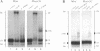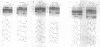Abstract
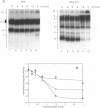
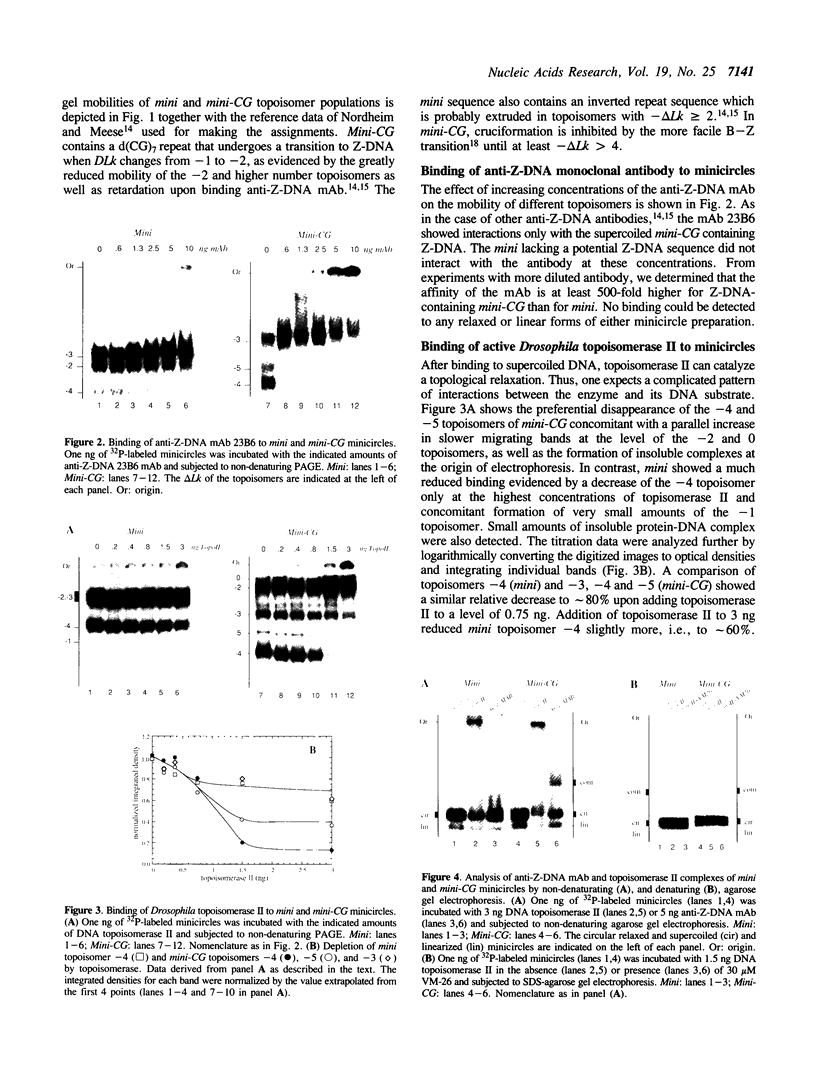
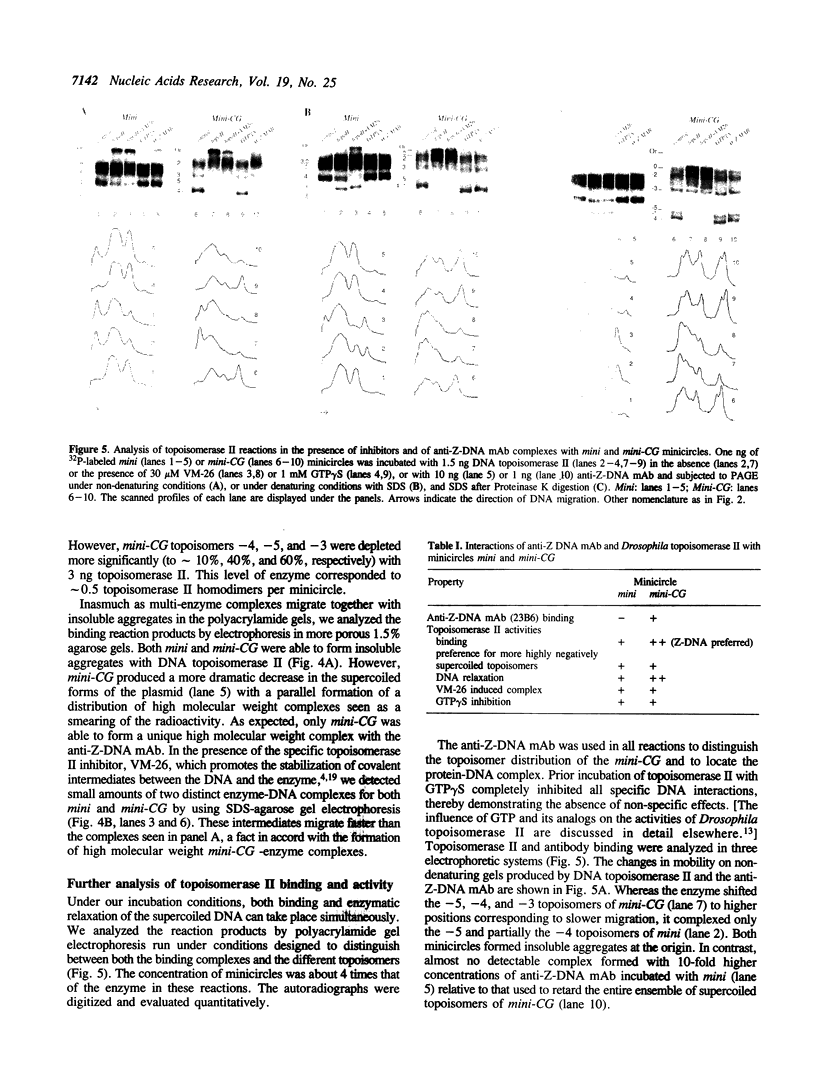
The native form of Drosophila melanogaster DNA topoisomerase II was purified from Schneider's S3 tissue culture cells and studied with two supercoiled minicircle preparations, mini and mini-CG, 354 bp and 370 bp in length, respectively. Mini-CG contains a d(CG)7 insert which assumes a left-handed Z-DNA conformation in negative supercoiled topoisomers with a negative linking number difference - delta Lk greater than or equal to 2. The interactions of topoisomerase II with topoisomer families of mini and mini-CG were studied by band-shift gel electrophoresis in which the individual topoisomers and their discrete or aggregated protein complexes were resolved. A monoclonal anti-Z-DNA IgG antibody (23B6) bound and aggregated only mini-CG, thereby confirming the presence of Z-DNA. Topoisomerase II bound and relaxed mini-CG more readily than mini. In both cases, there was a preference for more highly negatively supercoiled topoisomers. The topoisomerase II inhibitor VM-26 induced the formation of stable covalent DNA-protein intermediates. In addition, the non-hydrolyzable GTP analogue GTP gamma S inhibited the binding and relaxation activities. Experiments to detect topoisomerase cleavage sites failed to elicit specific loci on either minicircle preparation. We conclude that Drosophila topoisomerase II is able to bind and process small minicircles with lengths as short as 360 bp and negative superhelix densities, - sigma, which can exceed 0.1. Furthermore, the enzyme has a preferential affinity for topoisomers containing Z-DNA segments and relaxes these molecules, presumably by cleavage external to the inserts. Thus, a potentially functional relationship between topoisomerase II, an enzyme regulating the topological state of DNA-chromatin in vivo, and left-handed Z-DNA, a conformation stabilized by negative supercoiling, has been established.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Luke M., Laemmli U. K. Chromosome assembly in vitro: topoisomerase II is required for condensation. Cell. 1991 Jan 11;64(1):137–148. doi: 10.1016/0092-8674(91)90215-k. [DOI] [PubMed] [Google Scholar]
