Abstract
Learning theory and computational accounts suggest that learning depends on errors in outcome prediction as well as changes in processing of or attention to events. These divergent ideas are captured by models, such as Rescorla-Wagner (RW) and temporal difference (TD) learning on one hand, which emphasize errors as directly driving changes in associative strength, versus models such Pearce-Hall (PH) and more recent variants on the other hand, which propose that errors promote changes in associative strength by modulating attention and processing of events. Numerous studies have shown that phasic firing of midbrain dopamine neurons carries a signed error signal consistent with RW or TD learning theories, and recently we have shown that this signal can be dissociated from attentional correlates in the basolateral amygdala and anterior cingulate. Here we will review this data along with new evidence 1) implicating habenula and striatal regions in supporting error signaling in midbrain dopamine neurons and 2) suggesting that central nucleus of the amygdala and prefrontal regions process the amygdalar attentional signal. However while the neural instantiations of the RW and PH signals are dissociable and complementary, they may be linked. Any linkage would have implications for understanding why one signal dominates learning in some situations and not others and also for appreciating the potential impact on learning of neuropathological conditions involving altered dopamine or amygdalar function, such as schizophrenia, addiction, or anxiety disorders.
Keywords: attention, prediction error, dopamine, amygdala, learning
Over the past four decades, the development of animal learning models has been constrained by the need to account for certain cardinal traits of associative learning, such as cue selectivity (e.g. blocking) and cue interactions (e.g. overexpectation). One effective way of accounting for these traits has been to implement Kamin's dictum that, for learning to occur, the outcome of the trial must be surprising (Kamin, 1969). Formally, this dictum is captured in many influential models by the notion of prediction error, or discrepancy between the actual outcome and the outcome predicted on that trial (Rescorla & Wagner, 1972; Pearce & Hall, 1980; Pearce et al., 1982; Sutton, 1988; Lepelley, 2004; Pearce & Mackintosh, 2010; Esber & Haselgrove, 2011).
Despite their widespread use, prediction errors do not always serve the same function across different models. For example, the Rescorla-Wagner (RW; Rescorla & Wagner, 1972) and temporal difference learning models (TD; Sutton, 1988) use errors to drive associative changes in a direct fashion. Large errors will result in correspondingly large changes in associative strength, but no change will occur if the error is zero (i.e. the outcome is already predicted). Furthermore, the sign of the error determines the kind of learning that takes place. If the error is positive, as when the outcome is better than predicted, the association between the cues and the outcome will strengthen. If the error is negative, on the other hand, as when the outcome is worse than expected, this association will weaken or even become negative.
In contrast to these models, others such as Pearce and Hall's (PH; Pearce & Hall, 1980; Pearce et al., 1982), utilize the absolute value of the prediction error to modulate the amount of attention devoted to the cues on subsequent trials, which in turn dictates how much will be learned about them. Large errors will result in a boost in the attention paid to the cues that accompanied the errors, thereby facilitating subsequent learning, whereas small errors will weaken that attention, hampering learning. (Since the cue-specific attention learned by PH modulates subsequent associative learning about the cue, it is also known as the cue's associability. We use the terms interchangeably in this review.) On this view, therefore, by modulating associability, unsigned prediction errors ultimately determine the amount of learning.
Although initially conceived as mutually exclusive, evidence that prediction errors may well promote learning directly and by altering the attention to cues has gradually rendered these views compatible, as reflected in more recent learning models (Lepelley, 2004; Pearce & Mackintosh, 2010; Esber & Haselgrove, 2011). Indeed, the two mechanisms may serve complementary roles. For example, the degree to which associations in the RW and TD models are updated following a prediction error is scaled by free “learning rate” parameters, but the theories contain no explicit account of what factors or mechanisms influence them. The attentional allocation learned by PH could address this point, since PH's associabilities play the same role as learning rates, but are learned from experience rather than entirely free. Indeed, a separate family of Bayesian theories has studied how learning rules of this sort can be derived from principles of sound statistical reasoning; these considerations typically lead to models containing both a RW-like error-driven update, scaled by a PH-like associability mechanism (Sutton, 1992; Dayan & Long, 1998; Kakade & Dayan, 2002; Courville et al., 2006; Behrens et al., 2007; Bossaerts & Preuschoff, 2007). Yet even if RW and PH learning are viewed as competing rather than complementary, it might still be useful for the brain to employ multiple mechanisms to promote flexibility in different situations and robustness in the face of damage or other interference affecting either mechanism.
Consistent with this proposal, numerous studies have shown that phasic firing of midbrain dopamine neurons carries a RW or TD-like error signal (see Bromberg-Martin et al., 2010b for review), and recently we have shown that this signal can be dissociated from a PH attentional correlate in the basolateral amygdala (Roesch et al., 2010). Below we will describe this evidence along with new data implicating habenula and striatal regions in supporting error signaling in midbrain dopamine neurons and central nucleus and prefrontal regions in processing this attentional signal. We will also suggest that while the RW and PH signals are dissociable, they are likely linked. Thus while the brain utilizes both signals, they may not be fully independent. Interdependence has implications for understanding why one signal dominates learning in some situations versus others and also for appreciating the potential impact of pathological conditions such as schizophrenia, anxiety disorders, or even addiction on learning mechanisms.
Dopamine neurons signal bidirectional prediction errors
According to the influential RW (Rescorla & Wagner, 1972) model, prediction errors are calculated from the difference between the outcome predicted by all the cues available on that trial (ΣV) and the outcome that is actually received (λ). If the outcome is underpredicted, so that the value of λ is greater than that of ΣV, the error will be positive and learning will accrue to those stimuli that happened to be present. Conversely, if the outcome is overpredicted, the error will be negative and a reduction in learning will take place. Thus the magnitude and sign of the resulting change in the cues' associations (ΔV) is directly determined by prediction error according to the following equation:
| Eq. 1 |
wherein α and β are constants referring to the salience of the cue and the reinforcer, respectively, included to control the learning rate. This basic idea is also captured in temporal difference learning models – TD – which extend the idea to apply recursively to learning about cues from other cues' previously learned associations (Sutton, 1988).
