Abstract
Purpose
The aim of the study was to evaluate clinical and radiographic outcome of patients treated with a modified Grob technique analysing the advantages related to increased mechanical stability.
Methods
30 patients that underwent “in situ” fusion for L5-S1 spondylolisthesis were evaluated. All patients presented a low-dysplastic developmental L5-S1 spondylolisthesis. Patients were divided into two groups: A, in which L5-S1 pedicle instrumentation associated with transsacral screw fixation was performed, and B, in which L5-S1 pedicle instrumentation associated with a posterolateral interbody fusion (PLIF) was performed.
Results
Patients treated with transdiscal L5-S1 fixation observed a faster resolution of the symptoms and a more rapid return to daily activities, especially at 3–6 months’ follow-up. The technique is reliable in giving an optimal mechanical stability to obtain a solid fusion.
Conclusions
The advantages of this technique are lower incidence of neurologic complications, speed of execution and faster return to normal life.
Keywords: Spondylolisthesis, Surgical technique, Fusion, Back pain, Radicular pain
Introduction
The optimal treatment of high-grade spondylolisthesis is a controversial issue. It is well known that symptomatic high-grade slip, resistant to conservative management requires surgical stabilization [1, 2]. Good results may be obtained in low-dysplastic spondylolisthesis with “in situ” fusion [3]. In this type of spondylolisthesis a reduction is generally unnecessary to obtain good biomechanical and neurologic recovery. In situ fusion is a relatively safe and reliable procedure associated with a high rate of arthrodesis and at lower risk of neurologic injury [4, 5]. The main argument against reduction manoeuvers in spondylolisthesis is the high incidence of neurologic complications, as high as 31% [6]. Different surgical procedures could be used to obtain “in situ” spondylodesis: posterolateral fusion with or without instrumentation, posterior interbody fusion, combined anterior and posterior procedures and circumferential 360° fusion. Circumferential fusion as showed by Lamberg et al. [7], had better long-term results than isolated posterolateral fusion or anterior fusion alone. A few posterior surgical techniques could be used to achieve circumferential stabilization, including transvertebral pedicle screw fixation [8], posterior transsacral interbody fusion using a cortical bone graft with pedicle screw implantation [1], posterior interbody cage and pedicle screw fixation [9] and a posterior pediculo-body fixation alone or associated with fusion at the superior level to obtain a greater mechanical stability [10]. The last one technique combines the possibility of a three-column stabilization with the simplicity and speed of fixation; but presents limited indications: a significant reduction in the height of the interposed disc, a vertebral slippage of at least 25% and a good balance of the spine in the sagittal plane.
The aim of the study was to illustrate our modifications to Grob technique and to analyse the 5-year results obtained with this modified technique into surgical treatment of low-dysplastic L5-S1 spondylolisthesis compared with traditional posterior lumbar interboby fusion.
Methods
From January 2005 to September 2010, 30 patients underwent “in situ” fusion for L5-S1 spondylolisthesis. All patients presented a low-dysplastic developmental L5-S1 spondylolisthesis characterized from low back pain and leg pain and had a failed trial of conservative management. In all patients a pre-operative radiographic analysis with full length, plain, dynamic X-rays of the spine was performed. Spinopelvic parameters as pelvic tilt, sacral slope and pelvic incidence were analysed with dedicated software (Kodak DirectView Picture Archiving and Communication System). Severity index was also calculated following La Martina criteria [11]. A horizontal line was drawn through the centre of S2 on a standing lateral radiograph of the lumbar spine that includes the hips. A vertical line is drawn through the centre of the femoral heads. A second, vertical line is drawn through the middle of L5 inferior end plate. The distance from the centre of S2 to the vertical of the centre of the femoral heads is D2; the distance from the vertical of the middle of L5 inferior end plate to the vertical of the centre of the femoral heads is D1. The SI is calculated as follows: SI = D1 × 100/D2. A neuroradiological analysis with an MRI of the lumbar spine was also performed to evaluate neurological structures and L5-S1 disc degeneration according to Pfirrmann criteria [12]. Inclusion criteria were low back and radicular pain resistant to medical and physical treatment, a low-dysplastic developmental L5-S1 spondylolisthesis with severity index ≤20%, a L5 vertebral slip >25%, a Pfirrmann grade between IV b and V. Patients were divided in two groups: group A (15 patients) in which L5-S1 pedicle instrumentation associated with transsacral screw fixation was performed and group B (15 patients) in which a L5-S1 pedicle instrumentation associated with a posterolateral interbody fusion (PLIF) was performed. In both groups a full decompression and a posterolateral fusion were performed. For each group, we analyzed surgical time, intra-operative blood loss, perioperative complications and radiographical parameters. Pain was evaluated with the Visual Analogue Scale (VAS) preoperatively at 1 month, 3, 6 and 12 months and annually postoperatively. The “Short-Form 36 General Health Survey” was assessed preoperatively at 1 month, 3 months, 6 months, 12 months and annually after surgery. Standard X rays were performed at 30 days, 3, 6 and 12 months and annually postoperatively. All data were recorded and statistically analysed in a retrospective way.
