Abstract
The amyloidoses are a group of hereditary or acquired disorders caused by the extracellular deposition of insoluble protein fibrils that impair tissue structure and function. All amyloidoses result from protein misfolding, a common mechanism for disorders in older persons including Alzheimer's disease and Parkinson's disease. Cardiac amyloidoses in the elderly are most often caused by abnormalities in the protein transthyretin (TTR), a serum transporter of thyroxine and retinol. Mutations in TTR can result in familial amyloidotic cardiomyopathy, and wild-type TTR can result in senile cardiac amyloidosis. These underdiagnosed disorders are much more common than previously thought. The resulting restrictive cardiomyopathy can cause congestive heart failure, arrhythmias, and advanced conduction system disease. Although historically difficult to make, the diagnosis of TTR cardiac amyloidosis has become easier in recent years with advances in cardiac imaging and more widespread use of genetic analysis. While therapy to this point has largely involved supportive medical care, avoidance of potentially toxic agents, and rarely organ transplantation, the near future brings the possibility of targeted pharmacotherapies designed to prevent TTR misfolding and amyloid deposition. As these disease modifying agents are designed to prevent disease progression, it has become increasingly important that older persons with TTR amyloidosis be expeditiously identified and considered for enrollment in clinical registries and trials.
Keywords: amyloidosis, transthyretin, cardiomyopathy, heart failure, aging
A 75 year-old African-American man presents to your office with congestive heart failure. Over the past 18 months, he has noted increased fatigue, early satiety, and worsening lower extremity swelling. Although the patient has a history of hypertension, he has recently required much less pharmacotherapy due to symptomatic hypotension. He has no other medical problems. The patient lives independently and tries to restrict sodium intake though is often unsuccessful. Your examination is notable for a blood pressure of 98/70 mm Hg, regular heart rate of 90 beats per minute, marked jugular venous distention, bibasilar lung crackles, pulsatile liver three finger breaths below the costal margin and 16 cm in span, and 2+ lower extremity edema.
The patient is symptomatically managed with diuretics. Initial diagnostic testing includes a twelve-lead electrocardiogram (ECG) and standard 2-dimensional transthoracic echocardiogram (2D TTE). The ECG demonstrates low limb-lead voltage and precordial Q waves, and the 2D TTE shows significant thickening of the right ventricular free wall, interventricular septum, and left ventricular posterior wall. As cardiac amyloidosis could produce this presentation, genetic sequencing is performed of the transthyretin gene. A mutation is found whereby isoleucine is substituted for valine in codon 122 (Val122Ile), and the diagnosis is made of cardiac transthyretin amyloidosis. The patient enters a clinical trial testing a transthyretin stabilizer to prevent further amyloid formation.
BACKGROUND
The word amyloid was fashioned in 1834 by the German botanist Matthias Schleiden to describe the waxy starch in plants.1 Today, the amyloidoses refer to a large group of hereditary or acquired disorders caused by the extracellular deposition of insoluble protein fibrils that impair tissue structure and function. These deposits are easily seen with light microscopy using hematoxylin and eosin, sulfated Alcian blue, or Congo red staining.2 At this time, over 20 precursor proteins are known to form amyloid fibrils in vivo.3 The resulting disorder is classified according to the protein composition of the fibrils and the clinical features of the disease.4 Although almost every amyloidogenic protein may involve the heart, predilection for myocardium varies considerably, as some very uncommonly involve this organ and others do so almost exclusively. When cardiac involvement is present, resulting myocardial dysfunction is a frequent cause of disability, hospitalization, and death.5, 6, 7
From a pathobiological perspective, all amyloidoses result from abnormal protein folding and metabolism.8 Normally, following biosynthesis, the majority of proteins must be converted into tightly folded three-dimensional structures to function appropriately. Proper folding is determined in part by the protein's primary structure, the order of amino acids that contribute to a unique polypeptide chain. It is also determined by auxiliary proteins within the cell including folding catalysts and molecular chaperones such as heat shock proteins. Catalysts accelerate steps in the folding process that would otherwise occur extremely slowly, and chaperones bind vulnerable nascent polypeptides to prevent their inappropriate binding with other intracellular molecules.9 When folding occurs unsuccessfully despite these supportive measures, the nascent polypeptide is intentionally degraded by intracellular proteolysis. In the great majority of cases, proteins easily acquire their intended three-dimensional structure, which is the most energetically favorable conformation.8
However, this process of normal protein folding and conformational maintenance is subverted in the amyloidoses. Initial misfolding may result from genetic mutations that alter the primary structure of a polypeptide or the function of important auxiliary proteins, as well as from multiple potential epigenetic factors.10 Insufficient quality-control mechanisms in both intracellular and extracellular environments are unable to properly refold the misfolded protein, shield it to prevent aggregation, or target it for degradation.10 These misfolded proteins ultimately aggregate and organize into thread-like amyloid fibrils that deposit in tissues and become highly resistant to degradation. These fibrils, as well as their pre-fibrillar precursors, can provoke oxidative stress, cellular dysfunction, and apoptosis in cardiac myocytes.11, 12
This mechanism of protein misfolding with subsequent amyloid formation is a common disease pathway that injures post-mitotic cells in older persons. For example, accumulation of amyloid proteins is thought to contribute to common neurodegenerative diseases associated with aging including Alzheimer's disease and Parkinson's disease.8, 10 In Alzheimer's disease, cleavage of the amyloid precursor protein by gamma secretases results in multiple polypeptide fragments, once of which is named Aβ. Both fibrillar and pre-fibrillar aggregates of the Aβ protein have been implicated in disease pathogenesis.13 Similarly, in Parkinson's disease, significant alpha synuclein deposition within the dopaminergic neurons of the substantia nigra alongside dysfunction of the ubiquitin-proteasome system has been linked with neurotoxicity.10 The mechanisms driving the increased prevalence of amyloidoses in older persons are unclear, but may relate to microenvironmental changes associated with aging including alterations in pH and protein concentration, increased oxidative stress, proteasome dysfunction, and impairments in mechanisms that assist in extracellular aggregate clearance such as immune system pathways.9, 14, 15 As with neurons, cardiac myocytes are post-mitotic and may be particularly susceptible to the above age-related alterations.
The systemic amyloidoses that impact cardiac function are listed in Table 1. The subtypes shown to have clinically meaningful impact in predominantly older persons are the familial amyloidoses, most often due to mutations in the transthyretin protein, and senile cardiac amyloidosis, due to deposition of wild type (nonmutated) transthyretin. Cardiac AL amyloidosis (previously called primary amyloidosis), in contrast, while clearly associated with severe cardiac dysfunction and poor clinical prognosis, is not unique to older persons and can result from any plasma cell dyscrasia at any age.2, 5 Other forms of systemic amyloidoses such as AA amyloidosis (previously called secondary amyloidosis) resulting from deposition of proteolytic fragments of serum amyloid A and hemodialysis-related amyloidosis due to β2-microglobulin deposition only rarely affect the heart.2
Table 1.