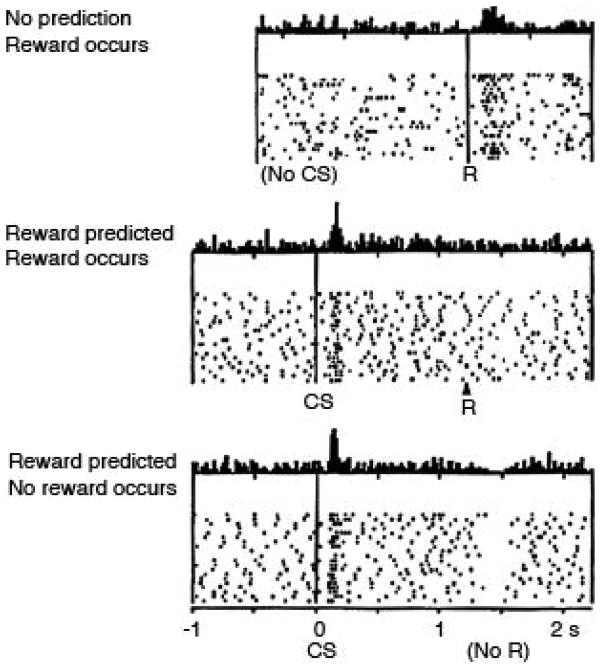
Dopamine neurons of the midbrain have been widely reported to signal signed errors in reward, and more recently punishment, prediction that are consistent with RW and TD models (Mirenowicz & Schultz, 1994; Houk et al., 1995; Montague et al., 1996; Hollerman & Schultz, 1998; Waelti et al., 2001; Fiorillo et al., 2003; Tobler et al., 2003; Ungless et al., 2004; Bayer & Glimcher, 2005; Pan et al., 2005; Morris et al., 2006; Roesch et al., 2007; D'Ardenne et al., 2008; Matsumoto & Hikosaka, 2009). Although this idea is not without critics, particularly regarding the precise timing of the phasic response (Redgrave & Gurney, 2006) and the classification of these neurons as dopaminergic (Ungless et al., 2004; Margolis et al., 2006), the evidence supporting this proposal is strong, deriving from multiple labs, species, and tasks. These studies demonstrate that a large proportion of putative dopamine neurons exhibit bidirectional changes in activity in response to rewards that are better or worse than expected. These neurons fire to an unpredicted reward and firing declines when the reward becomes predicted and is suppressed when the predicted reward is not delivered. This is illustrated in the single unit example in Figure 1. Moreover, the same neurons display activity in response to unpredicted cues, if those cues had been previously associated with reward. Although these cue-evoked responses might initially seem like they carry qualitatively different information from the error-related responses to primary rewards, TD models explain them both as instances of a common prediction error signal. In TD, unexpected reward-predictive cues induce a prediction error because they reflect a change from the expected value, just as delivery of a primary reward does earlier in learning. Of course, signed prediction errors do not necessarily have to be represented in the form of bidirectional phasic activity within the same neuron; however the occurrence of such a firing pattern in some dopamine neurons does serve to rule out other competing interpretations such as encoding of salience, attention or motivation, or even simple habituation.
Figure 1.
Changes in firing in response to positive and negative reward prediction errors (PE's) in a primate dopamine neuron. Figure shows spiking activity in a putative dopamine neuron recorded in the midbrain of a monkey performing a simple task in with a conditioned stimulus (CS) is used to signal reward. Data is displayed in a raster format in which each tic mark represents an action potential and each row a trial. Average activity per trial is summarized in a peri-event time histogram at the top of each panel. Top panel shows activity to an unpredicted reward (+PE). Middle panel shows activity to the reward when it is fully predicted by the CS (no PE). Bottom panel shows activity on trials in which the CS is presented but the reward is omitted (−PE). As described in the text, the neuron exhibits a bidirectional correlate of the reward prediction error, firing to unexpected but not expected reward and suppressing firing on omission of an expected reward. The neuron also fires to the CS; in theory such activity is thought to reflect the error induced by unexpected presentation of the valuable CS. This feature distinguishes a TDRL signal from the simple error signal postulated by Rescorla-Wagner. Figure adapted from Schultz, Dayan, and Montague (Schultz et al., 1997).
Although much of this evidence has come from primates, similar results have been reported in other species. For example, functional imaging results from many groups indicate that the BOLD response in the human ventral striatum also displays all the hallmarks of prediction errors seen in primate dopamine recordings, such as elevation for unexpected rewards and suppression when expected rewards are omitted (McClure et al., 2003; O'Doherty et al., 2003; Hare et al., 2008). Although the BOLD response is a metabolic signal and not specific to any particular neurochemical, several results support the inference that BOLD correlates of prediction errors in striatum may in part reflect the effect of its prominent dopaminergic input (Pessiglione et al., 2006; Day et al., 2007; Knutson & Gibbs, 2007). Also, although technically more challenging to image unambiguously, BOLD activity in the human midbrain dopaminergic nuclei also appears to reflect a prediction error (D'Ardenne et al., 2008).
Similarly, Hyland and colleagues have reported signaling of reward prediction errors in a simple Pavlovian conditioning and extinction task (Pan et al., 2005) in rodents. Putative dopamine neurons recorded in rat VTA initially fired to the reward. With learning, this reward-evoked response declined and the same neurons developed responses to the preceding cue. During extinction, activity was suppressed on reward omission. Parallel modeling revealed that the changes in activity closely paralleled theoretical error signals in their task.
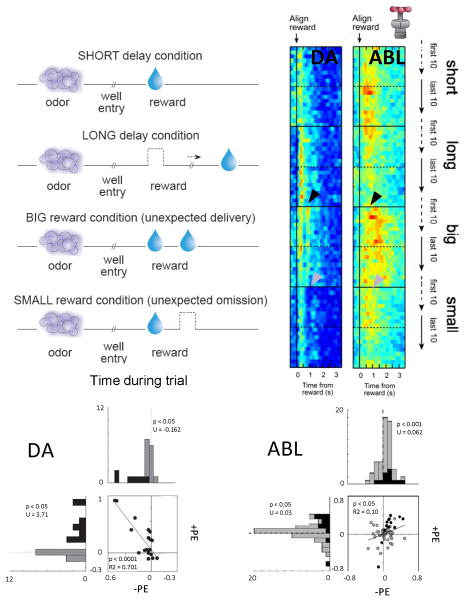
Prediction error signaling has also been reported in VTA dopamine neurons in rats performing a more complicated choice task. In this task, reward was unexpectedly delivered or omitted by altering the timing or number of rewards delivered (Roesch et al., 2007). This task is illustrated in Figure 2, along with a heat plot showing population activity on the subset of trials in which reward was delivered unexpectedly. Dopamine activity increased when a new reward was instituted and then declined as the rats learned to expect that reward. Activity in these same neurons was suppressed later, when the expected reward was omitted. In addition, activity was also high for delayed reward, consistent with recent reports in primates (Fiorillo et al., 2008; Kobayashi & Schultz, 2008). Furthermore, the same dopamine neurons that fired to unpredictable reward also developed phasic responses to preceding cues with learning, and this activity was higher when the cues predicted the more valuable reward. These features of dopamine firing are entirely consistent with prediction error encoding (Mirenowicz & Schultz, 1994; Montague et al., 1996; Hollerman & Schultz, 1998; Waelti et al., 2001; Fiorillo et al., 2003; Tobler et al., 2003; Bayer & Glimcher, 2005; Pan et al., 2005; Morris et al., 2006; Roesch et al., 2007; D'Ardenne et al., 2008; Matsumoto & Hikosaka, 2009).