- Benham C. J. Theoretical analysis of conformational equilibria in superhelical DNA. Annu Rev Biophys Biophys Chem. 1985;14:23–45. doi: 10.1146/annurev.bb.14.060185.000323. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Chung I. K., Muller M. T. Aggregates of oligo(dG) bind and inhibit topoisomerase II activity and induce formation of large networks. J Biol Chem. 1991 May 25;266(15):9508–9514. [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Halligan B., Cooke C. A., Heck M. M., Liu L. F. Topoisomerase II is a structural component of mitotic chromosome scaffolds. J Cell Biol. 1985 May;100(5):1706–1715. doi: 10.1083/jcb.100.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Heck M. M. Localization of topoisomerase II in mitotic chromosomes. J Cell Biol. 1985 May;100(5):1716–1725. doi: 10.1083/jcb.100.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M. T., Lee M. P., Hsieh T. S., Griffith J. D. Drosophila topoisomerase II-DNA interactions are affected by DNA structure. J Mol Biol. 1991 Jan 5;217(1):53–62. doi: 10.1016/0022-2836(91)90610-i. [DOI] [PubMed] [Google Scholar]
- Hsieh T. Knotting of the circular duplex DNA by type II DNA topoisomerase from Drosophila melanogaster. J Biol Chem. 1983 Jul 10;258(13):8413–8420. [PubMed] [Google Scholar]
- Hsieh T. Purification and properties of type II DNA topoisomerase from embryos of Drosophila melanogaster. Methods Enzymol. 1983;100:161–170. doi: 10.1016/0076-6879(83)00052-x. [DOI] [PubMed] [Google Scholar]
- Lee M. P., Sander M., Hsieh T. Nuclease protection by Drosophila DNA topoisomerase II. Enzyme/DNA contacts at the strong topoisomerase II cleavage sites. J Biol Chem. 1989 Dec 25;264(36):21779–21787. [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Miller K. G., Liu L. F., Englund P. T. A homogeneous type II DNA topoisomerase from HeLa cell nuclei. J Biol Chem. 1981 Sep 10;256(17):9334–9339. [PubMed] [Google Scholar]
