Abstract
An economic and cheap production of large amounts of recombinant allergenic proteins might become a prerequisite for the common use of microarray-based diagnostic allergy assays which allow a component-specific diagnosis. A molecular pharming strategy was applied to express the major allergen of Artemisia vulgaris pollen, Art v 1, in tobacco plants and tobacco cell cultures. The original Art v 1 with its endogenous signal peptide which directs Art v 1 to the secretory pathway, was expressed in transiently transformed tobacco leaves but was lost in stable transformed tobacco plants during the alternation of generations. Using a light-regulated promoter and “hiding” the recombinant Art v 1 in the ER succeeded in expression of Art v 1 over three generations of tobacco plants and in cell cultures generated from stable transformed plants. However, the amounts of the recombinant allergen were sufficient for analysis but not high enough to allow an economic production. Although molecular pharming has been shown to work well for the production of non-plant therapeutic proteins, it might be less efficient for closely related plant proteins.
Electronic supplementary material
The online version of this article (doi:10.1007/s00299-011-1199-3) contains supplementary material, which is available to authorised users.
Keywords: Artemisia vulgaris, Molecular pharming, Pollen, Allergy, Recombinant allergen
Introduction
Type I allergy, a hypersensitivity disease that is characterised by the production of IgE antibodies against the allergen (antigen), is now affecting more than 25% of the European population (Schmidt et al. 2008; Valenta et al. 2010) thus demanding new diagnostic and therapeutic tools. So far, native allergen extracts from allergen sources, e.g. pollen grains, cat dander and house dust mites, are used for diagnosis and also during specific immunotherapy (see Valenta et al. 2010 and references therein for general aspects of allergy and allergy treatments). Diagnostic tests using extracts allow the identification of the allergen-containing source whereas the application of purified allergens can identify the allergy-eliciting molecules (Cromwell et al. 2004; Valenta and Kraft 2002; Valenta et al. 1999). To improve diagnostic assays, the use of purified allergens is necessary (van Ree et al. 2008) but the purification of allergenic proteins from natural allergen sources is difficult as minute contaminations with other allergens cannot be excluded, and is far too expensive making their application in clinical diagnosis almost impossible. During the last 25 years, more and more allergens were identified by molecular biology techniques and recombinant allergens were produced in prokaryotic expression systems resembling most of the molecular properties of the native allergens including their IgE-binding capacity (Wallner et al. 2004). However, the majority of allergenic proteins are of plant origin and the prokaryotic expression systems fail to mimic plant-specific post-translational modifications identified in a number of major plant-derived allergens.
Molecular pharming is the production of important pharmaceutical and commercially valuable proteins in plants or plant cell cultures, and may become a common procedure to produce large amounts of recombinant allergens on a cost-effective base (Daniell et al. 2001, 2009; Hellwig et al. 2004; Horn et al. 2004; Obermeyer et al. 2004). Compared to animal or microbial expression systems, the plant-based expression has several advantages: potential for large-scale and low-cost biomass production, low risk of contaminants (mammalian viruses, prions, oncogenes or bacterial toxins), correct folding, post-translational modifications and assembly of multimeric proteins, low downstream processing costs, simultaneous production of multiple proteins and finally, less ethical problems than with transgenic animals and thus higher acceptance in the public (Doran 2000). Depending on the expressed protein and the plant system used for production up to 0.5 mg recombinant protein per gram plant tissue could be produced at very low costs (<100 US $ g−1 protein, Daniell et al. 2001). In addition, some plant-specific post-translational modifications, e.g. O-glycosylation which cannot be produced by bacteria or yeasts, have been suggested to be important for allergen recognition (Leonard et al. 2005), and therefore, plants are the expression system of choice for such modified proteins.
In the last few years, the suitability of recombinant allergens for clinical diagnostic assays has been investigated in vivo and in vitro (Astier et al. 2006; Schmid-Grendelmeier et al. 2003). Recombinant allergens were also spotted onto microarray carriers, which allow a component-specific diagnosis instead of the usual identification of the allergen source (Deinhofer et al. 2004; Hiller et al. 2002; Kim et al. 2002; Vigh-Conrad et al. 2010). These allergen microarrays allow a fast and specific allergen diagnosis at the molecular level. However, the production costs of these microarray-based assays will finally decide whether the clinical diagnosis of allergies will be performed with conventional extracts or with recombinant allergens. Recombinant allergens produced by molecular pharming techniques will help to dramatically reduce the production costs for allergen assays thus enabling these future perspectives in allergy diagnosis and may also be applicable for next generation allergy therapy.
To test the suitability of the plant-based production of allergens, we selected the major allergen of mugwort (Artemisia vulgaris), Art v 1, as a candidate for molecular pharming because earlier studies reported a lower IgE recognition of recombinant Art v 1 produced in E. coli probably due to post-translational glycosylation of the Art v 1 molecule (Himly et al. 2003; Schmid-Grendelmeier et al. 2003). Art v 1 is a 108 amino acid-long protein (apparent molecular weight in gel electrophoresis between 20 and 27 kDa) of unknown function with a cysteine-rich defensin-like domain at the N-terminus and a C-terminal proline-rich domain (Himly et al. 2003; Razzera et al. 2010). In addition, an N-terminal signal peptide predicts that Art v 1 enters the secretory pathway and is secreted into the external medium or cell wall of mugwort pollen. Two types of post-translational modifications were detected: hydroxylation of proline residues and a new type of an O-glycan composed of galactoses and arabinoses that were suggested to be important for the formation of IgE binding epitopes (Leonard et al. 2005). Using a molecular pharming strategy to produce sufficient amounts of recombinant Art v 1 might be applied to other allergens thus enhancing the quality of recombinant allergens and contemporaneously reducing production costs.
Materials and methods
Plant growth conditions
Wild-type tobacco plants (Nicotiana tabacum cv. SR1) were grown in a greenhouse under environmental conditions with artificial light (16 h light/8 h dark). Plants were fertilised weekly with commercial fertiliser. Surface sterilised seeds of transformed tobacco plants were cultivated on standard Murashige and Skoog (MS-) salts medium plus B5 vitamins, 3% (w/v) sucrose, 0.8% (w/v) agar, 100 mg l−1 kanamycin under aseptic conditions in growth chambers (16 h light/8 h dark, 24°C, Percival Scientific, model I-66HILQ, Perry, IA, USA) and finally transferred to soil, grown for 16 h light, 24°C and 8 h dark, 19°C (GroBanks, CLF, Emmersacker, Germany).
