Abstract
Objective
This study examined financial implications of CMS-Hierarchical Condition Categories (HCC) risk-adjustment model on Medicare payments for individuals with comorbid chronic conditions.
Study Design
The study used 1992-2000 data from the Medicare Current Beneficiary Survey and corresponding Medicare claims. The pairs of comorbidities were formed based on the prior evidence about possible synergy between these conditions and activities of daily living (ADL) deficiencies and included heart disease and cancer, lung disease and cancer, stroke and hypertension, stroke and arthritis, congestive heart failure (CHF) and osteoporosis, diabetes and coronary artery disease, CHF and dementia.
Methods
For each beneficiary, we calculated the actual Medicare cost ratio as the ratio of the individual’s annualized costs to the mean annual Medicare cost of all people in the study. The actual Medicare cost ratios, by ADLs, were compared to the HCC ratios under the CMS-HCC payment model. Using multivariate regression models, we tested whether having the identified pairs of comorbidities affects the accuracy of CMS-HCC model predictions.
Results
The CMS-HCC model underpredicted Medicare capitation payments for patients with hypertension, lung disease, congestive heart failure and dementia. The difference between the actual costs and predicted payments was partially explained by beneficiary functional status and less than optimal adjustment for these chronic conditions.
Conclusions
Information about beneficiary functional status should be incorporated in reimbursement models since underpaying providers for caring for population with multiple comorbidities may provide severe disincentives for managed care plans to enroll such individuals and to appropriately manage their complex and costly conditions.
Keywords: comorbidities, disability, functional impairment, Medicare, Activities of Daily Living, Hierarchical Conditions Categories
INTRODUCTION
Chronic conditions – such as heart disease, hypertension, arthritis, cancer, and diabetes – are the leading causes of disability and death in the United States for people older than age 651. Medicare beneficiaries with five or more chronic conditions account for 68% of the program’s spending 2. Co-occurrence of diseases increases markedly with age, with two thirds of non-institutionalized Medicare beneficiaries over the age of 65 reporting two or more chronic condition 3, with the prevalence of multiple comorbidities being even higher among Medicare population overall. Approximately 25% of those who experience chronic illness have some limitations in functional activity, and the percent of those with disability increases with the number of coexisting conditions 4. The presence of chronic disease has been consistently shown to be associated with functional dependence 5-7, with combinations of diseases showing different influence on physical functioning than would be expected of the sum of the individual conditions.8-10
Recognizing the increasing prevalence of chronic comorbid conditions in the Medicare population, and the need to adequately compensate Medicare managed care plans for the care they provide to this segment of the population, beginning in 2004 the Centers for Medicare and Medicaid Services (CMS) started to phase in a new risk-adjusted payment model. Known as the CMS-Hierarchical Condition Categories (CMS-HCC), this risk-adjustment model relies on demographic and diagnostic information available from administrative data to predict resource use. The model uses a selected subset of ICD-9-CM diagnostic codes from hospital and physician encounters to place beneficiaries into 70* disease groups, the HCCs11, 12. Each disease group includes conditions that are related clinically and have similar cost implications. In additional, the model accounts for the fact that having certain combinations of diseases may result in higher medical expenditures that simply the sum of the two. For instance, such disease interaction coefficients are allowed for diabetes and CHF, diabetes and cerebra-vascular disease, diabetes, CHF and renal failure, and a limited number of others12.
There have always been concerns regarding the accuracy of the HCC model in predicting Medicare payment13-16. The understanding of the relationship between functional limitations and cost of medical care in patients multiple chronic conditions is currently gaining importance and recognition. In 2003, the Center for Medicare and Medicaid Services (CMS) created Special Needs Plans (SNPs), allowing health care providers to accept full risk from the CMS for all medical and pharmacy health expenses for enrollees with specific chronic diseases.17 By the beginning of 2008, 775 plans have enrolled nearly 1 million beneficiaries 18
The HCC model does not (except for PACE) include adjustment for functional impairment. Studies have shown that this underestimates payments for enrollees with disabilities. We also know that people with comorbid conditions tend to be more functionally disabled, hence, our interest in examining the extent to which the HCC model may not pay appropriately for this segment of the population, very significantly represented in the SNPs. Furthermore, the HCC model does not account at all for a number of prevalent chronic conditions (e.g., dementia, osteoporosis), hence our interest in including those conditions in the analyses.
The effect of multiple comorbidities on disability and cost of care is poorly understood. Ettinger and colleagues (1994) 19 explored synergy for arthritis and four comorbidities (heart disease, pulmonary disease, obesity, and hypertension) and proposed a mechanism explaining increased disability resulting from multiple comorbidities. They suggested that an impairment from one disease (e.g., inactivity resulting from arthritis) may exacerbate the impairment from another comorbid condition (e.g., low work capacity caused by heart disease), hence, modifying disease-disability relationship. Prior studies also identified additional specific diseases such as cerebra-vascular disorders, diabetes, cancer, osteoporosis, atherosclerosis, and neurologic problems that may exacerbate disability resulting from other conditions 8, 7, 10, 19-23. Based on this evidence, we identified the following eleven target comorbidities to be examined in this study: arthritis, hypertension, heart disease, cancer, lung disease, stroke, osteoporosis, diabetes, coronary artery disease (CAD), congestive heart failure (CHF), and dementia and taking into account patient level of functional impairment.7
Furthermore, we hypothesized that certain combinations of the 11 target conditions we considered may have synergistic effects with respect to physical and cognitive functioning when evaluated longitudinally. This in turn would affect patient ADL performance, and furthermore, cost of medical care. These predictions were based on the previous cross-sectional (arthritis and hypertension, heart disease and cancer, lung disease and cancer, and stroke and hypertension) 10, 24-26 and longitudinal studies 7, 8, 16, 21, 27. In addition, dementia may accelerate functional decline and mortality and may exacerbate other chronic conditions as well. Osteoporosis could lead to more fractures and trauma in older patients that would increase temporary and permanent disability and may limit people’s ADL performance as they are trying to minimize their risks.
The purpose of this study is to assess the accuracy of the CMS-HCC Medicare capitation model in predicting Medicare expenditures for community-based beneficiaries with at least two target comorbidities identified above and various degrees of functional impairment. The population of Medicare beneficiaries with coexisting chronic conditions represents a good case for testing the accuracy of the CMS-HCC Medicare risk adjustment model: 1) that does not account for patient functional limitations that may exacerbated inaccuracy of predictions for patients with multiple chronic conditions, and 2) that does not account for all chronic conditions and this too may result in underestimation of payments.
METHODS
Data
The study used 9 years of data (1992-2000 Cost & Use files) from the Medicare Current Beneficiary Survey (MCBS) and corresponding Medicare claims data for the participating beneficiaries. The MCBS includes information about Medicare beneficiaries’ health and use of healthcare services, administrative data from the CMS, and Medicare claims for the survey participants for the corresponding calendar year. Several reports had been published describing the structure of the MCBS28 and the link between the survey and expenditure data29. Our total sample consisted of 46,790 community-dwelling Medicare beneficiaries who participated in the fee-for-service plans. We defined community population as those beneficiaries who did not stay in institutions for more than 90 days at a time according to Medicare Managed Care Manual30. We limited the sample to beneficiaries with continuous Part A and B enrollment for at least two calendar years. Beneficiaries with end stage renal disease were excluded.
Using the MCBS data, functional status was measured by the number of impairments in the Activities of Daily Living (ADL), 0 to 6, adding one point for the presence of each deficiency (e.g., whether the beneficiary got help with bathing, dressing, eating, walking, toileting and transferring or used assisted devices to perform these functions).
