Abstract
There is evidence that women may be less successful when attempting to quit smoking than men. One potential contributory cause of this gender difference is differential craving and stress reactivity to smoking-and negative affect/stress-related cues. The present human laboratory study investigated the effects of gender on reactivity to smoking and negative affect/stress cues by exposing nicotine dependent women (n=37) and men (n=53) smokers to two active cue types, each with an associated control cue: 1) in vivo smoking cues and in vivo neutral control cues, and 2) imagery-based negative affect/stress script and a neutral/relaxing control script. Both before and after each cue/script, participants provided subjective reports of smoking-related craving and affective reactions. Heart rate (HR) and skin conductance (SC) responses were also measured. Results indicated that participants reported greater craving and SC in response to smoking vs. neutral cues and greater subjective stress in response to the negative affect/stress vs. neutral/relaxing script. With respect to gender differences, women evidenced greater craving, stress and arousal ratings and lower valence ratings (greater negative emotion) in response to the negative affect/stressful script. While there were no gender differences in responses to smoking cues, women trended towards higher arousal ratings. Implications of the findings for treatment and tobacco-related morbidity and mortality are discussed.
Smoking cessation outcome is complex and multiply determined. Of the numerous factors associated with treatment outcome, gender is among the most empirically substantiated. Epidemiological data1 indicate that smoking rates of both sexes have substantially declined from their peak levels in the 1960s but the rate of decline in women has been half that observed for men (25% vs. 50% rate reduction, respectively). Additionally, lower rates of cessation in women have been reported in studies of self-quitters2–4, smokers in large population-based treatment trials5,6 and smokers in medication and nicotine replacement trials7–12. Thus, several studies of self-quitters and treatment-seekers echo the epidemiological data and collectively they suggest that women are less able to quit smoking than men, either alone or with the aid of treatment. Contributory factors that may explain this gender-related discrepancy in cessation include differential sensitivity to nicotine13–15, variation in responsiveness to social support16,17 and menstrual cycle effects18–20. The focus of the present study is on another potential contributor, gender-related differences in craving and reactivity to smoking cues.
Cue reactivity has been variously defined but commonly refers to laboratory procedures used to study a variety of responses to both drug-related and affective cues21. These responses include both subjective responses, such as self-reports of craving and mood, as well as objective responses, such as physiological responsiveness (e.g., heart rate). Subjective craving is a complex phenomenon22 that has served as the conceptual centerpiece of cue reactivity research. In general parlance, craving is a subjective state that refers to an internal experience of desire23,24. In the prototypical cue reactivity study with smokers, participants are exposed to cues that are commonly experienced during smoking, such as the sight and smell of the participant’s preferred brand of cigarettes, a lighter, etc. Several studies have also found that negative affect/stress cues also elicit smoking-related craving and cue reactivity25–28. The level of craving and reactivity that occurs in response to the cues is presumed to reflect, at least in part, the Pavlovian associative learning processes that result from numerous pairings between the cues and the rewarding effects of nicotine. Instrumental learning processes are also likely involved in cue reactivity; for example, the stress dampening effects of smoking can reinforce smoking behavior when stressors are encountered29. In general, associative learning processes likely play a larger role in the addictive process of dependent smokers, relative to other drug users, since the number of daily Pavlovian and/or instrumental pairings between cues and nicotine ingestion or smoking and reinforcement, respectively, are very high.
To date, seven laboratory studies examining gender differences in smoking-related cue reactivity have been published30–36 and a number of general observations can be derived from them. First, six of the seven studies reported evidence of gender differences and in the study that didn’t35, it seems likely that potential gender differences may have been obscured by medication effects (the majority of the participants, approximately 85%, received either nicotine replacement, naltrexone or both at the time of the cue reactivity assessment). Second, the effects of gender on smoking cue reactivity appear to vary across studies. This likely owes to several factors including the use of relatively small sample sizes and substantial between-study methodological variation. In the case of sample size, only two of the published studies30,34 were sufficiently powered to detect the small/medium effect sizes typically associated with gender. Methodologically, published studies have employed different types of stimuli (smoking cues, negative affect/stress cues) in a variety of modalities (in vivo, video, imagery) and examined their impact on a broad range of outcome measures including subjective craving, emotion and physiological measures such as heart rate, skin conductance, salivation and EEG. Third, despite these sample size and methodological disparities, a few tentative generalities can be gleaned from existing research. Four of the seven studies31–33,36 yielded evidence indicating that women are more craving responsive to smoking cues than men, but only one study has shown that this relatively inflated craving extends to cues that elicit negative affect/stress30. Additionally, it appears that smoking cue-elicited cardiovascular responses34,36 and EEG activity32,33 may differ between women and men, but the directionality of these differences has not been firmly established. These general observations are consistent with several laboratory studies that have identified gender differences in other substance abusing populations37–39 and suggest, as do emerging fMRI data40, that the effect of gender on craving and cue reactivity may be subserved by gender-based variation in neural activation.
The present study will contribute to extant research on gender effects in smoking cue reactivity by (a) employing a relatively large sample of women and men, (b) controlling for potential confounding factors such as level of nicotine dependence, order of stimulus presentation and menstrual cycle phase (follicular vs. luteal) of the female participants, and (c) examining the effects of gender on craving and cue reactivity to negative affect/stress cues. The findings reported here are drawn from a parent study41 that investigated the effects of menstrual cycle phase on smoking cue reactivity in which participants received four cue reactivity sessions, each one week apart. Since participant attrition increased over the course of the parent study, the present findings are from the first laboratory session as the relatively large sample offered the greatest power to detect gender differences if they were present.
Overview of Design and Hypotheses
Ninety nicotine dependent smokers (37 women and 53 men) that were otherwise free of major psychiatric and medical disorders served as participants. The laboratory session consisted of cues presented in one of two formats, in vivo vs. imagery scripts. Within each cue format, there were two cue types, an active cue and an associated control cue. Thus, the following four distinctive cues were presented: 1) an in vivo smoking cue; 2) an in vivo neutral control cue; 3) an imagery-based script consisting of negative affect/stress cue, and 4) imagery-based control script consisting of a neutral/relaxing imagery. Subjective ratings of craving, stress and other dimensions of emotionality (i.e., valence, arousal and dominance) were obtained immediately before and after each cue presentation whereas heart rate (HR) and skin conductance (SC) measures were obtained during a 90-sec baseline period prior to the start of the laboratory session and over the course of each 90-second cue presentation. While the present study incorporated two cue formats, in vivo vs. imagery, comparison of stimulus formats was not an intended purpose of the present study. Thus, the overall design of the study consisted of a single attribute between-subjects factor, gender, and two within-subjects factors corresponding to cue type (active vs. control cues) and time (time was a factor for HR and SC measures only). This design permitted the evaluation of the following hypotheses:
Regardless of gender, participants would evidence greater subjective (craving, stress and affect) and physiological (HR and SC) reactivity to the in vivo smoking cues and the negative affect/stress cues as compared to their respective control cues. This was necessary to establish the validity/potency of our paradigm to elicit cue reactivity.