Construction of plasmids for plant expression
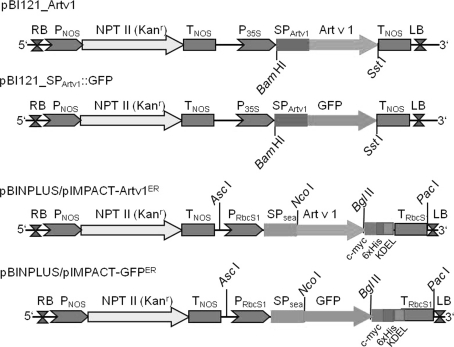
The plasmid pBI121-Artv1 was constructed by inserting the full-length Art v 1 coding sequence including its endogenous signal peptide (SPArtv1) between the BamHI and SstI cleavage sites of pBI121 (Fig. 1, Chen et al. 2003, access. no. AF485783), thus placing the Art v 1 coding sequence under the control of the CaMV 35S promoter and replacing the previous β-glucuronidase sequence. In addition, the Art v 1 signal peptide was fused to a GFP sequence using standard cloning and SOE (splicing by overlapping ends)-PCR (Horton et al. 1983) techniques and cloned into pBI121 between BamHI and SstI (Fig. 1). Vectors of the pIMPACT series were obtained from Plant Research International (Wageningen, The Netherlands (http://www.impactvector.com)). The sequence of Art v 1 without its endogenous signal peptide was inserted between the NcoI and BglII cleavage sites of pIMPACT 1.3 resulting in Art v 1 fused to a sea anemone signal peptide (SPsea) at the N-terminus (Outchkourov et al. 2003), a 6xHis tag and a c-myc tag at the C-terminus followed by a KDEL signal under the control of the chrysanthemum promoter rbcS1 (Outchkourov et al. 2003) resulting in pIMPACT-Artv1ER (Fig. 1, Fig. S1). In addition, a GFP coding sequence was also cloned into the respective pIMPACT vectors resulting in pIMPACT-GFPER which served as a control vector. The constructed expression cassettes, e.g. rbcS1 promoter-SPsea::Artv1::6xHis::c-myc::KDEL-rbcS1 terminator, were subcloned into pBINPLUS (van Engelen et al. 1995) by cutting both vectors with AscI and PacI. Digestion products were purified by agarose gel electrophoresis and ligated into BINPLUS giving the following vectors for plant transformation: pBINPLUS/pImpact-Artv1ER and pBINPLUS/pImpact-GFPER (Fig. 1).
Fig. 1.
Plasmid constructs used for the expression of Art v 1 in plants. Plasmids derived from pBI121: the entire ORF of Art v 1 including the endogenous signal peptide was placed between the restriction sites BamHI and SstI under the control of the 35S cauliflower mosaic promoter (P35S). Alternatively, the Art v 1 signal peptide was fused to the N-terminus of GFP (pBI121_SpArtv1::GFP), also under control of P35S. RB right border, LB left border, T NOS terminator of nopaline synthase, P NOS promoter of nopaline synthase, NPT II kanamycin resistance gene. Plasmids derived from pBINPLUS and pIMPACT series: coding sequence of Art v 1 without the signal peptide was cloned between NcoI and BglII sites in pIMPACT 1.3 resulting in an ER-localised protein. Expression is under control of the chrysanthemum promoter rbcS1 (PRbcS1). A signal peptide from sea anemona (SPsea) directs the protein of interest to the secretory pathway and the KDEL peptide retains the protein in the ER. All proteins are expressed with a C-terminal c-myc tag and 6xHis tag
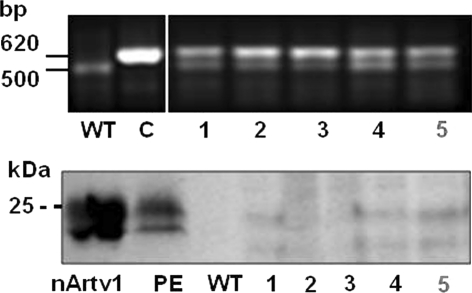
All cloning steps were monitored by control PCRs using combinations of primer pairs hybridizing to vector and insert sequences, respectively, and sequencing of the insert with its flanking regions. All vectors resulting from the various cloning steps were incorporated into E. coli (DH5α) for amplification, controlling, and storage. The incorporation of the Art v 1 sequence into the tobacco genome (Fig. 3) was detected by PCR with the forward primer 5′-ATGACGCACAATCCCACTATC-3′ and the reverse primer 5′-TCTACAGCAGCAGATCCA-3′ using 1 U of AmpliTaq DNA polymerase (Agilent Technologies, Santa Clara, CA, USA), 50°C annealing temperature and 35 cycles. Generally, standard molecular biology methods described by Sambrook et al. (2001) were performed with some adaptations for recombinant allergen expression described by Obermeyer et al. (2004).
Fig. 3.
Stable expression of Art v 1 in leaves of transformed tobacco plants. Transformed tobacco plants (Nicotiana tabacum) were generated by co-cultivation of leaf discs with Agrobacterium tumefaciens containing the pBI121_Artv1 plasmid and regeneration in the presence of kanamycin. Upper panel PCR with Art v 1-specific primer resulted in a specific product of 620 bp in the control plasmid (C, pBI121_Atrv1) and in plant nos. 1–5. An unspecific background signal of 500 bp is detectable in transformants but also in PCR experiments using DNA from wild-type tobacco. Lower panel Immunodetection of recombinant Art v 1 in leaf lysates of transformed tobacco plants 1–5. A typical double protein band of 20–25 kDa was detected by an anti Art v 1 antibody in the transformants 1–5. Purified, native Art v1 (nArtv1) and a mugwort pollen exudate (PE) served as positive controls. No protein signals were observed in the leaf lysate of wild-type plants (WT). 25 μg protein per lane. Plant no. 5 (in red) was used for further analysis
Growth and transformation of Agrobacteria
Agrobacterium tumefaciens (LBA 4404, Life Technologies, Rockville, USA) were grown in YM medium [0.04% (w/v) yeast extract, 1% (w/v) mannitol, 1.7 mM NaCl, 0.8 mM MgSO4, 2.2 mM K2PO4, pH 7.0 adjusted with NaOH] supplemented with 100 mg l−1 streptomycin and transformed with the appropriate pBINPLUS constructs by electroporation (GenePulser, Biorad, Vienna, Austria) as described by Obermeyer et al. (2004). Transformed Agrobacteria were selected on YM medium containing 100 mg l−1 kanamycin.
