Abstract
Uneconomical choices by humans or animals that evaluate reward options challenge the expectation that decision-makers always maximize the return currency. One possible explanation for such deviations from optimality is that the ability to sense differences in physical value between available alternatives is constrained by the sensory and cognitive processes for encoding profitability. In this study, we investigated the capacity of a nectarivorous bat species (Glossophaga commissarisi) to discriminate between sugar solutions with different concentrations. We conducted a two-alternative free-choice experiment on a population of wild electronically tagged bats foraging at an array of computer-automated artificial flowers that recorded individual choices. We used a Bayesian approach to fit individual psychometric functions, relating the strength of preferring the higher concentration option to the intensity of the presented stimulus. Psychometric analysis revealed that discrimination ability increases non-linearly with respect to intensity. We combined this result with a previous psychometric analysis of volume perception. Our theoretical analysis of choice for rewards that vary in two quality dimensions revealed regions of parameter combinations where uneconomic choice is expected. Discrimination ability may be constrained by non-linear perceptual and cognitive encoding processes that result in uneconomical choice.
Keywords: Nectarivory, Bat, Psychometric function, Uneconomical choice
Introduction
Value-based decision-making requires that the value of an option can be sensed and stored in memory. This makes a comparison between options possible. Decision-making processes can sometimes lead to outcomes that are not economical (Livnat and Pippenger 2008). This is the case when the decision-maker prefers an option with a lower caloric return over another with a higher caloric return, or when it fails to discriminate between options with different caloric contents. In order to understand how underlying mechanisms can lead to uneconomical choices, it is necessary to have a quantitative understanding of the steps involved in the evaluation process. Sensing the caloric value of a reward, and being able to discriminate between rewards, is the first step in such a process and the objective of our study.
Such considerations about value-based decision-making are also relevant for understanding the co-evolutionary development of energy rewards offered by plants to their pollinators. This is relevant in the context of this study, in which we investigated choice behavior of nectar-feeding bats. The evolution of flower traits in animal-pollinated plants is shaped in part by the selection pressure to offer attractive energy resources to potential pollen vectors (Zimmerman 1983; Real and Rathcke 1991; Sakai 1993). Among pollinator attractants, the most common are simple carbohydrates presented as nectars, i.e., sugar–water solutions (Baker and Baker 1983; Stiles and Freeman 1993). From the pollinators’ perspective, nectars with higher sugar concentrations represent richer energy sources that should be preferred by foragers seeking to optimize their energetic gains. Diverse groups of nectar-feeding animals such as bees (e.g., von Frisch 1927; Cnaani et al. 2006), birds (e.g., Hainsworth and Wolf 1976; Roberts 1996; Nicolson and Fleming 2003), and bats (e.g., Roces et al. 1993; Rodríguez-Peña et al. 2007) show a general pattern of preference for sweeter sugar solutions and more precise discrimination at low concentrations. In the case of nectar-feeding (glossophagine) bats, preference has been estimated by measuring differences in bat visitation and consumption rates at food sources with differing nectar qualities. However, our knowledge on the ability of bats to discriminate between nectar concentrations remains fragmentary and inconclusive, even though this ability has direct consequences on decision-making and on the selection pressures exerted by the bats.
A standardized method for estimating discrimination ability is fitting a psychometric function to data from alternative forced-choice tasks (Treutwein and Strasburger 1999). The psychometric function relates the behavioral response of the animal to the intensities of the physical stimuli. This methodology has been applied to estimate the ability of the flower-visiting and nectar-feeding bat Glossophaga soricina to discriminate between two volumes (Toelch and Winter 2007). Measuring the volume of nectar obtained from a feeding event is one necessary component for reward evaluation. With this study, we determined the second necessary component for a nectar-feeding animal: the ability to evaluate the concentration of sugar in a nectar reward. For this, we performed a psychometric analysis of concentration discrimination ability in a nectarivorous bat species. We obtained our data from a two-alternative free-choice test performed with a group of wild, free-flying Glossophaga commissarisi bats.