- Nordheim A., Meese K. Topoisomer gel retardation: detection of anti-Z-DNA antibodies bound to Z-DNA within supercoiled DNA minicircles. Nucleic Acids Res. 1988 Jan 11;16(1):21–37. doi: 10.1093/nar/16.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner K. J., Hobi R., Kuenzle C. C. Z-DNA-binding proteins. Identification critically depends on the proper choice of ligands. J Biol Chem. 1990 Nov 5;265(31):19112–19115. [PubMed] [Google Scholar]
- To R. Q., Kmiec E. B. Assembly of transcriptionally active chromatin in vitro: a possible role for topoisomerase II. Cell Growth Differ. 1990 Jan;1(1):39–45. [PubMed] [Google Scholar]
- Udvardy A., Schedl P., Sander M., Hsieh T. S. Novel partitioning of DNA cleavage sites for Drosophila topoisomerase II. Cell. 1985 Apr;40(4):933–941. doi: 10.1016/0092-8674(85)90353-8. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Caron P. R., Kim R. A. The role of DNA topoisomerases in recombination and genome stability: a double-edged sword? Cell. 1990 Aug 10;62(3):403–406. doi: 10.1016/0092-8674(90)90002-v. [DOI] [PubMed] [Google Scholar]
- Wood E. R., Earnshaw W. C. Mitotic chromatin condensation in vitro using somatic cell extracts and nuclei with variable levels of endogenous topoisomerase II. J Cell Biol. 1990 Dec;111(6 Pt 2):2839–2850. doi: 10.1083/jcb.111.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff E., Natalie D., Nolan J. M., Lee M., Hsieh T. Structure of the Drosophila DNA topoisomerase II gene. Nucleotide sequence and homology among topoisomerases II. J Mol Biol. 1989 Jan 5;205(1):1–13. doi: 10.1016/0022-2836(89)90361-6. [DOI] [PubMed] [Google Scholar]
- Zarling D. A., Arndt-Jovin D. J., Robert-Nicoud M., McIntosh L. P., Thomae R., Jovin T. M. Immunoglobulin recognition of synthetic and natural left-handed Z DNA conformations and sequences. J Mol Biol. 1984 Jul 5;176(3):369–415. doi: 10.1016/0022-2836(84)90495-9. [DOI] [PubMed] [Google Scholar]