Transformation and regeneration of tobacco plants
Prior to plant transformation, Agrobacteria were grown in YM medium with the appropriate antibiotics plus 10 mM MES, pH 5.6 and 200 μM acetosyringone to an OD600 of 0.6–0.8. Agrobacteria were pelleted at 4,000×g (20 min, 15°C) and resuspended in infiltration medium [MS-salts, 2% (w/v) sucrose, 200 μM acetosyringone, pH 5.8 adjusted with NaOH]. For transient expression, tobacco leaves from young plants (8 to 12-leaves) were vacuum-infiltrated with the Agrobacterium suspension (Kapila et al. 1997) or Agrobacteria suspension was injected into the intercellular space of leaves still connected to the plant via a syringe pressed gently at the lower leaf side. For stable expression, a leaf disc transformation protocol was followed (Horsch et al. 1985; Obermeyer et al. 2004). In brief, discs cut from surface-sterilised tobacco leaves, were co-cultivated with Agrobacterium cultures for 3 days in the light at 24°C in a sealed Petri dish on MS-agar. Leaf discs were washed with MS-medium plus 100 μg ml−1 timentin and cultivated on MS-agar, 100 mg l−1 timentin, 100 mg l−1 kanamycin, 0.1 mg l−1 α-naphthalene acetic acid (αNAA), 1 mg l−1 benzyl adenine (BA) at 24°C in a growth chamber (16 h light/8 h dark). After 4 weeks green shoots (approximately 10–15 mm) were transferred to solid MS-medium without hormones in Magenta boxes (Sigma, Vienna, Austria) and cultivated under the same conditions. After development of roots, the plantlets were transferred to soil and further cultivated for analysis.
Induction and maintenance of transgenic tobacco cell cultures
Leaf discs from wild type and transgenic tobacco plants growing under aseptic conditions, were cultivated on MS-medium supplemented with B5 vitamins and 1 mg l−1 2,4-dichlorophenoxy acetic acid (2,4-D) without (wild-type) or with 100 mg l−1 kanamycin (transformants) in the dark at 28°C to induce callus growth. Generation of pale yellow, friable calli was inspected weekly. After 4 weeks, calli were isolated and sub-cultivated for another 4 weeks before they were divided for propagation (24 h dark, 28°C). To generate cell suspension cultures, callus material (ca. 1–2 g) was gently cut into small pieces and transferred to liquid medium (same composition as above without agar) and incubated at 24°C and 100 rpm in an incubation shaker. Clones from the same calli or cell suspension cultures were cultivated in parallel in the dark or at day/night cycles of 16 h/8 h at 24°C.
Protoplast preparation and fluorescence microscopy
Wild-type and transgenic tobacco leaves (GFP expression) were cut into small pieces and infiltrated with 5 ml protoplast isolation medium (500 mM mannitol, 10 mM KCl, 10 mM CaCl2, 10 mM MES adjusted to pH 5.7 with Tris). Infiltrated leaf pieces were incubated in protoplast isolation medium plus 1% cellulase (Duchefa, Haarlem, The Netherlands) and 0.02% pectinase (Sigma) for 2 h at room temperature (RT) with another hour at RT under gentle shaking at 50 rpm. Protoplasts were washed twice with protoplast isolation medium. Protoplasts were inspected for GFP fluorescence using an epifluorescence microscope (Zeiss Axiovert 135, Oberkochen, Germany) equipped with a video camera (SPOT Insight, Visitron, Puchheim, Germany) with filter settings for GFP fluorescence (ex, 470 ± 20 nm; dichroic mirror, 495 nm; em, 525 ± 25 nm, AHF, Tübingen, Germany) or by confocal laser-scanning microscopy (Zeiss LSM 510).
Preparation of pollen exudates, leaf lysates, and intercellular fluids
Pollen exudates were prepared according to Hoidn et al. (2005). Cell lysates were prepared by freezing leaf or callus pieces in liquid nitrogen and grinding to a fine powder which was transferred into an equal volume of ice cold lysis buffer containing 100 mM sodium phosphate, pH 7.0, 150 mM NaCl, 2 mM ethylene glycol-bis(ß-amino ethyl ether) N,N,N′,N′-tetraacetic acid (EGTA), 2 mM ethylenediaminetetraacetic acid (EDTA), 2 mM 1,4-dithio-d-threitol (DTT), 0.5 mM 4-(2-amino-ethyl)benzenesulfonyl fluoride hydrochloride (AEBSF), 5 mg l−1 aprotinin and 5 mg l−1 leupeptin, mixed well and centrifuged at 10,000×g for 15 min at 4°C to remove cell debris and larger organelles. Leaf infiltrates were prepared by vacuum infiltration of leaves with infiltration buffer (100 mM sodium phosphate buffer, pH 7.0, 100 mM NaCl). The infiltrated leaves were placed in a 20 ml syringe in a 50 ml centrifugation tube and centrifuged at 800×g for 10 min at RT. The intercellular fluid was collected at the bottom of the centrifugation tube.
Enrichment of tagged recombinant proteins and immunodetection
The cell lysate (1 ml) was incubated with 1 ml Ni2+ nitriloacetic acid (Ni–NTA) agarose (Qiagen, Hilden, Germany) or Ni2+ iminodiacetic acid (Ni-IDA, Machery-Nagel, Düren, Germany) equilibrated with wash buffer (50 mM sodium phosphate, pH 7.0, 300 mM NaCl, 1 M urea) for 30 min at 4°C in a disposable 2 ml column (Biorad, Vienna, Austria). The Ni–NTA agarose beads with the bound His-tagged protein were washed three times with 1 ml wash buffer and eluted twice with 1 ml elution buffer (50 mM sodium phosphate, pH 7.0, 300 mM NaCl, 1 M urea, 250 mM imidiazole). The protein concentration of all fractions was determined (Bradford assay, Biorad) and fractions were aliquoted and stored at −20°C for further analysis.
Proteins were separated by SDS-PAGE and electro-blotted onto nitrocellulose membranes by standard methods. The membrane was blocked with 3% (w/v) casein in PBS-Tween (phosphate-buffered saline with 0.2% Tween 20) overnight at 4°C, washed three times with PBS-Tween, incubated for 1 h at RT with primary antibody diluted in PBS-Tween, washed three times in PBS-Tween, incubated for 1 h at RT with secondary antibody diluted with PBS-Tween, washed three times with PBS-Tween. Secondary antibodies conjugated with an alkaline phosphatase (AP) were detected in 100 mM Tris/HCl, pH 9.5, 100 mM NaCl, 50 mM MgCl2, 0.175 mg ml−1 5-bromo-4-chloro-3-indolyl-phosphate p-toluidine salt (BCIP) and 0.3375 mg ml−1 nitroblue tetrazolium chloride (NBT) at RT. Horse radish peroxidase (HRP)-conjugated antibodies were detected by chemiluminescence (ECL, GE Healthcare, Vienna, Austria). Human serum IgEs were detected as described previously (Himly et al. 2003) using sera of mugwort allergic patients with positive reactivity against the major allergen Art v 1. The following combinations of primary and secondary antibodies were used for immunodetection:
anti-Artv1 (1:100, Bauer et al. 2003) and anti-mouse IgG/IgM-AP (1:5,000, Dianova, Hamburg, Germany) or anti-mouse IgG/IgM-HRP (1:5,000, Dianova);
anti-Artv1arabinose (1:40) which is a single-chain variable fragment antibody directly conjugated with AP (scFv-AP) and recognises the alpha-arabinose of type III arabinogalactans which were found on Art v 1 proteins (Gruber et al. 2009);
anti-GFP (1:250, Clontech, Saint Germain, France) and monoclonal anti-rabbit IgG-AP (1:8,000, Sigma) or monoclonal anti-rabbit IgG-HRP (1:8,000, Sigma);
anti-His tag (1:1,000, Qiagen) and goat anti-mouse IgG-AP (1:8,000, Sigma) or goat anti-mouse IgG-HRP (1:5,000, Sigma);
anti-cmyc (1:2000, Invitrogen, Lofer, Austria) and goat anti-mouse IgG-AP (1:8,000, Sigma) or goat anti-mouse IgG-HRP (1:8,000, Sigma).