Systemic Amyloidoses Impacting Cardiac Function
| Familial Amyloidoses | Senile Cardiac Amyloidosis | Light chain Amyloidosis | |
|---|---|---|---|
| Nomenclature4 | ATTRmt | ATTRwt SCA, SSA | AL |
| Precursor Protein | Variant transthyretin most commonly (100+ known mutations)3 | Wild-type transthyretin2 | Monoclonal lambda or kappa immunoglobulin light chain1 |
| Very rarely mutations in apolipoprotein A-1, fibrinogen, and gelsolin2,5 | |||
| Epidemiology | ~3–3.9% of African-Americans in US with Val122Ile mutation (up to 150,00 older African Americans in US carry mutation)16, 17 | ~25% of Finnish study population >85 yrs old23 | 2000 – 2500 people per year in US5 |
| ~ 30% of Minnesota study population with CHF and preserved ejection fraction >75 yrs old24 | |||
| Uncertain population prevalence of other mutations | > 90% men19 | ||
| Cardiac Manifestations | Variable by specific mutation. CHF, atrial and ventricular arrhythmia, bradycardia, advanced heart block with Val122Ile mutation3, 28, 31 | CHF, atrial and ventricular arrhythmia, bradycardia, advanced heart block2, 5, 19, 24 | CHF, atrial and ventricular arrhythmia, bradycardia, advanced heart block2, 5 |
| Extra-cardiac Manifestations | Variable by specific mutation. Can Include polyneuropathy, autonomic neuropathy, ophthalmologic abnormalities3 | Carpal tunnel syndrome21 | Renal failure, proteinuria, polyneuropathy, autonomic neuropathy, GI symptoms1, 2, 5 |
| Definitive diagnosis | Genomic mutational analysis, endomyocardial biopsy18, 45 | Endomyocardial biopsy45 | Serum free light chain assay, urine protein electrophoresis, endomyocardial biopsy, fat pad biopsy1, 2, 5 |
| Treatment | Supportive care including avoidance of potential toxic therapies, orthotopic heart transplant, combined heart/liver transplant in younger adults, investigational therapy46, 48–52, 54–60 | Supportive care including avoidance of potential toxic therapies, orthotopic heart transplant, investigational therapy46, 48–54, 56–60 | Supportive care including avoidance of potential toxic therapies, treatment of underlying malignancy, combined heart/bone marrow transplant in younger adults2, 5, 46, 48–52, 54 |
Transthyretin Cardiac Amyloidoses in Older North Americans
Pathobiology and Epidemiology
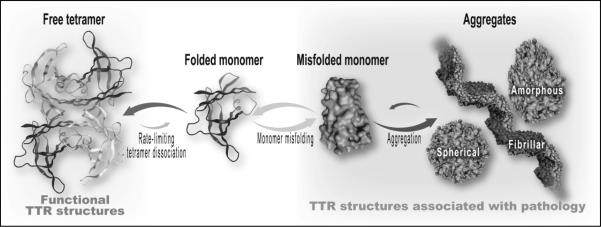
Transthyretin (TTR) is a 127 amino-acid transport protein primarily synthesized in the liver that functions as a tertiary carrier of both thyroxine and the retinol-retinol binding complex.3 TTR was formerly called prealbumin since it migrates closer to the anode than does albumin on serum protein electrophoresis. Due to its short half-life in plasma, TTR has been used as a nutritional marker in some settings when combined with clinical, anthropometric, and other laboratory measurements. It is important to note, however, that despite its name and use in nutritional assessment, TTR is not a precursor to albumin.3 TTR is an unrelated protein that circulates in its native state as a tetrameric complex composed of four single chain TTR monomers. Cardiac transthyretin amyloidosis occurs when the TTR protein misfolds, dissociates from its tetrameric form into four structurally abnormal monomers, oligomerizes (aggregates), and ultimately deposits in cardiac tissue as insoluble amyloid fibrils (Figure 1).
Figure 1.
Prevailing theory of pathogenesis of transthyretin amyloidosis. The tetrameric transthyretin molecule dissociates into four monomers (only one shown for simplicity). The monomer misfolds, aggregates with like molecules, and ultimately organizes into thread-like amyloid fibrils that are resistant to degradation and deposit in tissues. Image courtesy of Jeffery W. Kelly, PhD.
There are two distinct forms of cardiac TTR amyloidosis. The first occurs due to a mutation in the TTR gene. To date, over 100 TTR mutations have been identified, the great majority of which are amyloidogenic. Most persons with TTR amyloidoses are heterozygous carriers of mutations in TTR and express both normal and variant TTR to differing degrees.3, 5 In these individuals, deposition of large amounts of fibrillar TTR as amyloid can subvert the architecture of normal body tissues and consequently cause organ dysfunction. Some TTR mutations lead predominantly to non-cardiac disability including debilitating polyneuropathy, autonomic dysfunction, vitreous opacities, and ultimately, death at a young age.3 These persons are said to have familial amyloidotic polyneuropathy (FAP) and are predominantly of European or Japanese descent, though sporadic mutations occur worldwide. In North America, TTR mutation much more frequently causes cardiac disease that is inherited in an autosomal dominant fashion. This condition is referred to as a familial amyloidotic cardiomyopathy. Examples include Thr60Ala (Appalachia mutation), Ser77Tyr (FAP II), and Ile84Ser (Indiana mutation), the last two of which are relatively rare.3 The most common mutation causing cardiomyopathy is Val122Ile, whereby isoleucine is substituted for valine in codon 122. Pooled data on African-Americans from multiple epidemiologic studies indicate that approximately 3–3.9% are heterozygous carriers of the Val122Ile mutation.16, 17 Given this allele frequency, it is estimated that approximately 750,000 – 1,200,00 African-Americans in the US alone are carriers. Based on recent census data and in light of the age-dependent penetrance of this condition, it is estimated that 100,000 – 150,000 older African-Americans are affected with the disease. Homozygosity for the Val122Ile mutation is much more uncommon and is not required for cardiac amyloid deposition; heterozygosity is sufficient. Val122Ile is therefore the most common mutation associated with cardiac transthyretin amyloidosis worldwide despite its limitation to African-Americans and certain West African populations in which up to five percent of people carry the mutation.18
The second distinct pathway of cardiac TTR amyloidosis is via wild-type TTR deposition, previously called senile cardiac amyloidosis. In contrast to cases of familial TTR deposition, TTR in senile cardiac amyloidosis has a normal primary structure and has no known risk of familial inheritance.2, 5 While deposition also occurs to some degree in the aorta, brain, pancreas, liver, lung, and kidney, leading some to advocate the nomenclature senile systemic amyloidosis (SSA), the presentation of senile cardiac amyloidosis is always primarily as a cardiomyopathy.2 Wild-type TTR deposition has a clear association with both gender and aging and is almost exclusively seen in men older than 65 years of age.19, 20, 21, 22 For example, over 98% of persons with cardiomyopathy from wild-type disease in an international registry of transthyretin amyloidosis were men; mean age was 71 years.19 In a Finnish population older than 85 years, one-quarter had amyloid deposition in the heart on post-mortem examination.23 Almost 25% of this group had either moderate or severe degrees of intramyocardial amyloidosis, and the extent of deposition increased with advancing age and male sex. Additional data show that in a US community-based sample, approximately 30% of subjects over the age of 75 years with congestive heart failure and a preserved ejection fraction have cardiac deposits of wild-type TTR.24 In contrast, only five percent of subjects younger than 75 years old with heart failure and a preserved ejection fraction had cardiac amyloid deposits. It is unclear why this striking age and sex-association exists with senile cardiac amyloidosis. It is possible that post-translational modification of the TTR protein changes with aging, as cardiac TTR deposits in senile cardiac amyloidosis are composed of a combination of intact TTR and carboxy-terminal TTR fragments. This contrasts with certain forms of familial TTR cardiomyopathy where cardiac biopsy almost exclusively shows intact TTR protein.25 Wild type TTR deposition may also be related to age-related changes in chaperone and proteasome function, among other causes.26 The reasons for the association of senile cardiac amyloidosis with male gender are even more poorly understood, though may relate to sex hormone function.27
