Abstract
Purpose
The aim of the study was to report and discuss the preliminary data obtained in a homogeneous series of 50 patients affected by multiple myeloma treated with bisphosphonates.
Methods
Patients were followed for a minimum of 1 year. Main orthopaedic data were recorded. Visual Analogue Score and QLQ-C30 and MY 20 were used to assess the quality of life.
Results
Statistical analysis showed less lytic lesions in the group with zoledronate therapy and stable primary disease compared with a greater number of lesions in the non-treated group. Results regarding VAS score and QLQ-C30 and MY were statistically better in the first group than in the second.
Conclusions
Our results confirm the efficacy of zoledronate in ensuring an acceptable quality of life restraining the aggressiveness of the myeloma on bone tissue, especially in spine although further prospective studies have to be conducted to determine its correct use in myeloma patients.
Keywords: Bisphosphonate, Zoledronic acid, Lytic lesion, Myeloma, Spine
Introduction
With the increasing longevity of patients with oncologic disorders, an increased incidence of skeletal involvement and related complications is observed, most of which are in charge of a bad quality of life. Multiple myeloma (MM) commonly is characterised from skeletal-related complications that result from a shift in the normal balance between bone formation and bone resorption toward enhanced bone loss [1]. Hypercalcaemia and osteolytic lesions are two findings frequently associated with the monoclonal cells proliferation in MM. It is estimated that lesions of the bone affect 90% of the patients affected by this disease [2]. Bone lesions are the consequence of local damages due the pathologic tissue and/or fracture due to bone fragility. The first type of lesion frequently localized at the appendicular bones presents as a lytic area or pathologic fracture due to the aggressiveness of pathologic tissue localized in the bone. The second one, mainly localized to the spine, is fragility fracture due to osteoporosis that accompanies MM. Osteoporosis, also, in MM recognizes many pathogenetic aspects, the most important of which are the direct effect of the monoclonal plasma cells on the metabolism of the bone tissue and the indirect effects of high doses or long-time steroid therapies and radiation therapies [3]. Being the quality of life and the final outcome of the MM patients directly related to the occurrence of pathologic fractures, many attempts were made in the recent years to the prevention of these dramatic events. Concerning prevention of the fractures, today the role of the bisphosphonates (BPs), an interesting category of drugs widely used in other forms of osteoporosis, is highly debated. BPs have double major pharmacologic properties: they decrease resorptive effects of the metastatic disease process and correct tumoral hypercalcemia. Because of the capability to act directly on bone tissue metabolism they represent a very strategic drug to prevent the occurrence of pathologic fractures and, as a consequence, to provide a significant increase of the quality of life of these patients [4]. Zoledronic acid and pamidronate are the most commonly used intravenous BPs as a preventive treatment of bone complications encountered in multiple myeloma [5].
Although clinical effects of the drugs in oncologic patients are widely recognized no clear guidelines on BPs therapy have been published in literature. At the moment still lacking are the criteria to choose the optimal patient eligible for medical treatment with BPs as still lacking are other aspects such as doses and duration of the therapy. An attempt to establish the role of BPs in multiple myeloma was made in the past years by the American Society of Clinical Oncology and Clinical Practice [6]. Following studies have demonstrated improved clinical results for patients treated with chemotherapy associated with BP therapy versus patients treated only with chemotherapy [7]. BPs should probably be used very early in all patients, not only because of their beneficial skeletal effects, but also because they may slow tumour growth [7]. To better understand the complexity of the mechanisms of the BPs we started a project of surveillance of a homogenous series of patients affected by MM who are in charge by haematologists of our institution.
The aim of the present work was to evaluate the effect of the BP therapy in preventing new bone-related complications and in maintaining or improving the quality of life.