Results and discussion
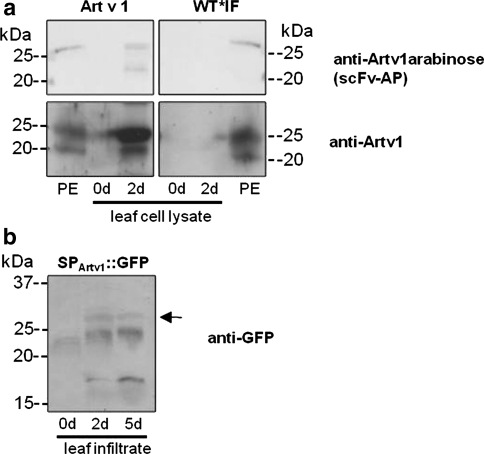
To test whether tobacco plants are a suitable expression system for the production of the recombinant Artemisia vulgaris pollen allergen Art v 1, the entire coding sequence including the endogenous signal peptide of Art v 1 (SPArtv1) was cloned into the plant transformation vector pBI121 (pBI121_Artv1, Fig. 1). Transiently transformed tobacco leaves expressed Art v 1 already 2 days after being infected with Agrobacteria whereas no Art v 1-specific signals could be observed in leaves injected with water (Fig. 2a). The Art v 1-specific antibody recognised the typical double band around 20 and 25 kDa which was also detectable in mugwort pollen exudates containing the native Art v 1. It has to be noted that the difference between the apparent molecular weight during SDS-PAGE (20–25 kDa) and the calculated molecular weight of Art v 1 (10.8 kDa) is caused by different intrinsic folding of the proline-rich C-terminus and various glycosylation levels (see Himly et al. (2003) and Leonard et al. (2005) for detailed discussion). In addition, both protein bands of the recombinant Art v 1 were also detected by an antibody fragment generated against the Art v 1-specific glycosylation pattern (single chain variable fragment antibody (scFv-AP = anti-Artv1arabinose), Fig. 2a). This may indicate a partial glycosylation of the recombinant protein although only the upper band of the native Art v 1 obtained from pollen exudates, shows a glycosylation signal. The scFv fragment recognises the α-arabinose of type III arabinogalactans which were found on Art v 1 proteins (Gruber et al. 2009). Recognition of two protein bands of the recombinant Art v 1 may indicate a partial glycosylation state of the lower band that contains α-arabinose but still lacks the hydroxy proline O-linked β arabinofuranosyl residues, and thus migrates faster during gel electrophoresis (Leonard et al. 2005). A second experiment, in which the signal peptide of Art v 1 was fused to the N-terminus of GFP (pBI121_SPArtv1::GFP, Fig. 1) revealed that GFP is secreted. A GFP-specific signal was detected in leaf infiltrates (Fig. 2b) proving the functionality of the Art v 1 signal peptide in tobacco and its role in targeting proteins to the secretory pathway. Note, that the lower molecular weight bands indicate degradation of the secreted GFP.
Fig. 2.
Transient expression of Art v 1 in leaves of transformed tobacco plants. Tobacco (Nicotiana tabacum) leaves were transiently transformed by pressure infiltration with Agrobacterium tumefaciens cultures at the lower leaf site. a Art v 1-specific glycosylation and the Art v 1 protein were detected in leaf cell lysates after 2 days in transformed leafs (Art v 1). Water-infiltrated leaves (WT*IF) did not show any signals. A pollen exudate (PE) from Artemisia vulgaris served as a positive control. b Infiltrates were harvested from leaves transformed with Agrobacteria containing the signal peptide of Art v 1 fused to GFP (SPArtv1::GFP) to verify targeting to the apoplast. GFP-specific signals at 27 kDa were observed at 2 and 5 days after transformation (arrow). 20 μg protein per lane, antibodies as indicated (see “Materials and methods”)
For in planta production of Art v 1, leaf discs were co-cultivated with Agrobacterium tumefaciens containing pBI121_Artv1 and tobacco plantlets were regenerated from calli to obtain transgenic plants that permanently express Art v 1. In a first screen, five different plants of the T0 generation were isolated which grew on kanamycin media and the Art v 1 sequence could be detected in their genomic DNA by PCR analysis (620 bp, Fig. 3, upper panel). Although a smaller product was also amplified from the DNA of wild-type plants, the transformants showed a PCR product of the same size as the control plasmid containing the Art v 1 sequence. In addition, the Art v 1-typical double band was recognised by an anti-Art v 1 antibody (Fig. 3, lower panel) with low signal intensities but verifying the presence of an Art v 1 protein in stable transformed plants and confirming a preliminary study (Gadermaier et al. 2003).
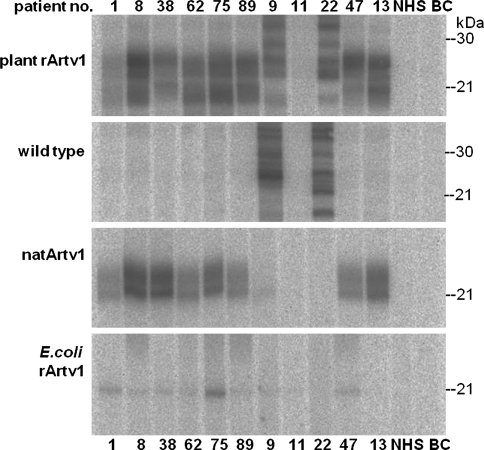
So far, the results indicated that the mugwort pollen allergen Art v 1 can be expressed in tobacco plants. Since a previous study on a bacterial-expressed recombinant Art v 1 demonstrated that some patient sera did not recognise the bacterial recombinant Art v 1, we tested whether the plant recombinant Art v 1 is recognised by IgE antibodies from sera of allergic patients. The leaf lysate proteins from plant no. 5 (Fig. 3) containing the plant recombinant Art v 1, were separated by SDS-PAGE, blotted onto a nitrocellulose membrane and tested for IgE binding (Fig. 4). In contrast to sera of non-allergic patients (NHS, non-atopic human sera), the various mugwort pollen-allergic patients’ sera contained IgEs against Art v 1 and recognised the native, the bacterial recombinant and the plant recombinant Art v 1 but no proteins in wild type tobacco plants except patient nos. 9 and 22. Generally, the bacterial recombinant Art v 1 was less recognised by the patients’ IgE than the native Art v 1 and plant recombinant Art v 1 with the exception of patient no. 75 who’s IgEs bound well to the bacterial recombinant Art v1, too. Note that patient no. 11 although classified as mugwort pollen allergic by clinical assays, did not recognise the mugwort pollen proteins. However, the IgEs of patient nos. 89 and 13 which hardly bound to the bacterial recombinant Art v 1, recognised the plant recombinant Art v 1 similar well as the native Art v 1.