Clinical Features and Prognosis
Both familial TTR amyloidosis and senile cardiac amyloidosis cause a restrictive cardiomyopathy characterized by thickened and stiffened ventricles that result in small increases in chamber volume despite large rises in ventricular pressure. TTR amyloid can infiltrate any and all cardiac structures including the atrial and ventricular myocardium, electrical conduction system, valvular tissue, and both small and large arteries.1, 5 The myocardial deposits cause decreased ventricular compliance and relaxation abnormalities with ensuing diastolic dysfunction. Eventual myocyte necrosis and fibrosis produces systolic dysfunction.1 The resultant heart failure syndrome is a product of biventricular involvement and often includes elements of both left and right heart failure including fatigue, hypotension, dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, hepatomegaly, ascites, early satiety, nausea, and lower extremity edema.
The cardiac conduction system is also frequently compromised and may result in rhythm disturbances including symptomatic bradycardia, atrial fibrillation, and complex ventricular arrhythmias. In series of 18 patients with senile cardiac amyloidosis and heart failure, 56% had first degree AV block, 21% had left bundle branch block, 71% had left anterior fascicular block, 50% had right bundle branch block, and more than one-third were not in sinus rhythm.21 These findings may result from direct fibril deposition in specialized conduction tissue but may also result from localized ischemia of conduction pathways due to microvascular amyloid deposits.1, 5 Although a common outcome, it is unknown what percentage of patients with cardiac TTR amyloidosis will ultimately require a permanent pacemaker, though over 20% had paced cardiac rhythms by electrocardiogram in a recent analysis of persons with TTR cardiomyopathy in an international amyloidosis registry.19 In addition, atrial involvement frequently results in the development of common atrial arrhythmias such as atrial fibrillation and atrial flutter with their associated sequelae including palpitations, chest discomfort, and cardioembolic stroke. Intra-atrial thrombus is common and far exceeds what would be expected in a matched control group without cardiac amyloidosis.28 Lastly, complex ventricular arrhythmias such as multiform ventricular ectopy and non-sustained ventricular tachycardia are also common and have been noted in up to half this population.29 While such arrhythmias may be a harbinger of subsequent sudden cardiac death, electromechanical dissociation is more often the cause of cardiac arrest in these persons. As a result, much uncertainty exists about the role of automated implanted cardioverter defibrillators (AICDs) in these individuals, as these devices primarily treat “shockable” rhythms such as ventricular tachycardia and ventricular fibrillation.
A unique feature of senile cardiac amyloidosis is the common association with bilateral carpal tunnel syndrome. A small series has shown that approximately 40% of people with senile amyloidosis and heart failure also have concomitant carpal tunnel syndrome.21 Carpal tunnel syndrome often precedes the diagnosis of congestive heart failure by three to five years and can be a useful diagnostic clue to the underlying pathology.5
The natural history of neither familial cardiac amyloidosis from the Val122Ile mutation nor senile systemic amyloidosis is very well defined. Prognostic estimates are based on small data sets. In a study of African-Americans with cardiomyopathy referred for systemic amyloidosis to the Boston Medical Center Amyloid clinic, those with the Val122Ile mutation had an average life expectancy of 27 months.30 Data from our institution suggest an even worse life expectancy once cardiomyopathy is apparent, as 50 percent survival was only 11 months.31 Most of these individuals with the Val122Ile mutation experience hospitalization for cardiovascular decompensation in the months preceding death.
The diagnosis of senile cardiac amyloidosis appears to carry a significantly better prognosis than that of familial amyloidotic cardiomyopathy due to the Val122Ile mutation, the reasons for which are unknown. A Mayo clinic retrospective review of patients with senile cardiac amyloidosis and heart failure found a median survival of five years despite a median age at diagnosis of 72 years.32 A second small series found a similar average life expectancy of approximately six years.21 Data from our institution is concordant with these findings.31 It may be that senile cardiac amyloidosis is truly a more benign entity than is cardiac amyloidosis from the Val122Ile mutation. However, it may also be that patients with wild type cardiac amyloidosis are being diagnosed earlier in their disease course than are persons with the Val122Ile mutation. In support of this, we note that at our institution, wild type TTR has constituted an increasing percentage of our biopsy-proven diagnoses of cardiac amyloidosis as our population has aged and clinical suspicion for the disease entity has increased with time.33
Ultimately, prognosis for patients with cardiac amyloidosis must incorporate functional status at the time of diagnosis. Little data exists for patients with the TTR amyloidoses. Extrapolating from patients with AL amyloidosis and cardiomyopathy, functional class is a significant predictor of overall mortality with greater potency than multiple diagnostic and imaging modalities including ECG, standard two-dimensional echocardiography, and cardiac magnetic resonance imaging when each is used in isolation.34
Diagnosis
The diagnosis of cardiac TTR amyloidosis is difficult to make on clinical grounds alone as congestive heart failure, atrial arrhythmia, and conduction abnormalities are all non-specific disease manifestations and are otherwise common in older persons. However, a few presentations are more suggestive of the underlying restrictive physiology including that of marked right-sided heart failure with increasing abdominal girth, early satiety, and lower extremity edema, as well as the development of relative hypotension in a person with longstanding hypertension. These findings are especially notable in a patient of African-American descent or with a history of idiopathic bilateral carpal tunnel syndrome. (Table 2)
Table 2.