Statistics
Descriptive statistics were calculated. The results obtained were analysed using the student’s t Test and χ2 Test and verified with Fisher’s exact test. Significance was accepted at p < 0.05. There are some limitations that need to be acknowledged and addressed regarding the present study. The number of cases is too limited for broad generalizations. Further empirical evaluations and greater patients’ series are needed to validate the present results.
Surgical technique
Group A
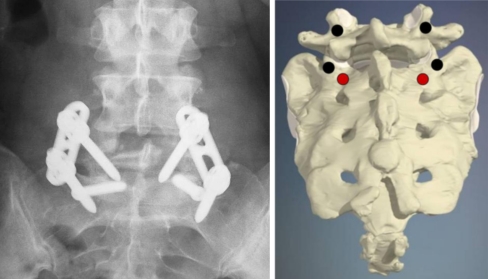
All patients were placed in a prone position on a carbon fibre operating table to have an optimal fluoroscopic visualization of the involved spine in the antero-posterior and lateral views. A lumbosacral longitudinal incision was made and a bilaterally subperiosteal dissection of the paravertebral muscles was performed to expose the affected level. Extensive decompression was performed and the affected nerve root decompressed adequately. The entry point of transdiscal screw was near S2 nerve root on the body of S1 and was identified 1 cm distally and 1 cm medially with respect to the standard S1 pedicle screw entry point. The drill was passed under fluoroscopic guidance into the S1 vertebral body, than traversing through L5-S1 disc space, and then into the L5 vertebral body. An AO 6.5 cancellous bone screw of appropriate length (Synthes Raynham, MA, USA) was implanted. (Fig. 1). The same procedure was repeated on the other side to stabilize the slip between L5 and S1. This posterior transdiscal L5-S1 fixation was implemented with pedicle screw instrumentation at L5 and S1 (EXPEDIUM SYSTEM, DePuy Spine, Raynham, MA, USA) and with a posterolateral fusion with autologous iliac crest bone graft, obtaining “in situ” fusion (Figs. 2.1, 2.2, 2.3, 3.1, 3.2).
Fig. 1.
Schematic view of the screw entry point in the Grob modified technique 1 cm medially and 1 cm inferiorly, the S1 pedicle screw and correspondent view in anteroposterior X-ray
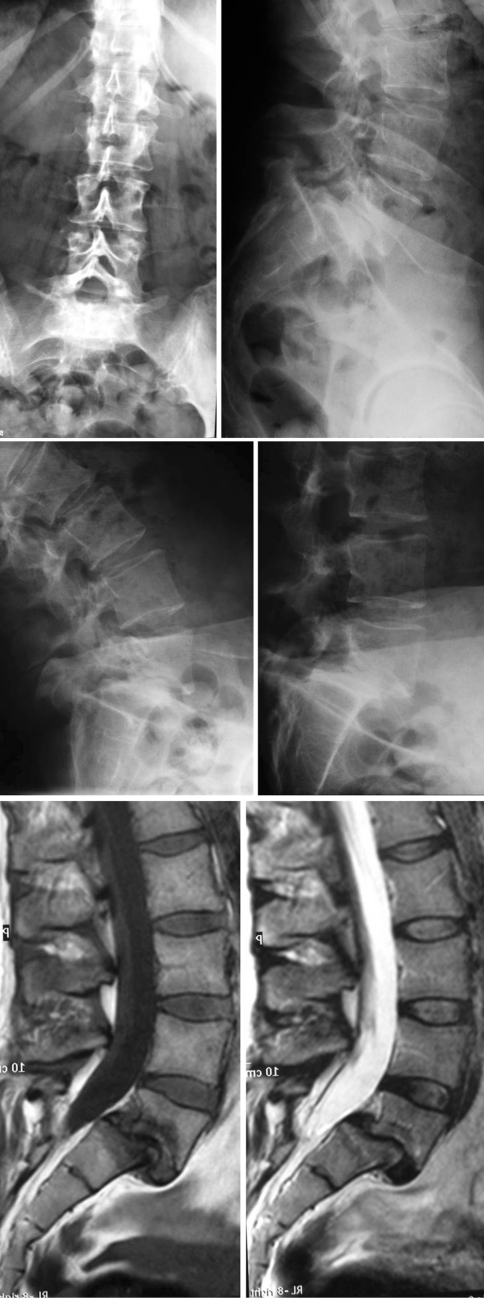
Fig. 2.