Fig. 4.
Recognition of plant recombinant Art v 1 by allergenic patient sera. Cell lysate proteins prepared from transformed plant no. 5 of Fig. 3 (transformed, pBI121_Artv1) were separated by SDS-PAGE and plant recombinant (plant rArtv1), bacteria recombinant (E.coli rArtv1) and native Art v 1 (natArtv1), respectively, were detected by Art v 1-specific IgEs from various patient sera as indicated by patient numbers. No Art v 1-specific signals were detectable in wild type leaf lysates or with a pool of non-atopic human sera (NHS). 25 μg protein per lane. BC buffer control
These promising results on the expression of Art v 1 that is well recognised by patients’ sera, would make the recombinant allergen an ideal tool in allergy diagnostic and therapy. However, these experiments were performed with transiently or stable transformed plants of the T0 generation. Selection of transgenic plants in the following generations resulted finally in a T3 generation with almost 100% kanamycin resistance. Unfortunately, during the generation cycles, the plants expressed less and less Art v 1 until it became undetectable in plants of the T3 generation which still grew on kanamycin (data not shown). In addition, the transgenic plants grew slower and seemed to be more sensitive against stress conditions. Since the physiological function of Art v 1 has not been elucidated, one may assume that its expression in plant tissues other than in the pollen might affect its own production or genomic silencing of the integrated transgene may have occurred. To minimise the effects of Art v 1 on its own production, a new expression strategy was designed in which the recombinant Art v 1 was “hidden” in the ER of the transgenic plants and a plant-derived promoter was chosen.
Expression of ER-localised Art v 1 and GFP
The vector pBINPLUS/pIMPACT-Artv1ER was constructed (Fig. 1) which (1) directs and retains the recombinant protein in the ER due to a signal peptide and a C-terminal KDEL sequence, respectively, (2) allows the detection and purification via 6xHis and c-myc tags and finally, (3) sets the expression under the control of the light-regulated promoter of the small RuBisCO subunit 1 (rbcS1) of Chrysanthemum morifolium Ramat. (Outchkourov et al. 2003). In parallel to transformation and expression of Art v 1, the same experiments were also performed with pBINPLUS/pIMPACT-GFPER, in which the Art v 1 sequence has been replaced by GFP to observe differences in the expression of the two proteins and detect putative problems with the expression of Art v 1. The strategy for the generation of Art v 1-expressing tobacco plants and cell cultures starts with transient transformations of tobacco leaves by injection of Agrobacteria to test the constructed plasmids, the generation of stable transformed plants by leaf disc regeneration and finally, the generation of transgenic cell cultures from transformed tobacco plants of the T1 generation. All experimental steps were first performed and optimised with the GFP-expression plasmids because the recombinant GFP could be detected not only via the fused tags and a specific antibody but also by its own fluorescence, and most importantly, its expression did not affect the plant expression system as has been proven by a large number of studies.
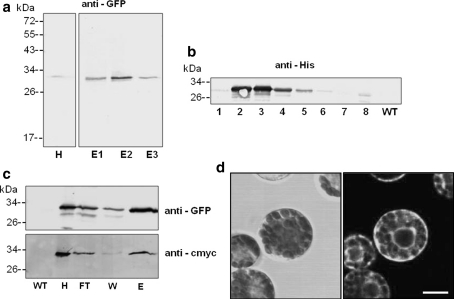
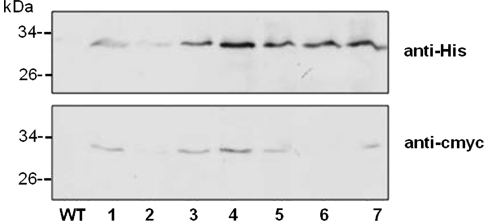
Pressure injection of Agrobacteria at the lower leaf side led to a transient expression of GFP after 5 days (Fig. 5a). Recombinant GFP was enriched by Ni–NTA agarose affinity chromatography and detected in the homogenate/lysate (H) and the elution fractions (E1–E3). Furthermore, GFP was still present in plants of the T1 generation, the kanamycin-resistant offspring of the leaf disc-regenerated T0-plantles (Fig. 5b). In every generation, the seeds from GFP-expressing plants were collected, selected on kanamycin-containing media and growing plants were tested for GFP. This time, GFP was still detectable in plants of the T3 generation: a leaf homogenate/lysate (H) was loaded onto a Ni–NTA column and the flow through (FT), washing (W) and elution (E) fractions were analysed for GFP (Fig. 5c). GFP could be detected indirectly by antibodies against the His and c-myc tag, respectively, in the leaf homogenates of transgenic but not of wild type plants. Finally, the expression of GFP was directly observed in mesophyll protoplasts prepared from leaves of a transgenic plant (Fig. 5d). Bright fluorescence signals are visible in ER strands surrounding the nucleus and the chloroplasts. This promising strategy was now applied to generate Art v 1-expressing plants. The transient transformations with the constructed plasmid pBINPLUS/pIMPACT-Artv1ER were successful, too (data not shown). The recombinant Art v 1 could be detected in a number of transgenic plants of the T1 generation via the attached His or c-myc tag with no signals detectable in wild type lysates (Fig. 6). The signals obtained by direct detection with the anti-Artv1 antibody were too weak for documentation. The recombinant Art v 1 was also recognised by an anti-Artv1 antibody (data not shown). These data indicate that the expression of the ER-localised Art v 1 under the control of the RuBisCO promoter was much more stable than that of the secreted protein controlled by the CaMV 35 S promoter and yielded detectable amounts of Art v 1 at least until the T1 generation. In this case, the targeting to the ER was probably the more important parameter.
Fig. 5.