Findings Suggestive of Cardiac Amyloidosis
| 1. Historical and Physical Findings |
|---|
| Heart failure with a normal/preserved ejection fraction in the absence of hypertension, particularly in male subjects |
| Hypotension in a person with previous hypertension |
| Evidence of right-sided heart failure including loss of appetite, hepatomegaly, ascites, and lower extremity edema |
| Intolerance to commonly used cardiovascular medications including digoxin, calcium channel blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers, or beta blockers |
| Bilateral carpal tunnel syndrome |
| 2. Imaging Findings |
| Low QRS voltage or pseudo-infarction pattern on electrocardiogram (ECG) |
| Progressive diminution in QRS voltage on serial ECGs over time |
| Increased interventricular septal thickness and refractile myocardium (granular sparkling) on standard two-dimensional echocardiogram (2D TTE) |
| The combination of low voltage on ECG and increased interventricular septal thickness on 2D TTE or cardiac MRI (low voltage to mass ratio) |
| Low tissue Doppler velocities, reduced strain, or diminished strain rate using more advanced echocardiographic techniques |
| Subendocardial late gadolinium enhancement on cardiac magnetic resonance imaging |
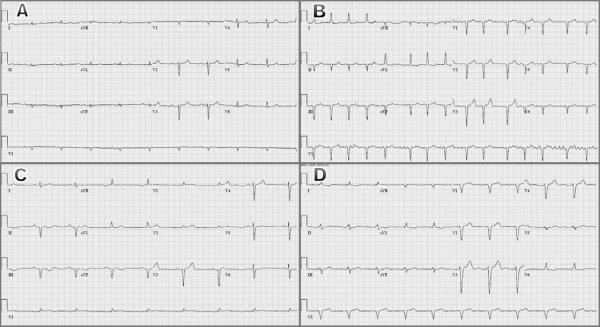
Additional diagnostic testing is always required. The most commonly ordered studies are the electrocardiogram (ECG) and two-dimensional transthoracic echocardiogram (2D TTE). Classic ECG findings in patients with cardiac amyloidosis include low QRS voltage, pseudo-infarction patterns, conduction abnormalities including bundle branch block and hemi-block, and rhythm disturbances such as atrial fibrillation. Figure 2 is a composite of four electrocardiograms, each of which illustrates common findings in persons with cardiac TTR amyloidosis.22, 30, 32 The ECG can be particularly helpful when serial cardiograms show progressive diminution in voltage over time.5 Ultimately however, electrocardiographic findings lack both sensitivity and specificity for persons with biopsy-proven cardiac amyloidosis. The combination of low voltage and pseudo infarction are seen in only a minority of patients.35 In addition, low voltage is seen in many other conditions including obesity, chronic obstructive pulmonary disease, pericardial effusion, and hypothyroidism.
Figure 2.
Representative electrocardiograms from patients with familial amyloidotic cardiomyopathy due to the Val122Ile mutation. (A) Sinus bradycardia with 1st degree AV block, low limb lead QRS voltage, poor precordial R-wave progression (cannot rule out anterior MI); (B) Atrial fibrillation, anterolateral and inferior infarcts (pseudo-infarcts); (C) Marked sinus bradycardia, inferior infarct (pseudo-infarct); (D) Sinus rhythm with marked 1st degree AV block, low limb lead QRS voltage, left bundle branch block.
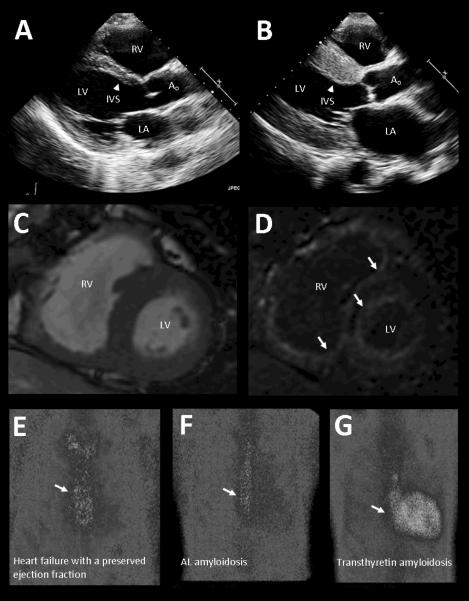
As with the electrocardiogram, classic echocardiographic patterns of cardiac amyloidosis do exist, but are neither sensitive nor specific. Persons with all cardiac amyloidoses are more likely to have thickened ventricular walls and refractile myocardium (Figure 3 panels A and B). As per the American Society of Echocardiography, the thickness of the normal left ventricular posterior wall and interventricular septum ranges between 6 and 11 millimeters. Persons with cardiac TTR amyloidosis of all etiologies may average wall thicknesses of 17 to 18 millimeters with concentric left ventricular hypertrophy.21, 32 They are also more likely to have echogenic myocardium that appears granular or “sparkling” with standard ultrasound assessment.36 However, in the early phases of the disease many persons with amyloid will have normal or just slightly elevated echocardiographic wall thickness, and only a minority will have characteristic granular echogenicity.35, 36 Higher degrees of accuracy can therefore be achieved when both ECG and 2D TTE are used in combination, especially when suspicion of cardiac amyloidosis is high. For example, the presence of low voltage in both limb and precordial ECG leads plus an interventricular septal thickness greater than 1.98 centimeters has a 79% positive predictive value for cardiac amyloidosis in a sample referred for cardiac biopsy.36 Sensitivity and specificity in this population was 72% and 91%, respectively, and the negative predictive value was 88%. At this time, the predictive power of voltage to mass ratios as a continuous variable has not been reliably demonstrated.
Figure 3.
Characteristic imaging findings in patients with cardiac transthyretin amyloidosis. (A) Echocardiogram in patient without cardiac disease. LA - left atrium, LV - left ventricle, RV - right ventricle, Ao - aorta. Interventricular septum (IVS), shown by arrowhead, is normal in thickness. (B) Echocardiogram in patient with familial amyloidotic cardiomyopathy due to Val122Ile mutation demonstrates markedly thickened Interventricular septum. (C) Cardiac MRI in patient with transthyretin amyloidosis showing short axis of heart. (D) Identical short axis view from the same patient demonstrates diffuse subendocardial late gadolinium enhancement of both ventricles that is characteristic of amyloid deposition. Panels (E), (F), and (G) show differences in cardiac retention of a technetium-pyrophosphate-99 radiotracer in subjects with heart failure and a preserved ejection fraction in hypertension (panel E) and AL amyloid (panel F) demonstrating no cardiac tracer retention compared to the subject with cardiac transthyretin amyloidosis in panel (G).