Methods
From February 2008 to February 2010 by Spine Surgery Division of Department of Orthopaedic Science and Traumatology of Catholic University the data of 50 patients affected by MM enrolled by our haematologic group were collected and analysed. All the patients enrolled in the present study were evaluated at base time and at 3, 6, 12 and 24 months. All patients underwent a complete diagnostic imaging investigation by total skeletal X-ray, femoral and spine bone densitometry, MRI of the involved spine region and PET CT. The data were divided as preeminent haematologic data and preeminent orthopaedic data. The following main orthopaedic data were recorded: duration of the disease, pain, functional ability, axial and appendicular lesions, previous orthopaedic treatment, steroid use, previous chemotherapy, previous radiotherapy, intravenous BP use and following oral BP use, BP therapy duration, surgical procedures and the presence of adverse events. All patients were clinically evaluated with regard to pain, quality of life and general condition, using the following self-administered questionnaire Visual Analogue Scale [8], QLQ-C30 and MY 20 [9]. Finally, the type and quantity of drugs taken by the patients to control pain, during the pre and post-treatments periods, were recorded. All data were registered and analysed in a retrospective way.
Statistics
Descriptive statistics were calculated. The results obtained were analysed using the Student’s t test and χ2 test and verified with Fisher’s exact test. Significance was accepted at P < 0.05. There are some limitations that need to be acknowledged and addressed regarding the present study. The number of cases is too limited for broad generalizations. Further empirical evaluations and greater patients’ series are needed to validate the present results, taking into account that these are only preliminary reports.
Results
50 patients, 17 males and 28 females, with a mean age of 67 years (range 40–76.3) were enrolled in our study and analysed in a retrospective way. The mean follow-up was 15 months (range 12–24). At the first evaluation 23 patients were in an active phase of primary pathology and with BP therapy on act, 25 were in a stable phase after a complete BP therapy, at least 12 administrations, and 2 were asymptomatic, at first diagnosis. All patients except the two asymptomatic underwent in different phases of the primary disease chemotherapy associated with steroid therapy and radiotherapy on the symptomatic bone lesions. 17 patients were at observation time with Thalidomide on act. In the first group (active phase), 11 patients out of 23 presented associated bone lesions, mostly skull, rib, femoral and humeral localizations and four underwent surgery for previous appendicular impending fractures or vertebral lesions. In the second group (stable phase) 9 patients out of 25 presented associated bone lesions, mostly skull, rib and pelvis localizations and 3 underwent previous kyphoplasty. 33 patients of the overall series underwent therapy with zoledronic acid in different phases of the myeloma and 17 patients including the 2 asymptomatic did not undergo the BP therapy. Only three patients were assuming at the day of the study therapy with oral BP after a cycle of intravenous therapy. At base time of the study 44 lytic lesions and 29 fragility vertebral collapses were identified in the group with a complete therapy of zoledronic acid and with a stable primary pathology. At 12 months’ minimum follow-up in the same group we observed an unchanged number of lytic lesions and 31 fragility vertebral collapses, P = 0.03. In the no-zoledronic therapy group we observed 37 lytic lesions and 24 fragility vertebral collapses. At 12 months’ minimum follow-up lytic lesions increased to 50 and fragility vertebral collapses to 35, P = 0.006. The two symptom-free patients have no lytic lesions but only osteoporotic vertebral collapses.
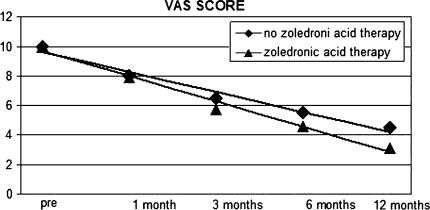
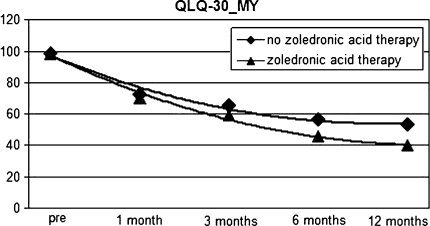
The analysis of the administered questionnaires demonstrated better results in the group of patients who were having the treatment with zoledronate compared with those who were not. VAS scores were 5.7 (range 3–8) P = 0.004 in the first group and 6.9 (range 4–8) P = 0.03 in the second one. QLQ-C30 and MY in the first group was 40.1% (range 23–65%) P = 0.003 compared with the second group QLQ-30 and MY20 = 53.4% (range 35–70%) P = 0.05 at 12 months’ minimum follow-up. (Figs. 1, 2)
Fig. 1.