Comorbidities were identified either according to self-reported disease status or through the Medicare claims of the survey participants. We have chosen 11 target chronic conditions to evaluate for the effect of comorbidity because of their prevalence among Medicare population as well as their reported association with disability 8, 7, 10, 19-23. Arthritis, hypertension, heart disease, cancer, lung disease, stroke, osteoporosis, diabetes, and coronary artery disease (CAD) were self-reported by the MCBS participants (with question “Has your doctor ever told you that you have…?), while congestive heart failure (CHF), and dementia were not addressed by the MCBS and therefore, were identified based on the ICD codes from the Medicare claims data (see Appendix A for the complete list of codes). Beneficiaries were identified to have CHF if they had any claims with ICD-9 codes 428-428.931. Dementia was identified based on having any Medicare claims containing ICD codes 290.0-290.3, 294.1, 294.8, 294.9, 298.9, 331.0, 331.2, 331.3, 331.4, 348.3, 797 and 780.932. Similar ICD-9-CM codes are used by Medicare risk-adjustment model for Part D prescription drug coverage, RxHCC 33
The MCBS survey reports life-long prevalence of chronic conditions, while claims-based approach identifies whether a patient had a condition-related utilization in a given year. Nevertheless, because CHF and dementia are chronic conditions that require ongoing treatment, we thought it was reasonable to use claims to identify patients with these conditions. In addition, the CMS-HCC model does not contain separate categories for hypertension or dementia, while the effect of CAD is reflected in several categories (HCC81-HCC83). It is assumed however, that the effect of hypertension and dementia on the costs of care would be accounted for by other related categories (e.g., acute or old myocardial infarction and angina for hypertension, and Parkinson’s disease for dementia).
Population Descriptive Statistics
Beneficiaries with the pairs of target conditions were compared to the general Medicare population on such characteristics as gender, race, frequency of each ADL, and place of residence using chi-square tests. T-tests were used to identify significant differences between these groups of patients by age, number of ADLs. Survey weights were incorporated into the comparisons to represent the entire Medicare population. All statistical tests were two-tailed and were performed using a significance level of 5%.
Comparing Actual Medicare costs with the CMS-HCC model predictions
We computed the Medicare annualized costs for each beneficiary by adjusting the reported annual Medicare costs for each person’s spell of eligibility. For each beneficiary, we calculated the actual Medicare cost ratio as the ratio of the individual’s annualized costs to the mean annual Medicare cost of all people in the study.
To calculate the HCC scores, we used the available CMS-HCC software11. The original CMS-HCC capitation payment approach was developed using year 1999 Medicare claims data to predict year 2000 medical expenditures, with three individual models developed to predict expenditures of new enrollees, community-based beneficiaries, and facility residents. Under the CMS-HCC model, individuals are assigned to multiple HCC groups based on the prior (base) year diagnoses. In addition, the model uses age, sex, original reason for Medicare entitlement (disability or age), Medicaid eligibility status, and whether the beneficiary resides in the community, facility, or is a new enrollee (enrolled in Medicare for less than 12 months in the prior year) to predict the next (prediction) year expenses12. The total individual HCC score is calculated as a sum of multiple HCC scores assigned to a person. For each person, the HCC score indicates how the predicted medical expenses compare to the average for the Medicare population. In this study, we focused only on the community model.
The relative error in the CMS-HCC model was computed as the percentage difference between the CMS-HCC predicted cost ratio and the actual Medicare cost ratio, with the positive difference suggesting model underprediction. The p-values less than 0.05 indicate relative errors significantly different from zero. The 95% confidence intervals were reported to illustrate robustness of the estimates.
Effect of multiple comorbidities and functional status on the accuracy of CMS-HCC model predictions
Using multiple regressions, we tested whether having the identified pairs of comorbidities affects the accuracy of CMS-HCC model predictions. The dependent variable was the residual Medicare expenditures ratio, defined as the difference between the actual cost ratio and the predicted cost ratio (the HCC score) for each individual (similar to the approach used by Kautter and Pope (2004) 13 and based on the work of Temkin-Greener and colleagues (2001) 16 and Riley (2000) 15. The residual ratio reflects the accuracy of CMS-HCC model prediction. The independent variables included the dummy variables for the different levels of physical disability (ADLs), target comorbidities, and the interactions between these comorbidities. Survey sampling weights were incorporated in the multiple regression analysis.
The analyses were conducted using STATA Statistical Software for Windows Release 8.034 and SAS for Unix Version 935.
RESULTS
Population characteristics
Nearly three quarters (72.55%) of all Medicare beneficiaries in our study had two or more of target comorbidities with the prevalence of different target comorbidities varying substantially. In Table 1, we compared the characteristics of the general Medicare population and beneficiaries with pairs of target chronic conditions. While more than a third of all beneficiaries had arthritis and hypertension, only about 1% of people had either CHF and osteoporosis or CHF and dementia. Patients with chronic illnesses were significantly older than the study population overall (72.75 years old). Conditions such as depression and CAD (69.22% women, p<0.01), osteoporosis and CHF (87.88% women, p<0.01), and arthritis and stroke (60.54%, women p<0.05) or hypertension (66.44% women, p<0.01) were more prevalent in women while cancer and heart (45.98% men, p<0.01) or lung disease (46.58%, p<0.01), diabetes and CAD (48.01%, p<0.01) were more likely to be present in men compared to general Medicare population (42.44% male). Except for the beneficiaries with cancer and heart disease, patients with the pairs of target comorbidities had lower income and were more likely to be on Medicaid compared to general Medicare population.
Table 1.
Population characteristics
| Variables | Total Population |
Arthritis & Hypertension |
Heart Disease & Cancer |
Lung Disease & Cancer |
Stroke & Hypertension |
Stroke & Arthritis |
CHF & Osteoporosis |
Diabetes & CAD |
CHF & Dementia |
|---|---|---|---|---|---|---|---|---|---|
| Number of Person Years | 46,790 | 16,844 | 6,936 | 2,455 | 3,760 | 3,508 | 795 | 2,499 | 598 |
| Prevalence (%) | 100 | 35.85 | 14.65 | 5.24 | 7.80 | 7.03 | 1.52 | 5.32 | 1.11 |
| Mean Age | 72.75 | 74.27** | 75.64** | 73.25* | 74.24** | 74.64** | 79.05** | 73.45** | 80.07** |
| Male, % | 42.44 | 33.56** | 45.98** | 46.58** | 42.75 | 39.46* | 12.12** | 48.01** | 38.67 |
| White, % | 87.64 | 84.09** | 93.88** | 93.94** | 83.39** | 85.49* | 89.85 | 84.90** | 83.57** |
| Married, % | 52.99 | 48.73** | 55.15** | 53.58 | 48.79** | 46.32** | 30.45** | 51.08 | 37.73** |
| Medicaid, % | 14.69 | 18.02** | 11.52** | 15.77 | 22.22** | 22.51** | 23.99** | 22.27** | 27.97** |
| Metropolitan Area (%) | 69.39 | 68.73 | 68.14 | 65.57 | 68.86 | 69.25 | 67.78 | 69.91 | 73.13 |
|
| |||||||||
| Mean Income (2002 $) | 34,015 | 29,677** | 36022* | 32936 | 28673** | 27689** | 23223** | 28601** | 24626** |
| < $15,000, % | 29.71 | 33.93** | 25.22** | 28.18 | 36.99** | 38.72** | 44.38** | 34.54** | 44.01** |
| $15,000 - $30,000, % | 31.82 | 33.20** | 32.21 | 34.21 | 32.86 | 32.98 | 33.60 | 34.10 | 32.47 |
| $30,000 - $50,000, % | 21.79 | 20.20** | 23.53** | 22.27 | 19.02** | 17.30** | 15.72** | 20.61 | 15.15** |
| > $50,000, % | 16.69 | 12.68** | 19.03** | 15.34 | 11.13** | 11.00** | 6.30** | 10.75** | 8.37** |
|
| |||||||||
| Mean ADLs | 0.29 | 0.38** | 0.39** | 0.38** | 0.87** | 0.89** | 1.22** | 0.58** | 1.85** |
| Difficulty in bathing (%) | 7.96 | 10.41** | 10.88** | 11.46** | 22.57** | 23.22 | 34.34** | 16.25** | 47.11** |
| Difficulty in dressing (%) | 5.52 | 7.09** | 6.83** | 6.56 | 16.95** | 17.01** | 21.52** | 10.72** | 37.28** |
| Difficulty in eating (%) | 1.43 | 1.60 | 1.64 | 1.28 | 5.44** | 5.61** | 5.31** | 2.24* | 12.91** |
| Difficulty in transferring (%) | 5.06 | 6.97** | 6.90** | 6.14 | 14.85** | 15.68** | 20.50** | 10.14** | 30.06** |
| Difficulty in walking (%) | 6.57 | 8.89** | 9.78** | 10.32** | 17.66** | 18.66** | 27.53** | 13.67** | 35.36** |
| Difficulty in toileting (%) | 2.63 | 3.18** | 3.12* | 2.64 | 9.10** | 9.30** | 12.59** | 5.14** | 22.21** |
|
| |||||||||
| Geographic Area | |||||||||
| New England (%) | 3.24 | 3.01* | 3.42 | 2.82 | 2.72* | 3.01 | 4.85 | 2.67 | 4.57* |
| Middle Atlantic (%) | 17.82 | 16.95* | 16.47 | 13.44** | 16.07* | 14.33** | 16.04 | 18.66 | 18.96 |
| East North Central (%) | 18.41 | 17.57 | 16.18** | 17.68 | 17.99 | 18.06 | 17.26 | 18.03 | 16.34 |
| West North Central (%) | 6.92 | 5.65** | 6.59 | 6.13 | 5.55** | 5.54** | 4.95* | 4.74** | 5.48 |
| South Atlantic (%) | 21.21 | 23.28** | 22.46 | 21.39 | 23.59** | 23.91** | 21.87 | 23.91* | 22.55 |
| East South Central (%) | 6.18 | 7.17** | 6.92* | 8.02** | 7.04 | 7.75** | 5.29 | 6.50 | 7.34 |
| West South Central (%) | 11.04 | 11.19 | 12.28 | 12.67 | 11.81 | 11.41 | 12.11 | 11.99 | 11.53 |
| Mountain (%) | 4.90 | 4.66 | 5.28 | 6.42** | 4.56 | 5.08 | 4.73 | 2.84** | 2.79** |
| Pacific (%) | 9.02 | 8.98 | 10.05 | 10.84 | 10.03 | 10.26 | 10.57 | 7.95 | 8.42 |
| Puerto Rico (%) | 1.27 | 1.55** | 0.36* | 0.58 | 0.65** | 0.64** | 2.32 | 2.73** | 2.01** |
Total number of person years is 46, 790, including those who participated in MCBS for at least two;
Compared with the total population,
Significant at 5% level,
Significant at 1% level.