Additional modalities can be used to further increase diagnostic accuracy. More advanced echocardiographic techniques such as tissue Doppler imaging as well as strain and strain rate measurements can be helpful. Normal 2D TTE measures cardiac systolic function predominantly by assessing contraction of the heart along its short axis. However, subendocardial myocytes are longitudinally oriented and are particularly susceptible to damage in amyloidosis, resulting in early impairment in longitudinal contraction not appreciated on standard 2D TTE.5, 37 In contrast, both strain and strain rate techniques can be used to measure cardiac systolic and diastolic deformation in longitudinal, radial, and circumferential directions. Characteristic impairments can lead to the early diagnosis of cardiac amyloidosis and help differentiate it from other more common conditions that cause thickened ventricular chambers and diastolic relaxation abnormalities like longstanding hypertension.35, 38
Cardiac magnetic resonance imaging (CMRI) can also be used to increase diagnostic sensitivity. Intravenous gadolinium contrast accumulates within amyloid infiltrated myocardium. As a result, the combination of myocardial late gadolinium enhancement and altered gadolinium blood pool kinetics can identify the presence of amyloid and localize it within the heart (Figure 3 panels C and D).39 Sensitivity can be as high as 90% with more diffuse late gadolinium enhancement, which is associated with greater interstitial amyloid infiltration on endomyocardial biopsy and worse contractile impairment.40, 41 Reported specificity, positive predictive value, and negative predictive value of diffuse gadolinium enhancement ranges between 88% and 90%.41 In contrast, patients with longstanding hypertension may have similarly thick ventricular walls on echocardiography but do not have late gadolinium enhancement absent prior myocardial infarction or other rare infiltrative diseases.39
Lastly, non-invasive scintigraphic imaging with technetium may be able to specifically identify cardiac amyloidosis from TTR as opposed to other precursor proteins. Two recent studies found that use of a particular technetium isotope (99mTc-DPD) accurately differentiated between cardiac amyloidosis due to monoclonal immunoglobulin light-chain deposition (AL amyloidosis) and that due to TTR deposition. Sensitivity and specificity were high.42, 43 We have also demonstrated that technetium pyrophosphate can be used to differentiate subjects with either heart failure and a preserved ejection fraction or AL amyloidosis from those with cardiac TTR amyloidosis. (Figure 3 panels E through GT). The ratio of cardiac to whole body technetium uptake has also been shown to identify patients at higher risk for major cardiac adverse events and was an independent predictor of outcomes in these subjects either alone or in combination with left ventricular wall thickness.44 Thus with the appropriate radiotracer, scintigraphy may therefore more accurately diagnose TTR cardiac amyloidosis than do echocardiography and cardiac MRI, both of which identify only non-specific patterns associated with all forms of cardiac amyloid deposition. Further confirmatory studies are needed to validate the utility of this modality.
However until such time that specific radiotracers become widely available, definite diagnosis of cardiac TTR amyloidosis can be achieved through endomyocardial biopsy. This technique is well suited for diagnosing cardiac amyloidosis, as amyloid deposits are usually deposited diffusely throughout the subendocardium.45 Once amyloid deposits are found, the precursor protein can be identified using histochemical and sequence analysis. At this time, endomyocardial biopsy is the only way to definitively diagnose senile cardiac amyloidosis via demonstration of cardiac TTR deposits in the absence of an amyloidogenic TTR mutation. In contrast, familial TTR amyloidosis can be diagnosed without cardiac tissue in cases where clinical suspicion is high and imaging results support the diagnosis. These patients can have their TTR gene sequenced with close to 100 percent accuracy for less than $500 dollars with results returned in 6–8 weeks. Sequence analysis is covered by Medicare. For evaluation of family members, testing for a specific mutant allele can be performed at lower cost and is available within 2–4 weeks from most laboratories. The names and contact information for specific international laboratories offering TTR sequencing for clinical use can be found at www.genetests.org.
Treatment
Until recently, treatment for cardiac amyloidosis has almost exclusively been supportive care. The most important of these interventions has been the combination of salt restriction and diuretic use to reduce high intracardiac filling pressures and improve New York Heart Association functional class. The use of either torsemide or bumetanide may be preferable to the more commonly used furosemide, as these two loop diuretics have increased oral bioavailability and potency.46 This higher oral bioavailability may be particularly important in patients with cardiac amyloidosis and right heart failure who have impaired drug absorption due to bowel wall edema. Moreover, the use of long acting diuretics from other classes including thiazide, thiazide-like, and aldosterone antagonists can provide additional receptor blockade within the nephron and increased natriuresis. Importantly, when increasing diuretic dosage and optimizing volume status in patients with restrictive cardiomyopathy, care must be taken to avoid excessive volume depletion given the preload-dependent nature of cardiac amyloidosis. Careful follow up of serum electrolytes and fastidious checking of daily weights by the patient or caregiver can help avoid complications and achieve fluid balance.
Supportive care includes a number of other interventions. For patients with atrial fibrillation, a rhythm control strategy using an agent such as amiodarone may be helpful as it can reestablish atrioventricular synchrony and manage excessive ventricular rates that further worsen diastolic filling. It is important to note, however, that this approach has not been validated in clinical trials and will require regular monitoring for known potential complications of amiodarone use such as thyroid, ophthalmologic, liver, and lung toxicities. In addition, in patients with advanced restrictive heart disease, atrial transit may contribute only minimally to ventricular filling.47 For patients with significant bradycardia or advanced conduction system disease and symptoms, a permanent pacemaker should be strongly considered. As isolated right ventricular pacing may result in ventricular dyssynchrony with further decline in stroke volume than is already present, biventricular pacing may be beneficial though awaits further study to support widespread recommendation.48 Progressive worsening of cardiac conduction is common, and autonomic neuropathy may compound with the effects of bradycardia to create significant hemodynamic instability. Finally, in patients with significant orthostasis, regular use of support stockings and counter-pressure maneuvers may help avoid falls. Fludrocortisone should be avoided in this population as it may cause significant volume retention. Pseudoephedrine may be proarrhythmogenic and midodrine, while purported to be beneficial, is of questionable efficacy based on recent FDA review.49, 50
Special considerations must be made in the general care of patients with cardiac amyloidosis including early anticoagulation for those in atrial fibrillation and avoidance of potentially toxic therapies commonly used in the heart failure population. Anticoagulation should be the rule in those with atrial fibrillation even if traditional stroke risk factors enumerated in validated risk prediction models such as the CHADS2 and CHA2DS2-VASc scores are not elevated. A retrospective analysis has shown that persons with cardiac TTR amyloidosis have an almost 20% prevalence of intracardiac thrombus. Both atrial fibrillation and diastolic relaxation abnormalities are associated with thrombus formation, and anticoagulation with warfarin appears protective.28 At this time, there is insufficient evidence to evaluate the safety and efficacy of novel oral anticoagulants such as dabigatran and apixaban in this population.