1 Standard A-P and LL X ray showing a L5-S1 low dysplastic developmental spondylolisthesis. 2 X ray at maximum flexion and extension. 3 Sagittal view MRI showing a degenerated L5-S1 (Pfirrmann V) intervertebral disc
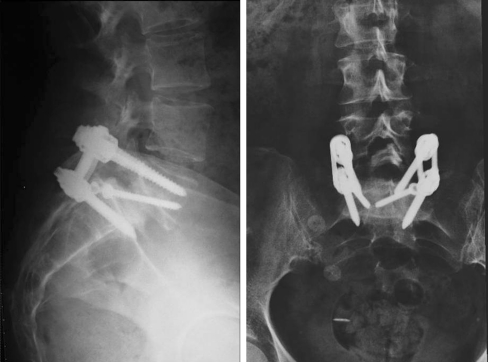
Fig. 3.
Anteroposterior and lateral view of post-operative L5-S1 low-dysplastic developmental spondylolisthesis X-ray treated with Grob modified technique
Group B
The same standard posterior approach described for the group A was used. Under fluoroscopic control four pedicle screws were inserted in L5 and S1 (EXPEDIUM SYSTEM, DePuy Spine, Raynham, MA, USA). Then a posterior L5 bilateral laminectomy and a L5-S1 discectomy were performed. The epiphyseal plates of the involved level were prepared and two interbody cages (Faber DePuy Spine, Raynham, MA, USA) filled with autologous bone graft were introduced using posterolateral approach. The rods were fixed to the screws in a compressive way. A posterolateral fusion with autologous iliac crest bone graft was performed in all patients.
Results
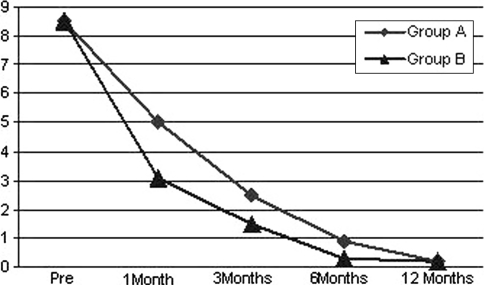
There were 17 males (56.6%) and 13 females (44.3%); the mean age was 52.5 years (range 33–69). All patients were affected by L5-S1 developmental spondylolisthesis with lysis in 16 cases and with pars interaricularis elongation in 14 cases. The mean operative time was 135 min (range 75–190) in group A versus 400 min (range 180–375) in group B. The mean intra-operative blood loss was 290 cc (range 210–370) in group A versus 520 cc (range 390–980) in group B. Both groups observed an improvement in radicular pain; however, in the first group we observed a faster resolution of the symptoms and a more rapid return to daily activities, especially at 3–6 months’ follow up. The mean follow-up was 3 year (min 1–max 5 years). A confirmation of this trend could be seen in VAS and SF-36 results with a faster decrease in the obtained values yet to one month’s follow-up. In the first group the average pre-operative VAS score was 8.5 (range 7–9.5) decreased to 5(range 3–6) at one month’s follow-up; to 2.5(range 1–3.5) at 3 months follow-up, to 0.9 (range 0–2) at 6 months’ follow-up; to 0.2 (range 0–0.5) at 12 months minimum follow-up (p = 0.003) (Fig. 4). In group B the average pre-operative VAS score was 8.5 (range 7–9.5) at base time, decreased to 3.1 (2.5–4.5) at 1 month’s follow up, a further decrease was seen at 3-, 6- and 12 months’ follow-up with a mean value, respectively, of 1.5 (range 1–3); 0.3 (range 0–1); 0.2 (0–0.5) (p = 0.004).