Functional status of Medicare beneficiaries with chronic conditions
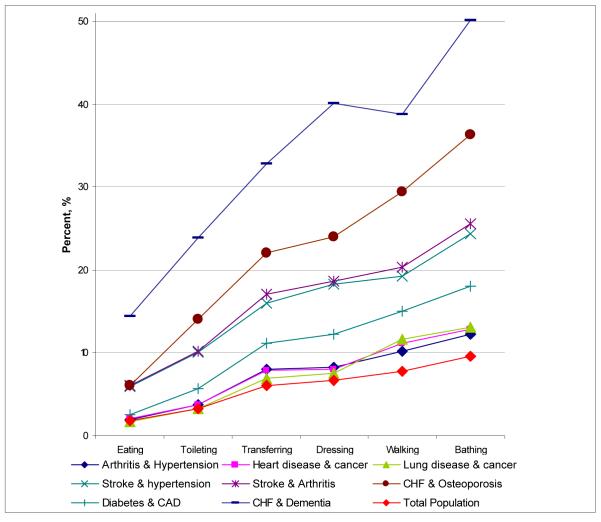
Patients with multiple comorbid conditions had a much greater level of ADL deficiencies than Medicare beneficiaries overall (Figure 1). The profiles of disability also varied substantially between patients with different chronic illnesses. Patients with CHF and dementia reported the highest level of deficiency across all ADL categories, 14.38% relied on others’ help with eating (feeding), and more than 50% used help or assisted devices for bathing. Other groups with high ADL deficiency level included patients with stroke combined with hypertension or arthritis, CHF and osteoporosis, and CAD and diabetes. However, the ranking of the prevalence of individual ADLs was consistent among all patient groups, with eating being the least common and bathing being the most common function for which beneficiaries received help.
Figure 1.
Prevalence of Activity of Daily Living deficiencies among Medicare beneficiaries with different comorbid conditions.
Comparing the actual Medicare costs with the CMS-HCC predicted payments
Overall, the CMS-HCC model significantly under-predicted medical expenses of patients with target single comorbidities, except for arthritis (p=0.13), cancer (p=0.21), and osteoporosis (p=0.32) (Tables 2-3). We found that for beneficiaries without functional limitations (ADL 0), the CMS-HCC predicted expenses were no different from the actual cost ratios except for patients with CHF (underpredicted by 18.47%, p=0.01). As the disability level increased, the model increasingly under-predicted the expenses – up to 43.65% (p<0.001) for patients with 6 ADLs.
Table 2.
Cost Ratios for Target Chronic Conditions Included in HCCs, by ADL
| Chronic Conditions | Number of ADLs |
Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Arthritis | 23,507 | 1,739 | 866 | 547 | 506 | 501 | 264 | 27,930 |
| Actual Cost | 0.93 | 1.71 | 2.19 | 2.57 | 2.54 | 2.69 | 3.54 | 1.11 |
| Predicted Cost | 0.96 | 1.47 | 1.63 | 1.80 | 1.93 | 2.26 | 2.61 | 1.07 |
| Predicted Error (%) | −3.77 | 19.06 | 41.09 | 42.94 | 44.39 | 31.76 | 56.71 | 1.27 |
| 95% CI | (−7.89; 0.35) | (5.90; 32.22) | (16.38; 65.80) | (19.22; 66.66) | (19.00; 69.78) | (9.97; 53.56) | (22.42; 91.00) | (−2.47; 5.02) |
| P Value | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.50 |
| Cancer | 12,009 | 801 | 406 | 236 | 222 | 197 | 128 | 13,999 |
| Actual Cost | 1.00 | 2.18 | 2.15 | 2.52 | 2.94 | 3.42 | 3.64 | 1.19 |
| Predicted Cost | 1.05 | 1.69 | 1.78 | 1.92 | 2.09 | 2.43 | 2.62 | 1.16 |
| Predicted Error (%) | −4.24 | 26.76 | 14.49 | 43.45 | 85.22 | 52.07 | 54.99 | 0.84 |
| 95% CI | (−9.52; 1.04) | (7.09; 46.44) | (−5.13; 34.10) | (15.56; 71.33) | (24.64; 145.80) | (28.10; 76.04) | (17.45; 92.54) | (−4.02; 5.69) |
| P Value | 0.12 | 0.01 | 0.15 | 0.00 | 0.01 | 0.00 | 0.00 | 0.74 |
| Lung Disease | 5,759 | 492 | 275 | 144 | 133 | 108 | 75 | 6,986 |
| Actual Cost | 1.23 | 2.48 | 2.56 | 3.17 | 3.40 | 2.79 | 3.81 | 1.49 |
| Predicted Cost | 1.19 | 1.70 | 1.89 | 2.01 | 2.22 | 2.63 | 2.88 | 1.32 |
| Predicted Error (%) | 2.40 | 32.00 | 70.31 | 50.80 | 69.25 | 53.22 | 44.05 | 10.00 |
| 95% CI | (−5.53; 10.33) | (9.56; 54.44) | (4.63; 135.99) | (−5.22; 106.83) | (5.43; 133.07) | (−31.69; 138.14) | (6.50; 81.60) | (2.51; 17.49) |
| P Value | 0.55 | 0.01 | 0.04 | 0.08 | 0.03 | 0.22 | 0.02 | 0.01 |
| Stroke | 3,663 | 459 | 294 | 202 | 232 | 251 | 206 | 5,307 |
| Actual Cost | 1.24 | 1.83 | 2.15 | 2.47 | 2.45 | 2.61 | 3.91 | 1.59 |
| Predicted Cost | 1.20 | 1.70 | 1.85 | 1.99 | 1.96 | 2.38 | 2.67 | 1.44 |
| Predicted Error (%) | 8.50 | −1.00 | 47.43 | 31.77 | 38.30 | 23.13 | 50.15 | 13.94 |
| 95% CI | (−2.22; 19.22) | (−16.17; 14.16) | (−10.56; 105.43) | (5.71; 57.83) | (−10.27; 86.88) | (1.87; 44.38) | (24.03; 76.27) | (5.30; 22.58) |
| P Value | 0.12 | 0.90 | 0.11 | 0.02 | 0.12 | 0.03 | 0.00 | 0.00 |
| CHF | 3,601 | 520 | 285 | 192 | 207 | 221 | 149 | 5,175 |
| Actual Cost | 2.56 | 3.23 | 3.50 | 4.21 | 3.39 | 3.37 | 5.44 | 2.87 |
| Predicted Cost | 2.16 | 2.55 | 2.73 | 2.94 | 3.17 | 3.38 | 3.62 | 2.38 |
| Predicted Error (%) | 10.89 | 19.34 | 23.56 | 42.31 | 18.47 | 5.30 | 54.44 | 14.64 |
| 95% CI | (−1.89; 23.67) | (2.20; 36.48) | (5.20; 41.91) | (−0.27; 84.89) | (−1.02; 37.96) | (−9.40; 20.00) | (20.01; 88.87) | (5.18; 24.10) |
| P Value | 0.09 | 0.03 | 0.01 | 0.05 | 0.06 | 0.48 | 0.00 | 0.00 |
| Diabetes | 5,991 | 571 | 287 | 210 | 176 | 174 | 88 | 7,497 |
| Actual Cost | 1.29 | 2.50 | 2.92 | 3.65 | 2.79 | 3.80 | 5.04 | 1.61 |
| Predicted Cost | 1.28 | 1.96 | 2.21 | 2.21 | 2.52 | 2.99 | 3.20 | 1.47 |