Care must be taken to avoid potentially harmful therapies commonly used in patients with atrial fibrillation and congestive heart failure such as digoxin, calcium channel blockers, ACE inhibitors, angiotensin receptor blockers, and beta blockers. Digoxin binds to amyloid fibrils and exerts unpredictable local effects with subsequent increased risk of arrhythmogenesis.51 Serum digoxin levels are an inaccurate estimate of tissue effects. Similarly, dihydropyridine calcium channel blockers also bind amyloid fibrils and can exert potentially deleterious negative inotropic effects and result in high degree atrioventricular block.52 Neither digoxin nor calcium channel blockers should be used in patients with cardiac amyloidosis. In contrast, ACE inhibitors, angiotensin receptor blockers, and beta blockers can be used, but with extreme caution. Both ACE inhibitors and angiotensin receptor blockers may induce hypotension, as angiotensin blockade can significantly reduce vascular tone in the setting of concomitant sympathetic dysfunction due to TTR deposition.5 Beta blockers may have undesirable negative inotropic and chronotropic effects. As patients with cardiac amyloidosis have a restrictive cardiomyopathy with a relatively fixed stroke volume, augmentation of cardiac output relies disproportionately on increased heart rate. This normal heart rate response is frequently impaired with normal aging and may be further exacerbated by beta blockade.
Disease modifying treatments have until recently been limited to organ transplantation in highly selected older persons up to the age of 77 years-old.53 Orthotopic heart transplant has been rarely performed for both familial and senile cardiac amyloidosis, often with the use of extended donor criteria.54 Post-transplant life expectancy is improved compared to non-transplanted controls with amyloidosis but worse than all comers who undergo heart transplantation. Although not an immediate complication, amyloidosis can theoretically recur in transplanted grafts. This is not surprising as the liver is the site of synthesis for mutated TTR and continues to produce the abnormal protein. The combination of orthotopic heart and liver transplants has therefore been used in highly selected younger patients.
Given the obvious limitations of organ transplantation, it is exciting that a number of new pharmacotherapies specifically designed to reduce amyloid burden are on the horizon. These agents are designed to either decrease production of the amyloidogenic protein or increase its clearance from the body. For example, a number of small molecules and pharmacologic agents have been shown to increase TTR's native state stability and kinetic barrier to misfolding and aggregation.1, 5, 55 Two of these compounds with the most robust clinical data are diflunisal and tafamadis. Diflunisal is a non-steroidal anti-inflammatory drug that binds to unoccupied thyroxine binding sites on TTR and reduces tetramer dissociation.56 Unpublished data from our institution demonstrates that when given for one year to a small group of elderly persons with cardiac TTR amyloidosis, diflunisal was associated with both echocardiographic stabilization of cardiac structure and function as well as worsening glomerular filtration rate and platelet count. Expected side effects of NSAIDs such as renal impairment, gastritis, peptic ulcer disease, and fluid retention may therefore limit prolonged use of the agent in older persons.
In contrast, tafamadis is a first in-class small molecule that stabilizes the TTR tetramer and prevents its dissociation without the side effects expected of NSAIDs. Tafamadis has had favorable results in phase II/III trials in patients with familial TTR polyneuropathy and was well tolerated.5, 57 Preliminary data from an open label trial also suggest efficacy in persons with familial TTR cardiomyopathy.58 However, randomized trials in persons with the Val122Ile mutation or wild type disease have not yet been performed.
Active clinical research programs are also testing strategies of RNA interference through the use of small interfering RNAs (siRNAs) and antisense nucleotides to silence the TTR gene. RNA interference is a natural cellular process within many eukaryotic cells that controls gene activity. In this process, a short segment of double-stranded RNA called an siRNA unwinds into a single-stranded RNA and ultimately pairs with a complementary strand of messenger RNA. This binding prevents the messenger RNA from being translated into a protein product. In this manner, siRNAs are being produced to selectively target the mutant TTR gene.59 Similarly, antisense nucleotides have been designed that specifically bind to messenger RNA transcripts of the TTR gene.60 Using these new technologies, phase I clinical studies are currently ongoing to test drug safety and tolerability in patients with transthyretin amyloidosis. As with tafamadis, none of these studies specifically test drug effects in persons with cardiac amyloidosis from either the Val122Ile mutation or wild type disease.
CONCLUSION
It is now apparent that a significant portion of older North Americans with heart failure and a preserved ejection fraction have significant transthyretin cardiac amyloidosis. As new targeted therapeutics become available in the future, it will be increasingly important that these persons be identified. As shown in Table 2, historical clues such as intolerance to commonly used cardiovascular medications can be combined with characteristic findings on physical examination and imaging studies to select patients with a high likelihood of disease. These patients can undergo endomyocardial biopsy and/or genetic testing to confirm or rule out the diagnosis. Those with cardiac TTR should be given the appropriate supportive care to improve functional status and avoid the most undesirable disease complications such as acute decompensated heart failure, syncope, and cardioembolic stroke. Where appropriate, patients should be given access to clinical trials testing potential disease modifying agents.
Our knowledge of the clinical syndromes due to the TTR amyloidoses is still incomplete. We must better understand the natural history of disease including its variable penetrance and progression, links between genotype and phenotype, and the response to both supportive and more specific therapies. One manner in which this is being done is via the Transthyretin Amyloidosis Outcomes Survey (THAOS, listed on clinicaltrials.gov), a multi-center longitudinal, observational survey open to all patients with transthyretin amyloidosis. THAOS is an open ended registry with hopes of enrolling several thousand people with TTR amyloidosis at more than 50 worldwide sites over a 10 year period. Registry management and operations will be performed by Pfizer, who is responsible for site identification, initiation, and support.
The patient described in the clinical vignette is typical for someone with cardiac TTR amyloidosis from the Val122Ile mutation. In his case, the diagnosis of cardiac TTR was delayed approximately 18 months following the first manifestation of congestive heart failure. However, once considered, the final diagnosis required only the two weeks necessary for targeted genetic sequencing of the mutant allele. The patient is currently participating in an open label trial of a transthyretin stabilizer. It is hoped that this novel agent will slow disease progression and improve quality of life for what has hitherto been a relentlessly progressive myopathic process.