Figure 2.
Changes in firing in response to positive and negative reward prediction errors (PE's) in rat dopamine (DA) and basolateral amygdala (ABL) neurons. Rats were trained on a simple choice task in which odors predicted different rewards. During recording, the rats learned to adjust their behavior to reflect changes in the timing or size of reward. As illustrated in the left panel, this resulted in delivery of unexpected rewards (+PE) and omission of expected rewards (−PE). Activity in reward-responsive DA and ABL neurons is illustrated in the heat plots to the right, which show average firing synchronized to reward in the first and last 10 trials of each block, and in the scatter/histograms below, which plot changes in firing for each neuron in response to +PE's and −PE's. As described in the text, neurons in both regions fired more to an unexpected reward (+PE, black arrow). However only the DA neurons also suppressed firing on reward omission; ABL neurons instead increased firing (−PE, gray arrows). This is inconsistent with the bidirectional error signal postulated by Rescorla-Wagner or TDRL and instead is more like the unsigned error signal utilized in attentional theories, such as Pearce-Hall. Figure adapted from Roesch, Calu, and Schoenbaum (Roesch et al., 2007) and Roesch, Calu, Esber, and Schoenbaum (Roesch et al., 2010).
Where do dopamine neurons get information relevant to predicting reward and where do these signals go?
The latter question is easier to address, since the working hypothesis for all intents and purposes has been “everywhere”. Error signals are found prominently in both substantia nigra and ventral tegmental area, and since dopamine neurons project widely and nearly every brain region is thought to be involved in some form of associative learning, a reasonable hypothesis might be that these signals could be important anywhere.
However while this may be a reasonable starting point, recent and not-so-recent evidence suggests it is an (obvious) over-simplification. First while dopamine neurons may project widely, their inputs are particularly concentrated on striatal regions, which are heavily implicated in associative learning. This combined with converging evidence from imaging and computational neuroscience have led to the suggestion that these regions are particularly receptive to the dopaminergic teaching signals. Second there has been increasing emphasis on whether the dopaminergic error signal (and dopamine neuron population it is contained in) is truly homogenous. While the signal seems to be present in the majority of dopamine neurons identified by waveform, this still leaves a substantial number of these neurons that are not coding errors. Further traditional waveform criteria may fail to identify at least some TH+ neurons (Margolis et al., 2006; Margolis et al., 2008); indeed there may be a particular sampling bias against those that project to prefrontal regions (Lammel et al., 2008). Given that prediction errors do not appear to be present in neurons that fail to show the classical waveform criteria (Roesch et al., 2007), this suggests an interesting possibility that dopaminergic prediction error signals may have their primary impact on subcortical associative learning nodes, such as striatum and amygdala, and much less impact on prefrontal executive regions. Of course this is largely speculative; until it is possible to directly tag specific neurons during extracellular recording, in order to identify their projection targets, it will be difficult to conclusively identify which projections carry these signals and for what purposes.
More developed ideas exist regarding where information to support dopaminergic error signals originates. One set of ideas has focused on the information content of this signal. In order to generate a prediction error, one must have a prediction - the overall expected value based on the summed values of the cues present in the environment. This summed value is similar to the overall expected value in RW. Based on anatomical and imaging data, it has been suggested that the ventral striatum serves this role, providing information about the summed expected value given current circumstances to the midbrain (O'Doherty et al., 2004). Other accounts have suggested that value predictions may also be derived from the amygdala (Belova et al., 2008). Notably, these are both limbic areas that are clearly implicated in signaling cue values. An interesting outstanding question is whether information impacting the dopamine signal also comes from prefrontal regions (Balleine et al., 2008), thought to represent task structure in so-called model-based reinforcement learning models (Daw et al., 2005). Some evidence exists suggesting that dopamine signals (or error-related BOLD responses in striatal regions) may reflect this type of information (Bromberg-Martin et al., 2010d; Daw et al., in press; Simon & Daw, in press). Consistent with this, we have recently shown that input from the orbitofrontal cortex, a key region in encoding such model-based associative structures (Pickens et al., 2003; Izquierdo et al., 2004; Ostlund & Balleine, 2007; Burke et al., 2008), is necessary for expectancy-related changes in phasic firing in midbrain dopamine neurons (Takahashi et al., in press).
A second set of ideas has focused on locating the error signal itself in upstream structures. In particular, considerable focus has been on lateral habenula (LHb)(Matsumoto & Hikosaka, 2007; Bromberg-Martin et al., 2010c; Hikosaka, 2010), which is thought to receive error information from globus pallidus (GP)(Hong & Hikosaka, 2008). Activity in LHb reflects signed prediction errors the same as activity of DA neurons, but remarkably, in the opposite direction. That is, activity is inhibited and excited by positive and negative prediction errors, respectively. Consistent with the idea that LHb is feeding DA neurons this information, activity of DA neurons is inhibited by LHb stimulation and prediction error signaling in LHb occurs earlier than those in DA neurons (Matsumoto & Hikosaka, 2007; Bromberg-Martin et al., 2010a). DA neurons are likely to receive this information via indirect connections through midbrain GABA neurons in VTA and the adjacent rostromedial tegmental nucleus (RMTg), which has similar response properties as LHb neurons and have heavy inhibitory projections to midbrain DA neurons (Ji & Shepard, 2007; Jhou et al., 2009; Kaufling et al., 2009; Omelchenko et al., 2009; Brinschwitz et al., 2010; Hong et al., 2011).
How to integrate these two stories is not clear. One possibility is that the GP-LHb-midbrain circuit forms an “error-signaling axis” that information relevant to determining the value expected in a particular state, provided by limbic and perhaps prefrontal regions, feeds into at multiple levels. Whatever the case, the emerging picture suggests a widespread error-signaling circuit, in position to receive a torrent of information regarding value, arising from nearly all the brain systems we would currently think of as important for valuation and decision-making.
Amygdala neurons signal shifts in attention
While the amygdala has often been viewed as critical for learning to predict aversive outcomes (Davis, 2000; LeDoux, 2000), the last two decades have revealed a more general involvement in associative learning (Gallagher, 2000; Murray, 2007; Tye et al., 2008). Although the mainstream view holds that amygdala is important for acquiring and storing associative information (LeDoux, 2000; Murray, 2007), there have been hints in the literature that amygdala may also support other functions related to associative learning and error signaling. For example, damage to central nucleus disrupts orienting and increments in attentional processing after changes in expected outcomes (Gallagher et al., 1990; Holland & Gallagher, 1993b; 1999), and other studies have proposed a critical role for amygdala – particularly central nucleus output to subcortical areas – in mediating vigilance or surprise (Davis & Whalen, 2001).