Based on the foregoing literature review, women (relative to men) would evidence higher subjective craving and stress ratings and physiological (HR and SC) reactivity to both smoking and negative affect/stress cues.
In accordance with hypothesis 2, it was expected that women would rate the smoking and negative affect/stress cues as more arousing and aversive and would report feeling less in control than men.
Methods
Participants
Non-treatment seeking men and women smokers between the ages of 18–40 who smoked an average of at least 10 cigarettes per day for the previous three months were eligible for study entry. Participants were excluded if they evidenced substance use disorders other than nicotine and caffeine (within past 30 days), were using psychotropic medication, or were using medications that might affect heart rate and skin conductance measurement (e.g. benzodiazepines, beta blockers). Medical conditions that could potentially impact physiological measurement were also exclusionary (e.g., hypertension, arrhythmia). Additionally, female participants were required to a) have a regular menstrual cycle lasting between 25 to 35 days, b) not meet criteria for premenstrual dysphoric disorder (PMDD) in the last 6 months, c) not use a hormonal form of birth control (e.g., birth control pills) or hormone replacement therapy, d) not be pregnant or have had a delivery within the past 3 months, e) not have been breast feeding within the last 3-months, and f) not have had a hysterectomy.
Female participants were also randomized to one of four different menstrual phase orders (Early Follicular, Mid-Late Follicular, Mid-Late Luteal and Late Luteal) based on self-report and biological verification via the use of an ovulation test kit provided to participants at the initial assessment. Luteal phase determination was based on date of ovulation and follicular phase determination was based on start of menses. Although this randomization was conducted primarily for purposes associated with the parent study, it benefits the present study by ensuring approximately equal distribution of female participants across phases of the menstrual cycle (i.e., follicular and luteal).
Participants that met study entrance criteria were scheduled for their first cue reactivity session. A total of 37 women and 53 men (n=90) qualified for the study and participated in the first cue reactivity session (See Table 1 for an overview of participant demographic and clinical data). All participants were required to smoke prior to the cue reactivity session to ensure equivalence with respect to time since last cigarette.
Table 1.
Means (standard error) or percentages (frequency) on key demographic and clinical measures.
| Variable | Men (n = 53) | Women (n = 37) |
|---|---|---|
| Age (continuous) | 29.1 (0.9) | 30.4 (1.1) |
| Race: | ||
| White | 84.9 (45) | 78.4 (29) |
| Other | 15.1 (8) | 21.6 (8) |
| Employed: | ||
| Yes | 75.5 (40) | 62.2 (23) |
| No | 24.5 (13) | 37.8 (14) |
| Education: | ||
| H.S. diploma or less | 26.4 (14) | 32.4 (12) |
| Some college or more | 73.6 (39) | 67.6 (25) |
| Menstrual Cycle Phase | ||
| N/A | 100 (53) | 0 (0) |
| Follicular | 0 (0) | 59.5 (22) |
| Luteal | 0 (0) | 40.5 (15) |
| FTND Score | ||
| Low (1–5) | 39.6 (21) | 43.2 (16) |
| High (6–10) | 60.4 (32) | 56.8 (21) |
| Mean Cigarettes Smoked per Day | 20.2 (1.1) | 18.1 (1.2) |
| Mean Years of Regular Smoking | 11.4 (0.8) | 11.2 (1.0) |
| Percent wanting to quit at the time of study entry | ||
| Yes | 79.2 (42) | 73.0 (27) |
| No | 20.8 (11) | 27.0 (10) |
| Percent with ≥ 1 quit attempt in the past | ||
| Yes | 67.9 (36) | 83.8 (31) |
| No | 32.1 (17) | 16.2 (6) |
Means were compared using a 2-sample t-test and proportions were compared using a chi-square tests of independence. All p’s > 0.05.
Description of Cues
Smoking (in vivo) cues
The smoking cues consisted of a pack of the participant’s preferred brand of cigarettes and a lighter. To control the onset of the cue administration, the cues were covered on a tray and placed on an adjustable table directly in front of the participant. At the beginning of the cue presentation, the participant was instructed (via headphones) to remove the cover and to (a) look at the pack of cigarettes and lighter, (b) remove one cigarette from the pack and hold it between his/her fingers as if smoking, and (c) smell the cigarette and to flick the lighter without actually lighting the cigarette. After the 90-second cue period elapsed, research staff entered the room, covered the cues, provided the participant with questionnaires and left the room.
Neutral (in vivo) cues
The neutral cues consisted of a pack of pencils and an eraser. The participant handled these cues in a manner similar to the smoking cues.
Negative affect/stress script
During the initial assessment, research staff used the Scene Development Questionnaire42,43 to obtain a detailed description of a recent life event that the participant perceived as unpleasant and stressful. The narrative was then used to create a 75-second audio recording that could be played back to each participant. During the cue presentation, the participant was instructed to close his/her eyes and listen to the recording (via sound-attenuating headphones) and to imagine the event as though it were currently taking place. Immediately following the 75-second recording, the participant continued imagining the event for an additional 15 seconds (to complete a 90 second cue period).
Neutral/relaxed script
The same procedures as immediately above were used to develop an idiographic script of a neutral or relaxing event that the participant had recently experienced.
Dependent Measures
Questionnaire of Smoking Urges–Brief
(QSU-B44) is a brief self-report craving assessment where participants respond to ten statements about their urges to smoke using 7-point scales anchored with 1 = strongly disagree and 7 = strongly agree. Participants completed the QSU-B prior to and following each of the four cue presentations (in order to evaluate changes in craving).
Stress visual analog scales
A single analog scale was used to evaluate stress reactivity to the cue presentations. Participants provided a self-rating for the questions “How much stress do you feel at present?” on a scale measuring 10 centimeters (range 0–10). The distance of the respondent’s mark from zero was used as the score for the scale. The scale was administered at the same time points as the QSU-B.