ACKNOWLEDGMENTS
Sponsor's Role: No funding was received
Footnotes
Conflict of Interest Dr. Maurer is funded by a K24 Award from the National Institute on Aging (AG036778-02) Mathew S. Maurer received research funding support from FoldRx Pharmaceuticals, now owned by Pfizer, Inc. He also holds a non-compensated position on the executive board of the Transthyretin Amyloidosis Outcomes Survey Registry (THAOS). THAOS is administered by Pfizer, Inc.
Author contributions: Kumar Dharmarajan and Mathew S. Maurer both contributed substantially to manuscript conception and design, preparation, critical revision, and final approval.
REFERENCES
- 1.Shah KB, Inoue Y, Mehra MR. Amyloidosis and the heart: A comprehensive review. Arch Intern Med. 2006;166:1805–1813. doi: 10.1001/archinte.166.17.1805. [DOI] [PubMed] [Google Scholar]
- 2.Desai HV, Aronow WS, Peterson SJ, et al. Cardiac amyloidosis: Approaches to diagnosis and management. Cardiol Rev. 2010;18:1–11. doi: 10.1097/CRD.0b013e3181bdba8f. [DOI] [PubMed] [Google Scholar]
- 3.Rapezzi C, Quarta CC, Riva L, et al. Transthyretin-related amyloidoses and the heart: A clinical overview. Nat Rev Cardiol. 2010;7:398–408. doi: 10.1038/nrcardio.2010.67. [DOI] [PubMed] [Google Scholar]
- 4.Westermark P, Benson MD, Buxbaum JN, et al. Amyloid: toward terminology clarification. Report from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2005;12:1–4. doi: 10.1080/13506120500032196. [DOI] [PubMed] [Google Scholar]
- 5.Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52:347–361. doi: 10.1016/j.pcad.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Buxbaum J, Alexander A, Koziol J, et al. Significance of the Amyloidogenic Transthyretin Val 122 Ile allele in African-Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J. 2010;159:864–870. doi: 10.1016/j.ahj.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson D, Tagoe C, Schwartzbard A, et al. Relation of clinical, echocardiographic and electrocardiographic features of cardiac amyloidosis to the presence of the transthyretin V122I allele in older African-American men. Am J Cardiol. 2011 May 18; doi: 10.1016/j.amjcard.2011.03.069. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–909. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- 9.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 10.Dohm CP, Kermer P, Bahr M. Aggregopathy in neurodegenerative diseases: Mechanisms and therapeutic implication. Neurodegener Dis. 2008;5:321–338. doi: 10.1159/000119459. [DOI] [PubMed] [Google Scholar]
- 11.Brenner DA, Jain M, Pimentel DR, et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004;94:1008–1010. doi: 10.1161/01.RES.0000126569.75419.74. [DOI] [PubMed] [Google Scholar]
- 12.Shi J, Guan J, Jiang B, et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc Natl Acad Sci USA. 2010;107:4188–4193. doi: 10.1073/pnas.0912263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 14.Cohen E, Bieschke J, Perciavalle RM, et al. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 15.Macario AJ, Conway de Macario E. Sick chaperones and ageing: A perspective. Ageing Res Rev. 2002;1:295–311. doi: 10.1016/s1568-1637(01)00005-8. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson DR, Pastore R, Pool S, et al. Revised transthyretin Ile 122 allele frequency in African-Americans. Hum Genet. 1996;98:236–238. doi: 10.1007/s004390050199. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336:466–473. doi: 10.1056/NEJM199702133360703. [DOI] [PubMed] [Google Scholar]
- 18.Sekijima Y, Yoshida K, Tokuda T, et al. Familial transthyretin amyloidosis. In: Pagon RA, Bird TD, Dolan CR, et al., editors. GeneReviews – NCBI Bookshelf (Internet) University of Washington, Seattle; Seattle: 1993. [Accessed June 12, 2011]. present (online). Available at: http://www.ncbi.nlm.nih.gov/books/NBK1194/tfap.Molecular_Genetics. [Google Scholar]
- 19.Dharmarajan K, Salomon S, Helmke S, et al. Genotype and phenotypic characteristics of persons with cardiac amyloidosis from the multinational Transthyretin Amyloidosis Outcomes Survey (THAOS) registry. J Cardiac Fail. 2011;17:S69. [Google Scholar]
- 20.Bhuiyan T, Helmke S, Patel AR, et al. Pressure-volume relationships in patients with transthyretin (ATTR) cardiac amyloidosis secondary to V122I mutations and wild-type transthyretin: Transthyretin Cardiac Amyloid Study (TRACS) Circ Heart Fail. 2011;4:121–128. doi: 10.1161/CIRCHEARTFAILURE.109.910455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng B, Connors LH, Davidoff R, et al. Senile systemic amyloidosis presenting with heart failure: A comparison with light chain-associated amyloidosis. Arch Intern Med. 2005;165:1425–1429. doi: 10.1001/archinte.165.12.1425. [DOI] [PubMed] [Google Scholar]
- 22.Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 23.Tanskanen M, Peuralinna T, Polvikoski T, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: A population-based autopsy study. Ann Med. 2008;40:232–239. doi: 10.1080/07853890701842988. [DOI] [PubMed] [Google Scholar]
- 24.Sultan AM, Edwards WD, Mohammed SF, et al. Cardiac amyloid deposition is common in elderly patients with heart failure and preserved ejection fraction. Circulation. 2010;122:A17926. [Google Scholar]
- 25.Bergstrom J, Gustavsson A, Hellman U, et al. Amyloid deposits in transthyretin-derived amyloidosis: Cleaved transthyretin is associated with distinct amyloid morphology. J Pathol. 2005;206:224–232. doi: 10.1002/path.1759. [DOI] [PubMed] [Google Scholar]
- 26.Greene MJ, Sam F, Soo Hoo PT, et al. Evidence for a functional role of the molecular chaperone clusterin in amyloidotic cardiomyopathy. Am J Pathol. 2011;178:61–68. doi: 10.1016/j.ajpath.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goncalves I, Alves CH, Quintela T, et al. Transthyretin is up-regulated by sex hormones in mice liver. Mol Cell Biochem. 2008;317:137–142. doi: 10.1007/s11010-008-9841-2. [DOI] [PubMed] [Google Scholar]
- 28.Feng D, Syed IS, Martinez M, et al. Intracardiac thrombus and anticoagulation therapy in cardiac amyloidosis. Circulation. 2009;119:2490–2497. doi: 10.1161/CIRCULATIONAHA.108.785014. [DOI] [PubMed] [Google Scholar]
- 29.Falk RH, Rubinow A, Cohen AS. Cardiac arrhythmias in systemic amyloidosis: correlation with echocardiographic abnormalities. J Am Coll Cardiol. 1984;3:107–113. doi: 10.1016/s0735-1097(84)80436-2. [DOI] [PubMed] [Google Scholar]