Consistent with this idea, Salzman and colleagues (Belova et al., 2007) have reported that amygdala neurons in monkeys are responsive to unexpected outcomes. However this study showed minimal evidence of negative prediction error encoding or transfer of positive prediction errors to conditioned stimuli in amygdala neurons, and many neurons fired similarly to unexpected rewards and punishments. This pattern of firing does not meet the criteria for a RW or TDRL prediction error signal.
Similar firing patterns have also been reported in rat basolateral amygdala (ABL). For example, Janak and colleagues examined activity of neurons in ABL during unexpected omission of reward during the extinction of reward-seeking behavior (Tye et al., 2010). Rats were first trained to respond at a nosepoke operandum for partial reinforcement (50%). After several training sessions, the sucrose reward was withheld during extinction. Rats quickly learned to stop nosepoking during extinction, and many neurons in ABL started to fire to the empty port. Importantly, these changes were correlated with the response intensity and the extinction resistance of the rat and fit well with several lesion studies demonstrating the importance of the ABL in altering behavior when expected values change (Corbit & Balleine, 2005; McLaughlin & Floresco, 2007; Ehrlich et al., 2009)
Modulation of neural activity in lateral and basal nucleus of amygdala by expectation has also been described during fear conditioning in rats (Johansen et al., 2010). In this procedure shock to the eye lid was induced after presentation of an auditory conditioned stimulus. Recordings were conducted during initial CS-UCS pairings and showed that shocks elicited stronger neural responses early during learning than later after learning. For these cells, responses evoked by the shock were inversely correlated with freezing to the conditioned stimulus, suggesting that diminution of shock-evoked activity was related to the changes in expectation. Overall, activity was stronger for unsignalled versus signaled shock, consistent with amygdala encoding the surprise induced by unexpected shock.
The patterns observed in these studies do not appear to be fully consistent with what is predicted by RW or TD, and instead seem more like a correlate of surprise or attention. However it is unclear if they fully comply with predictions of the PH theory for an attentional correlate, since in most cases they were recorded in settings that make it difficult to identify whether they are unsigned, either because recordings were done across days or without both types of errors or due to potential confounds between aversive positive errors with appetitive negative errors (or vice versa). In order to fully address this question, it would be useful to record these neurons in a task already applied to isolate RW or TD-like signed errors in midbrain dopamine neurons.
Data from such a study are presented in Figure 2. In this experiment, neural activity was recorded from ABL neurons in rats during performance of the same choice task used to isolate prediction error signaling in rat dopamine neurons (Roesch et al., 2010). As in monkeys, many ABL neurons increased firing when reward was delivered unexpectedly. However such activity differed markedly in its temporal specificity from what was observed in VTA dopamine neurons. This is evident in Figure 2, where the increased firing in ABL occurs somewhat later and is much broader than that in dopamine neurons.
Moreover activity in these ABL neurons was not inhibited by omission of an expected reward. Instead, activity was actually stronger during reward omission, and those neurons that fired most strongly for unexpected reward delivery also fired most strongly after reward omission.
Activity in ABL also differed from that in VTA in its onset dynamics. While firing in VTA dopamine neurons was strongest on the first encounter with an unexpected reward and then declined, activity in the ABL neurons continued to increase over several trials after a shift in reward. These differences and the overall pattern of firing in the ABL neurons are inconsistent with signaling of a signed prediction error as envisioned by RW and TD, at least at the level of individual single-units. Instead such activity in ABL appears to relate to an unsigned error signal, or more particularly, to an associability or attention variable derived from unsigned errors.
That is, theories of associative learning have traditionally employed unsigned errors to drive changes in stimulus processing or attention, operationalized as an associability parameter controlling learning rate (Mackintosh, 1975; Pearce & Hall, 1980). According to this idea, the attention that a cue receives is equal to the weighted average of the unsigned error generated across the past few trials, reflecting the idea that cues that have been accompanied by either positive or negative errors in the past are more likely to be responsible for errors in the future, and thus should be updated preferentially. Mathematically, according to one variant of PH (Pearce et al., 1982), the associability of a cue on trial n (αn) is updated relative to its previous level (αn-1) using the absolute value of the prediction error on that trial. Thus:
| Eq. 2 |
where (λn-1 - ΣV n-1) is defined as the difference between the value of the reward predicted by all cues in the environment (ΣV n-1) and the value of the reward that was actually received (λn-1), and γ is a weighting factor ranging between 0 and 1, which serves to account for the observed gradual change in attention or associability. The resultant quantity— termed attention (α)—can then be used to scale the update of a cue's value. PH's version of this rule is:
| Eq. 3 |
where S and λ represent the intrinsic salience (e. g. intensity) of the cue (S) and the reward, respectively.
Of course, the same associability term can be used, in principle, with RW (Eq. 1) to modulate learning rate (Lepelley, 2004). Interestingly, hybrid RW/PH mechanisms of this sort also emerge in Bayesian models that attempt to explain learning in conditioning as arising from normative principles of statistical reasoning. In these models, learning about a cue is often driven by prediction error (as in RW) but the learning rate is not a free parameter. Instead, it should be determined by uncertainty about the cue's associative strength: all else equal, if you are more uncertain about a cue's value, you should be more willing to update it. In these models, then, uncertainty plays a role like associability, in controlling learning rates. In these models, uncertainty is determined by a rule strikingly similar to Eq 2. This is because an important determinant of uncertainty is the variance of the prediction error, of which the unsigned (in this case, squared rather than absolue) error is a sample (Dayan & Long, 1998; Courville et al., 2006; Bossaerts & Preuschoff, 2007). Thus, Bayesian models offer a normative interpretation for Eq 2 and the attentional factor it describes, and motivate its use in hybrid models together with RW-like mechanisms.
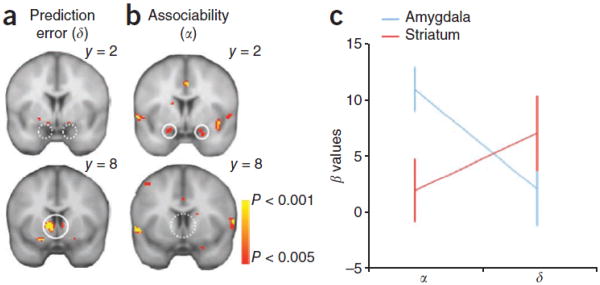
The activity of the ABL neurons in Figure 2 appears to provide an associability signal similar to that proposed by PH and these other models to underlie surprise and attentional modulation in the service of associative learning. As predicted by these theoretical accounts, this pattern is clearly unlike that of a signed error contained in the firing of the VTA dopamine neurons. A similar dissociation between the BOLD signal in ventral striatum and amygdala has also been reported in humans during aversive learning (Li et al., 2011). In this study, subjects performed a reversal task in which two visual cues were initially partially paired with an electrical shock or nothing, respectively, and then the associations were reversed. Changes in skin conductance response during presentation of the two cues indicated that subjects learned not only the value of the cues but also represented the cues associability or salience as predicted by the PH model. Subsequent analyses of BOLD response at the time of cue termination (when the outcome was delivered or not) showed that activity in the ventral striatum was positively correlated with the aversive prediction error (Figure 3), whereas activity in the amygdala was positively correlated with associability (Figure 3). Given results implicating the dopamine system in signaling aversive errors (Ungless et al., 2004; Matsumoto & Hikosaka, 2009; Mileykovskiy & Morales, 2011), these results are at least consistent with a neural dissociation between dopaminergic error signaling and amygdalar signaling of attention.