Methods
Study site and subjects
Experiments were conducted from February to April 2009, at La Selva Biological Station, Province Heredia, Costa Rica. Wild bats were initially recruited to visit the experimental site by setting up nectar feeders filled with 20% sugar solution and equipped with a dimethyl disulfide reservoir giving off odor plumes to act as a far-range attractant to the bats (von Helversen et al. 2000). Feeders were mounted on an aluminum rectangular frame (2 × 4 m), suspended below a 3 × 6-m steel frame canopy, which provided cover from the rain. The frame was parallel to and 1.6 m above the ground. Using mist-nets we caught and marked 63 adult individuals, 39 males, and 24 females, of the common (Tschapka 1998) nectarivore Glossophaga commissarisi Gardner. Bats were weighed, sexed, marked with RFID collars, and released at the site of capture. Over the course of the study, 54 of these bats were registered visiting the artificial flowers, along with an unknown number of unmarked bats and other visitors. Permission for experimentation and RFID-tagging was obtained from Sistema Nacional de Areas de Conservación (SINAC) at the Ministerio de Ambiente y Energía (MINAE).
Artificial flowers
For the purpose of this study, a model was developed that incorporates some characteristics of typical Neotropical chiropterophilic plants, e.g., the bromeliad Werauhiagladioliflora, a common bat-pollinated plant in the area of this study (Tschapka and von Helversen 2007). Individual plants were represented by computer-controlled feeders (Winter and Stich 2005; Santoso et al. 2006; Thiele 2006), which delivered sugar water (hereafter “nectar”). We used twenty-four feeders mounted under the steel frame canopy. The distance between flower “corollas” in the same row was about 40 cm and the distance between rows about 60 cm. Such plant density is not unusual for W. gladioliflora (personal observation). The control computer, hardware interface, power supply units, and nectar reservoirs were all placed in an air-conditioned shed, some 5 meters away from the canopy set-up and connected to it via signal cables, power leads, and main nectar tubes. Visits to the feeders were registered with an infrared beam detector, and transponder-reading devices identified individuals carrying RFID tags. Each feeder was equipped with two solenoid pinch valves and connected to two nectar delivery systems via tubing systems (Fig. 1). Nectar reward delivery was controlled by two syringe pumps using two gas-tight Hamilton glass syringes (Series 1025). Feeders delivered 55–60 μL rewards on every visit.
Fig. 1.
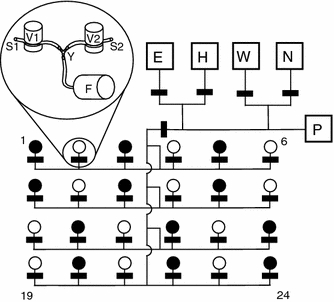
Pump and tubing system of the 24-feeder array. Lines represent the tubes, and black rectangles the pinch valves. Feeders are numbered 1–24. Boxes represent the following liquid reservoirs: ethanol (E), water (H), waste (W), nectar (N), and stepping-motor syringe pump (P), as described in Winter and Stich (2005). Length of tubes not drawn to scale. Two identical tubing systems were connected to the feeders. The merging point of the tubing systems is illustrated in the inset: magnetic pinch valves for the first (V1) and second tubing systems (V2), with their corresponding tubes (S1 and S2), a Y connector (Y) and feeder head (F). Feeders represented by black circles only received nectar from S1, and feeders shown in white were only fed by S2. The two pumping systems were filled with different sugar concentrations on different days. See “Methods” for further details
Nectar consisted of fructose and sucrose (2:1 parts) dissolved in water, with a hexose to sucrose ratio similar to the ratio in natural nectars of glossophagine-pollinated plants (Baker et al. 1998). Half of the feeders received nectar from one pumping system, and the other half from the other system (Fig. 1). The two systems were filled with nectars with different concentrations. Thus, during a single night, the concentration offered at each feeder was fixed and did not change. In order to prevent bacterial and fungal growth inside the tubing systems, they were rinsed with water and 70% ethanol every 3–4 days.