Fig. 4.
Visual Analogue Scale score results after a 12-months’ follow-up; patients (n = 15) group A and B
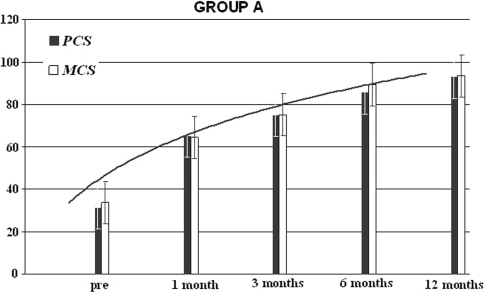
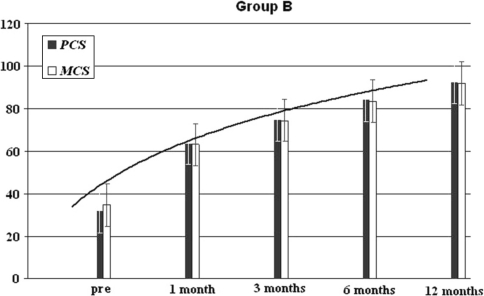
The Short-Form 36 Physical Health in the group A was equal to 35% (range 22–48%), to 64% (range45–52%) at 1 month, of 75.4% (65.3–86.4%) after 3 months, of 82% (71.4–92.2%) after 6 months and of 92% (83–98%) after 12 months’ minimum follow-up (p < 0.001) (Fig. 5). The Short-Form 36 Physical Health in the group B was equal to 28% (range 23–41%) at base time, to 43% (range 35–55%) at 1 month, of 60% (range 53–71%) after 3 months, of 75% (range 59–85%) at 6 months and of 85% (69–91%) (p = 0.003) at 12 months’ minimum follow-up (Fig. 6).
Fig. 5.
Group A results of the SF-36 physical and mental component after a 12-months’ follow-up; patients (n = 15)
Fig. 6.
Group B results of the SF-36 physical and mental component after a 12-months’ follow-up; patients (n = 15)
The Short-Form 36 Mental Health was in the group A of 38% (range 35–43%) at base time, of 64% (range54–71%) at 1 month, of 76% (range 71–83%) at 3 months, of 90% (range 82–94%) at 6 months and of 92% (83–97%) at 12 months’ minimum follow-up, p < 0.001 (Fig. 3). The Short-Form 36 Mental Health SF-36 mental was in the group B of 35% (range 28–46%) at base time, of 46% (range31–67%) at 1 month, of 62% (range 48–73%) at 3 months, of 78% (range 66–91%) at 6 months and at 85% (69–91%) at 12 months minimum follow-up p = 0.005 (Fig. 4).
The severity index was in group A 19.2% at base time unchanged at 1-year medium follow-up, 19.7% at base time in the group B unchanged at 1-year minimum follow-up.
The pre-operative spinopelvic parameters were unchanged in both groups at 1-year medium follow-up. In group A the mean pre-operative SS was 42.2° (range 38–65°) and the mean pre-operative PT was 27.7° (range 16–32°) unchanged at 1-year minimum follow-up. In the group B the mean pre-operative SS was 46.3° (range 37–68°) and the mean pre-operative PT was 25.9° (range 15–34° unchanged at 1-year minimum follow-up.
We observed one deep wound infection in the group A, which required revision surgery and one superficial wound infection in the group B resolved with antibiotic therapy administration. In group A one S1 pedicle screw misplacement was observed in a patients showing radicular leg pain which required revision surgery. In group B we observed one transitory L5 neurological deficit resolved with physiotherapy. No hardware failure, or slip increase was observed.
