Abstract
Purpose
The aim of the study was to evaluate the efficacy of an injectable and partly absorbable calcium bone cement (CERAMENT™, Bone Support, Sweden) in the treatment of osteoporotic or traumatic vertebral fractures by percutaneous vertebroplasty.
Methods
From March 2009 to October 2010 an open, prospective study in two centres was performed. 33 patients with symptomatic vertebral fractures were enrolled. Patients were included based on evaluation by X-ray, CT, and MRI. Clinical evaluation by Visual Analogue Scale (VAS, 0–10) and Oswestry Disability index test (ODI, 0–100 %) was performed before the operation as well as 1, 6 and 12 months after the procedure. Radiology assessment post-procedure was carried out by X-ray, CT, and MRI at 1, 6 and 12 months post-op. Intake of analgesic medications pre- and post-procedure was monitored.
Results
66 vertebral bodies underwent percutaneous vertebroplasty. VAS score demonstrated a significant decrease from 8.61 (SD 19.8) pre-operatively to 2.48 (SD 2.36) at 1 month. The score was 2.76 (SD 2.68) at 6 months and 1.36 (SD 1.33) at the latest follow up. ODI score dropped significantly from 58.86 pre-op to 26.94 at 6 months and further down to 7.61 at 12 months. No re-fractures or adjacent level fractures were reported.
Conclusion
Data show that CERAMENT can be a substitute of PMMA in the treatment of osteoporotic and traumatic vertebral fractures, especially in young patients.
Keywords: PMMA, Calcium–phosphate cement, Bone substitute, Biomaterials, Biocompatibility, Vertebroplasty
Introduction
Percutaneous vertebral body augmentation, using a minimally invasive procedure, has been used, for decades, for vertebral fracture pain management in patients where conservative treatments, such as analgesics, bed rest, and bracing had failed [1–3].
Two different procedures have been developed to restore vertebral body stability and avoid further height compression: vertebroplasty (VP) where the fracture lines and cancellous bone are filled with injectable bone cement, and kyphoplasty (KP) where the created void and cancellous bone are filled with injectable bone cement [1, 4, 5]. Both procedures are today proven and accepted as standard of care for pain caused by compression fractures of the spine, such as traumatic non-osteoporotic vertebral fractures and traumatic compressive vertebral collapses, especially in younger patients [3, 6, 7].
The bone cement most commonly used in the above-described procedures is polymethylmethacrylate (PMMA), its proper function consisting stabilization of the fracture and increased strength of the collapsed vertebra [1]. The success of this material is based on immediate relief of pain and mechanical stabilization improving physical functions [3, 8] as well as low cost [9]. However, clinical trial results have exposed a high complication rate in percutaneous vertebroplasty with PMMA range from 1 to 10 % [10, 11], highlighting potential weaknesses such as thermal injury to surrounding tissues, potentially causing neurological damage, increasing fracture risk at adjacent levels due to the high inherent stiffness, and potential toxicity caused by the reactive material [2, 4, 9, 12].
The optimal design for an ideal cement includes adequate mechanical support, good radiopacity, injectability, biocompatibility and bioactivity [8, 9]. New injectable materials such as calcium–phosphate (CaP), bioactive glass and calcium–sulphate cement (CaS) are being developed, and they seem suitable for vertebral stabilization and augmentation in younger patients [9, 12, 13]. However, these materials have poor injectability, poor handling, lower viscosity and/or too fast absorption [9, 12, 14]. To bypass these drawbacks, a new kind of biphasic cement has been developed consisting of 60 % α-CaS hemihydrates and 40 % hydroxyapatite (HA) [2, 4], marked as CERAMENT Spine Support (CSS) (Bone Support, Sweden). This product allows delayed absorbable of the CaS and controlled bone in-growth by osteoconductivity of the HA.
The aim of this study was to investigate safety and efficacy in percutaneous vertebroplasty performed with CSS as an alternative to PMMA [15, 16].
Methods
Thirty-three patients were enrolled from March to October 2009. Inclusion criteria were historical and clinical evidence of symptomatic compressive spine fracture caused by osteoporosis and trauma, confirmed by radiological signs of spine fracture based on X-ray and MRI (T1, T2 fat sat); moreover, patients had previously been examined with CT scan. Only fracture type A1–A3 (AO classification) was included. Only patients with a clear correlation between affected vertebral and symptoms were included.
Exclusion criteria included patients affected by neurological deficits, complete burst fracture, retropulsion of fracture fragment, concomitant local tumour, anaphylaxis reaction history, acute infection, coagulation disorders such bleeding disease or anticoagulant medication intake.
All treatments were administered on an outpatient surgery basis. Written informed consent was obtained from all patients. Pre-operatively, all patients received appropriate analgesic medication and a prophylactic broad-spectrum antibiotic.