Self-Assessment Manikin
(SAM45–47) is a 3-item self-report instrument that was used to directly assess three dimensions of emotion using 9-point scales: 1. Arousal (9 = excited/nervous; 1 = calm/relaxed), 2. Valence (9 = Happy and 1 = Sad), and 3. Dominance (9 = full control; 1 = no control). Each of the points on the scales was graphically represented by a cartoon manikin that exhibited varying degrees of a physical attribute associated with each of the three dimensions of emotion (i.e., the pleasure manikin had a facial expression ranging from smile to a frown, the arousal manikin ranged a wide-eyed excited figure to a sleepy figure, and the dominance manikin ranged from a small submissive figure to a large dominant figure). The SAM ratings were obtained before and after each cue presentation.
Heart rate (HR) and skin conductance (SC) data were collected continuously during each 90-second cue presentation as well as a 90 second baseline measurement prior to the start of the cue session23. HR data were collected via Lab Linc V series V71-01 bioamplifier. SC data were collected using a Coulbourn Lab Linc V Series V71-23 Isolated Skin Conductance Coupler. Sensors were placed on the right collarbone, lower left ribcage, and the left mid-forearm for collection of HR data and two sensors were placed on the hypothenar eminence of the non-dominant hand for SC data collection. The units of measure for the HR and SC measures were beats per minute (BPM) and microsiemens, respectively. All SC values were log transformed to normalize the data.
Procedure
Following an initial phone screening, participants were scheduled for a general clinical evaluation session in which demographic, smoking history, psychiatric (via SCID-IV48) and menstrual cycle data were collected to determine study eligibility. Level of nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND49).
The cue reactivity (CR) session was scheduled Monday through Friday, between 9:30am and 2:30pm at the outpatient Clinical and Translational Research Center (CTRC). Men were scheduled for their CR session within one week after their initial assessment whereas the timing for women varied according to which menstrual cycle phase they were randomized. The randomization by menstrual phase meant that women could receive the first CR session between 3 and 30 days after their initial evaluation. Prior to conducting the CR session, all participants completed an alcohol breathalyzer assessment and a urine drug screen (UDS) and women received a urine pregnancy screening. If the participant’s breath alcohol level was greater than 0.01 or if the participant had a positive UDS, the participant was excused from the session and rescheduled for a later date. A positive pregnancy test resulted in withdrawal from the study. Participants were instructed to smoke a cigarette immediately prior to attendance at their CR session and a carbon monoxide breathalyzer (CO) level was collected within 30 minutes after the last reported cigarette was smoked for biological verification.
CR sessions were conducted in a laboratory setting specifically designed for the administration of cue reactivity procedures. A wall-mounted surveillance camera in the testing room was connected to a monitor in the control room that enabled study staff to monitor cue presentation. Participants were seated in a reclining chair for the duration of the testing session. Prior to the CR session, participants completed baseline self report measures. After baseline questionnaires were completed and verified by study personnel, HR and SC sensors were attached. For purposes related to the parent study, an IV catheter was placed in the non-dominant forearm for biological assay collection. Baseline HR and SC measures were collected 45 minutes after catheter insertion to allow time for acclimation to novel environment.
All participants were exposed to a series of four counterbalanced cue presentations during the CR session, each lasting 90 seconds. Immediately following each cue presentation, participants provided subjective ratings (described above) and then were instructed to watch a 10-minute nature slideshow presented on a computer monitor directly in front of them. Methodologically, the slideshow was designed to mitigate any carryover effects from the cue presentations. Instructions given to participants during cue presentations were pre-recorded and presented using DMDX stimulus presentation software50 implemented on an IBM-compatible computer. These instructions (and script cues described above) were delivered to participants via Bose©, noise-canceling headphones.
Data Analysis
Assessment of the distributional properties of the subjective measures (i.e., QSU, VAS stress and SAM) revealed distributions that departed appreciably from normality. Accordingly, all analyses were performed using both appropriate nonparametric (e.g., proportional odds models with quartiles of subjective response serving as dependent variables) and parametric (e.g., ANCOVA) procedures. Since both approaches yielded identical results, we elected to present the findings obtained with the more conventional parametric approach. Thus, the primary analytic strategy was ANCOVA, with gender serving as the primary between-groups factor and each of the subjective measures serving as the dependent measure. All ANCOVAs, included a covariate consisting of each participant’s response to either the neutral in vivo cues or the relaxed script cues, with the former serving as a control for responding to the smoking in vivo cues and the latter serving as a control for responding to the negative affect/stress script cues. Two additional covariates included in each analysis were the order of stimulus presentation (smoking vs. script cues) and the level of nicotine dependence (as measured by the FTND).
In contrast to the subjective measures, HR and SC levels were obtained continuously over a 90-sec period prior to administration of all cues, and again during each stimulus presentation. For the purposes of analysis, 30-sec means (0–30 sec, 31–60 sec and 61–90 sec) were computed to yield a measure of responding across time. The SC means were log transformed to address potential violations of normality in the distribution of this variable. The primary data analytic strategy was the repeated-measures ANOVA, with gender as a between factor; cue type (smoking vs. neutral or negative affect/stress script vs. relaxed script) and time (as above) served as within factors. Greenhouse-Geisser degrees freedom adjustment was employed when evidence of sphericity assumption violation was present (as indicated by Mauchly’s Test of Sphericity).
Since physiological measures may vary prior to any stimulus presentation, it was necessary to compute a baseline estimate of HR and SC and then assess differences between men and women. The baseline measure consisted of a mean of the 90-sec data collection that occurred prior to any stimulus presentation (i.e., this was the first measure of HR and SC obtained in the laboratory session). Analyses indicated that while women and men did not differ on mean baseline SC (women M = .21, SE = .08; men M = .21, SE = .09; t(82) = −.01, p = .99), women did evidence a higher mean baseline HR than men (women M = 76.0, SE = 1.5; men M = 72.4, SE = 1.1; t(86) = 2.0, p = .050). Accordingly, mean baseline heart rate was used as a covariate in the analysis of the heart rate data.
Results
Demographic and Clinical Characteristics
Table 1 depicts the summary values for all relevant demographic and clinical variables. Men and women were essentially equivalent with respect to the tabled characteristics and therefore, none of the variables were used as covariates in the analyses described below (with the exception of FTND score which could be expected to covary with some of the subjective measures such as craving).