Experimental schedule
We recorded data between 18:00 and 06:00 h. The nectar concentrations ranged from 5 to 50% weight/weight (or 148–1,796 mmol L−1 sucrose equivalents, Bolten et al. 1979) and were presented in two series of two-alternative free-choice tests, with 12 feeders per option. The first series of tests consisted of nine different conditions with a difference between the two options of 5% (from 5% vs. 10% to 45% vs. 50%). The second series consisted of seven conditions with a difference of 15% (from 5% vs. 20% to 35% vs. 50%) between options. The sequence of conditions within both series was random. However, every condition was presented twice on consecutive nights on which the feeder positions for each concentration were exchanged (Fig. 1, black and white feeders), as a control for positional biases. The choice of pumping system for the higher nectar concentration during the first night of each condition was random.
Data analysis
Recorded data consisted of the time-stamped visitation events of marked bats and unknown unmarked visitors. Analysis was limited to the hours between 20:00 and 03:00 h. We excluded the hours before 20:00 h in order to focus on plateau performance, after the initial sampling and exploration phase. The visits after 03:00 h were excluded because of unexpectedly high visit numbers and premature depletion of the nectar supply on some nights. (This only occurred during two nights in the 5% series and three nights in the 15% series.) For each bat and each condition, we calculated the stimulus intensity and the discrimination strength. The stimulus intensity was calculated as the absolute difference between the two sugar concentrations, divided by the mean concentration. Over the two presentations of the same condition, discrimination strength was calculated as the number of visits to higher sugar concentration feeders divided by the total number of visits. If a bat showed a perfect “preference” for one feeder type without having made any visits to the other type during a whole night, including the time before 20:00 h, its data for that night were eliminated from the analysis.
Psychometric analysis
We performed individual psychometric analyses on the data from each animal and fitted Weibull psychometric functions using the algorithm proposed by Kuss et al. (2005) using R 2.10.1 (R Development Core Team 2009). A similar application of this method is presented in Toelch and Winter (2007). In this Bayesian approach, Markov Chain Monte Carlo (MCMC) sampling is used to estimate the threshold, slope, and lapse rate of the psychometric functions, along with their confidence intervals. The point on the curve halfway between the lower and upper asymptote (corresponding to a discrimination performance of ca. 75%) is referred to as the threshold. The slope of the function at the threshold is interpreted as a reliability measure of sensory performance (Treutwein and Strasburger 1999). Finally, the lapse rate is a measure of the frequency of errors (in this case, visits to the low concentration feeders) due to distraction, motivational problems, and other factors of a non-perceptual nature. In this particular application, it may also be interpreted as a base rate of exploration. As prior function for the lapse rate, we chose a beta distribution (2; 50). For the threshold we chose a normally distributed prior with a mean of 1 and a standard deviation of 0.5, and for the slope a log-normal prior with a mean of 2 and a standard deviation of 1. We performed 5,000 MCMC sampling runs with a leapfrog step size of 100. From the individual psychometric functions obtained using this method, we calculated the mean and 95% confidence intervals for the threshold, slope, and lapse rate.
Researchers have shown that the fit of the psychometric function is very sensitive to the sampling scheme, i.e., the choice of stimulus intensities and their distribution (Wichmann and Hill 2001). The intensities resulting from the chosen sugar concentrations in the 5% series were clustered in a region of lower to medium intensities without critical values around the threshold. On the other hand, the intensities resulting from the 15% series resulted in a wider range of intensities and included two points around the threshold. The theoretical expectation was that relative differences rather than absolute differences would predict bat choice. This relative difference is captured by our intensity measure expressed in terms of the absolute difference divided by the mean stimulus magnitude. We therefore restricted individual psychometric analysis to the 23 most active bats, which made on average more than 50 visits per night during the 15% series and were absent for no more than a single test condition from that series. With the exception of three bats, these 23 animals were also detected during the 5% series and analysis was performed on the pooled data from both series. From the individually fitted psychometric functions, we calculated the mean of the lapse rate, threshold, and slope across animals. For visualization purposes, we also fitted a psychometric function on the pooled data from all 23 bats.
