to an attenuated immune response, no significant differences in serum anti-rabbit IgG or glomerular Ig deposition were found as compared to WT mice. Moreover, NGAL-WT mice with NTN treated with exogenous NGAL had accelerated renal disease and decreased survival, supporting a direct effect of NGAL on renal tissue. NGAL can facilitate renal damage induced by antibody deposition by promoting the release of IP-10, VCAM-1 and MCP-1, and enhancing apoptosis of resident kidney cells.
Our results demonstrating a tight correlation between uNGAL levels and the severity of renal disease are in line with many other studies validating the use of uNGAL as a biomarker of kidney damage (5,9–11). Moreover, our previous observations indicate that increasing NGAL levels can predict an impending LN flare months before this would be diagnosed clinically (16–18). While in the current study we found that increases in NGAL expression appeared concurrently with renal damage, measuring changes in NGAL expression more frequently may have demonstrated that a rise in NGAL precedes functional and/or structural lesions. At least following a defined ischemic insult in a NGAL reporter mouse described recently, an increase in NGAL could be found already after 3–6 hours (28). It is also possible that we would have seen more accelerated NGAL kinetics in a mouse strain more sensitive to NTN. Alternatively, this may be an instance where NTN diverges from LN, where NGAL increases heralds other markers of nephritis.
In investigating possible mechanisms by which NGAL may be contributing to kidney injury in NTN, we determined that NGAL promotes apoptosis of resident kidney cells in vitro and in vivo. Moreover, NGAL deficient mice showed less upregulation of apoptosis related genes following induction of NTN. Several studies have previously demonstrated that apoptosis is detrimental in NTN (29–30). While we have yet to conclusively show that the pro-apoptotic effects of NGAL are directly responsible for more severe nephritis, these and other published studies do suggest that excessive cell death contributes to disease severity in this model (31).
Chemokine-mediated recruitment of macrophages and T cells is instrumental in the pathogenesis of NTN. Kelley et al has shown that T cells and macrophages expressing CXCR3, the receptor for IP-10, are increased with NTN. Indeed, CXCR3-KO mice had improved renal function and decreased renal pathology following NTN induction (32). Therefore, we believe that the decreased intrarenal chemokine expression in NGAL deficient mice shown here may inhibit inflammatory cell recruitment, and thus prevent subsequent amplification of renal injury. Indeed, treatment of anti-endothielial cell antibody-induced nephritis with a neutralizing antibody to IP-10 reduced the number of kidney-infiltrating T cells and improved renal function (33). Finally, although we focused our mechanistic studies on the apoptotic and inflammatory effects of NGAL on resident kidney cells, it remains to be determined whether any possible effects of NGAL on infiltrating cells, or immune cell interactions, may also be relevant to NGAL’s detrimental effects. While outside the scope of the current investigation, we plan to continue to explore these, and other possible contributions of NGAL to the pathogenesis of nephritis, in future studies.
A recent study provides support for an important role for NGAL in progression of chronic kidney disease (34). Viau et al showed that NGAL is essential for the development of renal insufficiency in FVB/N mice following 75% nephron reduction. Ineffective iron clearance did not explain the progressive renal dysfunction, while iron chelation surprisingly exacerbated renal disease. In line with our finding of a role for NGAL in apoptosis, following nephron reduction NGAL-KO mice showed significantly fewer apoptotic glomerular and tubular cells. In this model, activation of the EGF receptor increased NGAL expression, an effect mediated via HIF1α. It will be interesting to see whether EGFR and HIF1α are similarly involved in antibody-mediated nephritis.