Assessment of Cue Potency
A precondition for assessing gender differences in cue reactivity was the need to demonstrate basic differential cue reactivity effects (i.e., establish that the smoking cues elicited more craving than the neutral comparison cues and that the negative affect/stress script elicited greater subjective stress than the neutral/relaxed script). Paired samples t-tests were applied to the mean difference between these cue pairs to determine if this precondition had been met. The results verified that participants reported substantially greater craving and subjective stress to the smoking cues and negative affect/stress script, respectively (p’s < 0.001). The smoking cues also elicited greater arousal ratings than the neutral cues (p < .001) and the negative affect/stress cues elicited higher craving and arousal ratings, and lower valence and dominance ratings than the neutral/relaxed script cues (all p’s < .001). In sum, the smoking cues were relatively powerful elicitors of subjective craving and arousal whereas the negative affect/stress script was a relatively powerful elicitor of subjective stress, craving, and arousal and was perceived as affectively negative (i.e., negatively valenced) and producing feelings of diminished self-control (i.e., low dominance).
Smoking Prior to the Cue Reactivity Session
While requiring participants to smoke immediately prior to the laboratory session should have served to equate the participants with respect to cigarette/nicotine exposure, it remains possible that there was significant variation in exposure. To rule out the possibility that potential gender differences might be explained by differences in smoking/nicotine exposure, we compared the expired CO levels of women (M = 19.1 ppm, SE = 1.9) and men (M = 23.4 ppm, SE = 1.8) obtained just prior to the cue reactivity session. An independent samples t-test failed to identify a group difference, t(87) = 1.6, p = .11), suggesting that women and men did have equivalent exposure to cigarettes/nicotine. Additionally, correlational analyses failed to identify a significant association between CO level and any of the subjective or physiological responses to either the smoking or negative affect/stressful script cues (all r’s < .1, all p’s > .4). Thus, these findings diminish the likelihood that any of the gender differences reported below were due to variation in smoking prior to the laboratory session.
Primary Findings
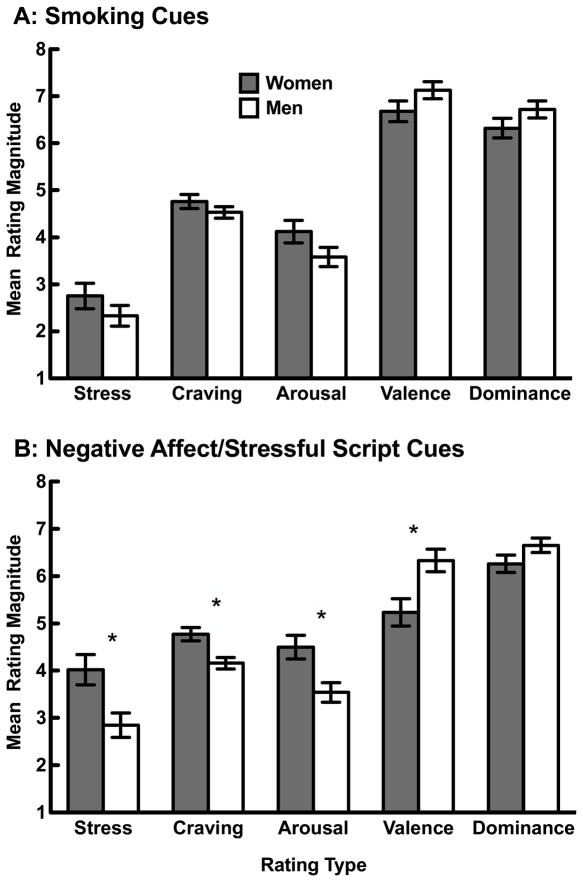
Figure 1 depicts the mean (±SE) stress, craving, arousal, valence and dominance rating magnitudes of women vs. men smokers in response to smoking cues (panel A) and negative affect/stressful script cues (panel B). In the case of smoking cue reactivity, women and men did not significantly differ (controlling for neutral cue responding, order of stimulus presentation and FTND score), but women trended toward higher arousal ratings than men, F(1,84) = 2.94, p =.09. In the case of negative affect/stress script reactivity, women reported higher stress, craving, and arousal ratings but lower valence ratings than men, all F’s > 7.0, all p’s < .01. The dominance ratings of women and men did not differ, F(1, 85) = 2.55, p = .11. Thus, women appear to experience greater subjective stress, craving, arousal and negative emotion in response to the stress cues. With respect to effect size, the partial eta squared (PES) statistics associated with the stress, craving, arousal and valence effects were .09, .11, .09 and .09, respectively. These PES values indicate that gender accounted for approximately 10 percent of the variability in the outcome measures. In all analyses, response to the control cues (i.e., covariate) was a significant predictor of response to smoking and negative affect/stressful cues (all F’s > 4.5, all p’s < 0.01), which validates our use of neutral in vivo and relaxed script cues as controls, respectively.
Figure 1.
Panels A and B depict the mean stress, craving, arousal, valence and dominance rating magnitudes of women vs. men smokers in response to smoking cues (A) and negative affect/stressful script cues (B). Means are adjusted for (i) response to control cues (neutral in vivo cues for smoking cues and relaxed script cues for negative affect/stress script cues), (ii) order of smoking vs. script cues presentation, and (iii) level of nicotine dependence (as measured by the Fagerström Test for Nicotine Dependence (FTND). * indicates p < .01.
Repeated measures ANOVA applied to the three, 30-sec epochs of HR data obtained after the onset of the smoking and neutral cues (controlling for baseline variation between these two cue types) revealed a marginal gender × time interaction, F(1.9,155.3) = 3.1, p = .05, which reflected a trend towards a higher heart rate in women (means were 75.6, 73.6 and 73.3) vs. men (73.3, 72.2 and 72.5). No other main effects or interactions in this or the analysis of the HR data collected during the negative affect/stress vs. neutral/relaxed script cues exceeded statistical threshold. ANOVA results from the SC analysis of the smoking vs. neutral cue data did not reveal any main effects or interactions involving gender but did identify a significant main effect for cue and time, F(1,84) = 8.1, p < .01 and F(2,168) = 8.4, p < .01, respectively. The cue effect verified that the mean log transformed SC response to the smoking cues (M = .71, SE = .04) was greater than to the neutral cues (M = .66, SE = .04) while the time effect verified that SC responses first increased from the 1st to the 2nd, 30-sec epoch and then was unchanged from the 2nd to 3rd epoch (M(1–30) = .67, M(31–60) = .69, M (61–90) = .69; all SEs = .04).