Results
Between 20:00 and 03:00 h, the bats selected for analysis made an average of 75 visits per bat per night (excluding bats which made no visits, SD = 58, N = 23 bats). These visits represented 26% of the total registered visits between 20:00 and 03:00 h. Discrimination performance decreased with increasing average concentration of the presented stimuli in both experimental series (Fig. 2a). Bats showed no discrimination between concentrations at low stimulus intensities and good discrimination at high intensities (Fig. 2b). As a reminder, the lowest stimulus intensity presented was 45% versus 50% and the highest stimulus intensity was 5% versus 20%. The average threshold (ca. 75% discrimination performance) of the psychometric functions calculated for the individual bats was 0.50 ± 0.073 SD, N = 23. The average lapse rate and slope were 0.04 ± 0.023 SD, N = 23, and 3.41 ± 1.34 SD, N = 23, respectively. Restricting the analysis only to the animals that were present during every single night (N = 6) produced similar results: the values for the threshold, lapse rate, and slope were 0.50 ± 0.049 SD, 0.04 ± 0.025 SD, and 2.66 ± 0.93 SD, respectively. Fitting a psychometric function to the pooled data from all visitors, including unmarked animals, also produced similar results: the values for the threshold, lapse rate, and slope were 0.52 ± 0.002 SD, 0.06 ± 0.002 SD, and 2.02 ± 0.03 SD, respectively, (N = 5,000 MCMC simulation runs).
Fig. 2.
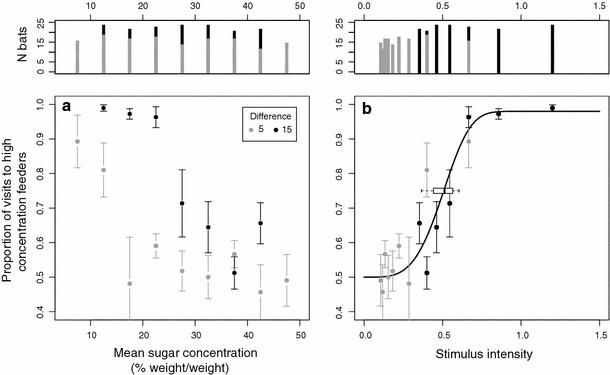
Bat visitation patterns to feeders of different concentrations. a Relative visitation rate versus mean sugar concentration. Circles represent the median proportion of visits to higher concentration feeders for the series with 5% difference (gray) and with 15% difference (black). Upper panel shows the number of bats in the tested conditions for the 5% difference (gray) and 15% difference series (black). Error bars give the median absolute deviation calculated over the respective number of bats. b Psychometric curve for concentration discrimination. Same data and symbols as in a, but with stimulus intensities on the abscissa (for intensity calculations, see “Methods”). The line represents the psychometric function fitted to the pooled data. The box gives the median (here at 0.5) and 95% confidence interval of the threshold values (ca. 75% discrimination performance) calculated for the individual bats
Discussion
The ability of G. commissarisi to discriminate between sugar concentrations can be described with the psychometric function presented in this study (Fig. 2). Within the tested range of concentration pairs, bats either made more visits to the feeder with more concentrated nectar or showed no preference. The psychometric function can also be used to predict discrimination performance of bats for any pair of sugar concentrations between 5 and 50%. For example, for intensities higher than the threshold (x > 0.5), the psychometric function predicts that the options with the more concentrated nectars will receive at least 75% of all visits. The predicted relative visitation rate to the sweeter option of two concentrations with intensity x can be calculated with the following equation:
| 1 |
where m is the threshold, s is the slope at the threshold, and π l is the lapse rate (from equations (1) and (11) in Kuss et al. 2005). If we have a given standard concentration c 2 and we want to obtain the concentration c 1 (c 1 > c 2) that paired with the standard will result in discrimination at some intensity level i, we can use the formula for intensity calculation to obtain the following ratio, which is constant for any chosen i:
| 2 |
The ratio of the difference of the two concentrations and the standard is also constant:
| 3 |
Our results indicate that the evaluation of sugar concentrations by G. commissarisi is affected by two systematic biases. Discrimination performance improves as the difference between alternative choices increases (distance effect). Discrimination performance declines as distance (the absolute difference between two concentrations) is kept constant but the average concentration of the two options increases (magnitude effect). Stimulus comparison in the case of sugar concentrations cannot occur simultaneously. Instead, the currently experienced concentration must be compared with a sample retrieved from memory. Presumably the distance and magnitude effects are consequences of sensory transfer functions and the memory representation of sweetness or caloric value. In essence, these effects and Eq. 3 above are consistent with Weber-Fechner’s law, which states that physical stimuli are scaled on a logarithmic internal representation over a major part of their perceptible range and that a differential threshold such as the just-noticeable difference (jnd) is a constant fraction of the magnitude of the stimulus (Kacelnik and Brito e Abreu 1998; Deco and Rolls 2006; Kang et al. 2010). We suggest that a non-linear perception and encoding process can also explain the biases described in this study.