Figure 3.
Neural correlates of associability and prediction error term. (a) BOLD in the ventral striatum, but not amygdala, correlated with prediction error. (b) BOLD in the bilateral amygdala, but not ventral striatum, correlated with associability regressor (p < 0.05, SVC). (c) Differential representations of associability (α) and prediction error (δ) in striatum and amygdala BOLD (± s.e.m). Figure adapted from Li, Schiller, Schoenbaum, Phelps, and Daw (Li et al., 2011).
Where do amygdala neurons get information relevant to modulating attention and where do these signals go?
As with the dopamine neurons, it is simpler to address where the signal might go, and what its impact might be than to identify where the information to support the signal originates. Two major circuits appear to be the most likely recipients of the attentional signal from the basolateral amygdala. The first is a circuit running through the central nucleus of the amygdala (CeA). The central nucleus receives information from the basolateral areas and it has been strongly implicated in surprise and vigilance (Davis & Whalen, 2001) as well as in modulating attention for learning during unblocking procedures (Holland & Gallagher, 1993a; b). These and other related findings (Holland & Gallagher, 1993a; b; Holland & Kenmuir, 2005) demonstrate that CeA is essential for the enhancement of attention that results from the unexpected omission of an outcome (negative error). The specificity of this effect suggests a special role of CeA in processing the absence of an expected event; consistent with this we have found that neurons in central nucleus provide a signal that specifically increases when an expected reward is omitted (Calu et al., 2010). Whether the partial representation of a PH signal in the central nucleus reflects a filtering of the fuller signal from basolateral amygdala will require a causal disconnection to demonstrate, although recent data from Holland and colleagues implicating the ABL in attentional function during unblocking procedures would favor this hypothesis (Chang et al., 2010).
The second circuit that may be receive information from basolateral amygdala regarding attentional modulation are prefrontal areas. As outlined above, these regions may not receive a dopaminergic error signal, since it has been reported that prefrontal-projecting dopamine neurons lack the characteristic waveform features that characterize error-signaling dopaminergic neurons in our work (Roesch et al., 2007; Lammel et al., 2008). Yet prefrontal regions - particularly the orbital and cingulate regions that receive input from amygdala - are generally implicated in learning and processes that would benefit from input highlighting particularly cues for attention.
The anterior cingulate (ACC) is a particularly likely candidate to receive error information from ABL; it has strong reciprocal connections with ABL (Sripanidkulchai et al., 1984; Cassell & Wright, 1986; Dziewiatkowski et al., 1998) and has already been shown to be involved in number of functions related to error processing and attention (Carter et al., 1998; Scheffers & Coles, 2000; Paus, 2001; Holroyd & Coles, 2002; Ito et al., 2003; Rushworth et al., 2004; Walton et al., 2004; Amiez et al., 2005; 2006; Kennerley et al., 2006; Magno et al., 2006; Matsumoto et al., 2007; Oliveira et al., 2007; Rushworth et al., 2007; Sallet et al., 2007; Quilodran et al., 2008; Rudebeck et al., 2008; Rushworth & Behrens, 2008; Kennerley et al., 2009; Kennerley & Wallis, 2009; Totah et al., 2009; Hillman & Bilkey, 2010; Wallis & Kennerley, 2010; Hayden et al., 2011; Rothe et al., 2011).
Although most of this research has focused on the role of the ACC in the detection of errors of commission (Ito et al., 2003; Amiez et al., 2005; Quilodran et al., 2008; Rushworth & Behrens, 2008; Totah et al., 2009), more recent work has suggested that ACC does not simply detect errors, but is important for signaling other aspects of behavioral feedback (Kennerley et al., 2006; Oliveira et al., 2007; Rothe et al., 2011), including prediction error encoding similar to what we find in ABL. For example, Hayden and colleagues showed that activity in monkey ACC was high when rewards were delivered and omitted unexpectedly in a task in which rewards were delivered at predetermined probabilities. These changes in ACC firing occurred regardless of valence at the single cell level, consistent with encoding of surprise or attentional variables (Hayden et al., 2011).
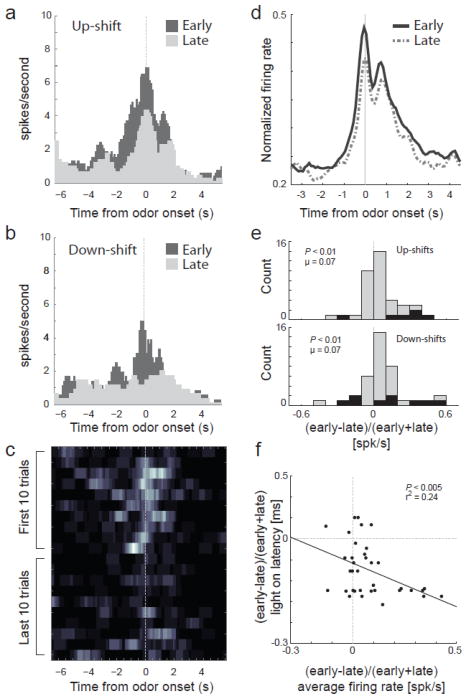
To more directly address how activity related to changes in reward in ACC compares to that in other areas, we have recently recorded in ACC in rats in the same task in which we characterized firing in ABL and VTA. Importantly this task allows us to examine neural activity in ACC during trials after reward prediction error signals occur in a setting in which animals learn from detection of such errors. Consistent with previous findings we found that ACC detects errors of commission and reward prediction at the time of their occurrence (Bryden et al., in press). However, we also found that activity in ACC was elevated in anticipation of and during cue presentation on trials after reward contingences changed. Such an elevation, which was not observed in ABL, could reflect increased processing of cues on subsequent trials, as predicted by the PH theory. Consistent with this proposal, changes in firing were correlated with behavioral measures of attention to the cues evident on these trials. These effects are illustrated in a single cell example and across the population in Figure 4. Thus ACC is not only involved in detecting errors but in utilizing that information to drive attention and learning on subsequent trials. Detection of prediction errors and the subsequent changes in attention critical for learning might be dependent on connections between ABL and ACC.