Discussion
The present study examined the effects of gender on subjective (craving and affect ratings) and physiological reactions (heart rate and skin conductance) to smoking and negative affect/stress cues in a human laboratory study of cue reactivity. A necessary precondition for examining gender differences was a demonstration of differential cue reactivity and this precondition was met in the present study; smoking cues were found to elicit greater craving and arousal than the neutral control cues and the negative affect/stress script cues elicited greater subjective stress, arousal, craving and were perceived as more aversive and elicited a perception of being less in control than the neutral/relaxing script cues. The observation that skin conductance level was larger in response to the smoking vs. neutral cues is consistent with previous research51 indicating that smoking cues can elevate sympathetic activity and further substantiates the potency of the smoking cues. Collectively, these observations are consistent with hypothesis 1 (i.e., that smoking and negative affect/stress cues would elicit greater craving, stress and emotional reactivity than relevant control cues).
With respect to gender differences, it was observed that women, relative to men, evidenced greater subjective craving, stress and arousal ratings in response to the negative affect/stress script cues. Women also evidenced lower valence ratings to the negative affect/stress script cues, indicating that they perceived the cues as more aversive than men. Unexpectedly, there were no significant gender differences in response to the smoking cues, though women did trend towards greater arousal ratings in response to the smoking cues. While women evidenced a trend towards greater heart rate reactivity than men during the presentation of the smoking and neutral control cues, this marginal gender difference occurred irrespective of cue type. Skin conductance responding did not vary by gender. Overall, the gender differences in subjective reactivity to the negative affect/stress script cues provide partial support for hypothesis 2 and 3 whereas the failure to find gender differences in subjective reactivity to the smoking cues was inconsistent with expectation. The absence of gender differences on physiological measures of cue reactivity was also contrary to prediction.
The differential smoking cue reactivity observed in the present study is generally consistent with a larger body of research demonstrating similar craving and skin conductance effects23,52–55. It should be noted that there is considerable between-study variation in the pattern of findings reported with regard to smoking cue reactivity52, with most studies reporting differential craving to smoking cues and less robust findings with respect to physiological reactivity. The less robust findings observed with physiological response systems is at least partly due to the relatively small effect sizes observed with these measures and the small sample sizes generally employed in cue reactivity studies52.
Despite the presence of a marginal gender-related heart rate difference that was not cue specific, the present study did not yield evidence of gender differences on any of the subjective ratings or measures of physiological reactivity to smoking vs. neutral cues (although a trend was present on one subjective measure). By contrast, four of the seven previously reviewed studies found some evidence of greater craving reactivity in women vs. men31–33,36, two studies reported evidence of gender differences on cardiovascular measures34,36 and one study reported greater salivary reactivity in women vs. men31. Since four of the six studies that reported gender differences employed sample sizes considerably smaller than used in the present study, it is unlikely that sample size alone contributed to our failure to observe gender-related effects. There were some potentially important procedural differences between the present study and those that found evidence of gender differences in reactivity to smoking cues. For example, the smoking cues in the present study consisted of the participant’s preferred brand of cigarettes whereas three of the six studies noted above employed either scripted imagery cues or video segments of people smoking. Furthermore, while Field & Duka31 used in vivo cues similar to ours, theirs were presented relatively briefly, in the absence of negative affect inducing cues and under conditions of perceived smoking availability/unavailability. Thus, it remains possible that identification of gender differences in reactivity to smoking cues may depend on important methodological aspects of a study.
The observation that the negative affect/stress cues can elicit both negative affect, stress and arousal reactions and craving in smokers and other substance users is well established25,27,28,56–62. However, the present study appears to stand alone in demonstrating that women, relative to men, report higher craving in response to negative affect/stress cues. Since the women in this study also reported greater subjective stress to these cues, a finding consistent with studies of stress reactivity63,64, it is possible that the craving-related gender difference was a product heightened ambient negative affect.
The primary finding of the present study highlights the potentially unique vulnerability that women smokers may have with respect to the experience of negative affect, stress and craving and it points to several potentially important clinical implications. Research linking the experience of negative affect directly to smoking behavior suggests a pathway to smoking relapse that may be especially challenging for quit-attempting women. In their study of the negative mood dampening effects of smoking, Conklin & Perkins65 demonstrated that negative mood induction prior to an opportunity to smoke both decreased latency to smoke and increased smoking (number of puffs) relative to positive mood induction. If, as suggested by the present study, women are more prone than men to experience craving in response to negative affect/stress induction, then the findings of Conklin & Perkins suggests the possibility that women may also have a unique liability in terms of impulsively initiating smoking and increasing smoking behavior when experiencing negative emotion. Likewise, negative emotion and stress are frequent occurrences in the lives of most people. The present research suggests that women smokers who are trying to quit may be especially disadvantaged because negative emotional experiences are linked inextricably to smoking craving. One common negative affect/stress scenario that may be challenging for women is living in a discordant relationship with a partner who smokes. Cessation under these circumstances may be especially difficult for women not only because of a lowered threshold for relapse under conditions of discord but also women whose partners smoke are at greater risk to relapse after initiating a quit attempt17. In this case, there is a synergy between two gender-specific liabilities that put quit attempting women at greater risk of relapse. Once relapsed, the abstinence violation effect66 that so often accompanies cessation failure may lead to additional negative affect that continues to leave women uniquely prone to either diminished motivation to make another quit attempt or a repetitive cycle of quitting and lapsing that eventually gives way to psychological defeat and a return to baseline smoking levels. Although speculative, causal processes such as these may, in part, explain why women have more trouble quitting than men67–69. The present work also serves to underscore the importance of adjunctive behavioral therapy for treatment-seeking women that have a special emphasis on the management of negative mood, stress and craving that occurs in the context of negative emotional experience.
The notion that addicted women may have unique vulnerabilities related to negative emotion and treatment outcome has also gained support in the greater (non-nicotine) substance abuse literature. A recent controlled drinking treatment study70 of individuals who report alcohol misuse during dysphoric mood states indicated that from the period between treatment termination and 12-month follow-up, women evidenced a consumption increase (1.2 alcohol units/week) whereas men evidenced and additional decrease in drinking (3.9 alcohol units/week). Likewise, McKay, et al.,71 found in their study of relapse differences in women and men cocaine users that relapse was more often associated with negative emotion in women than men. Rubonis, et al38 found that negative mood induction prior to alcohol cue presentations resulted in greater craving reactivity in alcohol dependent women than men. It is perhaps not surprising that cue reactivity under these conditions differs between sexes since there appears to be growing evidence of a sexual dimorphism in the neural activation of several brain structures (e.g., amygdala) that are directly involved in the processing of emotional cues72,73 and substance cues40,74. Together, these studies appear consistent with the smoking cessation literature and highlight the possibility that the interface between negative emotion, stress and substance use may be different for women and men.