| Predicted Error (%) | 1.29 | 21.57 | 34.29 | 60.24 | 22.53 | 47.93 | 64.67 | 7.37 |
| 95% CI | (−6.28; 8.86) | (2.18; 40.95) | (5.05; 63.53) | (13.59 ;106.89) | (−9.99; 55.04) | (20.96; 74.90) | (22.86; 106.48) | (0.80; 13.95) |
| P Value | 0.74 | 0.03 | 0.02 | 0.01 | 0.17 | 0.00 | 0.00 | 0.03 |
| CAD | 8,486 | 759 | 404 | 251 | 202 | 246 | 155 | 10,503 |
| Actual Cost | 1.24 | 2.02 | 2.56 | 2.75 | 2.45 | 2.88 | 3.51 | 1.45 |
| Predicted Cost | 1.18 | 1.70 | 1.87 | 2.00 | 2.25 | 2.47 | 2.69 | 1.32 |
| Predicted Error (%) | 4.79 | 21.55 | 34.49 | 45.73 | 10.39 | 32.33 | 44.09 | 8.96 |
| 95% CI | (−1.56; 11.14) | (0.03; 43.07) | (15.78; 53.19) | (7.67; 83.79) | (−18.93; 39.71) | (7.48; 57.17) | (16.07; 72.11) | (3.38; 14.55) |
| P Value | 0.14 | 0.05 | 0.00 | 0.02 | 0.49 | 0.01 | 0.00 | 0.00 |
Note: Total number of person years is 46,790, including those who participated in MCBS for at least two years.
Actual costs are presented as the ratio of the individual’s annualized costs to the mean annual Medicare cost of all people in the study. Predicted costs are estimated by HCC scores.
Table 3.
Actual/Predicted Cost Ratios for Target Chronic Conditions NOT Included in HCCs, by ADLs
| Chronic Conditions | Number of ADLs |
Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Hypertension | 21,238 | 1,493 | 726 | 484 | 467 | 393 | 247 | 25,048 |
| Actual Cost Ratio | 0.97 | 1.85 | 2.13 | 2.67 | 2.94 | 2.92 | 4.07 | 1.16 |
| Predicted Cost Ratio | 0.98 | 1.52 | 1.72 | 1.85 | 1.99 | 2.36 | 2.69 | 1.10 |
| Predicted Error (%) | −0.72 | 21.85 | 34.45 | 45.02 | 67.56 | 45.22 | 71.10 | 4.52 |
| 95% CI | (−5.19; 3.76) | (5.47; 38.24) | (8.10; 60.79) | (17.82; 72.21) | (32.48; 102.64) | (17.54; 72.90) | (33.24;108.95) | (0.40; 8.64) |
| P Value | 0.75 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 |
| Heart Disease | 16,170 | 1,306 | 677 | 412 | 393 | 430 | 251 | 19,639 |
| Actual Cost Ratio | 1.16 | 2.15 | 2.44 | 2.66 | 2.65 | 2.69 | 3.96 | 1.37 |
| Predicted Cost Ratio | 1.13 | 1.69 | 1.85 | 1.99 | 2.13 | 2.37 | 2.82 | 1.26 |
| Predicted Error (%) | 2.80 | 31.72 | 33.90 | 46.42 | 38.32 | 26.86 | 60.10 | 8.04 |
| 95% CI | (−2.00; 7.60) | (12.79; 50.66) | (14.57; 53.23) | (18.00; 74.83) | (2.29; 74.34) | (9.66; 44.06) | (24.80; 95.39) | (3.69; 12.39) |
| P Value | 0.25 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 |
| Osteoporosis | 4,136 | 427 | 222 | 158 | 160 | 184 | 109 | 5,396 |
| Actual Cost Ratio | 0.95 | 2.04 | 2.23 | 2.81 | 2.24 | 2.18 | 3.00 | 1.23 |
| Predicted Cost Ratio | 1.01 | 1.64 | 1.57 | 1.81 | 1.94 | 2.05 | 2.27 | 1.17 |
| Predicted Error (%) | −6.71 | 31.56 | 25.72 | 43.49 | 33.93 | 36.74 | 97.40 | 2.74 |
| 95% CI | (−14.22; 0.80) | (5.14; 57.98) | (0.73; 50.71) | (−5.69; 92.68) | (−14.82; 82.67) | (−14.83; 88.31) | (22.33; 172.48) | (−4.16; 9.63) |
| P Value | 0.08 | 0.02 | 0.04 | 0.08 | 0.17 | 0.16 | 0.01 | 0.44 |
| Dementia | 1,421 | 218 | 165 | 118 | 143 | 177 | 158 | 2,400 |
| Actual Cost Ratio | 1.79 | 2.43 | 3.18 | 3.33 | 3.03 | 3.03 | 4.13 | 2.31 |
| Predicted Cost Ratio | 1.61 | 2.09 | 2.19 | 2.40 | 2.48 | 2.60 | 3.08 | 1.93 |
| Predicted Error (%) | 6.86 | 7.79 | 46.81 | 45.82 | 22.27 | 31.87 | 32.43 | 15.72 |
| 95% CI | (−5.33; 19.06) | (−15.94; 31.53) | (6.89; 86.72) | (5.64; 86.00) | (−3.59; 48.13) | (1.37; 62.36) | (8.29; 56.58) | (6.84; 24.59) |
| P Value | 0.27 | 0.52 | 0.02 | 0.03 | 0.09 | 0.04 | 0.01 | 0.00 |
Note: Total number of person years is 46, 790, including those who participated in MCBS for at least two years.
The discrepancy between the actual and predicted cost ratios was larger for beneficiaries with multiple comorbidities than for those with a single target condition. For example, the CMS-HCC model underpredicted the expenses of the beneficiaries with CHF and osteoporosis by 30.02% (Tables 4-5) while the predictions were 20.60% lower (p<0.001) for patients with CHF only and no different from actual costs (p=0.32) for osteoporosis only (Tables 2-3). The model also underpredicted medical expenses for the beneficiaries with arthritis and hypertension by 7.08% (p=0.01), while underpredicting by 5.70% for the patients with hypertension (p=0.01) only; expenditures of the patients with diabetes and CAD were underpredicted by 18.70% (p<0.001), but only by 9.77% (p<0.001) for diabetes and 10.40% (p<0.001) for the patients with CAD. Moreover, the magnitude of the prediction error was greater for the pairs that included conditions without corresponding HCCs than for the conditions that have corresponding HCCs (e.g., CHF, cancer) or those accounted for by other HCCs (e.g., hypertension, heart disease) (Tables 4-5).
Table 4.