Discussion
The most useful classification system for spondylolisthesis, which gives also information about prognosis and therapy, is that of Marchetti and Bartolozzi [13]. In this system, spondylolisthesis is divided in two major groups, developmental and acquired. Developmental are divided in two subgroups: low dysplastic and high dysplastic, including that with lysis and elongation. The low-dysplastic type is characterized by normal S1 and L5 vertebral shape, a normal lumbosacral profile and a balanced pelvis without retroversion. Because of the absence of bony morphologic changes and of spinopelvic inbalance this type of spondylolisthesis is at lower risk of slip progression compared with high-dysplastic [14]. Spinopelvic imbalance can modify the biomechanical load at the lumbosacral junction and creates compensatory mechanism to maintain adequate posture and gait. In clinical practice is difficult to differentiate low-dysplastic from high dysplastic spondylolisthesis, especially in young subjects [11]. A first attempt to differentiate these two pathologies was made by Vidal and Marnay introducing the Index C (couple-charnière) [14]. This index was a calculation of the opposing torque generated by the anterior displacement of the hips consequent to the loosening of the auditory meatus travel, L5-S1 and the centre of femoral head alignment. In 2009 Lamartina et al. [11] gave a numerical value to this torque introducing the Severity Index (SI) obtained as follows: SI = D1 × 100/D2. The SI became a simple criterion in the characterization and assessment of slip progression and in differentiating low from high dysplastic spondylolisthesis. As demonstrated by Vidal and Marney [14] a SI <20% is present in normal subjects and also in low-dysplastic spondylolisthesis patients since there was no pelvic retroversion. A SI >20% and pelvic retroversion characterized the high-dysplastic spondylolisthesis. In this type of spondylolisthesis reduction is mandatory to restore the spine physiological alignment, the sagittal balance and to correct the pelvic retroversion, avoiding non union and slip progression seen in this group with an “in situ” fusion [15]. Reduction is gravened by a great risk of neurologic complication with an incidence of 31% [6]. In low-dysplastic developmental spondylolisthesis reduction is unnecessary since there is no pelvic retroversion and sagittal unbalance [11]. “In situ” fusion has reported in this type of spondylolisthesis satisfactory clinical outcomes and good fusion rate. [11, 16]. Different surgical techniques to obtain “in situ” fusion have been described. Grob et al. [10] suggest a pediculo-body fixation with two cancellous screws inserted from the S1 pedicle to the L5 vertebral body. This technique was also reported by Zagra et al. with satisfactory long-term outcome [17]. Bartolozzi et al. [9] described an in situ interbody fusion with a titanium cage inserted according to the Bohlman and Cook [18] technique by a transacral approach associated with pedicle screw fixation. In this study we introduce a modification to Grob technique obtaining a new “in situ” fusion. The modification was introduced to obtain a higher mechanical stability using a six-screw fixation at one level. We modified the entry point of the trandiscal-transvertebral screw. The entry point of transdiscal screw has to be identified meticulously 1 cm medially and 1 cm distally to S1 pedicle screw entry point to avoid impingement between these screws. The identification of the sacral foramen is mandatory to avoid neurologic complications due to transdiscal screw misplacement. The analysis of our data showed a great reliability of this surgical technique, a lower operative time, lower intraoperative blood loss resulting in better clinical outcome and faster return to normal day-life compared with traditional interbody fusion group. At 5-year follow-up we observed no hardware failure. The complications were not statistically significant. The success of this technique is due to the correct indication: a SI <20%, a slip >25% and a Pfirrmann grade of at least IVB.
Conclusion
The analysis of the 5-year follow-up data of low-dysplastic developmental spondylolisthesis with this modified technique gave satisfactory results. The technique is reliable in giving an optimal mechanical stability to obtain a solid fusion. The advantages are the lower incidence of neurologic complications, the speed of execution and the faster return to normal life of treated patients. The limits of the study are the retrospective analysis and the small number of cases.
Conflict of interest
None.