As with all studies, the results of the present study should be interpreted in the context of several cautionary notes. First, if it is assumed that a minimal threshold of activation is a necessary precondition for the expression of gender differences in cue reactivity, then the failure to observe gender differences in reactivity to smoking cues may be partly due to the moderate salience of the smoking cues. Accordingly, if participants were nicotine deprived and/or a lit cigarette was used as a cue, it is possible that a more robust reactivity profile may have permitted the detection of gender differences. Second, the participants in this study were exposed to all four stimulus conditions in a single cue reactivity session and, as a consequence, there was a possibility of carryover effects across the cues. Although a 10-min nature slide show administered between each cue presentation was used to diminish carryover effects, it is unclear to what extent this end was achieved. Furthermore, the counterbalancing of cue order would not only minimize order effects but also potentially limit carryover effects associated with any specific cue order. Nonetheless, it remains possible that the multiple cue presentations did increase variability in the outcome measures, thereby potentially contributing to the failure to identify gender effects on responding to smoking cues. This issue might be resolved in future studies if the smoking and negative affect/stress cues were presented in separate cue reactivity sessions. Third, the use of scripted negative affect/stress inducing cues, despite their personalized content, may not adequately capture the nature and quality of naturally occurring negative affect and stress-related mood states75. This may partly explain why some studies find an association between negative affect, stress, craving and/or smoking behavior while others do not. Relatedly, the relationship between the findings of the present study and actual smoking behavior remains purely speculative. Future investigations of the interface between gender, mood and craving might benefit from adopting a methodology similar to Conklin & Perkins65 where exposure to smoking and/or mood inducing cues are followed by a smoking opportunity in which various topographical features of smoking behavior could be measured (e.g., latency to smoke, puff frequency, puff duration, etc.). Fourth and finally, the present results might be partly explained by the greater tendency of women vs. men to self-report psychological stress while experiencing relatively similar levels of physiological reactivity. The absence of gender differences in differential physiological reactivity (HR, SC) is consistent with this interpretation. Even so, to the extent that the subjective interpretation of the physiological responses may relate to smoking behavior and relapse, the observed gender differences in subjective craving and stress reactivity may be clinically meaningful.
In summary, the primary findings of the present human laboratory investigation of cue reactivity indicate that while women and men smokers did not appear to substantially differ in their response to smoking cues, they did differ significantly in the magnitude of craving and stress reactivity to negative affect/stress eliciting cues. Specifically, women vs. men reported higher stress, arousal and craving reactions to a scripted imagery account of an unpleasant/stressful personal experience. This observation points to a potentially important gender-related liability that may contribute significantly to poorer smoking cessation outcome among women, with its consequent elevated risk of morbidity and/or mortality76–79. Accordingly, the present authors concur with Cahill80 who noted, “… in addiction, as in so many other domains of neuroscience, investigators are increasingly realizing that they can no longer assume that essentially identical processes occur in men and women, nor that identical therapeutics will apply.” We would add that continued failure to systematically investigate the role of sex differences in addiction science might ultimately compromise treatment development and refinement for both women and men.
Acknowledgments
The authors thank Christine Horne, Ashley McCullough, Erin Klintworth and Gina Frattarolli for their invaluable contributions to completion of this project. This research was supported by NIDA grants P50 DA016511-02 (Dr. Upadhyaya, component PI; Drs. Kathleen T. Brady and Ronald See, Center PIs), K23 DA020482 (Dr. Carpenter), K12 DA000357 (Dr. Gray), and by USPHS grant M01 RR01070 (CTRC, MUSC). Dr. Upadhyaya is now Medical Advisor at Eli Lilly & Company, Indianapolis, IN, 46285, Drop Code 6112.
Footnotes
There are no conflicts of interest pertaining to the present work. However, Dr. Upadhyaya has been a consultant and/or advisory board member of Eli Lilly and Company and Shire Pharmaceuticals. Dr. Upadhyaya is an ex-stockholder of New River Pharmaceutical Company, was on the Speakers’ Bureau of Shire Pharmaceuticals and Pfizer, Inc., and has received research support from Cephalon, Inc., Eli Lilly and Company, and Pfizer, Inc. Dr. Upadhyaya is now employed at Eli Lilly and Company. Dr. Gray has received research support from Pfizer, Inc. (medication and placebo supply for NIH-funded research).
References
- 1.Giovino GA. Epidemiology of tobacco use in the United States. Oncogene. 2002 Oct 21;21(48):7326–7340. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- 2.Fortmann SP, Killen JD. Who shall quit? Comparison of volunteer and population-based recruitment in two minimal-contact smoking cessation studies. Am J Epidemiol. 1994 Jul 1;140(1):39–51. doi: 10.1093/oxfordjournals.aje.a117157. [DOI] [PubMed] [Google Scholar]
- 3.Husten CG, Shelton DM, Chrismon JH, Lin YC, Mowery P, Powell FA. Cigarette smoking and smoking cessation among older adults: United States, 1965–94. Tobacco Control. 1997;6(3):175–180. doi: 10.1136/tc.6.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward KD, Klesges RC, Zbikowski SM, Bliss RE, Garvey AJ. Gender differences in the outcome of an unaided smoking cessation attempt. Addict Behav. 1997 Jul-Aug;22(4):521–533. doi: 10.1016/s0306-4603(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 5.Bjornson W, Rand C, Connett JE, et al. Gender differences in smoking cessation after 3 years in the Lung Health Study. Am J Public Health. 1995 Feb;85(2):223–230. doi: 10.2105/ajph.85.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COMMIT. Community Intervention Trial for Smoking Cessation (COMMIT): I. cohort results from a four-year community intervention. Am J Public Health. 1995 Feb;85(2):183–192. doi: 10.2105/ajph.85.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis LJ, Jr, Hurt RD, Offord KP, Lauger GG, Morse RM, Bruce BK. Self-administered Nicotine-Dependence Scale (SANDS): item selection, reliability estimation, and initial validation. J Clin Psychol. 1994 Nov;50(6):918–930. doi: 10.1002/1097-4679(199411)50:6<918::aid-jclp2270500617>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Killen JD, Fortmann SP, Newman B, Varady A. Evaluation of a treatment approach combining nicotine gum with self-guided behavioral treatments for smoking relapse prevention. J Consult Clin Psychol. 1990 Feb;58(1):85–92. doi: 10.1037//0022-006x.58.1.85. [DOI] [PubMed] [Google Scholar]