It is instructive to compare our results in NTN with the effects of NGAL in ischemia-reperfusion, where prophylactic administration of NGAL decreased azotemia and improved tubular pathology (7). However, NGAL administration 1 hour after reperfusion was much less effective. Moreover, NGAL-KO mice did not have more severe disease following a similar ischemia-perfusion injury (22), perhaps since endogenous NGAL did not increase early enough post injury to exert a protective effect. Nevertheless, the discrepancy between the reported protective effect of exogenous NGAL in ischemia-reperfusion and our findings in NTN where NGAL was detrimental deserves comment. In the ischemia-reperfusion model, the protective effect of NGAL is dependent on delivery of a NGAL:siderophore:iron complex to the proximal tubules, while non-iron loaded NGAL was much less protective. Interestingly, the NGAL found protective in the ischemia-reperfusion study was cloned in XL1-Blue cells, and contains bacterial enterochelin (a iron chelating siderophore). In contrast, the BL21 derived NGAL which we used does not contain enterochelin and is not iron loaded. Therefore, it is possible that the differential effect of NGAL in NTN and ischemia-reperfusion is iron dependent. But even if iron-loaded NGAL would be detrimental as well (as suggested by our studies with NGAL-KO mice), it is reasonable to postulate that NGAL may very well have a differential role in various types of renal injury (e.g. glomerular vs. tubular). However, before reaching such a conclusion in future studies it will be crucial to exclude a possible contribution of differences in NGAL preparations used in the various models.
In conclusion, renal deposition of pathogenic antibodies directed against glomerular antigens lead to upregulation of kidney NGAL expression, which then exacerbates the resulting damage. Mechanisms by which NGAL contributes to disease include enhancing apoptosis of kidney resident cells and promoting the secretion of inflammatory mediators, although other pathways may be operative as well. While in ischemia-reperfusion a beneficial effect of NGAL was dependent on iron delivery, this may not be true in NTN where both endogenous and exogenous NGAL increased disease severity. The direct contribution of NGAL to renal injury in antibody-mediated nephritis lends further strong support to the continued development of NGAL as a valuable biomarker for LN and related conditions. Moreover, our study raises the interesting possibility that blocking NGAL may be a novel approach to the treatment of nephritis mediated by pathogenic antibodies.
Supplementary Material
Acknowledgments
Funding: This work was supported by a National Institutes of Health Grant, RO1 AR048692 (to C.P.), and a Postdoctoral Fellowship Award from the Arthritis Foundation (to R.D.P.).
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Fu Y, Du Y, Mohan C. Experimental anti-GBM disease as a tool for studying spontaneous lupus nephritis. Clin Immunol. 2007;124:109–18. doi: 10.1016/j.clim.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45:17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414–20. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinstein T, Pitashny M, Putterman C. The novel role of neutrophil gelatinase-B associated lipocalin (NGAL)/Lipocalin-2 as a biomarker for lupus nephritis. Autoimmun Rev. 2008;7:229–34. doi: 10.1016/j.autrev.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Mori K, Li JY, Barasch J. Iron, lipocalin, and kidney epithelia. Am J Physiol Renal Physiol. 2003;285:F9–18. doi: 10.1152/ajprenal.00008.2003. [DOI] [PubMed] [Google Scholar]
- 7.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–21. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307–15. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 9.Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21:856–63. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- 10.Trachtman H, Christen E, Cnaan A, Patrick J, Mai V, Mishra J, et al. Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediatr Nephrol. 2006;21:989–94. doi: 10.1007/s00467-006-0146-y. [DOI] [PubMed] [Google Scholar]
- 11.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S. Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am J Nephrol. 2006;26:287–92. doi: 10.1159/000093961. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH, 3rd, Ma Q, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22:2089–95. doi: 10.1007/s00467-007-0601-4. [DOI] [PubMed] [Google Scholar]
