Abstract
AIM: To investigate the impact of phosphatase and tensin homolog (Pten) in the specification of intestinal enteroendocrine subpopulations.
METHODS: Using the Cre/loxP system, a mouse with conditional intestinal epithelial Pten deficiency was generated. Pten mutant mice and controls were sacrificed and small intestines collected for immunofluorescence and quantitative real-time polymerase chain reaction. Blood was collected on 16 h fasted mice by cardiac puncture. Enzyme-linked immunosorbent assay was used to measure blood circulating ghrelin, somatostatin (SST) and glucose-dependent insulinotropic peptide (GIP) levels.
RESULTS: Results show an unexpected dual regulatory role for epithelial Pten signalling in the specification/differentiation of enteroendocrine cell subpopulations in the small intestine. Our data indicate that Pten positively regulates chromogranin A (CgA) expressing subpopulations, including cells expressing secretin, ghrelin, gastrin and cholecystokinin (CCK). In contrast, Pten negatively regulates the enteroendocrine subtype specification of non-expressing CgA cells such as GIP and SST expressing cells.
CONCLUSION: The present results demonstrate that Pten signalling favours the enteroendocrine progenitor to specify into cells expressing CgA including those producing CCK, gastrin and ghrelin.
Keywords: Phosphatase and tensin homolog, Enteroendocrine cells, Intestinal epithelial cell specification, Chromogranin A
INTRODUCTION
The phosphatase and tensin homolog (PTEN) tumour suppressor gene is one of the most frequently mutated/deleted genes in various human cancers[1,2]. PTEN is a lipid and protein phosphatase. Its best-known substrate, the phosphatidylinositol 3,4,5-trisphosphate (PIP3), is a lipid second messenger mainly produced by class IA phosphatidylinositol 3-kinases (PI3Ks)[3]. PTEN dephosphorylates PIP3 to produce phosphatidylinositol 4,5-bisphosphate, which inhibits PI3K-dependent effectors such as the downstream kinases Akt and pyruvate dehydrogenase kinase 1. PI3Ks have been implicated in many signalling pathways that regulate cell survival, growth, proliferation, migration, phagocytosis, and metabolism[4]. PTEN has also been shown to regulate genomic stability[5,6], stem cell renewal[7,8], senescence[9] and cell differentiation[10-12].
The multiple cellular functions of PTEN suggest that this protein plays major roles in overall system homeostasis. Indeed, homozygous deletion of Pten in the mouse causes early embryonic lethality by embryonic day (E) 9.5, whereas Pten heterozygous mice (Pten+/-) develop, over a period of time, various dysplasia and hyperplasia in organs such as the breast, thyroid, prostate and intestine[1,2,13]. As reviewed by Knobbe et al[14], Pten has also been conditionally deleted in many specific tissues. These models have established the tumour suppressive function of Pten but have also unravelled its important role in the maintenance of normal physiological functions in various tissues such as the immune system, skin, lung, liver, pancreas and hypothalamus[14].
In a previous study, we reported that Pten is important for intestinal homeostasis[10]. The villin-Cre system was used to specifically inactivate Pten in the mouse intestinal epithelium. Pten mutant mice developed an intestinalomegaly associated with an increase in epithelial cell proliferation. Histological analysis also demonstrated significant perturbation of the crypt-villus architecture, a marked increase in goblet cells and a decrease in enteroendocrine cells, suggesting a role for Pten in the commitment of the multipotential-secretory precursor cell[10].
Enteroendocrine cells are hormone-secreting epithelial cells that are scattered throughout the gastrointestinal epithelium and although they represent only 1% of the intestinal epithelium, taken together, they constitute the major endocrine organ of the body[15,16]. At least 10 different enteroendocrine cell types have been identified in the small intestine and are classified based on their main hormonal products[16,17]. The various hormones produced by these endocrine cells [ghrelin (GHR), gastrin-releasing peptide (GRP), glucose-dependent insulinotropic peptide (GIP), secretin (SCT), peptide YY (PYY), glucagon-like peptide-1 (GLP-1), glucagon-like peptide-2 (GLP-2), cholecystokinin (CCK), neurotensin, serotonin, substance P, somatostatin (SST) and motilin] control important physiological functions, such as gastrointestinal motility, glycaemia, exocrine pancreatic secretion, biliary secretion, digestion, gut epithelial renewal and appetite[16,18,19]. Most enteroendocrine cell types secrete chromogranin A (CgA), a soluble glycoprotein stored with hormones and neuropeptides in secretory granules of endocrine cells. The important role of enteroendocrine cells in whole body homeostasis prompted us to further analyze the effect of intestinal epithelial deletion of Pten on the specification of the various enteroendocrine subpopulations. Using our Cre/loxP Pten conditional knock out mouse model[10], we report herein an unexpected dual regulatory role for epithelial Pten signalling in the specification of enteroendocrine cells. Our data indicate that Pten positively influences the determination and specification of CgA-expressing cell subpopulations in the small intestine including those expressing secretin, ghrelin, gastrin and CCK. Conversely, Pten limits determination and specification of non-expressing CgA endocrine cell subpopulations, including GIP and SST.
MATERIALS AND METHODS
Animals
BALB/c-Ptenfx/fx mice were purchased from The Jackson Laboratory (Bar Harbor, ME, United states). The C57BL/6 12.4KbVilCre transgenic line was provided by Dr. Deborah Gumucio (University of Michigan, Ann Arbor, MI, United states)[20]. Genomic DNA was isolated using the Spin Doctor genomic DNA kit from Gerard Biotech according to the manufacturer’s protocol. Both mutations were genotyped following protocols already published[20] or as directed by The Jackson Laboratory. For this study, the BALB/c-Ptenfx/fx mice mice were first crossed with the C57BL/6 12.4KbVilCre to generate F1-generation heterozygous animals. F1-generation heterozygous animals were then backcrossed with BALB/c-Ptenfx/fx mice to produce F2-generation experimental animals. All experiments were conducted in F2-generation experimental animals. All mice were maintained on regular diet in the transgenic mouse facility at the Faculty of Medicine and Health Sciences of the Université de Sherbrooke. All experiments were approved by the animal research committee of the Faculty of Medicine and Health Sciences of the Université de Sherbrooke.