GFP-expression in transformed tobacco plants. a Leaves of a transformed tobacco plant (Agro-injection) were homogenised after 5 days, purified via Ni–NTA agarose and detected by an anti-GFP antibody (H: 40 μg, E1–E3: 4 μg protein per lane). b Leaf homogenates of various stable transformed plants of the T1 generation cultivated on kanamycin-containing media (numbers indicate individual plants) and wild type (WT) were prepared and analysed by immunodetection using an anti-His antibody (15 μg protein per lane). c Detection of GFP in leaves harvested from tobacco plants of the T3 generation. GFP was purified from leaf homogenates by affinity chromatography (Ni–NTA IDA) and detected by an anti-GFP as well as an anti-cmyc antibody directed against the C-terminal cmyc-tag (27, 13, 6 and 1 μg protein per lane for fractions H, FT, W and E, respectively). d Mesophyll protoplasts prepared from leaves of transformed tobacco plant were observed by confocal laser scanning microscopy. Bright field image on the right and corresponding fluorescence image (left) Bar 10 μm. All plants were transformed with pBINPLUSpIMPACT-GFPER. WT wild type homogenate, H homogenate, FT flow through of column, W wash fraction, E eluate
Fig. 6.
Stable expression of ER-localised Art v 1 in tobacco plants. Tobacco plants of the T1 generation were analysed by immunodetection of their respective tag (numbers indicate individual plants). Recombinant Art v 1 was purified from leaf homogenates via affinity chromatography (Ni-IDA) and detected by antibodies directed to the C-terminal tags, 6xHis and c-myc. 21 μg (WT) and 10 μg (transformants) protein per lane
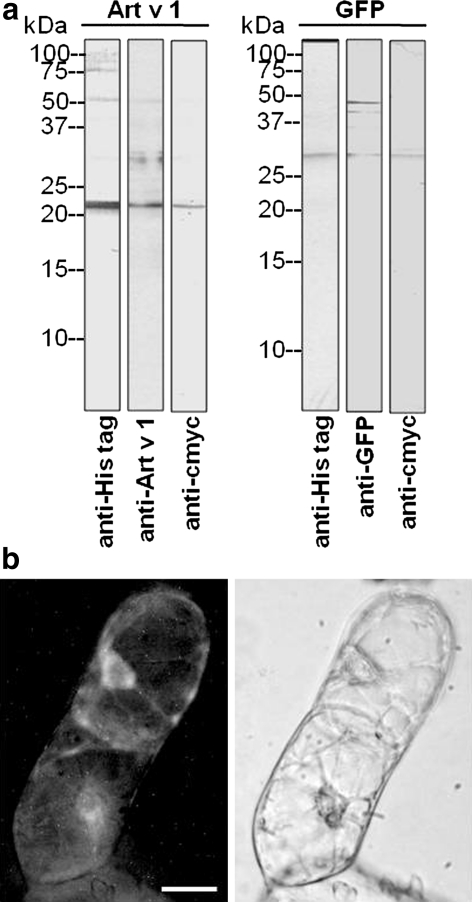
A cell culture was now generated from transgenic tobacco plants with the aim to use the transgenic tobacco cell culture for a stable and reliable production of recombinant Art v 1 in a closed fermenter system. Leaf discs of surface-sterilised leaves from GFP- and Art v 1-expressing plants (T1 generation) were incubated on Murashige and Skoog medium supplemented with B5 vitamins, kanamycin and 2,4-D in the dark at 24°C. Visible calli were isolated and cultivated on the same medium which was changed every 2–3 weeks. Calli from both transgenic plants were friable, showed a pale yellow colour when cultivated in the dark and could be transferred to cell suspension cultures. The expression of GFP and Art v1 in the respective calli grown in the dark, was very low and almost at the detection limits (data not shown) due to the light-regulated promoter. When calli were exposed to light, they developed chloroplasts and turned green (Fig. S2). Cell suspension cultures were generated from these calli and grown at 16 h light/8 h dark cycles at 24°C with weekly sub-culturing. The recombinant proteins GFP and Art v 1 could now be detected in these cultures (Fig. 7). Crude cell homogenates/lysates were analysed by immunodetection and antibodies against the protein itself and against the fused tags recognised a protein band of the appropriate molecular weight (Fig. 7a). In intact suspension cells, GFP fluorescence was observed in the ER around the nucleus (Fig. 7b).
Fig. 7.
Stable expression of ER-localised Art v 1 and GFP in cell suspension cultures generated from transgenic tobacco plants. Cell cultures were obtained from transformed tobacco plants via leaf disc cultivation in the dark, selection with kanamycin and 2,4-D as hormone. Calli were suspended in liquid media to obtain suspension cultures and grown under artificial light. Cells were harvested for analysing the recombinant proteins. a Cell homogenate analysed with antibodies against the C-terminal tag and against the Art v 1 and GFP. 17 μg protein per lane. b Fluorescence image of a suspension cell expressing GFP in the ER with the corresponding bright field image. Bar 20 μm
Hence, these results provide a proof-of-principle for the expression of the mugwort pollen allergen Art v 1 in plants. But is this system suitable for a plant-based production of recombinant Art v 1 to supply sufficient amounts for clinical allergy tests or even therapies? Unfortunately, the answer at present is no due to several reasons. First, the amounts of the recombinant allergen were very low. In most experiments, no extra protein band not to mention a prominent band was visible in Coomassie-stained protein gels of all cell homogenates and the amount of 25 ml cell culture was only sufficient for detection. We estimate the amount of recombinant Art v 1 as ≤1 μg per litre cell culture which is far from a real overexpression (approximately 1 mg l−1 cell culture) that might allow a profitable purification of the protein. Second, the recombinant Art v 1 was retained in the ER thus making any purification laborious in comparison to secreted recombinant proteins even when up-scaling the cultures to litre volumes. It has to be noted that tobacco plants and cell cultures expressing a secreted form of Art v 1 and GFP were also generated but preliminary experiments showed that the secreted recombinant proteins were hardly detectable in the cell culture medium, even after including protein precipitation and partial purification steps.
The idea of producing proteins of plant origin in a plant expression system seems intriguing especially for plant allergens because plant-specific post-translational modifications might contribute to the recognition by the human immune system and therefore might be import for successful therapies (Schmidt et al. 2008; Schmidt and Hoffman 2002; Singh and Bhalla 2006). Unfortunately, only a few studies have been performed until today which succeeded in the expression of pollen allergens in plants (for review see Schmidt et al. 2008). However, none of these studies reported an overproduction of the recombinant allergen that will facilitate the purification and makes plants or plant cell cultures an economical production platform. Most of the plant allergens belong to families of pathogenesis-related (PR) proteins (Hoffmann-Sommergruber 2002; Radauer et al. 2008; Sels et al. 2008) which are induced upon stress in plants. For instance, the latex allergen Hev b 2, a 1,3 β-glucanase from Hevea brasiliensis, belongs to the PR-2 family and lipid transfer proteins [e.g. Art v 3 (mugwort), Hev b 12 (rubber tree), Ole e 7 (olive)] belong to the PR-14 family. Expression of these proteins and directing them to their site of action, the “external” cell wall, may trigger various processes in plants during the stress response and therefore, an overexpression of these proteins probably disturbs the normal development and growth of the plant. Although, the physiological function of Art v 1 is not known and its expression and targeting to the cell wall is not lethal for tobacco plants, the N-terminal defensin domain (Himly et al. 2003) may finally disturb the normal growth and expression pattern of the host plant although no phenotype was observed under standard growth conditions. A similar effect has been observed with the latex allergen Hev b 2 similar to tobacco 1,3 β-glucanases, which could be expressed in tobacco plants only at a low amount (data not shown). To circumvent any interference of plant allergen proteins with the expression system, one might direct the expression of the allergen protein to a very late state of plant development, e.g. during the development of seeds or fruits (Horvarth et al. 2000; Takaiwa et al. 2007). This strategy has been tested for the expression of a peptide constructed from cedar pollen allergen sequences, which was then used for oral vaccination of mice (Takagi et al. 2005).