The capacity of G. commissarisi to discriminate between nectar concentrations appears to be very similar to that of congeneric G. soricina (Fig. 3). In contrast, data obtained from the larger, more specialized nectarivore Leptonycterisyerbabuenae (formerly L. curasoae) imply that its psychometric function has as a lower threshold compared with the two Glossophaga species (Fig. 3). This may indicate a general trend among phyllostomids that the degree of diet specialization on sugar-rich flower nectar will negatively correlate with the psychometric function threshold. Such an evolutionary trend could be driven by the costs associated with sensory processing and the resulting energy-information trade-off (Isler and van Schaik 2006; Niven et al. 2007; Niven and Laughlin 2008). Increasing signal-to-noise ratio or bandwidth causes disproportionate increases in energetic cost at the cellular level which in turn constitutes a severe penalty on excess functional capacity (Niven et al. 2007; Niven and Laughlin 2008). Furthermore, even a theoretically optimal decision-maker produced by natural selection is still expected to make systematic mistakes (Livnat and Pippenger 2008).
Fig. 3.
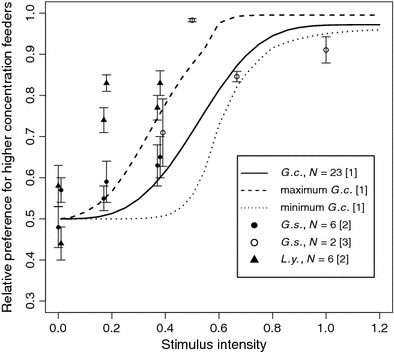
Concentration discrimination in three different glossophagine bat species. Stimulus intensities are given on the abscissa (for intensity calculations, see “Methods”). The continuous line is the psychometric function estimated from the mean threshold, slope, and lapse rate of the individuals tested in this study. The dashed lines represent the maximum (longer dashes) and minimum (shorter dashes) values from the individually fitted psychometric functions of the 23 animals tested in this study. The dashed lines give the range of the individual psychometric curves. Symbols represent mean preferences for the more concentrated feeder type, and whiskers represent one standard error. For clarity in the graph, horizontal jitter of 0.1 is added to points with the same intensity in order to prevent overlap. G.c. = Glossophaga commissarisi, G.s. = G. soricina, L.y. = Leptonycterisyerbabuenae. Sources: [1] = This study; [2] = Rodríguez-Peña et al. (2007); [3] = Roces et al. (1993)
We now have made available the two psychometric functions that describe the perception of sugar concentration and nectar volume in Glossophaga. These functions can serve as the basis for any decision that evaluates nectar reward quality. The mathematical nature of the psychometric functions makes it possible to predict theoretically the parameter space where uneconomical choice is expected. For the following, let us first assume that the psychometric function for volume perception estimated in G. soricina has the same parameters (threshold = 0.75, lapse rate = 0.05, slope = 1.6, average values from Table 2 in Toelch and Winter 2007) in G. commissarisi. Second, we assume equal lapse rates for both functions at 0.05. Finally, we assume that when the psychometric functions for concentration and volume predict different choices, choice is determined by the reward dimension predicting the higher relative visitation rate. In case of a tie, the opposite predictions neutralize each other and choice becomes random. Consider for example a reference reward type with 20% concentration and 30 μL volume. The predicted relative preference for alternative options with the same volume as the reference and different concentrations is given in Fig. 4a. The predicted relative preference for alternative options with the same concentration as the reference and different volumes is given in Fig. 4b. The gray areas in Fig. 4c indicate the combinations of volumes and concentrations that—paired with the reference—are predicted to result in non-profitable choices.
Fig. 4.