Figure 4.
Activity in ACC is stronger on trials after errors in reward prediction. a-b, Histogram represents firing of one neuron during the first 10 (dark gray) and last 10 (light gray) trials after up- and down-shifts in value aligned on odor onset. c, Heat plot shows the average firing, of the same neuron, across shifts during the first and last 10 trials after reward contingencies change. d, Average normalized neural activity for all task-related neurons (odor onset to fluid well entry) comparing the first 10 trials in all blocks (early; solid black) to the last 10 trials in all blocks (late; dashed gray). e, Distribution reflecting the difference in task related firing rate between early and late in a trial block (early-late)/(early+late) following either up-shifts (top) or down-shifts (bottom). f, Correlation between light-on latency (house light on until nose poke; y-axis) and firing rate (x-axis) either early or late within a block ((early-late)/(early+late)). N = 4 rats. Figure adapted from Bryden and Roesch (Bryden et al., in press).
Where information supporting these signals originates is again a more complex question, particularly since these signals have only been recently uncovered. Like RW or TD error signals, the attentional signals carried by neurons in basolateral and central amygdala also depend on information about expected value, in order to calculate the prediction error that forms the basis of the PH effect. The amygdala is a major associative learning node, thus it is well positioned to compute an error signal internally, utilizing associative information coded locally and sent to it by other regions, such as prefrontal regions or hippocampus.
However an alternative, intriguing possibility is that the PH signal in the amygdala is dependent on an external prediction error signal. An obvious candidate to provide this signal would be the midbrain dopamine neurons. These neurons already have access to the information necessary to compute this quantity and project strongly into basolateral amygdala. Indeed, although learning as in Eq. 2 could in principle be implemented using a signed error signal of the sort associated with dopamine (e.g., if the rules governing plasticity at target neurons effect the absolute value), recent work suggests that some dopamine neurons may provide an unsigned error signal. Specifically, in monkeys, some putative dopamine neurons increase firing when either an appetitive (juice) or aversive (air-puff) outcome is delivered unexpectedly (Matsumoto & Hikosaka, 2009). These results have yet to be shown clearly in rats, but would be consistent with a linkage between dopaminergic and amygdalar teaching signals.
Although speculative, we recently repeated our earlier experiment, recording in both controls and rats with ipsilateral 6-OHDA lesions (Esber et al., Society for Neuroscience Abstracts, 2010). These rats performed normally on the task, presumably utilizing the intact hemisphere, and amygdala neurons recorded in these rats showed robust reward-evoked activity. However unlike neurons in controls and in our prior experiment, neurons deprived of dopamine input failed to modulate firing in response to surprising upshifts or downshifts in reward. Thus removal of dopamine input disrupted error-related modulations in the activity of these neurons. This result provides evidence linking the RW signal computed by the dopamine neurons with the PH attentional signal in basolateral amygdala neurons.
The significance for normal and pathological learning
If single-unit activity in amygdala contributes to attentional changes, then the role of this region in a variety of learning processes may need to be reconceptualized or at least modified to include this function. This is particularly true for ABL. ABL appears to be critical for encoding associative information properly; associative encoding in downstream areas requires input from ABL (Schoenbaum et al., 2003; Ambroggi et al., 2008). This has been interpreted as reflecting an initial role in acquiring the simple associative representations (Pickens et al., 2003). However an alternative account – not mutually exclusive – is that ABL may also augment the allocation of attentional resources to directly drive acquisition of the same associative information in other areas. As noted above, the signal in ABL, identified here, may serve to augment or amplify the associability of cue-representations in downstream regions, so that these cues are more associable or salient on subsequent trials. Such an effect may be evident in neural activity prior to and during cue sampling reported here in ACC.
A role in attentional function for ABL would also affect our understanding of how this region is involved in neuropsychiatric disorders. For example, amygdala has long been implicated in anxiety disorders such as post-traumatic stress disorder (Davis, 2000). While this involvement may reflect abnormal storage of information in ABL, it might also reflect altered attentional signaling, affecting storage of information not in ABL but in other brain regions. This would be consistent with accounts of amygdala function in fear that have emphasized vigilance (Davis & Whalen, 2001).
Understanding how RW and PH signaling are linked in the brain will also be important. One possibility, which we have suggested several times here and elsewhere (Lepelley, 2004; Courville et al., 2006; Li et al., 2011), is that the RW/TD and PH mechanisms effectively comprise the associative learning and learning rate control components of a single hybrid learner. Thus the associabilities learned by PH may serve to scale the associative TD updates driven by dopaminergic prediction errors. The demonstration that signaling related to associability in ABL depends on a dopaminergic input would support such an integrated view, since it is consistent with the possibility that the error terms in Eqs. 1 and 2 derive from a common source.
Even if the two mechanisms are tightly interacting in many circumstances, they may also contribute separately or independently to other behaviors. Indeed, there do exist some behavioral phenomena that are difficult to explain by the simple composition of RW and PH mechanisms into a single hybrid learner, at least in the form described here, but instead may be consistent in various circumstances with the mechanism described by one or the other model can operate more or less independently. For example, when rewards are omitted unexpectedly, rats typically show increased responding to added cues (Holland & Gallagher, 1993a), which is consistent with the original PH models but not RW or TD learning (even when augmented by PH attentional updates; see Dayan and Long 1996). However if switches involving omission or reward downshift occur repeatedly, rats show less or slower responding (Roesch et al., 2007; Roesch et al., 2010), consistent with RW but not with naked PH. These considerations suggest that the two classical conditioning mechanisms may contribute to different degrees in different circumstances, much as has been suggested for different instrumental learning mechanisms (Dickinson & Balleine, 2002; Daw et al., 2005). In the case of PH and RW, the factors governing the relative contribution of the mechanisms are not yet as well understood.
Finally it is worth noting that a linkage (or lack thereof) between dopaminergic RW signals and PH signals in ABL and elsewhere has significance for our understanding of altered learning in neuropathological conditions. For example, some symptoms of schizophrenia have been proposed to reflect the spurious attribution of salience to cues (Kapur, 2003). These symptoms might be secondary to altered signaling in ABL (Taylor et al., 2005) under the influence of aberrant dopaminergic input. Such alterations could drive frank attentional problems as well as positive symptoms such as hallucinations and delusions. The amygdala has also been central to ideas about addiction, where it is proposed to mediate craving and the abnormal attribution of motivational significance to drug-associated cues and contexts. Again such aberrant learning may reflect, in part, disrupted attentional processing, potentially under the influence of altered dopamine function.