Actual/Predicted Cost Ratios for Pairs of Target Chronic Conditions Included in HCCs, by ADLs
| Chronic Conditions | Number of ADLs |
Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Lung Disease and Cancer | 1,983 | 205 | 102 | 60 | 48 | 39 | 18 | 2,455 |
| Actual Cost Ratio | 1.35 | 2.15 | 2.69 | 2.84 | 3.64 | 3.61 | 4.44 | 1.59 |
| Predicted Cost Ratio | 1.29 | 1.86 | 1.95 | 2.26 | 2.69 | 2.87 | 3.28 | 1.44 |
| Predicted Error (%) | 8.63 | 19.92 | 25.23 | 28.30 | 72.16 | 34.68 | 39.83 | 12.30 |
| 95% CI | (−3.84; 21.10) | (−7.37; 47.21) | (−8.88; 59.34) | (−15.04; 71.63) | (−29.43; 173.74) | (−9.23; 78.58) | (−15.63; 95.29) | (1.57; 23.03) |
| P Value | 0.17 | 0.15 | 0.15 | 0.20 | 0.16 | 0.12 | 0.15 | 0.02 |
| Stroke and Arthritis | 2,357 | 330 | 219 | 153 | 163 | 159 | 127 | 3,508 |
| Actual Cost Ratio | 1.25 | 1.93 | 2.52 | 2.67 | 2.43 | 2.46 | 4.31 | 1.64 |
| Predicted Cost Ratio | 1.26 | 1.78 | 1.89 | 2.04 | 2.11 | 2.39 | 2.80 | 1.51 |
| Predicted Error (%) | 7.30 | 1.76 | 75.92 | 44.00 | 13.10 | 6.22 | 53.97 | 13.98 |
| 95% CI | (−3.80; 18.41) | (−16.92; 20.43) | (−2.09; 153.94) | (11.07; 76.92) | (−8.31; 34.50) | (−12.32; 24.77) | (21.35; 86.59) | (4.66; 23.30) |
| P Value | 0.20 | 0.85 | 0.06 | 0.01 | 0.23 | 0.51 | 0.00 | 0.00 |
| Diabetes and CAD | 1,869 | 234 | 132 | 83 | 70 | 74 | 37 | 2,499 |
| Actual Cost Ratio | 1.67 | 2.46 | 3.85 | 4.21 | 3.57 | 4.59 | 5.78 | 2.10 |
| Predicted Cost Ratio | 1.54 | 2.14 | 2.37 | 2.29 | 3.06 | 3.23 | 3.86 | 1.77 |
| Predicted Error (%) | 12.12 | 14.14 | 61.33 | 46.36 | 41.18 | 68.79 | 44.26 | 18.33 |
| 95% CI | (−0.71; 24.95) | (−12.92; 41.20) | (30.61; 92.06) | (−39.33; 132.04) | (−31.55; 113.92) | (27.82; 109.77) | (0.35; 88.18) | (7.59; 29.07) |
| P Value | 0.06 | 0.30 | 0.00 | 0.28 | 0.26 | 0.00 | 0.05 | 0.00 |
Note: Total number of person years is 46, 790, including those who participated in MCBS for at least two years.
Table 5.
Actual/Predicted Cost Ratios for Pairs of Target Chronic Conditions, with one of the conditions NOT included in HCCs, by ADLs
| Chronic Conditions | Number of ADLs |
Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Arthritis and Hypertension | 13,885 | 1,178 | 556 | 388 | 353 | 299 | 185 | 16,844 |
| Actual Cost Ratio | 1.02 | 1.73 | 2.31 | 2.74 | 2.85 | 2.91 | 4.11 | 1.23 |
| Predicted Cost Ratio | 1.02 | 1.53 | 1.69 | 1.82 | 2.01 | 2.37 | 2.76 | 1.14 |
| Predicted Error (%) | 0.82 | 19.63 | 47.61 | 40.46 | 54.71 | 43.78 | 79.86 | 6.57 |
| 95% CI | (−4.55; 6.20) | (3.49; 35.77) | (14.23; 80.99) | (11.67; 69.26) | (22.11; 87.32) | (10.89; 76.67) | (33.17; 126.55) | (1.70; 11.43) |
| P Value | 0.76 | 0.02 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 |
| Heart Disease and Cancer | 5,657 | 528 | 252 | 150 | 137 | 128 | 84 | 6,936 |
| Actual Cost Ratio | 1.27 | 2.42 | 2.66 | 2.81 | 3.03 | 3.46 | 4.28 | 1.52 |
| Predicted Cost Ratio | 1.24 | 1.82 | 1.87 | 2.08 | 2.30 | 2.64 | 2.94 | 1.38 |
| Predicted Error (%) | 6.91 | 34.67 | 33.29 | 48.81 | 75.05 | 47.28 | 56.97 | 12.83 |
| 95% CI | (−1.06; 14.87) | (7.56; 61.78) | (8.07; 58.52) | (14.90; 82.71) | (−11.01; 161.12) | (18.27; 76.29) | (13.08; 100.86) | (5.66; 20.00) |
| P Value | 0.09 | 0.01 | 0.01 | 0.01 | 0.09 | 0.00 | 0.01 | 0.00 |
| Stroke and Hypertension | 2,590 | 342 | 210 | 146 | 164 | 171 | 137 | 3,760 |
| Actual Cost Ratio | 1.31 | 1.78 | 2.29 | 2.40 | 2.97 | 2.82 | 4.53 | 1.68 |
| Predicted Cost Ratio | 1.24 | 1.74 | 1.92 | 1.97 | 2.17 | 2.51 | 2.87 | 1.49 |
| Predicted Error (%) | 13.06 | −3.33 | 62.94 | 27.61 | 60.26 | 34.07 | 62.74 | 19.27 |
| 95% CI | (0.95; 25.16) | (−20.88; 14.23) | (−15.34;141.22) | (−1.97; 57.19) | (−6.55; 127.07) | (7.64; 60.51) | (30.00;95.47) | (9.10; 29.43) |
| P Value | 0.03 | 0.71 | 0.11 | 0.07 | 0.08 | 0.01 | 0.00 | 0.00 |
| CHF and osteoporosis | 441 | 114 | 53 | 46 | 46 | 63 | 32 | 795 |
| Actual Cost Ratio | 2.96 | 3.12 | 4.35 | 5.86 | 3.07 | 2.48 | 3.27 | 3.23 |
| Predicted Cost Ratio | 2.24 | 2.64 | 2.58 | 2.84 | 3.00 | 2.86 | 3.25 | 2.48 |
| Predicted Error (%) | 12.60 | 13.00 | 54.56 | 75.83 | 10.38 | −10.84 | 18.57 | 17.49 |
| 95% CI | (−14.32; 39.51) | (−18.67; 44.66) | (3.00; 106.12) | (−70.67; 222.32) | (−29.42; 50.17) | (−35.51; 13.82) | (−19.36; 56.51) | (−0.88; 35.87) |
| P Value | 0.36 | 0.42 | 0.04 | 0.30 | 0.60 | 0.38 | 0.33 | 0.06 |
| CHF and Dementia | 259 | 65 | 60 | 33 | 57 | 59 | 65 | 598 |
| Actual Cost Ratio | 4.06 | 3.96 | 4.66 | 4.40 | 3.53 | 3.66 | 6.14 | 4.23 |
| Predicted Cost Ratio | 3.04 | 3.03 | 3.20 | 3.46 | 3.61 | 3.66 | 4.24 | 3.30 |
| Predicted Error (%) | 17.64 | 24.02 | 19.60 | 31.78 | 7.74 | 17.50 | 54.09 | 21.80 |
| 95% CI | (−7.70; 42.97) | (−14.29; 62.32) | (−14.63; 53.83) | (−23.74;87.31) | (−27.95; 43.43) | (−12.63;47.64) | (10.78; 97.39) | (7.63; 35.97) |
| P Value | 0.17 | 0.21 | 0.26 | 0.25 | 0.67 | 0.25 | 0.02 | 0.00 |
Note: Total number of person years is 46, 790, including those who participated in MCBS for at least two years.
The 95% confidence intervals around the error estimates demonstrated that the study sample size was generally sufficient to make robust prediction. In some cases, where the predicted error was not statistically significantly different from zero, the analysis of confidence intervals illustrated clinically or practically substantial error (e.g., p=0.08, 95% CI [−5.69; 92.68] for osteoporosis with 3 ADLs, p=0.15, 95% CI [−7.37; 47.21] for lung disease and cancer with 1 ADL).
Effect of functional status and comorbidity on medical expenses
Since the majority of beneficiaries in our sample had more than one of the target comorbidities and various levels of functional impairment, we examined the joint impact of the multiple comorbidities and disability on the accuracy of the CMS_HCC capitation model (Table 3).