- 9.Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008 Jul;10(7):1245–1250. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- 10.Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction. 2004 Nov;99(11):1462–1469. doi: 10.1111/j.1360-0443.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 11.Wetter DW, Fiore MC, Young TB, McClure JB, de Moor CA, Baker TB. Gender differences in response to nicotine replacement therapy: objective and subjective indexes of tobacco withdrawal. Experimental and Clinical Psychopharmacology. 1999 May;7(2):135–144. doi: 10.1037//1064-1297.7.2.135. [DOI] [PubMed] [Google Scholar]
- 12.Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. J Consult Clin Psychol. 1999 Aug;67(4):555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- 13.Perkins KA. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Experimental and Clinical Psychopharmacology. 1996 May;4(2):166–177. [Google Scholar]
- 14.Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berl) 2002 Sep;163(2):194–201. doi: 10.1007/s00213-002-1168-1. [DOI] [PubMed] [Google Scholar]
- 15.Robinson JD, Cinciripini PM, Tiffany ST, Carter BL, Lam CY, Wetter DW. Gender differences in affective response to acute nicotine administration and deprivation. Addict Behav. 2007 Mar;32(3):543–561. doi: 10.1016/j.addbeh.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray RP, Johnston JJ, Dolce JJ, Lee WW, O’Hara P. Social support for smoking cessation and abstinence: the Lung Health Study. Lung Health Study Research Group. Addict Behav. 1995 Mar-Apr;20(2):159–170. doi: 10.1016/s0306-4603(99)80001-x. [DOI] [PubMed] [Google Scholar]
- 17.Walsh PM, Carrillo P, Flores G, Masuet C, Morchon S, Ramon JM. Effects of partner smoking status and gender on long term abstinence rates of patients receiving smoking cessation treatment. Addict Behav. 2007 Jan;32(1):128–136. doi: 10.1016/j.addbeh.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine Tob Res. 2000 Aug;2(3):231–241. doi: 10.1080/14622200050147493. [DOI] [PubMed] [Google Scholar]
- 19.Allen SS, Hatsukami DK, Christianson D, Nelson D. Withdrawal and pre-menopausal symptomatology during the menstrual cycle in short-term smoking abstinence: Effects of menstrual cycle on smoking abstinence. Nicotine and Tobacco Research. 1999;1:129–142. doi: 10.1080/14622299050011241. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine & Tobacco Research. 2006;8(5):627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- 21.Drummond DC, Tiffany ST, Glautier S, Remington B. Addictive Behavior: Cue Exposure Theory and Practice. Chichester: John Wiley & Sons Ltd; 1995. [Google Scholar]
- 22.Shadel WG, Niaura R, Brown RA, Hutchison KE, Abrams DB. A content analysis of smoking craving. J Clin Psychol. 2001 Jan;57(1):145–150. doi: 10.1002/1097-4679(200101)57:1<145::aid-jclp15>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.LaRowe SD, Saladin ME, Carpenter MJ, Upadhyaya HP. Reactivity to nicotine cues over repeated cue reactivity sessions. Addict Behav. 2007 Dec;32(12):2888–2899. doi: 10.1016/j.addbeh.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohsenow DJ, Monti PM, Abrams D, et al. Cue elicited urge to drink and salivation in alcoholics: Relationship to individual differences. Advances in Behaviour Research and therapy. 1992;14:195–210. [Google Scholar]
- 25.Maude-Griffin PM, Tiffany ST. Production of smoking urges through imagery: The impact of affect and smoking abstinence. Experimental and Clinical Psychopharmacology. 1996;4(2):198–208. [Google Scholar]
- 26.Niaura R, Shadel WG, Britt DM, Abrams DB. Response to social stress, urge to smoke, and smoking cessation. Addict Behav. 2002 Mar-Apr;27(2):241–250. doi: 10.1016/s0306-4603(00)00180-5. [DOI] [PubMed] [Google Scholar]
- 27.Payne TJ, Schare ML, Levis DJ, Colletti G. Exposure to smoking-relevant cues: effects on desire to smoke and topographical components of smoking behavior. Addict Behav. 1991;16(6):467–479. doi: 10.1016/0306-4603(91)90054-l. [DOI] [PubMed] [Google Scholar]
- 28.Tiffany ST, Drobes DJ. Imagery and smoking urges: the manipulation of affective content. Addict Behav. 1990;15(6):531–539. doi: 10.1016/0306-4603(90)90053-z. [DOI] [PubMed] [Google Scholar]
- 29.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004 Jan;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 30.Colamussi L, Bovbjerg DH, Erblich J. Stress- and cue-induced cigarette craving: effects of a family history of smoking. Drug Alcohol Depend. 2007 May 11;88(2–3):251–258. doi: 10.1016/j.drugalcdep.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Field M, Duka T. Cue reactivity in smokers: the effects of perceived cigarette availability and gender. Pharmacol Biochem Behav. 2004;78(3):647–652. doi: 10.1016/j.pbb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Knott VJ, Cosgrove M, Villeneuve C, Fisher D, Millar A, McIntosh J. EEG correlates of imagery-induced cigarette craving in male and female smokers. Addict Behav. 2008 Apr;33(4):616–621. doi: 10.1016/j.addbeh.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Knott VJ, Naccache L, Cyr E, et al. Craving-induced EEG reactivity in smokers: effects of mood induction, nicotine dependence and gender. Neuropsychobiology. 2008;58(3–4):187–199. doi: 10.1159/000201716. [DOI] [PubMed] [Google Scholar]
- 34.Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: effects of gender and cue type. Addict Behav. 1998;23(2):209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- 35.Rohsenow DJ, Monti PM, Hutchison KE, et al. High-dose transdermal nicotine and naltrexone: effects on nicotine withdrawal, urges, smoking, and effects of smoking. Experimental and Clinical Psychopharmacology. 2007 Feb;15(1):81–92. doi: 10.1037/1064-1297.15.1.81. [DOI] [PubMed] [Google Scholar]
- 36.Tong C, Bovbjerg DH, Erblich J. Smoking-related videos for use in cue-induced craving paradigms. Addict Behav. 2007 Dec;32(12):3034–3044. doi: 10.1016/j.addbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53(3):223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- 38.Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD. Alcohol cue reactivity and mood induction in male and female alcoholics. J Stud Alcohol. 1994 Jul;55(4):487–494. doi: 10.15288/jsa.1994.55.487. [DOI] [PubMed] [Google Scholar]