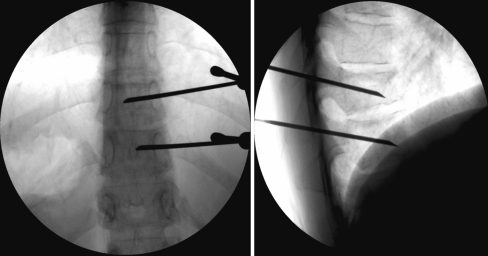
All patients received local analgesia into the skin, subcutaneous tissues and the periosteum, and treatments were carried out with patients in the prone position on the fluoroscopy table with the upper part of the body slightly elevated. The vertebroplasty procedures were performed with unilateral trans-pedicular vertebral approach under fluoroscopic guidance with lateral and oblique views (Figs. 1, 2). A 10–13 Gauge needle was introduced into the vertebral pedicle through a small skin incision, one for each level. CSS (Bone Support; Sweden) was prepared; this is a composite material based on two phases, consisting of 60 % calcium sulphate α-hemihydrate and 40 % hydroxyapatite, mixed together with iohexol 300 mg/ml in water to a viscous past. CSS was then instilled under close imaging guidance until the anterior two-thirds of the vertebral body were filled and cement was equally distributed inside the bone.
Fig. 1.
Anteroposterior and latero-lateral intraprocedural fluoroscopic view
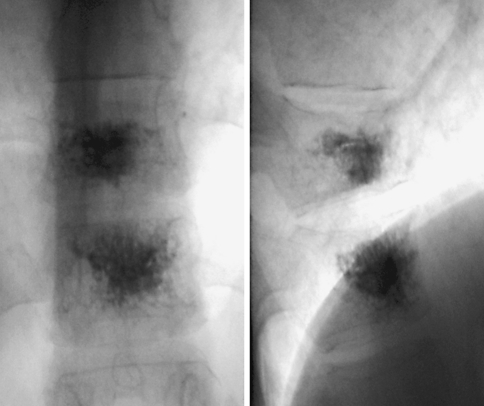
Fig. 2.
Anteroposterior and latero-lateral intraprocedural fluoroscopic view shows the good opacity of the bone cement
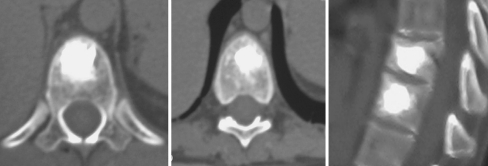
Following the procedure, patients require bed rest for 2 h before mobilization, and they were discharged the same day. CT scan was carried out post-procedure to assess intra-osseous cement distribution and signs of any cement leakages (Fig. 3).
Fig. 3.
CTms check after procedure
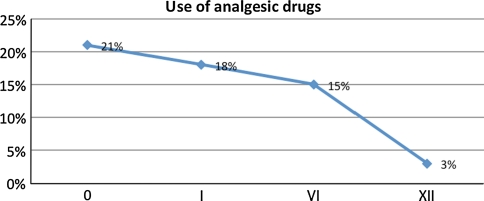
Clinical outcome was to quantify using VAS score (0–100) and ODI questionnaire based on the self-administered score, and was performed before the operation as well as 1, 6 and 12 months after the procedure. Radiology assessment post-procedure was carried out by X-ray, CT, and MRI at 1, 6 and 12 months post-op (Figs. 4, 5, 6, 7, 8, 9). Intake of analgesic medications pre- and post-procedure was also registered (Fig. 10).
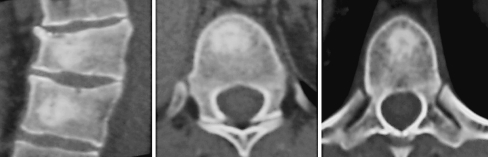
Fig. 4.
CTms check 1 month after procedure: poor visualization of the bone cement into vertebral bodies
Fig. 5.
X-ray film check 1 month after procedure: poor visualization of the bone cement into vertebral bodies
Fig. 6.
MRI check 1 month after procedure: reduction of hyperintensity in T2Fat Sat sequences
Fig. 7.
X-ray film check 12 months after procedure: no visualization of the bone cement into vertebral bodies
Fig. 8.
CTms check 12 months after procedure: the bone cement has been progressively included into the vertebral bodies
Fig. 9.
MRI check 12 months after procedure: further reduction of hyperintensity in T2Fat Sat sequences
Fig. 10.
Changes in terms of analgesic drug use
Statistical methodology
Data are presented using as average, standard deviation, and t test at baseline as well as during follow-up at 1, 6, 12 months. Statistical significance was accepted as significant at P < 0.001.