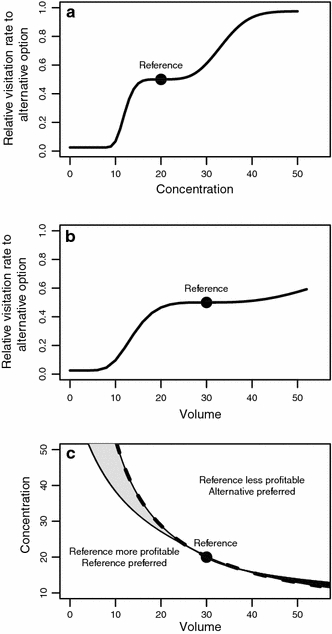
Relative preference for nectar sources in a choice between a reference option and alternatives with different volumes and concentrations. The reference option (point, all panels) has 20% concentration and 30 μL volume. a Relative visitation rates to alternative options with different concentrations and volumes equal to the reference. b Relative visitation rates to alternative options with different volumes and concentrations equal to the reference. The flat portion of the curves around the reference (a, b) is an “indifference zone” and indicates options for which bats are indifferent with respect to the reference. Preference increases more slowly for values higher than the reference (slope of curve to the right of the indifference zone) than it decreases for values lower than the reference (slope of curve to the left of the indifference zone). Curves in a and b calculated with Eq. 1. c Preference for less profitable nectar sources when both volume and concentration are changed in the alternative options. Points on the dashed line have the same energetic value (Bolten et al. 1979) as the reference. Options above the dashed line have higher and below the line lower energetic values than the reference. The continuous line marks the options for which bats are indifferent with respect to the reference, based on psychometric predictions from Eq. 1. The light gray area between the two curves contains the options that are less profitable and preferred over the reference. The dark gray area denotes the cases in which the reference is preferred even though it is less profitable than the alternative option
Food choice experiments in which both volume and concentration are manipulated indicate, as one would expect, that nectar-feeding animals estimate sugar concentration and nectar volume using different mechanisms, rather than evaluating overall sugar intake over time (Bateson et al. 2003; Cnaani et al. 2006). In these two studies, animals were presented with equicaloric options differing in volume and concentration. However, contrary to expectations of equal preference, animals made more visits to the options with the higher concentration. Such preferences, which remain to be tested in Glossophaga, could also be explained by corresponding psychometric functions for volume and concentration if for hummingbirds and bumblebees the mechanisms discussed here also apply. If our functions for volume and concentration discrimination are recalculated in Joules, then the function for concentration has a threshold of 0.55, which is lower than the threshold for volume at 0.75. That means that for equivalent changes in caloric value, bats are predicted to be more sensitive to changes in concentration than to changes in volume (Fig. 5).
Fig. 5.
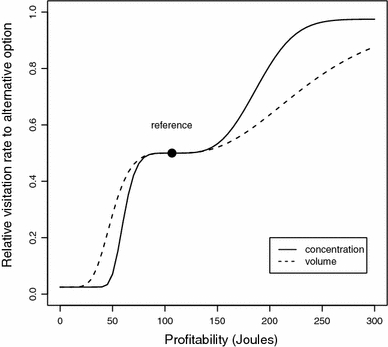
Psychometric functions for discrimination of sugar concentration (Fig. 4a) and nectar volume (Fig. 4b) recalculated in Joules. Although the two curves have roughly equal indifference zones, the concentration curve is steeper and has a lower threshold than the volume curve. Consequently, for sufficiently large changes in energetic content, bats are predicted to respond more strongly when the change is associated with a change in sugar concentration than with a change in volume. Data calculated with Eq. 1
Deviations from optimality can be even stronger if evaluation of reward properties takes place sequentially and if one dimension is given priority over the others. For example, Cnaani et al. (2006) suggest that bumble bees perceive sugar concentration first, and may reject a nectar reward if it is too dilute without consuming it completely and obtaining information about the available volume. Similarly, an emptied flower provides no information about its nectar concentration. Since bats’ discrimination of feeders differing in their likelihood of being empty exhibits the same distance and magnitude effects described above (Nachev and Winter, unpubl.), variance-sensitive foraging behavior can also result in non-profitable choices. In summary, because of the shape of the psychometric functions, negative changes in nectar reward properties along one dimension, e.g., concentration, might be masked by positive changes along the remaining dimensions, reward probability, and volume, even when these changes decrease overall profitability.