Among the pairs of comorbid conditions, having arthritis and hypertension (0.079, p=0.05), diabetes and CAD (0.260, p=0.01), and CHF and dementia (0.783, p=0.01) led to substantial underpayments calculated by the CMS-HCC model. However, these differences were mainly due to the underpayment for the single conditions (lung disease, CHF and dementia) rather than additional error due to having multiple comorbidities since adding single conditions improved the explanatory power of the model (R2=0.34 compared to 0.21, Table 6) and reduced the significance of p-values (>0.05) for the variables identifying pairs of conditions. Functional status helped explained even more of the difference between the actual costs and the predicted amount based on the capitation model (R2=0.46). Number of ADLs was highly significant (p<0.01) in explaining the variation between actual costs and predicted payment, and so was the presence of hypertension, lung disease, and CHF (p<0.05).
Table 6.
Impact of Chronic Conditions and Physical Disabilities on Cost Predictions.
| Chronic Conditions or ADLs | Corresponding HCC |
Model with Disease Interactions Only |
Model without ADL |
Model with ADL |
|---|---|---|---|---|
| Having 1 or 2 ADLs | + | -- | -- | 0.257** |
| Having 3 or more ADLs | + | -- | -- | 0.542** |
| Arthritis | + | -- | 0.069 | 0.065 |
| Cancer | + | -- | 0.011 | 0.015 |
| Lung Disease | + | -- | 0.175* | 0.169* |
| Stroke | + | -- | 0.151 | 0.070 |
| CHF | + | -- | 0.384* | 0.339* |
| Diabetes | + | -- | 0.042 | 0.026 |
| CAD | + | -- | −0.004 | −0.006 |
|
| ||||
| Hypertension | Other HCC | -- | 0.087 | 0.094* |
| Heart Disease | Other HCC | -- | 0.024 | 0.021 |
| Osteoporosis | − | -- | −0.022 | −0.051 |
| Dementia | − | -- | 0.257** | 0.154 |
|
| ||||
| Lung Disease and Cancer | +/+ | 0.073 | −0.097 | −0.104 |
| Stroke and Arthritis | +/+ | −0.077 | −0.171 | −0.159 |
|
| ||||
| Diabetes and CAD | +/+ | 0.260** | 0.157 | 0.154 |
| Arthritis and Hypertension | +/− | 0.079* | −0.032 | −0.045 |
| Heart Disease and Cancer | +/− | 0.092 | 0.057 | 0.058 |
| Stroke and Hypertension | +/− | 0.164 | 0.033 | 0.040 |
| CHF and osteoporosis | +/− | 0.576 | 0.286 | 0.246 |
| CHF and Dementia | +/− | 0.783** | 0.235 | 0.211 |
|
| ||||
| Year 1999 | −0.103 | −0.106 | −0.100 | |
| Intercept | −0.074** | −0.185** | −0.201** | |
| R2 (%) | 0.21 | 0.34 | 0.46 | |
The dependent variable is the difference between the actual cost ratio and predicted cost ratio; the number of observations is 46,790; sampling weights are used in the model estimation;
Significant at 5% level;
Significant at 1% level.
DISCUSSION
While several studies have examined the effect of multiple comorbidities on physical functioning and disability, less is known about the financial implications of the Medicare capitation payment model on health plans serving enrollees with comorbid chronic conditions and functional impairment. Our results demonstrate that the CMS-HCC model is likely to underpredict expenses for such Medicare beneficiaries, and that the disability level accounts for a substantial portion of the difference between actual and predicted expenses. The CMS-HCC model significantly underpredicts expenses for patients with hypertension, lung disease, CHF, and dementia after adjusting for patients’ disability level. This is supported by other studies reporting that the accuracy of expenditure models varies by medical condition36.
Currently, the CMS-HCC model does not account for additional costs of functional impairments that often accompany chronic health conditions. However, the CMS has always accounted for beneficiary ADL levels when calculating reimbursement for the Program of All-Inclusive Care for the Beneficiaries (PACE) plans27.
Our results demonstrate that unless a special disability-adjustment is introduced for patients with comorbidities, entering into risk arrangements with Medicare for services provided to people with multiple comorbid conditions may be more risky for health plans serving this population than anticipated. Capitation payments for SNPs are calculated based on the HCCs just as for the Medicare Advantage plans. It is anticipated that if the existing SNPs perform well over time, new disease management SNPs will be established for patients with a wider range of chronic conditions. Currently SNPs do not receive frailty adjustments, while some demonstration programs do. If the SNPs are not qualified for the frailty adjustment, then to the extent that comorbid conditions result in greater disability and thus, higher medical expenses, these plans are financially at a disadvantage in providing care to the very frail and disabled. The Medicare Advocacy commission report demonstrated that beneficiaries in private fee-for-service plans have had difficulties receiving care.37 This could be partially explained by financial disincentives resulting from low reimbursement that providers receive for these patients and serve as evidence that financial incentives play a role in determining providers’ behavior. Similar effects are expected in managed care plans.
In addition to the SNPs, hospitals and physicians are developing clinical specialty-services lines as well, competing for patients and looking to ways to maximize profits24. By focusing on specific patient populations, currently those with heart disease, cancer, or orthopedic problems, providers are trying to avoid really sick patients for whom they do not receive a sufficient reimbursement, a practice that can be minimized by proper risk-adjustment of payments.
There could be several possible explanations why adjusting for disability decreases the prediction error of the CMS-HCC risk-adjustment model. Some combinations of chronic conditions are more likely to lead to disability and worsening of health. For instance, patients with cardiovascular conditions who have a disease of bone, muscles, and joints are likely to have a worse prognosis because of limited possibility for physical activity that is essential for preventing worsening and maintaining their cardio-vascular health. Having dementia would exacerbate any existing heath problems because of limited ability of the patient to participate in his/her own care.
The study has several limitations. First, we used the 1992-2000 MCBS data to verify the performance of the CMS-HCC model that was developed using 1999-2000 data only. It is conceivable that the main discrepancy between the actual and the HCC-based cost ratios is explained by the different relationship between the risk factors and the healthcare costs in early and late 1990s. We included a time dummy variable in the model (Table 6) but did not find any significant time trends. Also, to use the CMS-HCC software, we had to have at least two years of data for each beneficiary included in our study. Hence, it is conceivable, that by excluding subjects who did not have two years of the claims data, we limited our sample to healthier individuals that could result in the underestimation of underpayments.
Second, we did not control for other potential comorbidities in our sample population that could also bias our estimates. We chose to concentrate on these heterogeneous groups rather than limiting the sample to beneficiaries with only the target comorbidities because this could introduce a different type of selection bias. While disability status and comorbidities were significant predictors of the discrepancy between the actual expenses and HCC-based reimbursement, together they explain less than 1% of variation in the cost difference. Similarly, Kautter and Pope (2004) 13showed that frailty explains about 1% of the variation. One reason we may not see more statistically significant results is because we use cost ratios (on the scale of 0 and 6) rather than expenditures ($0 to $100,000) thus resulting in fairly small effect size.
Finally, the accuracy and the specificity of the ICD codes vary by condition. Our results demonstrated that having CHF, in addition to other chronic conditions, resulted in substantial underpredictions of the CMS-HCC model. Since there is a great variation in the severity of the CHF that is not reflected in the ICD codes (ICD 428 is predominantly used to code for HF, Appendix A), it is conceivable is that our sample by chance had a higher prevalence of severe CHF than the population for whom HCC was developed. In contrast, there is a variety of codes for diabetes (Appendix B) that reflect the severity of the condition and associated expenditures. Hence, HCC predictions for diabetic patients (9.77% underprediction error) are more accurate than for patients with CHF (20.60% underprediction).
For this study we used both self-reported disease status from the MCBS (for arthritis, hypertension, heart disease, cancer, lung disease, stroke, osteoporosis, diabetes, and CAD) and claims-based identification of patients with specific conditions (for CHF and dementia). Prior reports suggested that information elicited from subjects face-to-face is generally of high accuracy38,39. Numerous studies that examined sensitivities of claims-based identification algorithms compared to a variety of gold standards reported satisfactory results that varied, however, by disease (PD using MCBS40 or VA and medical records41; diabetes using MCBS42; chronic kidney disease using charts and Medicare claims43; breast cancer using SEER-Medicare25, 44-46; dementia using medical records32; cardiovascular disease and stroke, Medicare using medical records47).