Despite the general application of molecular strategies for the production of therapeutic proteins, the production of plant-derived allergens might be particularly difficult due to the close relation between production system and product.
Conclusion
The mugwort pollen allergen Art v 1 could be expressed in plants and plant cell cultures using conventional Agrobacterium-mediated transformation methods. The expression was stable during a number of plant generations when controlled by a light-regulated promoter and by targeting the Art v 1 to the ER. Otherwise, the expression of Art v 1 was lost after a two generation cycles. So far, the amounts of the recombinant protein were sufficient for analysis but would not meet the requirements for an economical production of the recombinant allergen for clinical assays. However, screening of much larger numbers of transgenic plant lines (>1,000) might result in a plant line suitable for economical production.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1 Nucleotide and amino acid sequence of Art v 1 cloned into pIMPACT 1.3. The signal peptide the tags of the vector and the two domains of Art v 1 are colour-coded.
Fig. S2 Transgenic tobacco calli. Calli were generated from leaf discs of transgenic tobacco plants expressing Art v 1. (a) Callus cultured at day/night cycles (16 h light/8 h darkness). (b) Callus grown in the dark. Bar = 5 mm
Acknowledgments
The project was financed by grants to GO from the Austrian Science Fund (FWF): S8804 and L189-B03, the OeNB Jubiläumsfond (10210) and the University priority programme “Biosciences and Health”. We thank Ana Gimeno and Aniela Sommer for technical assistance, Marta Novak, Wolfgang Sebesta, Nichola Hamilton and Petra Gruber for an early involvement in the project, and Thomas Hawranek (SALK, Salzburg, Austria) for the patients’ sera.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
A contribution to the Special Issue: Plant Molecular Pharming in 2012 and Beyond.
References
- Astier C, Morisset M, Roitel O, Codreanu F, Jacquenet S, Proust B, Moneret-Vautrin D-A, Burks AW, Bihain B, Sampson HA, Kanny G. Predictive value of skin prick tests using recombinant allergens for diagnosis of peanut allergy. J Allergy Clin Immunol. 2006;118:250–256. doi: 10.1016/j.jaci.2006.04.053. [DOI] [PubMed] [Google Scholar]
- Bauer R, Himly M, Dedic A, Ferreira F, Thalhamer J, Hartl A. Optimization of codon usage is required for the effective genetic immunization against Art v 1, the major allergen of mugwort pollen. Allergy. 2003;58:1–8. doi: 10.1034/j.1398-9995.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- Chen P-Y, Wang C-K, Soong S-C, To K-Y. Complete sequence of the binary vector pBI121 and its application in cloning T-DNA insertion from transgenic plants. Mol Breed. 2003;11:287–293. doi: 10.1023/A:1023475710642. [DOI] [Google Scholar]
- Cromwell O, Suck R, Kahlert H, Nandy A, Weber B, Fiebig H. Transition of recombinant allergens from bench of clinical application. Methods. 2004;32:300–312. doi: 10.1016/j.ymeth.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Daniell H, Streatfield SJ, Wycoff K. Medical molecular farming: production of antibodies, biopharmaceuticals and edible vccines in plants. Trends Plant Sci. 2001;6:219–226. doi: 10.1016/S1360-1385(01)01922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Singh ND, Mason H, Streatfield SJ. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009;14:669–679. doi: 10.1016/j.tplants.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhofer K, Sevcik H, Balic N, Harwanegg C, Hiller R, Rumpold H, Müller MW, Spitzauer S. Microarrayed allergens for IgE profiling. Methods. 2004;32:249–254. doi: 10.1016/j.ymeth.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Doran PM. Foreign protein production in plant tissue cultures. Curr Opin Biotechnol. 2000;11:199–204. doi: 10.1016/S0958-1669(00)00086-0. [DOI] [PubMed] [Google Scholar]
- Gadermaier G, Gehwolf R, Sebesta W, Pertl H, Hamilton N, Hoidn C, Ferreira F, Obermeyer G. In-planta production of pollen allergens. Expression of Art v 1 in virus- and Agrobacterium-transformed tobacco plants. Allergy Clin Immunol Int Suppl. 2003;2:63–66. [Google Scholar]
- Gruber P, Gadermeier G, Bauer R, Weiss R, Wagner S, Leonard R, Breiteneder H, Ebner C, Ferreira F, Egger M. Role of the polypeptide backbone and post-translational modifications in cross-reactivity of Art v 1, the major mugwort pollen allergen. Biol Chem. 2009;390:445–451. doi: 10.1515/BC.2009.063. [DOI] [PubMed] [Google Scholar]
- Hellwig S, Drossard J, Twyman RM, Fischer R. Plant cell cultures for the production of recombinant proteins. Nat Biotechnol. 2004;22:1415–1422. doi: 10.1038/nbt1027. [DOI] [PubMed] [Google Scholar]
- Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, Barletta B, Becker WM, Blaser K, Breiteneder H, Chapman M, Crameri R, Duchene M, Ferreira F, Fiebig H, Hoffmann-Sommergruber K, King TP, Kleber-Janke T, Kurup VP, Lehrer SB, Ligholm J, Müller U, Pini C, Reese G, Scheiner O, Scheynius A, Shen H-D, Spitzauer S, Suck R, Swoboda I, Wayne T, Tinghino R, van Hage-Hamsten M, Virtanen T, Kraft D, Müller MW, Valenta R. Micro-arrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–416. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- Himly M, Jahn-Schmid B, Dedic A, Kelemen P, Wopfner N, Altmann F, van Ree R, Briza P, Richter K, Ebner C, Ferreira F. Art v 1, the major allergen of mugwort pollen, is a modular glycoprotein with a defensin-like and a hydroxylproline-rich domain. FASEB J. 2003;17:106–108. doi: 10.1096/fj.02-0472fje. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Sommergruber K. Pathogenesis-related (PR)-proteins identified as allergens. Biochem Soc Transact. 2002;30:930–935. doi: 10.1042/BST0300930. [DOI] [PubMed] [Google Scholar]
- Hoidn C, Puchner E, Pertl H, Holztrattner E, Obermeyer G. Non-diffusional release of allergens from pollen grains of Artemisia vulgaris and Lilium longiflorum depends mainly on the type of the allergen. Int Arch Allergy Immunol. 2005;137:27–36. doi: 10.1159/000084610. [DOI] [PubMed] [Google Scholar]