Visual analogue scale score results after a 12-month minimum follow-up in group with a complete zoledronic acid therapy and group with no therapy
Fig. 2.
QLQ-30 and MY score results after a 12-month minimum follow-up in group with a complete zoledronic acid therapy and group with no therapy
According to these data a better clinical outcome and quality of life is observed in the group with a complete therapy with zoledronic acid. Finally, we observed too an empirical reduction of analgesics’ consumption and a faster disposal of orthesis in the group with a complete zoledronic therapy compared with the other group. No cases of osteonecrosis of the jaw were reported.
Discussion
Multiple myeloma is characterized by the accumulation of malignant plasma cells in the bone marrow followed by impaired haematopoiesis and bone disease, which includes mainly lytic lesions, pathological fractures, hypercalcaemia and osteoporosis [10]. Increased osteoclast activity plays a major role in the development of bone impairment. Osteoclast activation and proliferation is due to the interactions between myeloma cells and bone marrow microenvironment, which lead to an increased bone resorption through the production of different cytokines with OAF (osteoclasts activating function) activity, mostly through the up-regulation of RANKL expression, the inhibition of the osteoprothegerin (OPG) and the over expression of IL-6 [10]. MM presents also typical osteoblast inhibition. Silvestris et al. [11] suggested that, in the myeloma bone microenvironment, both high cytokine levels and physical interaction between malignant plasma cells with osteoblasts are responsible for the accelerated apoptosis of osteoblasts and the defective new bone formation. The presence of this double mechanism, in which both osteoclasts and osteoblasts are involved, could explain why myeloma bone lesions do not heal even in patients in complete remission. There is a complete change in the marrow microenvironment, which maintains the bone effect of the MM cells also in their absence. The BPs have demonstrated a double effect on bone lesions both on osteoclast inhibition as on myeloma cell apoptosis. They inhibit osteoclast recruitment and maturation, prevent the development of monocytes into osteoclasts, induce osteoclasts apoptosis and interrupt their attachment to the bone [10]. In addition, several studies demonstrated their effect on normalisation of cytokines patterns, which helps to interrupt several feedback loops that would otherwise potentiate the growth of the malignant clone and further osteoclast development and activity [12, 13]. A particular inhibition effect is recognised on the production of IL-6, the most potent survival factor for the MM clone, from the diseased bone-marrow stroma. This inhibition has been shown to occur with pamidronate and zoledronate, suggesting an inhibitory effect at concentrations of 1 μmol/L or less [14, 15]. The most complete overall analysis of the effects of BPs on multiple myeloma has been published in a Cochrane review, in which was demonstrated a reduction of the absolute risk of vertebral fracture and experiencing of pain by 10 and 9%, respectively [16]. In this systematic review clodronate and pamidronate are currently the preferred agents according to the guidelines produced by the British Committee for Standards in Haematology [17]. However, the clinical practice guidelines of the American Society of Clinical Oncology [6] recommend the use of either intravenous pamidronate or zoledronate as the treatments of choice and suggest the continuation of treatment until a significant decline in performance status occurs. Discussed in medical literature remain the optimal starting point of treatment with BPs. Both guidelines recommend treatment with BPs once the diagnosis of multiple myeloma is made or in presence of severe osteopenia or after lytic lesions of the bone have been identified [6]. The recognized goal of BP therapy is to prevent the onset and recurrence of bone involvement, to palliate bone pain, to reduce use of analgesics and palliative therapies such as surgery and radiation to the bone. BP therapy presents several advantages compared with other palliative therapies. They are not