Results
Thirty-three patients were enrolled from March to October 2009 (6 males and 27 females, aged between 29 and 84, average age 59 years), totally 66 vertebral body underwent percutaneous vertebroplasty. The mean volume of CSS injected was 3 ml (SD 0.35) (Table 1).
Table 1.
Demographic data of the patients
| Total | Women | Male | |
|---|---|---|---|
| Patient number | 33 | 26 | 7 |
| Mean age (years) | 59 | 63 | 43 |
| Time from fracture to operation (days) | 30 | 30 | 30 |
| Fracture type (AO classification, %) | |||
| A1.1 | 18 | ||
| A1.2 | 21 | ||
| A1.3 | 61 | ||
| Levels treated | 66 | 51 | 11 |
| Mean CERAMENT injected (ml) | 3 | 3 | 3 |
86 % of the patients were affected by osteoporotic fractures (85 % female, 29 % male), and 27 % of the patients were affected by non-osteoporotic fractures (15 % female, 71 % male). The procedure was performed at a mean of 30 days after injury.
An overall amount treated was 55 levels for women and 11 levels for men with the distribution peak of prevalence at D7, D8 and L1 (12, 10, 12 %), respectively.
The type of fracture (AO Classification system) was gathered as follows: A1.1 (18 %), A1.2 (21 %) and A1.3 (61 %).
At CT scan post-procedure no cement leakage into the spinal canal or into discal space was observed (Fig. 3).
At X-ray check 12 months after procedure shows no evidence of the bone cement into vertebral bodies (Fig. 7).
At the CT control, starting already after 1 month, we found a good distribution of the bone cement, partial reabsorption and visible new bone formation.
MRI examinations after 1, 6 and 12 months did not show expected signals of vertebral body oedema, confirming fracture stabilization and excluding re-fracture (Figs. 6, 9).
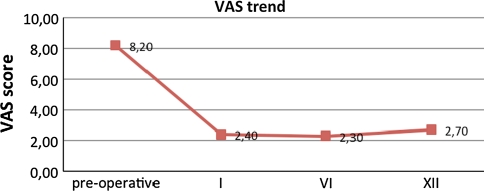
The VAS pain trend demonstrated a decrease over time from a mean of 8.4 (SD 2.02) pre-operatively, to 2.4 (SD 2.36) at 1 month and 2.3 (SD 2.68) at 6 months and 2.7 (SD 1.33) at the latest follow up (Fig. 11).
Fig. 11.
VAS score shows a decrease over the 12-month follow-up
Quality assessment by ODI scoring over the follow-up also reported a statistically clear improvement of functional conditions in terms of reduction in pain intensity and of quality of life comparing to baseline data starting from 50.6 % (SD 14.82) dropping to 20.6 % (SD 11.53) after 12 months (Fig. 12).
Fig. 12.
ODI score shows a significant improvement over the 12-month follow-up
Both cases showed significant levels with P < 0.0005 (Figs. 1, 2). Neither periprocedural as long-term clinical complication nor any infections resulting from the procedure were noted. At the end of 1 year follow-up, no cases of adjacent fracture were reported.
Conclusion
The results of this work, according to our data, highlight that CERAMENT bone cement can be used as an ideal bone substitute in treatments of both osteoporotic and/or traumatic vertebral fracture.
As potential alternative the Cerement can be adopted instead of PMMA overlapping its well-know drawbacks.
Over 1 year of study demonstrated the own Cerement effectiveness in terms of vertebral stabilization and augmentation properties, bioactivity and reabsorbability, feasibility to inject and radio-opacity.
Other strengths could be seen as patients’ short hospitalization and period of inactivity, better quality of life.
We reported total absence of complications, including new incidental adjacent fracture.
Acknowledgments
Conflict of interest
None.
Contributor Information
Stefano Marcia, Phone: +39-0706092486, FAX: +39-0706092205, Email: stemarcia@gmail.com.
Claudia Boi, Email: claudiaboi@yahoo.it.
Mario Dragani, Phone: +39-0854252898, Email: mariodragani@yahoo.it.
Stefano Marini, Email: stemarini@gmail.com.
Mariangela Marras, Email: mariangela.marrasmd@gmail.com.
Emanuele Piras, Email: emanuele.piras@yahoo.it.
Giovanni Carlo Anselmetti, Phone: +39-0119933307, Email: giovanni.anselmetti@ircc.it.
Salvatore Masala, Phone: +39-0620902401, Email: salva.masala@tiscali.it.