Acknowledgments
This work was supported by grants to GS from NIDA, NIMH, and NIA and to MR from NIDA. This article was prepared, in part, while GS was employed at UMB. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government
References
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Joseph JP, Procyk E. Anterior cingulate error-related activity is modulated by predicted reward. Eur J Neurosci. 2005;21:3447–3452. doi: 10.1111/j.1460-9568.2005.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex. 2006;16:1040–1055. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Daw ND, O'Doherty JP. Multiple forms of value learning and the function of dopamine. In: Glimcher PW, Camerer CF, Fehr E, Poldrack RA, editors. Neuroeconomics: Decision Making and the Brain. Elsevier; Amsterdam: 2008. [Google Scholar]
- Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nature Neuroscience. 2007;10:1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belova MA, Patton JJ, Salzman CD. Moment-to-moment tracking of state value in the amygdala. Journal of Neuroscience. 2008;28:10023–10030. doi: 10.1523/JNEUROSCI.1400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaerts P, Preuschoff K. Adding prediction risk to the theory of reward learning in the dopaminergic system. Annals of the New York Academy of Sciences. 2007;1104:135–146. doi: 10.1196/annals.1390.005. [DOI] [PubMed] [Google Scholar]
- Brinschwitz K, Dittgen A, Madai VI, Lommel R, Geisler S, Veh RW. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience. 2010;168:463–476. doi: 10.1016/j.neuroscience.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Distinct tonic and phasic anticipatory activity in lateral habenula and dopamine neurons. Neuron. 2010a;67:144–155. doi: 10.1016/j.neuron.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive and alerting. Neuron. 2010b;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010c;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hong S, Hikosaka O. A pallidus-habenula-dopamine pathway signals inferred stimulus values. Journal of Neurophysiology. 2010d;104:1068–1076. doi: 10.1152/jn.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden DW, Johnson EE, Tobia SC, Kashtelyan V, Roesch MR. Attention for learning signals in anterior cingulate cortex. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.4715-11.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke KA, Franz TM, Miller DN, Schoenbaum G. The role of the orbitofrontal cortex in the pursuit of happiness and more specific rewards. Nature. 2008;454:340–344. doi: 10.1038/nature06993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Roesch MR, Haney RZ, Holland PC, Schoenbaum G. Neural correlates of variations in event processing during learning in central nucleus of amygdala. Neuron. 2010;68:991–1001. doi: 10.1016/j.neuron.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Wright DJ. Topography of projections from the medial prefrontal cortex to the amygdala in the rat. Brain Res Bull. 1986;17:321–333. doi: 10.1016/0361-9230(86)90237-6. [DOI] [PubMed] [Google Scholar]
- Chang SE, Wheeler DS, McDannald M, Holland PC. The effects of basolateral amygdala lesions on unblocking. Society for Neuroscience Abstracts. 2010 doi: 10.1037/a0027576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courville AC, Daw ND, Touretzky DS. Bayesian theories of conditioning in a changing world. Trends in Cognitive Sciences. 2006;10:294–300. doi: 10.1016/j.tics.2006.05.004. [DOI] [PubMed] [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. Oxford University Press; Oxford: 2000. pp. 213–287. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans' choices and striatal prediction errors. Neuron. doi: 10.1016/j.neuron.2011.02.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nature Neuroscience. 2005;8:1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nature Neuroscience. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Dayan P, Long T. Statistical models of conditioning. NIPS. 1998;10:117–123. [Google Scholar]
- Dickinson A, Balleine BW. The role of learning in the operation of motivational systems. In: Pashler H, Gallistel R, editors. Stevens' Handbook of Experimental Psychology (3rd Edition, Vol 3: Learning, motivation, and emotion) John Wiley & Sons; New York: 2002. pp. 497–533. [Google Scholar]
- Dziewiatkowski J, Spodnik JH, Biranowska J, Kowianski P, Majak K, Morys J. The projection of the amygdaloid nuclei to various areas of the limbic cortex in the rat. Folia Morphol (Warsz) 1998;57:301–308. [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Esber GR, Haselgrove M. Reconciling the influence of predictiveness and uncertainty on stimulus salience: a model of attention in associative learning. Proceedings of the Royal Society B. 2011;278:2553–2561. doi: 10.1098/rspb.2011.0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo CD, Newsome WT, Schultz W. The temporal precision of reward prediction in dopamine neurons. Nat Neurosci. 2008 doi: 10.1038/nn.2159. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1856–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Gallagher M. The amygdala and associative learning. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. Oxford University Press; Oxford: 2000. pp. 311–330. [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. Journal of Neuroscience. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Heilbronner SR, Pearson JM, Platt ML. Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. J Neurosci. 2011;31:4178–4187. doi: 10.1523/JNEUROSCI.4652-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman KL, Bilkey DK. Neurons in the rat anterior cingulate cortex dynamically encode cost-benefit in a spatial decision-making task. J Neurosci. 2010;30:7705–7713. doi: 10.1523/JNEUROSCI.1273-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behavioral Neuroscience. 1993a;107:246–253. doi: 10.1037//0735-7044.107.2.246. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Effects of amygdala central nucleus lesions on blocking an d unblocking. Behavioral Neuroscience. 1993b;107:235–245. doi: 10.1037//0735-7044.107.2.235. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends in Cognitive Sciences. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Holland PC, Kenmuir C. Variations in unconditioned stimulus processing in unblocking. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:155–171. doi: 10.1037/0097-7403.31.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nature Neuroscience. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–729. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31:11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk JC, Adams JL, Barto AG. A model of how the basal ganglia generate and use neural signals that predict reinforcement. In: Houk JC, Davis JL, Beiser DG, editors. Models of information processing in the basal ganglia. MIT Press; Cambridge: 1995. pp. 249–270. [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Izquierdo AD, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. Journal of Neuroscience. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Tarpley JW, LeDoux JE, Blair HT. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nature Neuroscience. 2010;13:979–986. doi: 10.1038/nn.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakade S, Dayan P. Acquisition and extinction in autoshaping. Psychological Review. 2002;109:533–544. doi: 10.1037/0033-295x.109.3.533. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. Predictability, suprise, attention, and conditioning. In: Campbell BA, Church RM, editors. Punishment and Aversive Behavior. Appleton-Century-Crofts; New York: 1969. pp. 242–259. [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Americal Journal of Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Dahmubed AF, Lara AH, Wallis JD. Neurons in the frontal lobe encode the value of multiple decision variables. J Cogn Neurosci. 2009;21:1162–1178. doi: 10.1162/jocn.2009.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Wallis JD. Evaluating choices by single neurons in the frontal lobe: outcome value encoded across multiple decision variables. Eur J Neurosci. 2009;29:2061–2073. doi: 10.1111/j.1460-9568.2009.06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Knutson B, Gibbs SEB. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology. 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Schultz W. Influence of reward delays on responses of dopamine neurons. J Neurosci. 2008;28:7837–7846. doi: 10.1523/JNEUROSCI.1600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The amygdala and emotion: a view through fear. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. Oxford University Press; New York: 2000. pp. 289–310. [Google Scholar]