Acknowledgments
We thank Arne Jungwirth for fieldwork assistance and Alexej Schatz for software programming. Ulf Toelch and Lutz Wiegrebe gave helpful comments on earlier drafts of the manuscript. During the preparation of this manuscript, V. N. received funding by the Volkswagen Foundation. Field study funded by the National Geographic Society and further support was received by DFG Exc257 and Exc277.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
Treatment of the experimental animals complied with the national laws on animal care and experimentation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Vladislav Nachev, Phone: +49(0)30-450-539748, FAX: +49(0)30-450-539937, Email: vladislav.nachev@charite.de.
York Winter, Email: york.winter@charite.de.
References
- Baker HG, Baker I. Floral nectar sugar constituents in relation to pollinator type. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold; 1983. pp. 117–141. [Google Scholar]
- Baker HG, Baker I, Hodges SA. Sugar composition of nectars and fruits consumed by birds and bats in the tropics and subtropics. Biotropica. 1998;30:559–586. doi: 10.1111/j.1744-7429.1998.tb00097.x. [DOI] [Google Scholar]
- Bateson M, Healy SD, Hurly TA. Context-dependent foraging decisions in rufous hummingbirds. Proc Biol Sci. 2003;270:1271–1276. doi: 10.1098/rspb.2003.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolten AB, Feinsinger P, Baker HG, Baker I. On the calculation of sugar concentration in flower nectar. Oecologia. 1979;41:301–304. doi: 10.1007/BF00377434. [DOI] [PubMed] [Google Scholar]
- Cnaani J, Thomson JD, Papaj DR. Flower choice and learning in foraging bumblebees: effects of variation in nectar volume and concentration. Ethology. 2006;112:278–285. doi: 10.1111/j.1439-0310.2006.01174.x. [DOI] [Google Scholar]
- Deco G, Rolls ET. Decision-making and Weber’s law: a neurophysiological model. Eur J Neurosci. 2006;24:901–916. doi: 10.1111/j.1460-9568.2006.04940.x. [DOI] [PubMed] [Google Scholar]
- Hainsworth FR, Wolf LL. Nectar characteristics and food selection by hummingbirds. Oecologia. 1976;25:101–113. doi: 10.1007/BF00368847. [DOI] [PubMed] [Google Scholar]
- Isler K, van Schaik CP. Metabolic costs of brain size evolution. Biol Lett. 2006;2:557–560. doi: 10.1098/rsbl.2006.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacelnik A, Brito e Abreu F. Risky choice and Weber’s law. J Theor Biol. 1998;194:289–298. doi: 10.1006/jtbi.1998.0763. [DOI] [PubMed] [Google Scholar]
- Kang J, Wu J, Smerieri A, Feng J. Weber’s law implies neural discharge more regular than a Poisson process. Eur J Neurosci. 2010;31:1006–1018. doi: 10.1111/j.1460-9568.2010.07145.x. [DOI] [PubMed] [Google Scholar]
- Kuss M, Jäkel F, Wichmann FA. Bayesian inference for psychometric functions. J Vision. 2005;5:478–492. doi: 10.1167/5.5.8. [DOI] [PubMed] [Google Scholar]
- Livnat A, Pippenger N. Systematic mistakes are likely in bounded optimal decision-making systems. J Theor Biol. 2008;250:410–423. doi: 10.1016/j.jtbi.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Nicolson SW, Fleming PA. Nectar as food for birds: the physiological consequences of drinking dilute sugar solutions. Plant Syst Evol. 2003;238:139–153. [Google Scholar]
- Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211:1792–1804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- Niven JE, Anderson JC, Laughlin SB. Fly photoreceptors demonstrate energy-information trade-offs in neural coding. PLoS Biol. 2007;5:e116. doi: 10.1371/journal.pbio.0050116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2009. [Google Scholar]