- 39.Yu J, Zhang S, Epstein DH, et al. Gender and stimulus difference in cue-induced responses in abstinent heroin users. Pharmacol Biochem Behav. 2007 Mar;86(3):485–492. doi: 10.1016/j.pbb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 40.McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008 Aug;33(9):2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- 41.Gray KM, DeSantis SM, Carpenter MJ, Saladin ME, LaRowe SD, Upadhyaya HP. Menstrual cycle and cue reactivity in women smokers. Nicotine Tob Res. 2010 Feb;12(2):174–178. doi: 10.1093/ntr/ntp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller G, Levin D, Kozak M, Cook E, McLean A, Lang P. Individual differences in imagery and the psychophysiology of emotion. Cognit Emot. 1987;1:122–126. [Google Scholar]
- 43.Sinha R, Lovallo WR, Parsons OA. Cardiovascular differentiation of emotions. Psychosom Med. 1992 Jul-Aug;54(4):422–435. doi: 10.1097/00006842-199207000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001 Feb;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 45.Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry. 1994 Mar;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 46.Hodes RL, Cook EW, Lang PJ. Individual differences in autonomic response: conditioned association or conditioned fear? Psychophysiology. 1985 Sep;22(5):545–560. doi: 10.1111/j.1469-8986.1985.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 47.Lang PJ, Kozak MJ, Miller GA, Levin DN, McLean A., Jr Emotional imagery: conceptual structure and pattern of somato-visceral response. Psychophysiology. 1980 Mar;17(2):179–192. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 48.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis I Disorders, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 49.Heatherton T, Kozlowski L, Frecker R, Fagerstrom K-O. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 50.Forster KI, Forster JC. DMDX: a windows display program with millisecond accuracy. Behav Res Methods Instrum Comput. 2003 Feb;35(1):116–124. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
- 51.Burton SM, Tiffany ST. The effect of alcohol consumption on craving to smoke. Addiction. 1997 Jan;92(1):15–26. [PubMed] [Google Scholar]
- 52.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999 Mar;94(3):327–340. [PubMed] [Google Scholar]
- 53.Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001 May;9(2):183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- 54.Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J Abnorm Psychol. 1997 Feb;106(1):15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- 55.Morgan MJ, Davies GM, Willner P. The Questionnaire of Smoking Urges is sensitive to abstinence and exposure to smoking-related cues. Behav Pharmacol. 1999 Nov;10(6–7):619–626. doi: 10.1097/00008877-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 56.Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: the process of relapse. Addict Behav. 1990;15(2):105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- 57.Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997 May;106(2):243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- 58.Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict Behav. 1995 Sep-Oct;20(5):657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- 59.Hutchison KE, Niaura R, Swift R. Smoking cues decrease prepulse inhibition of the startle response and increase subjective craving in humans. Exp Clin Psychopharmacol. 1999 Aug;7(3):250–256. doi: 10.1037//1064-1297.7.3.250. [DOI] [PubMed] [Google Scholar]
- 60.Litt MD, Cooney NL, Kadden RM, Gaupp L. Reactivity to alcohol cues and induced moods in alcoholics. Addict Behav. 1990;15(2):137–146. doi: 10.1016/0306-4603(90)90017-r. [DOI] [PubMed] [Google Scholar]
- 61.Shiffman S. A cluster-analytic classification of smoking relapse episodes. Addict Behav. 1986;11(3):295–307. doi: 10.1016/0306-4603(86)90057-2. [DOI] [PubMed] [Google Scholar]
- 62.Shiffman S, Read L, Jarvik ME. Smoking relapse situations: a preliminary typology. Int J Addict. 1985 Feb;20(2):311–318. doi: 10.3109/10826088509044913. [DOI] [PubMed] [Google Scholar]
- 63.Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcoholism: Clinical & Experimental Research. 2008 Jul;32(7):1242–1250. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmaus BJ, Laubmeier KK, Boquiren VM, Herzer M, Zakowski SG. Gender and stress: differential psychophysiological reactivity to stress reexposure in the laboratory. Int J Psychophysiol. 2008 Aug;69(2):101–106. doi: 10.1016/j.ijpsycho.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Conklin CA, Perkins KA. Subjective and Reinforcing Effects of Smoking During Negative Mood Induction. Journal of Abnormal Psychology. 2005 Feb;114(1):153–164. doi: 10.1037/0021-843X.114.1.153. [DOI] [PubMed] [Google Scholar]
- 66.Marlatt GA, Gordon JR. Relapse Prevention. New York: The Guilford Press; 1985. [Google Scholar]
- 67.Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002 Nov;164(2):121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- 68.Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999 Dec;1(4):301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- 69.Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004 Oct;28(6):533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Kavanagh DJ, Sitharthan G, Young RM, et al. Addition of cue exposure to cognitive-behaviour therapy for alcohol misuse: a randomized trial with dysphoric drinkers. Addiction. 2006 Aug;101(8):1106–1116. doi: 10.1111/j.1360-0443.2006.01488.x. [DOI] [PubMed] [Google Scholar]
- 71.McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis. 1996 Oct;184(10):616–622. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001 Feb 12;12(2):427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- 73.Killgore WD, Yurgelun-Todd DA. Sex differences in amygdala activation during the perception of facial affect. Neuroreport. 2001 Aug 8;12(11):2543–2547. doi: 10.1097/00001756-200108080-00050. [DOI] [PubMed] [Google Scholar]
- 74.Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004 Feb;161(2):233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- 75.Shiffman S, Gwaltney CJ. Does heightened affect make smoking cues more salient? J Abnorm Psychol. 2008 Aug;117(3):618–624. doi: 10.1037/0021-843X.117.3.618. [DOI] [PubMed] [Google Scholar]
- 76.Henschke CI, Yip R, Miettinen OS. Women’s susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA. 2006 Jul 12;296(2):180–184. doi: 10.1001/jama.296.2.180. [DOI] [PubMed] [Google Scholar]
- 77.Risch HA, Howe GR, Jain M, Burch JD, Holowaty EJ, Miller AB. Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol. 1993 Sep 1;138(5):281–293. doi: 10.1093/oxfordjournals.aje.a116857. [DOI] [PubMed] [Google Scholar]
- 78.Zang EA, Wynder EL. Cumulative tar exposure. A new index for estimating lung cancer risk among cigarette smokers. Cancer. 1992 Jul 1;70(1):69–76. doi: 10.1002/1097-0142(19920701)70:1<69::aid-cncr2820700112>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 79.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst. 1996 Feb 21;88(3–4):183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 80.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006 Jun;7(6):477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]