- 30.Connors LH, Prokaeva T, Lim A, et al. Cardiac amyloidosis in African Americans: Comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. 2009;158:607–614. doi: 10.1016/j.ahj.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Noumi B, Latif F, Helmke S, et al. Differences between transthyretin (ATTR) cardiac amyloidosis secondary to V122I mutations and wild type TTR presenting to a tertiary referral center. J Card Fail. 2010;16:S40. [Google Scholar]
- 32.Kyle RA, Spittell PC, Gertz MA, et al. The premortem recognition of systemic senile amyloidosis with cardiac involvement. Am J Med. 1996;171:395–400. doi: 10.1016/S0002-9343(96)00229-X. [DOI] [PubMed] [Google Scholar]
- 33.Latif F, Delisle S, Helmke S, et al. Changes in the type of cardiac amyloidosis diagnosed at a tertiary referral center: An impact of an aging population. J Am Coll Cardiol. 2011;57:E247. [Google Scholar]
- 34.Austin BA, Duffy B, Tan C, et al. Comparison of functional status, electrocardiographic, and echocardiographic parameters to mortality in endomyocardial-biopsy proven cardiac amyloidosis. Am J Cardiol. 2009;103:1429–1433. doi: 10.1016/j.amjcard.2009.01.361. [DOI] [PubMed] [Google Scholar]
- 35.Selvanayagam JB, Hawkins PN, Paul B, et al. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol. 2007;50:2101–2110. doi: 10.1016/j.jacc.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 36.Rahman JE, Helou EF, Gelzer-Bell R, et al. Non-invasive diagnosis of biopsy proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43:410–415. doi: 10.1016/j.jacc.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 37.Hosch W, Kristen AV, Libicher M, et al. Late enhancement in cardiac amyloidosis: correlation of MRI enhancement pattern with histopathological findings. Amyloid. 2008;15:196–204. doi: 10.1080/13506120802193233. [DOI] [PubMed] [Google Scholar]
- 38.Sun JP, Stewart WJ, Yang XS, et al. Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol. 2009;103:411–415. doi: 10.1016/j.amjcard.2008.09.102. [DOI] [PubMed] [Google Scholar]
- 39.Maceira AM, Joshi J, Prasad SK, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–193. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 40.Syed IS, Glockner JF, Feng D, et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2010;3:155–164. doi: 10.1016/j.jcmg.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Austin BA, Tang WHT, Rodriguez ER, et al. Delayed hyperenhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. J Am Coll Cardiol Img. 2009;2:1369–1377. doi: 10.1016/j.jcmg.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-Diphosphono-1,2-Propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076–1084. doi: 10.1016/j.jacc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 43.Rapezzi C, Quarta CC, Guidalotti PL, et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2011;38:470–478. doi: 10.1007/s00259-010-1642-7. [DOI] [PubMed] [Google Scholar]
- 44.Rapezzi C, Quarta CC, Guidalotti PL, et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging. 2011;4:659–670. doi: 10.1016/j.jcmg.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 45.Ardehali H, Qasim A, Cappola T, et al. Endomyocardial biopsy plays a role in diagnosing patients with unexplained cardiomyopathy. Am Heart J. 2004;147:919–923. doi: 10.1016/j.ahj.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 46.Wargo KA, Banta WM. A comprehensive review of the loop diuretics: should furosemide be first line? Ann Pharmacother. 2009;43:1836–1847. doi: 10.1345/aph.1M177. [DOI] [PubMed] [Google Scholar]
- 47.Nihoyannopoulos P, Dawson D. Restrictive cardiomyopathies. Eur J Echocardiogr. 2009;10:iii23–33. doi: 10.1093/ejechocard/jep156. [DOI] [PubMed] [Google Scholar]
- 48.Holzmeister J, Leclercq C. Implantable cardioverter defibrillators and cardiac resynchronization therapy. Lancet. 2011;378:722–730. doi: 10.1016/S0140-6736(11)61228-2. [DOI] [PubMed] [Google Scholar]
- 49.Low PA, Gilden JL, Freeman R, et al. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA. 1997;277:1046–1051. [PubMed] [Google Scholar]
- 50.FDA news release: FDA proposes withdrawal of low blood pressure drug. [Accessed November 16, 2011];United States Food and Drug Administration (online) Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm222580.htm.
- 51.Rubinow A, Skinner M, Cohen AS. Digoxin sensitivity in amyloid cardiomyopathy. Circulation. 1981;63:1285–1288. doi: 10.1161/01.cir.63.6.1285. [DOI] [PubMed] [Google Scholar]
- 52.Gertz MA, Falk RH, Skinner M, et al. Worsening of congestive heart failure in amyloid heart disease treated by calcium channel-blocking agents. Am J Cardiol. 1985;55:1645. doi: 10.1016/0002-9149(85)90995-6. [DOI] [PubMed] [Google Scholar]
- 53.Kang GH, Dong RR, Song PS, et al. A case of a senile systemic amyloidosis patient presenting with angina pectoris and dilated cardiomyopathy. Korean Circ J. 2011;41:209–212. doi: 10.4070/kcj.2011.41.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maurer MS, Raina A, Hesdorffer C, et al. Cardiac transplantation using extended-donor criteria organs for systemic amyloidosis complicated by heart failure. Transplantation. 2007;83:539–545. doi: 10.1097/01.tp.0000255567.80203.bd. [DOI] [PubMed] [Google Scholar]
- 55.Bartolini M, Andrisano V. Strategies for the inhibition of protein aggregation in human diseases. Chembiochem. 2010;11:1018–1035. doi: 10.1002/cbic.200900666. [DOI] [PubMed] [Google Scholar]
- 56.Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;13:236–249. doi: 10.1080/13506120600960882. [DOI] [PubMed] [Google Scholar]
- 57.Coelho T, Maia M, Martins da Silva A, et al. Tafamadis (Fx-1006A): A first-in-class disease-modifying therapy for transthyretin type familial amyloid polyneuropathy. Abstract presented at American Academy of Neurology annual meeting.2010. [Google Scholar]
- 58.Falk RH, Maurer MS, Fedson SE, et al. Tafamadis stabilizes transthyretin and improves clinical outcomes in transthyretin amyloid cardiomyopathy. J Cardiac Fail. 2011;17:S56. [Google Scholar]
- 59.Zimmermann TS, Lee AC, Akinc A, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 60.Benson MD, Kluve-Beckerman B, Zeldenrust SR, et al. Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve. 2006;33:609–618. doi: 10.1002/mus.20503. [DOI] [PubMed] [Google Scholar]