- Real LA, Rathcke BJ. Individual variation in nectar production and its effect on fitness in Kalmia latifolia. Ecology. 1991;72:149–155. doi: 10.2307/1938910. [DOI] [Google Scholar]
- Roberts MW. Hummingbirds’ nectar concentration preferences at low volume: the importance of time scale. Anim Behav. 1996;52:361–370. doi: 10.1006/anbe.1996.0180. [DOI] [Google Scholar]
- Roces F, Winter Y, von Helversen O. Concentration preference and water balance in a flower visiting bat, Glossophaga soricinaantillarum. In: Barthlott W, Naumann CM, Schmidt-Loske K, Schuchmann K, editors. Animal-plant Interactions in tropical environments. Bonn: Zoologisches Forschungsinstitut und Museum Alexander Koenig; 1993. pp. 159–165. [Google Scholar]
- Rodríguez-Peña N, Stoner KE, Schondube JE, Ayala-Berdón J, Flores-Ortiz CM, Martínez del Río C. Effects of sugar composition and concentration on food selection by Saussure’s long-nosed bat (Leptonycteriscurasoae) and the long-tongued bat (Glossophaga soricina) J Mammal. 2007;88:1466–1474. doi: 10.1644/06-MAMM-A-353R1.1. [DOI] [Google Scholar]
- Sakai S. A model for nectar secretion in animal-pollinated plants. Evol Ecol. 1993;7:394–400. doi: 10.1007/BF01237870. [DOI] [Google Scholar]
- Santoso A, Kaiser A, Winter Y. Individually dosed oral drug administration to socially-living transponder-tagged mice by a water dispenser under RFID control. J Neurosci Meth. 2006;153:208–213. doi: 10.1016/j.jneumeth.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Stiles FG, Freeman CE. Patterns in floral nectar characteristics of some bird-visited plant species from Costa Rica. Biotropica. 1993;25:191–205. doi: 10.2307/2389183. [DOI] [Google Scholar]
- Thiele J (2006) Nahrungssuchstrategien der nektarivoren Fledermaus Glossophaga commissarisi (Phyllostomidae) im Freiland—eine individuenbasierte Verhaltensstudie unter Verwendung von Transpondertechnik. Dissertation, Ludwig-Maximilians-Universität, Munich
- Toelch U, Winter Y. Psychometric function for nectar volume perception of a flower-visiting bat. J Comp Physiol A. 2007;193:265–269. doi: 10.1007/s00359-006-0189-3. [DOI] [PubMed] [Google Scholar]
- Treutwein B, Strasburger H. Fitting the psychometric function. Percept Psychophys. 1999;61:87–106. doi: 10.3758/BF03211951. [DOI] [PubMed] [Google Scholar]
- Tschapka M (1998) Koexistenz und Ressourcennutzung in einer Artengemeinschaft von Blumenfledermäusen (Phyllostomidae: Glossophaginae) im atlantischen Tieflandregenwald Costa Ricas. Dissertation, Friedrich-Alexander-Universität, Erlangen–Nürnberg
- Tschapka M, von Helversen O. Phenology, nectar production and visitation behaviour of bats on the flowers of the bromeliad Werauhiagladioliflora in a Costa Rican lowland rain forest. J Trop Eco. 2007;23:385–395. doi: 10.1017/S0266467407004129. [DOI] [Google Scholar]
- von Frisch K. Versuche über den Geschmackssinn der Bienen. Naturwissenschaften. 1927;15:321–327. doi: 10.1007/BF01504545. [DOI] [Google Scholar]
- von Helversen O, Winkler L, Bestmann HJ. Sulphur-containing “perfumes” attract flower-visiting bats. J Comp Physiol A. 2000;186:143–153. doi: 10.1007/s003590050014. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001;63:1293–1313. doi: 10.3758/BF03194544. [DOI] [PubMed] [Google Scholar]
- Winter Y, Stich KP. Foraging in a complex naturalistic environment: capacity of spatial working memory in flower bats. J Exp Biol. 2005;208:539–548. doi: 10.1242/jeb.01416. [DOI] [PubMed] [Google Scholar]
- Zimmerman M. Plant reproduction and optimal foraging: experimental nectar manipulations in Delphinium nelsonii. Oikos. 1983;41:57–63. doi: 10.2307/3544346. [DOI] [Google Scholar]