- 13.Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT. Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis. 2008;52:425–33. doi: 10.1053/j.ajkd.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Di Grande A, Giuffrida C, Carpinteri G, Narbone G, Pirrone G, Di Mauro A, et al. Neutrophil gelatinase-associated lipocalin: a novel biomarker for the early diagnosis of acute kidney injury in the emergency department. Eur Rev Med Pharmacol Sci. 2009;13:197–200. [PubMed] [Google Scholar]
- 15.Qing X, Zavadil J, Crosby MB, Hogarth MP, Hahn BH, Mohan C, et al. Nephritogenic anti-DNA antibodies regulate gene expression in MRL/lpr mouse glomerular mesangial cells. Arthritis Rheum. 2006;54:2198–210. doi: 10.1002/art.21934. [DOI] [PubMed] [Google Scholar]
- 16.Pitashny M, Schwartz N, Qing X, Hojaili B, Aranow C, Mackay M, et al. Urinary lipocalin-2 is associated with renal disease activity in human lupus nephritis. Arthritis Rheum. 2007;56:1894–903. doi: 10.1002/art.22594. [DOI] [PubMed] [Google Scholar]
- 17.Rubinstein T, Pitashny M, Levine B, Schwartz N, Schwartzman J, Weinstein E, et al. Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. Rheumatology. 2010;49:960–71. doi: 10.1093/rheumatology/kep468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunner HI, Mueller M, Rutherford C, Passo MH, Witte D, Grom A, et al. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2006;54:2577–84. doi: 10.1002/art.22008. [DOI] [PubMed] [Google Scholar]
- 19.Hinze CH, Suzuki M, Klein-Gitelman M, Passo MH, Olson J, Singer NG, et al. Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity. Arthritis Rheum. 2009;60:2772–81. doi: 10.1002/art.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki Y, Shirato I, Okumura K, Ravetch JV, Takai T, Tomino Y, et al. Distinct contribution of Fc receptors and angiotensin II-dependent pathways in anti-GBM glomerulonephritis. Kidney Int. 1998;54:1166–74. doi: 10.1046/j.1523-1755.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 21.Corna D, Morigi M, Facchinetti D, Bertani T, Zoja C, Remuzzi G. Mycophenolate mofetil limits renal damage and prolongs life in murine lupus autoimmune disease. Kidney Int. 1997;51:1583–9. doi: 10.1038/ki.1997.217. [DOI] [PubMed] [Google Scholar]
- 22.Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2006;103:1834–9. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saiga H, Nishimura J, Kuwata H, Okuyama M, Matsumoto S, Sato S, et al. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J Immunol. 2008;181:8521–7. doi: 10.4049/jimmunol.181.12.8521. [DOI] [PubMed] [Google Scholar]
- 24.Xie C, Sharma R, Wang H, Zhou XJ, Mohan C. Strain distribution pattern of susceptibility to immune-mediated nephritis. J Immunol. 2004;172:5047–55. doi: 10.4049/jimmunol.172.8.5047. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–13. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 26.Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 27.Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Lipocalin 2 functions as a negative regulator of red blood cell production in an autocrine fashion. FASEB J. 2005;19:1881–3. doi: 10.1096/fj.05-3809fje. [DOI] [PubMed] [Google Scholar]
- 28.Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17:216–22. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tesch GH, Schwarting A, Kinoshita K, Lan HY, Rollins BJ, Kelley VR. Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J Clin Invest. 1999;103:73–80. doi: 10.1172/JCI4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinozaki M, Hirahashi J, Lebedeva T, Liew FY, Salant DJ, Maron R, et al. IL-15, a survival factor for kidney epithelial cells, counteracts apoptosis and inflammation during nephritis. J Clin Invest. 2002;109:951–60. doi: 10.1172/JCI14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niranjan T, Murea M, Susztak K. The pathogenic role of Notch activation in podocytes. Nephron Exp Nephrol. 2009;111:e73–9. doi: 10.1159/000209207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menke J, Zeller GC, Kikawada E, Means TK, Huang XR, Lan HY, et al. CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. J Am Soc Nephrol. 2008;19:1177–89. doi: 10.1681/ASN.2007111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panzer U, Steinmetz OM, Reinking RR, Meyer TN, Fehr S, Schneider A, et al. Compartment-specific expression and function of the chemokine IP-10/CXCL10 in a model of renal endothelial microvascular injury. J Am Soc Nephrol. 2006;17:454–64. doi: 10.1681/ASN.2005040364. [DOI] [PubMed] [Google Scholar]
- 34.Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120:4065–76. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.