On the basis of our findings, we conclude that the CMS-HCC model quite fairly calculates the Medicare capitation payments for beneficiaries with most chronic conditions except for patients with functional impairments and those with hypertension, lung disease, CHF, and dementia. The discrepancy between the predicted and actual expenditures was larger for patients with CHF and dementia than for beneficiaries with other pairs of target comorbidities. However, more research is needed to understand the pathophysiology of physical disability in these chronic conditions and what makes medical expenses of patients with chronic illnesses so much higher than expenses of beneficiaries without such conditions.
SUMMARY.
Our findings indicate that information about beneficiary functional status should be incorporated in Medicare reimbursement models since without functional status adjustment such models are likely to underestimate costs of caring for patients with disability and multiple comorbidities. Underpaying providers for caring for population with multiple comorbidities may provide severe disincentives for managed care plans to enroll such individuals and to appropriately manage their complex and costly conditions.
Acknowledgement
The authors would like to thank the National Institute of Aging for their support of the study. The use of the Medicare Current Beneficiary Survey was covered by the Data Use Agreement #12874.
We gratefully acknowledge financial support from the National Institute of Health, Grant K01 AG20980. This study does not have any potential conflicts of interest in the past three years.
KN and HL were in part supported in part by a K01 AG 20980 from the National Institute of Aging. The use of the Medicare Current Beneficiary Survey was covered by the Data Use Agreement #12874.
Appendix A.
ICD 9 Diagnosis Codes for Identifying Patients with Different Comorbidities Using Claims Data
| Comorbidities | ICD9 Codes | Description |
|---|---|---|
| Heart failure | ||
| 428 | Heart failure | |
| 428.0 | Congestive heart failure | |
| 428.1 | Left heart failure | |
| 428.2 | systolic heart failure | |
| 428.3 | diastolic heart failure | |
| 428.4 | Combined systolic and diastolic heart failure | |
| 428.9 | Heart failure, unspecified | |
| Dementia | ||
| 290.0 | Senile dementia | |
| 290.1 | Pre-senile dementia | |
| 290.3 | Senile dementia with delirium | |
| 294.1 | Dementia in conditions classified elsewhere | |
| 294.8 | Other persistent mental disorders due to conditions classified elsewhere |
|
| 294.9 | Unspecified persistent mental disorders due to conditions classified elsewhere |
|
| 298.9 | Unspecified psychosis | |
| 331.0 | Alzheimer’s disease | |
| 331.2 | Senile degeneration of brain | |
| 331.3 | Communicating hydrocephalus | |
| 331.4 | Obstructive hydrocephalus | |
| 348.3 | Encephalopathy, unspecified | |
| 797 | Senility without mention of psychosis | |
| 780.9 | Other general symptoms |
Appendix B.
ICD9 Codes associated with diabetes Based on the MDS http://www.e-mds.com/services/icd9/index.html and STATA software
| 250.00 | Diabetes, type II. |
| 250.01 | Diabetes, type I. |
| 250.02 | Diabetes, type II, uncontrolled. |
| 250.03 | Diabetes, type I, uncontrolled. |
| 250.10 | Diabetes with ketoacidosis, type II. |
| 250.11 | Diabetes with ketoacidosis, type I. |
| 250.12 | Diabetes with ketoacidosis, type II, uncontrolled. |
| 250.13 | Diabetes with ketoacidosis, type I, uncontrolled. |
| 250.20 | Diabetes with hyperosmolarity, type II. |
| 250.21 | Diabetes with hyperosmolarity, type I. |
| 250.22 | Diabetes with hyperosmolarity, type II, uncontrolled. |
| 250.23 | Diabetes with hyperosmolarity, type I, uncontrolled. |
| 250.40 | Diabetes with renal manifestations, type II. |
| 250.41 | Diabetes with renal manifestations, type I. |
| 250.42 | Diabetes with renal manifestations, type II, uncontrolled. |
| 250.43 | Diabetes with renal manifestations, type I, uncontrolled. |
| 250.50 | Diabetes with ophthalmic manifestations, type II. |
| 250.51 | Diabetes with ophthalmic manifestations, type I. |
| 250.52 | Diabetes with ophthalmic manifestations, type II, uncontrolled. |
| 250.53 | Diabetes with ophthalmic manifestations, type I, uncontrolled. |
| 250.60 | Diabetes with neurological manifestations, type II. |
| 250.61 | Diabetes with neurological manifestations, type I. |
| 250.62 | Diabetes with neurological manifestations, type II, uncontrolled. |
| 250.63 | Diabetes with neurological manifestations, type I, uncontrolled. |
| 250.70 | Diabetes with peripheral vascular disease, type II. |
| 250.8 | Diabetes with manifestsations, nec |
| 250.9 | Diabetes with complications, nos |
| 253.5 | Diabetes insipidus |
| 357.2 | Neuropathy in diabetes |
| 588.1 | Nephrogen diabetes insipidus |
| 648.0 | Diabetes mellitus in pregnancy |
| 648.00 | Diabetes in pregnancy, nos |
| 648.01 | Diabetes-delivered |
| 648.02 | Diabetes-delivered with postpartum |
| 648.03 | Diabetes-antepartum |
| 648.04 | Diabetes-postpartum |
| 775.1 | Neonat diabetes mellitus |
| V18.0 | Family history-diabetes mellitus |
| V77.1 | Screening-diabetes mellitus |
Footnotes
The original model was developed using 1999-2000 claims. Starting 2007, the HCC model has been recalibrated using 2002-2003 data.
Study Description: The study examines financial implications of risk-adjustment on Medicare payments for individuals with co-morbid conditions and functional impairment and demonstrates importance of controlling for disability.
REFERENCES
- (1).Hoffman C, Rice D, Sung H. Persons with chronic conditions: their prevalence and costs. Journal of American Medical Association. 1996;276:1473–9. [PubMed] [Google Scholar]
- (2).Anderson GF. Medicare and chronic conditions. New England Journal of Medicine. 2005;353(3):305–9. doi: 10.1056/NEJMsb044133. [DOI] [PubMed] [Google Scholar]
- (3).Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Rep. 2004;119(3):263–70. doi: 10.1016/j.phr.2004.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Partnership for Solutions JHU Chronic Conditions and Disability: Analysis of 2000 Census. 2003. 2003.