limited to an anatomic site but dispersed systemically and delivered at sites of active bone remodelling are able to attach rapidly to mineralized bone surfaces [18, 19]. Another advantage is that BPs can be used in combination with other anticancer treatments, without an associated increase in myelotoxicity [12]. It has been demonstrated that treatment with 4 mg zoledronic acid significantly decreased the proportion of patients who required palliative bone radiotherapy and analgesic use [20]. Our preliminary data seem to confirm this finding; the group with a complete administration of zoledronic therapy reports a minor use of analgesics, shorter radiation therapy cycles and a better quality of life as showed by the self-administered questionnaire results. The mechanism of action enables BPs to stop bone resorption, strengthening bone and providing an overall improvement in bone pain. Different classes of BPs have different abilities to protect against skeletal-related events (SKEs), but nitrogen-containing BPs and particularly zoledronic acid present considerable higher efficacy in this regard and it may provide benefits to patients for up to 2 years of therapy, even after the onset of SREs [21, 22]. Attractive is the possible use of zoledronic acid not only to control bone pain but also to prevent future SREs, in this prospective crucial became to clarify when to start BPs therapy. The starting point of BP therapy plays a key role in increasing its clinical efficacy. In our series, we observed that there was no univocal indication about the use of the drug, the optimal starting time of administration and duration of the therapy, and this aspect may have influenced our results. Despite the great variability of the protocols of therapy used, the effect of the zoledronate on the amelioration of the quality of life and on the control the number of SKEs remains unquestioned. In fact skeletal lesions in the treatment group remained unchanged while a significant number of new lesions (from 37 to 50) occurred in the no-therapy group during the time of surveillance. Certainly the most surprising aspects emerging from our work regard the great differences of treatments. The lack of widely accepted guidelines at the start of our survey together with a certain degree of concern of possible adverse events (AEs) of medical therapy may have induced a less aggressive behavior by the haematologists. Potential AEs from BPs therapy for MM include inflammatory reactions at the injection site, acute phase reactions following i.v. use, hyperthermia and hypocalcemia. Additionally, renal impairment and avascular osteonecrosis of the jaw are infrequent but serious complications of this treatment [23]. In particular, this last event, more than others seems to be a main concern: not so much how often it occurs, rather the severity of the consequences. Concerns about these complications are justified but not sufficient to limit the use of such an effective therapy. Physicians should be proactive in asking patients about AE symptoms in monitoring typical indicators of serious problems while selecting patients considering their renal and hepatic function and especially their odontostomathologic status.
Conclusion
Bisphosphonate therapy proved to be effective in the treatment of bone localizations in multiple myeloma in our series. The quality of life of patients who received zoledronate was significantly better than that of patients who did not. The drug seems to be effective too in limiting the aggressiveness of the disease. In fact, in the treated group only two new skeletal lesions occurred during the short surveillance time compared with the seven cases in the no-treatment group. Results observed in this study are only preliminary, and so it is difficult to assure a certain effect of zoledronic acid to prevent new osteolytic lesions. The major limits of the present study are the short time of observation and the amount of the samples. Although the efficacy of the drug is indisputable in improving clinical condition of patients with bone lesions other prospective studies are needed particularly to elucidate its role in preventing skeletal-related events in patients without bony lesions.
Conflict of interest
None.