- Horn ME, Woodard SL, Howard JA. Plant molecular farming: systems and products. Plant Cell Rep. 2004;22:711–720. doi: 10.1007/s00299-004-0767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch BB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Frley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1983;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Horvarth H, Huang J, Wong O, Kohl E, Okita T, Kannangara CG, von Wettstein D. The production of recombinant proteins in transgenic barley grains. Plant Biol. 2000;97:1914–1919. doi: 10.1073/pnas.030527497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapila J, De Rycke R, Van Montagu M, Angenon G. An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci. 1997;122:101–108. doi: 10.1016/S0168-9452(96)04541-4. [DOI] [Google Scholar]
- Kim T-E, Park S-W, Cho N-Y, Choi S-Y, Yong T-S, Nahm B-H, Lee SS, Noh G. Quantitative measurement of serum allergen-specific IgE on protein chip. Exp Mol Med. 2002;34:152–158. doi: 10.1038/emm.2002.22. [DOI] [PubMed] [Google Scholar]
- Leonard R, Petersen BO, Himly M, Kaar W, Wopfner N, Kolarich D, Van Ree R, Ebner C, Duus JO, Ferreira F, Altmann F. Two novel types of O-glycans on the mugwort pollen allergen Art v 1 and their role in antobody binding. J Biol Chem. 2005;280:7932–7940. doi: 10.1074/jbc.M410407200. [DOI] [PubMed] [Google Scholar]
- Obermeyer G, Gehwolf R, Sebesta W, Hamilton N, Gadermaier G, Ferreira F, Commandeur U, Fischer R, Bentrup F-W. Over-expression of plant allergens by molecular farming strategies. Methods. 2004;32:235–240. doi: 10.1016/j.ymeth.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Outchkourov NS, Peters J, de Jong J, Rademakers W, Jongsma MA. The promoter-terminator of chrysanthemum rbcS1 directs very high expression levels in plants. Planta. 2003;216:1003–1012. doi: 10.1007/s00425-002-0953-8. [DOI] [PubMed] [Google Scholar]
- Outchkourov NS, Rogelj B, Strukelj B, Jongsma MA. Expression of sea anemona equistatin in potato. Effects of plant proteases on heterologous protein production. Plant Physiol. 2003;133:379–390. doi: 10.1104/pp.102.017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radauer C, Bublin M, Wagner S, Mari A, Breiteneder H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J Allergy Clin Immunol. 2008;121:847–852. doi: 10.1016/j.jaci.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Razzera G, Gadermaier G, de Paula V, Almeida MS, Egger M, Jahn-Schmid B, Almeida FC, Ferreira F, Valente AP. Mapping the interactions between a major pollen allergen and human IgE antibodies. Structure. 2010;11:1011–1021. doi: 10.1016/j.str.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning. 3. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Schmid-Grendelmeier P, Holzmann D, Himly M, Weichel M, Tresch S, Rückert B, Menz G, Ferreira F, Blaser K, Wüthrich B, Crameri R. Native Art v 1 and recombinant Art v 1 are able to induce humoral and T cell-mediated in vitro and in vivo responses in mugwort allergy. J Allergy Clin Immunol. 2003;111:1328–1336. doi: 10.1067/mai.2003.1495. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Hoffman DR. Expression systems for production of recombinant allergens. Int Arch Allergy Immunol. 2002;128:265–270. doi: 10.1159/000063865. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Gadermeier G, Pertl H, Siegert M, Oksman-Caldentey K-M, Ritala A, Himly M, Obermeyer G, Ferreira F. Production of recombinant allergens in plants. Phytochem Rev. 2008;7:539–552. doi: 10.1007/s11101-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sels J, Mathys J, De Coninck BMA, Cammune BPA, De Bolle MFC. Plant pathogenesis-related (PR) proteins: a focus on PR peptides. Plant Physiol Biochem. 2008;46:941–950. doi: 10.1016/j.plaphy.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Singh MB, Bhalla PL. Recombinant expression systems for allergen vaccines. Inflamm Allergy Drug Targets. 2006;5:53–59. doi: 10.2174/187152806775269312. [DOI] [PubMed] [Google Scholar]
- Takagi H, Hiroi T, Yang L, Tada Y, Takamura K, Ishimitsu R, Kawauchi H, Kiyono H, Takaiwa F (2005) A rice-based edible vaccine expressing multiple T cell epitopes induces oral tolerance for inhibition of Th2-mediated IgE responses. Proc Natl Acad Sci USA 102:17525–17530 [DOI] [PMC free article] [PubMed]
- Takaiwa F, Takagi H, Hirose S, Wakasa Y. Endosperm tissue is good production platform for artificial recombinant proteins in transgenic rice. Plant Biotechnol J. 2007;5:84–92. doi: 10.1111/j.1467-7652.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- Valenta R, Kraft D. From allergen structure to new forms of allergen-specific immunotherapy. Curr Opin Immunol. 2002;14:718–727. doi: 10.1016/S0952-7915(02)00402-8. [DOI] [PubMed] [Google Scholar]
- Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Grönlund H. The recombinant allergen-based concept of component-resolved dianostic and immunotherapy (CRD and CRIT) Clin Exp Allergy. 1999;29:896–904. doi: 10.1046/j.1365-2222.1999.00653.x. [DOI] [PubMed] [Google Scholar]
- Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, Swoboda I, Vrtala S. From allergen genes to allergy vaccines. Ann Rev Immunol. 2010;28:211–241. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- van Engelen FA, Molthoff JW, Conner AJ, Nap JP, Pereira A, Stiekma WJ. pBINPLUS: an improved plant transformation vector based on pBIN19. Transgenic Res. 1995;4:288–290. doi: 10.1007/BF01969123. [DOI] [PubMed] [Google Scholar]
- van Ree R, Chapman MD, Ferreira F, Vieths S, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008;63:310–326. doi: 10.1111/j.1398-9995.2007.01612.x. [DOI] [PubMed] [Google Scholar]
- Vigh-Conrad KA, Conrad DF, Preuss D. A protein allergen microarray detects specific IgE to pollen surface, cytoplasmic, and commercial allergen extracts. PLoS One. 2010;5:e10174. doi: 10.1371/journal.pone.0010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Gruber P, Radauer C, Maderegger B, Susani M, Hoffmann-Sommergruber K, Ferreira F. Lab scale and medium scale production of recombinant allergens in E. coli. Methods. 2004;32:219–226. doi: 10.1016/j.ymeth.2003.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.