- Lepelley ME. The role of associative history in models of associative learning: a selective review and a hybrid model. Quarterly Journal of Experimental Psychology. 2004;57:193–243. doi: 10.1080/02724990344000141. [DOI] [PubMed] [Google Scholar]
- Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND. Differential roles of human striatum and amygdala in associative learning. Nature Neuroscience. 2011;14:1250–1252. doi: 10.1038/nn.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82:276–298. [Google Scholar]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The anterior cingulate and error avoidance. J Neurosci. 2006;26:4769–4773. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: Is there an electrophysiological marker for dopaminergic neurons? Journal of Physiology. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa Y, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. Journal of Neuroscience. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Matsumoto K, Abe H, Tanaka K. Medial prefrontal cell activity signaling prediction errors of action values. Nat Neurosci. 2007;10:647–656. doi: 10.1038/nn1890. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Floresco SB. The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking behavior. Neuroscience. 2007;146:1484–1494. doi: 10.1016/j.neuroscience.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy B, Morales M. Duration of inhibition of ventral tegmental area dopamine neurons encodes a level of conditioned fear. Journal of Neuroscience. 2011;31:7471–7476. doi: 10.1523/JNEUROSCI.5731-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. Journal of Neurophysiology. 1994;72:1024–1027. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive hebbian learning. Journal of Neuroscience. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Nevet A, Arkadir D, Vaadia E, Bergman H. Midbrain dopamine neurons encode decisions for future action. Nat Neurosci. 2006;9:1057–1063. doi: 10.1038/nn1743. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends in Cognitive Sciences. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Friston KJ, Critchley H, Dolan RJ. Temporal difference learning model accounts for responses in human ventral striatum and orbitofrontal cortex during Pavlovian appetitive learning. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston KJ, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Oliveira FT, McDonald JJ, Goodman D. Performance monitoring in the anterior cingulate is not all error related: expectancy deviation and the representation of action-outcome associations. J Cogn Neurosci. 2007;19:1994–2004. doi: 10.1162/jocn.2007.19.12.1994. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental learning. Journal of Neuroscience. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Schmidt R, Wickens JR, Hyland BI. Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network. Journal of Neuroscience. 2005;25:6235–6242. doi: 10.1523/JNEUROSCI.1478-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. 1980;87:532–552. [PubMed] [Google Scholar]
- Pearce JM, Kaye H, Hall G. Predictive accuracy and stimulus associability: development of a model for Pavlovian learning. In: Commons ML, Herrnstein RJ, Wagner AR, editors. Quantitative Analyses of Behavior. Ballinger; Cambridge, MA: 1982. pp. 241–255. [Google Scholar]
- Pearce JM, Mackintosh NJ. Two theories of attention: a review and a possible integration. In: Mitchell CJ, LePelley ME, editors. Attention and Associative Learning: From Brain to Behaviour. Oxford University Press; Oxford, UK: 2010. pp. 11–39. [Google Scholar]
- Pessiglione M, Seymour P, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Setlow B, Saddoris MP, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. Journal of Neuroscience. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilodran R, Rothe M, Procyk E. Behavioral shifts and action valuation in the anterior cingulate cortex. Neuron. 2008;57:314–325. doi: 10.1016/j.neuron.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nature Reviews Neuroscience. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. Appleton-Century-Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Roesch MR, Calu DJ, Esber GR, Schoenbaum G. Neural correlates of variations in event processing during learning in basolateral amygdala. Journal of Neuroscience. 2010;30:2464–2471. doi: 10.1523/JNEUROSCI.5781-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Schoenbaum G. Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nature Neuroscience. 2007;10:1615–1624. doi: 10.1038/nn2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe M, Quilodran R, Sallet J, Procyk E. Coordination of High Gamma Activity in Anterior Cingulate and Lateral Prefrontal Cortical Areas during Adaptation. J Neurosci. 2011;31:11110–11117. doi: 10.1523/JNEUROSCI.1016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Bannerman DM, Rushworth MF. The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci. 2008;8:485–497. doi: 10.3758/CABN.8.4.485. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Sallet J, Quilodran R, Rothe M, Vezoli J, Joseph JP, Procyk E. Expectations, gains, and losses in the anterior cingulate cortex. Cogn Affect Behav Neurosci. 2007;7:327–336. doi: 10.3758/cabn.7.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG. Performance monitoring in a confusing world: error-related brain activity, judgments of response accuracy, and types of errors. J Exp Psychol Hum Percept Perform. 2000;26:141–151. doi: 10.1037//0096-1523.26.1.141. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate for prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Simon DA, Daw ND. Neural correlates of forward planning in a spatial decision task in humans. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.4647-10.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripanidkulchai K, Sripanidkulchai B, Wyss JM. The cortical projection of the basolateral amygdaloid nucleus in the rat: a retrograde fluorescent dye study. J Comp Neurol. 1984;229:419–431. doi: 10.1002/cne.902290310. [DOI] [PubMed] [Google Scholar]
- Sutton RS. Learning to predict by the method of temporal difference. Machine Learning. 1988;3:9–44. [Google Scholar]
- Sutton RS. Adapting bias by gradient descent: An incremental version of delta-bar-delta. Proceedings of the Tenth National Conference on Artificial Intelligence. 1992:171–176. [Google Scholar]
- Takahashi YK, Roesch MR, Wilson RC, Toreson K, O'Donnell P, Niv Y, Schoenbaum G. Expectancy-related changes in firing of dopamine neurons depend on orbitofrontal cortex. Nature Neuroscience. doi: 10.1038/nn.2957. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Britton JC, Liberzon I. Neural responses to emotional salience in schizophrenia. Neuropsychopharmacology. 2005;30:984–995. doi: 10.1038/sj.npp.1300679. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Dickinson A, Schultz W. Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. Journal of Neuroscience. 2003;23:10402–10410. doi: 10.1523/JNEUROSCI.23-32-10402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totah NK, Kim YB, Homayoun H, Moghaddam B. Anterior cingulate neurons represent errors and preparatory attention within the same behavioral sequence. J Neurosci. 2009;29:6418–6426. doi: 10.1523/JNEUROSCI.1142-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Cone JJ, Schairer WW, Janak PH. Amygdala neural encoding of the absence of reward during extinction. J Neurosci. 2010;30:116–125. doi: 10.1523/JNEUROSCI.4240-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Stuber GD, De Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–1257. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Kennerley SW. Heterogeneous reward signals in prefrontal cortex. Curr Opin Neurobiol. 2010;20:191–198. doi: 10.1016/j.conb.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. 2004;7:1259–1265. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]