References
- 1.Mundy GR, Bertoline DR. Bone destruction and hypercalcemia in plasma cell myeloma. Semin Oncol. 1986;13:291–299. [PubMed] [Google Scholar]
- 2.Yeh HS, Berenson JR. Treatment for myeloma bone disease. Clin Cancer Res. 2006;12:6279s–6284s. doi: 10.1158/1078-0432.CCR-06-0681. [DOI] [PubMed] [Google Scholar]
- 3.Rodmann GD. Pathogenesis of myeloma bone diseases. Leukemia. 2009;23:435–441. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- 4.Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–1575. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Lacerna L, Hohneker J. Zoledronic acid for the treatment of bone metastases in patients with breast cancer and other solid tumors. Semin Oncol. 2003;30:150–160. doi: 10.1053/j.seminoncol.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Berenson JR, Hillner BE, Kyle RA, et al. American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2002;20:3719–3736. doi: 10.1200/JCO.2002.06.037. [DOI] [PubMed] [Google Scholar]
- 7.Body JJ, Mancini I. Bisphosphonates for cancer patients: why, how and when? Support Care Cancer. 2002;10:399–407. doi: 10.1007/s005200100292. [DOI] [PubMed] [Google Scholar]
- 8.Knop C, Oeser M, Bastian L, Lange U, Zdichavsky M, Blauth M. Development and validation of the visual analogue scale (VAS) spine score. Unfallchirurg. 2001;104(6):488–497. doi: 10.1007/s001130170111. [DOI] [PubMed] [Google Scholar]
- 9.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organitation for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 10.Terpos E, Dimopoulos MA. Myeloma bone disease pathophisiology and management. Ann Oncology. 2005;16:1223–1231. doi: 10.1093/annonc/mdi235. [DOI] [PubMed] [Google Scholar]
- 11.Silvestris F, Cafforio P, Calvani N, Dammacco F. Impaired osteoblastogenesis in myeloma bone disease: role of upregulated apoptosis by cytokines and malignant plasma cells. Br J Haematol. 2004;126:475–486. doi: 10.1111/j.1365-2141.2004.05084.x. [DOI] [PubMed] [Google Scholar]
- 12.Gordon S, Helfrich MH, Sati HI, et al. Pamidronate causes apoptosis of plasma cells in vivo in patients with multiple myeloma. Br J Haematol. 2002;119:475–483. doi: 10.1046/j.1365-2141.2002.03824.x. [DOI] [PubMed] [Google Scholar]
- 13.Croucher PI, Hendrik R, Perry MJ, et al. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res. 2003;18:482–492. doi: 10.1359/jbmr.2003.18.3.482. [DOI] [PubMed] [Google Scholar]
- 14.Savage AD, Belson DJ, Vesico RA et al (1996) Pamidronate reduces IL-6 production by bone marrow stroma from myeloma patients. Blood 88:105A
- 15.Derenne S, Amiot M, Barille S, et al. Zoledronate is a potent inhibitor of myeloma cell growth and secretion of IL-6 and MMP-1 by the tumoral environment. J Bone Miner Res. 1999;14:2048–2056. doi: 10.1359/jbmr.1999.14.12.2048. [DOI] [PubMed] [Google Scholar]
- 16.Djulbegovic B, Wheatley K, Ross J et al (2002) Bisphosphonates in multiple myeloma (Cochrane Review). Cochrane Database Syst Rev 3:CD003188 [DOI] [PubMed]
- 17.British Committee for Standards in Haematology (UK Myeloma Forum) Diagnosis and management of multiple myeloma. Br J Haematol. 2001;115:522–540. doi: 10.1046/j.1365-2141.2001.03206.x. [DOI] [PubMed] [Google Scholar]
- 18.Licata AA. Discovery, clinical development, and therapeutic uses of bisphosphonates. Ann Pharmacother. 2005;39:668–677. doi: 10.1345/aph.1E357. [DOI] [PubMed] [Google Scholar]
- 19.Hansen-Algenstaedt N, Johschek C, Wolfram L, et al. Sequential changes in vessel formation and micro-vascular function during bone repair. Acta Orthop. 2006;77:429–439. doi: 10.1080/17453670610046361. [DOI] [PubMed] [Google Scholar]
- 20.Sabino MA, Mantyh PW. Pathophysiology of bone cancer pain. J Support Oncol. 2005;3:15–24. [PubMed] [Google Scholar]
- 21.Hirsh V, Tchekmedyian NS, Rosen LS, Zheng M, Hei YJ. Clinical benefit of zoledronic acid in patients with lung cancer and other solid tumors: analysis based on history of skeletal complications. Clin Lung Cancer. 2004;6:170–174. doi: 10.3816/CLC.2004.n.030. [DOI] [PubMed] [Google Scholar]
- 22.Costa L, Major PP. Effect of bisphosphonates on pain and quality of life in patients with bone metastases. Nat Clin Prat Oncol. 2009;6:163–174. doi: 10.1038/ncponc1323. [DOI] [PubMed] [Google Scholar]
- 23.Terpos E, Sezer O, Croucher PI et al (2009) The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol 20:1303–1317 [DOI] [PubMed]