- (5).Gijsen R, Hoeymans N, Schellevis F, et al. Causes and consequences of co morbidity: A review. J Clin Epidemiol. 2001;54:661–74. doi: 10.1016/s0895-4356(00)00363-2. [DOI] [PubMed] [Google Scholar]
- (6).Stuck A, Walthert J, Nikolaus T, Bula C, Hoffman C, Beck J. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Social Science & Medicine. 1999;48:445–69. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- (7).Boult C, Kane RL, Louis TA, Boult L, McCaffrey D. Chronic conditions that lead to functional limitation in the elderly. Journal of Gerontology. 1994;49(1):M28–M36. doi: 10.1093/geronj/49.1.m28. [DOI] [PubMed] [Google Scholar]
- (8).Kriegsman D, Deeg D, Stalman W. Comorbidity of somatic chronic diseases and decline in physical functioning: the Longitudinal Aging Study Amsterdam. Journal of Clinical Epidemiology. 2004;57:55–65. doi: 10.1016/S0895-4356(03)00258-0. [DOI] [PubMed] [Google Scholar]
- (9).Fried LP, Ettinger WH, Lind B, Newman AB, Gardin J. Physical disability in older adults: a physiologic approach. Cardiovascular Health Study Research Group. Journal of Clinical Epidemiology. 1994;47(7):747–60. doi: 10.1016/0895-4356(94)90172-4. [DOI] [PubMed] [Google Scholar]
- (10).Fried LP, Bandeen-Roche K, Kasper J, Guralnik JM. Association of comorbidity with disability in older women: The Women’s Health and Aging Study. Journal of Clinical Epidemiology. 1999;52:27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- (11).Centers for Medicare and Medicaid Services (CMS) CMS-HCC Payment Model software and data for 2004. Centers for Medicare and Medicaid Services; 2003. Available at: URL: http://www.cms.hhs.gov/healthplan/rates/.2003. [PubMed] [Google Scholar]
- (12).Pope GC, Kautter J, Elllis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financing Review. 2004;25(4):119–141. [PMC free article] [PubMed] [Google Scholar]
- (13).Kautter J, Pope GC. The CMS frailty adjustment model. Health Care Financing Review. 2004;26(2):1–201. [PMC free article] [PubMed] [Google Scholar]
- (14).McCall N, Khatutsky G, Smith K, Pope GC. Estimation of non-response bias in the Medicare FFS HOS. Health Care Financing Review. 2004;25(4):27–41. [PMC free article] [PubMed] [Google Scholar]
- (15).Riley GF. Risk adjustment for health plans disproportionately enrolling frail Medicare beneficiaries. Health Care Financing Review. 2000;21(3):135–148. [PMC free article] [PubMed] [Google Scholar]
- (16).Temkin-Greener H, Meiners MR, Gruenberg L. PACE and the Medicare+Choice risk-adjusted payment model. Inquiry. 2001;38(1):69–72. doi: 10.5034/inquiryjrnl_38.1.60. [DOI] [PubMed] [Google Scholar]
- (17).Centers for Medicare and Medicaid Services (CMS) Special needs plans. CMS; [2007 August 10]. http://www.cms.hhs.gov/SpecialNeedsPlans/ [Google Scholar]
- (18).Medicare Advantage Special Needs Plans: A Beneficiary Perspective. SNP Conference; 10-18-2007; http://www.medicareadvocacy.org/SNP%20Conference/Home.htm. [Google Scholar]
- (19).Ettinger WH, Davis M, Neuhaus J, Mallon K. Long-term physical functioning in persons with knee osteoarthritis from NHANES-1: effect of comorbid medical conditions. Journal of Clinical Epidemiology. 1994 November 1;47:809–15. doi: 10.1016/0895-4356(94)90178-3. [DOI] [PubMed] [Google Scholar]
- (20).Fillenbaum G, Pieper C, Cohen H, Cornoni-Huntley JC, Guralnik JM. Comorbidity of five chronic health conditions in elderly community residents: determinants and impact on mortality. Journal of Gerontology: Medical Sciences. 2000;55A:M84–M89. doi: 10.1093/gerona/55.2.m84. [DOI] [PubMed] [Google Scholar]
- (21).Guralnik JM, LaCroix AZ, Abbott RD, et al. Maintaining mobility in late life. I. Demographic characteristics and chronic conditions. American Journal of Epidemiology. 1993;137:845–57. doi: 10.1093/oxfordjournals.aje.a116746. [DOI] [PubMed] [Google Scholar]
- (22).Newschaffer CJ, Bush TL, Penberthy LT. Comorbidity measurement in elderly female breast cancer patients with administrative and medical records data. Journal of Clinical Epidemiology. 1997;50:725–33. doi: 10.1016/s0895-4356(97)00050-4. [DOI] [PubMed] [Google Scholar]
- (23).van den Bos GAM. The burden of chronic illness in terms of disability, use of health care and health life expectancies. European Journal of Public Health. 1995;5:29–34. [Google Scholar]
- (24).Berenson RA, Bodenheimer T, Pham HH. Specialty-service lines: salvos in the new medical arms race. Health Affairs. 2006;25:w337–w343. doi: 10.1377/hlthaff.25.w337. [DOI] [PubMed] [Google Scholar]
- (25).Freeman JL, ZD, Freeman D, Goodwin JS. An approach to identifying incident breast cancer cases using Medicare claims data. Journal of Clinical Epidemiology. 2000;53(6):605–14. doi: 10.1016/s0895-4356(99)00173-0. [DOI] [PubMed] [Google Scholar]
- (26).Verbrugge LM, Lepkowski JM, Imanka Y. Comorbidity and its impact on disability. Milbank Quarterly. 1989;67:450–84. [PubMed] [Google Scholar]
- (27).Centers for Medicare and Medicaid Services (CMS) CMS Announcement of Calendar Year (CY) 2008 Medicare Advantage Capitation Rates and Payment Policies. CMS. 2007 July 2; Available at: URL: http://www.cms.hhs.gov/MedicareAdvtgSpecRateStats/Downloads/Advance2008.pdf.
- (28).Adler GS. A profile of the Medicare Current Beneficiary Survey. Health Care Financing Review. 1994;15(4):153–163. [PMC free article] [PubMed] [Google Scholar]
- (29).Eppig F, Chulis GS. Matching MCBS and Medicare data: the best of the both worlds. Health Care Financing Review. 1997;18(3):211–229. [PMC free article] [PubMed] [Google Scholar]
- (30).Centers for Medicare and Medicaid Services (CMS) Medicare Managed Care Manual. 2004 http://www cms hhs gov/manuals/116_mmc/mc86toc asp.
- (31).Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970-1974 and 1990-1994. Circulation. 2006;113(6):799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- (32).Pippenger M, Holloway RG, Vickrey BG. Neurologists’ use of ICD-9CM codes for dementia. Neurology. Neurology. 2001;56:1206–9. doi: 10.1212/wnl.56.9.1206. [DOI] [PubMed] [Google Scholar]
- (33).CMS Drug Coverage Claim Data 02 Rx Claims Payment Risk Adjustment. 2008 http://www.cms.hhs.gov/DrugCoverageClaimsData/02_RxClaims_PaymentRisk Adjustment.asp.
- (34).STATA for Windows Version 8/SE. College TX: 2006. [Google Scholar]
- (35).SAS Institute . SAS for Unix. Cary, NC: 2004. [Google Scholar]
- (36).Mark TL, Ozminkowski RJ, Kirk A, Ettner SL, Drabek J. Risk Adjustment for people with chronic conditions in private sector health plans. Medical Decision Making. 2003;23:397–405. doi: 10.1177/0272989x03257264. [DOI] [PubMed] [Google Scholar]
- (37).CMS The Center for Medicare Advocacy Releases a Report on Medicare Advantage private Fee-for-Service Plans. 2008 http://www.medicareadvocacy.org/MA_PFFSPrimerForAdvocates.pdf.
- (38).Marder K, Levy G, Louis ED, et al. Accuracy of family history data on Parkinson’s disease. Neurology. 2003;61(1):18–23. doi: 10.1212/01.wnl.0000074784.35961.c0. [DOI] [PubMed] [Google Scholar]
- (39).Strickland D, Bertoni JM. Parkinson’s prevalence estimated by a state registry. Movement Disorders. 2004;19(3):318–323. doi: 10.1002/mds.10619. [DOI] [PubMed] [Google Scholar]
- (40).Noyes K, Liu H, Holloway RG, Dick AW. Accuracy of Medicare Claims Data in Identifying Parkinsonism Cases. Movement Disorders. 2006;9(6):339–348. doi: 10.1002/mds.21299. [DOI] [PubMed] [Google Scholar]
- (41).Swarztrauber K, JA, Peters D. Identifying and distinguishing cases of Parkinsonism and Parkinson’s disease using ICD-9 CM codes and pharmacy data. Movement Disorders. 2005;20(8):964–70. doi: 10.1002/mds.20479. [DOI] [PubMed] [Google Scholar]
- (42).Hebert PI, Geiss LS, Tierney EF, Engelgau MM, Yawn BP. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14(6):270–7. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- (43).Winkelmayer WC, SS, Mogun H, Patrick AR, Avorn J, Soloman DH. Identification of individuals with CKD from Medicare claims data: a validation study. American Journal of Kidney Disease. 2005;46(2):225–32. doi: 10.1053/j.ajkd.2005.04.029. [DOI] [PubMed] [Google Scholar]
- (44).Gold HT, Do HT. Evaluation of Algorithms to Identify Breast Cancer Cases in Medicare Claims Data. Medical Decision Making. 2005;25:6. [Google Scholar]
- (45).Nattinger AB, Laud PW, Bajorunaite R, Sparapani RA, Freeman JL. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Services Research. 2004;39(6 Pt 1):1733–49. doi: 10.1111/j.1475-6773.2004.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Warren JL, Feuer E, Potosky AL, Riley GF, Lynch CF. Use of Medicare hospital and physician data to assess breast cancer incidence. Medical Care. 1999;37(5):445–56. doi: 10.1097/00005650-199905000-00004. [DOI] [PubMed] [Google Scholar]
- (47).Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Medical Care. 2005;43:480–5. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]