References
- 1.Boxall D, Bradford D, Winter R, et al. Management of severe spondylolisthesis. J Bone Joint Surg Am. 1979;61:479–495. [PubMed] [Google Scholar]
- 2.Bradford D. Spondylolysis and spondylolisthesis. Curr Pract Orthop Surg. 1979;8:12–37. [PubMed] [Google Scholar]
- 3.Harris IE, Weinstein SL. Long-term follow-up of patients with grade III and IV spondylolisthesis. Treatment with and without posterior fusion. J Bone Joint Surg Am. 1987;69:960–969. [PubMed] [Google Scholar]
- 4.Watkins M. Posterolateral fusion of the lumbar and lumbosacral spine. J Bone Joint Surg Am. 1953;35:1014–1018. [PubMed] [Google Scholar]
- 5.Harris I, Weinstein S. Long-term follow-up of patients with grade III and IV spondylolisthesis. J Bone Joint Surg Am. 1987;71:1098–1106. [PubMed] [Google Scholar]
- 6.Bradford D, Gotfried Y. Staged salvage reconstruction of grade IV and V spondylolisthesis. J Bone Joint Surg Am. 1987;69:191–202. [PubMed] [Google Scholar]
- 7.Lamberg T, Remes V, Helenius I, et al. Uninstrumented in situ fusion for high-grade childhood and adolescent isthmic spondylolisthesis: long-term outcome. J Bone Surg Am. 2007;89:512–518. doi: 10.2106/JBJS.E.00545. [DOI] [PubMed] [Google Scholar]
- 8.Abdu WA, Wilber RG, Emery SE. Pedicular transvertebral screw fixation of the lumbosacral spine in spondylolisthesis. A new technique for stabilization. Spine (Phila Pa 1976) 1994;19:710–715. doi: 10.1097/00007632-199403001-00011. [DOI] [PubMed] [Google Scholar]
- 9.Bartolozzi P, Sandri A, Cassini M, Ricci M. One-stage posterior decompression-stabilization and trans-sacral interbody fusion after partial reduction for severe L5-S1 spondylolisthesis. Spine (Phila Pa 1976) 2003;28:1135–1141. doi: 10.1097/01.BRS.0000067274.38273.5C. [DOI] [PubMed] [Google Scholar]
- 10.Grob D, Humke T, Dvorak J. Direct pediculo-body fixation in cases of spondylolisthesis with advanced intervertebral disc degeneration. Eur Spine J. 1996;5:281–285. doi: 10.1007/BF00301335. [DOI] [PubMed] [Google Scholar]
- 11.Lamartina C, Zavatsky M, Petruzzi M, Specchia N. Novel concepts in the evaluation and treatment of high-dysplastic spondylolisthesis. Eur Spine J. 2009;18:S133–S142. doi: 10.1007/s00586-009-0984-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfirrmann CV, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;17:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 13.Marchetti PG, Bartolozzi P. Classification of spondylolisthesis as a guideline for treatment. In: Bridwell K, DeWald R, editors. The textbook of spinal surgery. 2. Philadelphia: Lippincott-Raven; 1997. pp. 1211–1254. [Google Scholar]
- 14.Vidal J, Marnay T. Morphology and anteroposterior body equilibrium in spondylolisthesis L5/S1. Rev Chir Orthop. 1983;69:17–28. [PubMed] [Google Scholar]
- 15.Umehara S, Zindrick MR, Patwardhan AG, et al. The biomechanical effect of postoperative hypolordosis in instrumented lumbar fusion on instrumented and adjacent spinal segments. Spine. 2000;25:1617–1624. doi: 10.1097/00007632-200007010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Moller H, Hellund R. Surgery versus conservative management in adult isthmic spondylolisthesis: a prospective randomized study part 1. Spine. 2000;25:1711–1715. doi: 10.1097/00007632-200007010-00016. [DOI] [PubMed] [Google Scholar]
- 17.Zagra A, Giudici F, Minoia L, Corriero AS, Zagra L. Long-term results of pediculo-body fixation and posterolateral fusion for lumbar spondylolisthesis. Eur Spine J. 2009;18:S151–S155. doi: 10.1007/s00586-009-0997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohlman HH, Cook SS. One-stage decompression and posterolateral interbody fusion for lumboscral spondyloptosis through a posterior approach: report of two cases. J Bone Joint Surg Am. 1982;64:415–418. [PubMed] [Google Scholar]