References
- 1.Calvo-Fernandez T, Parra J, Fernandez-Gutierrez M, Vazquez-Lasa B, Lopez-Bravo A, Collia F, Perez de la Cruz MA, San RJ. Biocompatibility of alendronate-loaded acrylic cement for vertebroplasty. Eur Cell Mater. 2010;20:260–273. doi: 10.22203/ecm.v020a21. [DOI] [PubMed] [Google Scholar]
- 2.Rauschmann M, Vogl T, Verheyden A, Pflugmacher R, Werba T, Schmidt S, Hierholzer J. Bioceramic vertebral augmentation with a calcium sulphate/hydroxyapatite composite (Cerament SpineSupport): in vertebral compression fractures due to osteoporosis. Eur Spine J. 2010;19:887–892. doi: 10.1007/s00586-010-1279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmelzer-Schmied N, Cartens C, Meeder PJ, Dafonseca K. Comparison of kyphoplasty with use of a calcium phosphate cement and non-operative therapy in patients with traumatic non-osteoporotic vertebral fractures. Eur Spine J. 2009;18:624–629. doi: 10.1007/s00586-008-0880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu XS, Zhang ZM, Mao HQ, Geng DC, Zou J, Wang GL, Zhang ZG, Wang JH, Chen L, Yang HL. A novel sheep vertebral bone defect model for injectable bioactive vertebral augmentation materials. J Mater Sci Mater Med. 2011;22:159–164. doi: 10.1007/s10856-010-4191-5. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez L, Parra J, Vazquez B, Bravo AL, Collia F, Goni I, Gurruchaga M, San RJ. Injectable acrylic bone cements for vertebroplasty based on a radiopaque hydroxyapatite. Bioactivity and biocompatibility. J Biomed Mater Res B Appl Biomater. 2009;88:103–114. doi: 10.1002/jbm.b.31156. [DOI] [PubMed] [Google Scholar]
- 6.Folman Y, Shabat S (2011) A comparison of two new technologies for percutaneous vertebral augmentation: confidence vertebroplastys. Sky kyphoplasty. BMC Musculoskelet Disord 12:206. doi:10.1186/1471-2474-12-206 (published online 2011 September 22)
- 7.Chen LH, Lai PL, Chen WJ. Current status of vertebroplasty for osteoporotic compression fracture. Chang Gung Med J. 2011;34(4):352–359. [PubMed] [Google Scholar]
- 8.Belkoff SM, Mathis JM, Jasper LE. Ex vivo biomechanical comparison of hydroxyapatite and polymethylmethacrylate cements for use with vertebroplasty. Am J Neuroradiol. 2002;23:1647–1651. [PMC free article] [PubMed] [Google Scholar]
- 9.Lim TH, Brebach GT, Renner SM, Kim WJ, Kim JG, Lee RE, Andersson GB, An HS (2002) Biomechanical evaluation of an injectable calcium phosphate cement for vertebroplasty. Spine (Phila Pa 1976) 27:1297–1302 [DOI] [PubMed]
- 10.Robinson Y, Heyde CE, Försth P, Olerud C (2011) Kyphoplasty in osteoporotic vertebral compression fractures—guidelines and technical considerations. J Orthop Surg Res 6:43. doi:10.1186/1749-799X-6-43 (published online 2011 August 19) [DOI] [PMC free article] [PubMed]
- 11.Al-Nakshabandi NA. Percutaneous vertebroplasty complications. Ann Saudi Med. 2011;31(3):294–297. doi: 10.4103/0256-4947.81542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grafe IA, Baier M, Noldge G, Weiss C, Da FK, Hillmeier J, Libicher M, Rudofsky G, Metzner C, Nawroth P, Meeder PJ, Kasperk C (2008) Calcium–phosphate and polymethylmethacrylate cement in long-term outcome after kyphoplasty of painful osteoporotic vertebral fractures. Spine (Phila Pa 1976) 33:1284–1290 [DOI] [PubMed]
- 13.Maestretti G, Cremer C, Otten P, Jakob RP. Prospective study of standalone balloon kyphoplasty with calcium phosphate cement augmentation in traumatic fractures. Eur Spine J. 2007;16:601–610. doi: 10.1007/s00586-006-0258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burguera EF, Xu HH, Sun L. Injectable calcium phosphate cement: effects of powder-to-liquid ratio and needle size. J Biomed Mater Res B Appl Biomater. 2008;84:493–502. doi: 10.1002/jbm.b.30896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu KS, Shim JH, Heo HY, Park CK. Therapeutic efficacy of injectable calcium phosphate cement in osteoporotic vertebral compression fractures: prospective nonrandomized controlled study at 6-month follow-up. World Neurosurg. 2010;73:408–411. doi: 10.1016/j.wneu.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Masala S, Nano G, Marcia S et al (2011) Osteoporotic vertebral compression fractures augmentation by injectable partly resorbable ceramic bone substitute (Cerament™ Spine Support): a prospective nonrandomized study. Neuroradiology. doi:10.1007/s00234-011-0940-5 [DOI] [PubMed]