Tissue collection, tissue preparation, RNA extraction and gene expression analysis
Digestive tracts from 120-d-old Pten∆IEC mice and control littermates were fixed in 4% paraformaldehyde (PFA) overnight at 4 °C, then dehydrated and embedded in paraffin. Sections of 5 μm were applied to Probe-On Plus slides (Fisher Scientific, Ottawa, ON, Canada) and kept at room temperature until used[10,21]. Total RNA was isolated and processed using the Totally RNA extraction kit (Ambion, Grand Island, NY, United states). Reverse-transcription polymerase chain reaction (RT-PCR) and quantitative real-time PCR were performed as described previously[21]. Quantitative real-time PCR conditions were as follows: one cycle of 15 min at 95 °C; 50 cycles at 95 °C for 15 s; 59 °C for 30 s and 72 °C for 30 s. The following forward and reverse primers were used: Hairy and enhancer of split 1 (NM_008235), 5′-TTCCAAGCTAGAGAAGGCAGA-3′, 5′-GTTGATCTGGGTCATGCAGTT-3′; Atonal homolog 1 (NM_007500), 5′-GCTTCCTCTGGGGGTTACTC-3′, 5′-ACAACGATCACCACAGACCA-3′; Neurogenin 3 (NM_009719), 5′-CGGATGACGCCAAACTTACAAAG-3′ 5′-CACAAGAAGTCTGAGAACAACAG-3′; Growth factor independent 1 (NM_010278), 5′-TCCGAGTTCGAGGACTTTTG-3′, 5′-CATGCATAGGGCTTGAAAGG-3′; Neurogenic differentiation 1 (NM_010894), 5′-AGCCACGGATCAATCTTCTCT-3′, 5′-GACGTGCCTCTAATCGTGAAA-3′; Pancreatic and duodenal homeobox 1 (NM_008814), 5′-AACCCGAGGAAAACAAGAGG-3′, 5′-TTCAACATCACTGCCAGCTC-3′; Forkhead box O1 (NM_019739), 5′-CCGGAGTTTAACCAGTCCAA-3′, 5′-TGCTCATAAAGTCGGTGCTG-3′; Forkhead box a1 (NM_008259), 5′-CAAGGATGCCTCTCCACACTT-3′, 5′-TGACCATGATGGCTCTCTGAA-3′; Forkhead box a2 (NM_010446), 5′-GAGCACCATTACGCCTTCAAC-3′, 5′-GGCCTTGAGGTCCATTTTGT-3′; PDGB (NM_013551), 5′-TGCACGATCCTGAAACTCTG-3′, 5′-TGCATGCTATCTGAGCCATC-3′.
Immunofluorescence
Immunofluorescence staining was performed as previously described[21]. The following antibodies were used at the indicated dilutions: FITC-conjugated anti-mouse IgG (1:200, Vector, Burlingame, CA, United states), FITC-conjugated anti-rabbit IgG (1:200, Vector), AlexaFluor 568 donkey anti-goat (1:400, Invitrogen, Grand Island, NY, United states), AlexaFluor 488 donkey anti-goat (1:400, Invitrogen), AlexaFluor 488 donkey anti-rabbit (1:400, Invitrogen), rabbit anti-SP-1 CgA (1:1000, ImmunoStar, Hudson, WI, United states), goat anti-CgA (1:50, SantaCruz, Santa Cruz, CA, United states), rabbit anti-gastrin (1:200, Chemicon, Billerica, MA, United states), goat anti-GIP (1:100, SantaCruz), mouse anti-serotonin (1:200, LabVision, Kalamazoo, MI, United states), rabbit anti-secretin (1:1000, Phoenix pharmaceuticals, Burlingame, CA, United states), goat anti-SST (1:100, SantaCruz), goat anti-ghrelin (1:100, SantaCruz), rabbit anti-CCK (1:100, ab92128 gift from Rehfeld JF)[22].
Measurement of circulating hormone levels
Blood was collected on 16 h fasted mice by cardiac puncture. Serum levels of total ghrelin and GIP were measured using Millipore ELISA kits (EZRGRT-91K, EZRMGIP-55K) (Millipore, Billerica, MA, United states) according to manufacturer’s instructions. Serum levels of SST were measured using the Phoenix Pharmaceuticals ELISA kit EK-060-03, according to the manufacturer’s instructions.
Statistical analysis
All cell count analyses were performed using continuous serial sections from low-powered fields of well-oriented intestinal cross-sections in a blind manner on an average of 10 independent fields per animal. Three different intestinal sections were evaluated: duodenum, jejunum and ileum. The total number of enteroendocrine cells was counted per crypt-villus axis. Image magnification was calibrated by comparison with a stage micrometer (graticulesTM Ltd., Tonbridge, Kent, England). Statistical analyses were performed using two-way ANOVA. For qRT-PCR, data were analyzed using the Mann Whitney-test for abnormal distribution. Differences were considered significant with a P value of < 0.05. All statistical analyses were carried out using Graph Pad Prism 5 (Graph Pad Inc., San Diego, CA).
RESULTS
CgA is not expressed in all enteroendocrine cell subtypes of the mouse small intestinal epithelium
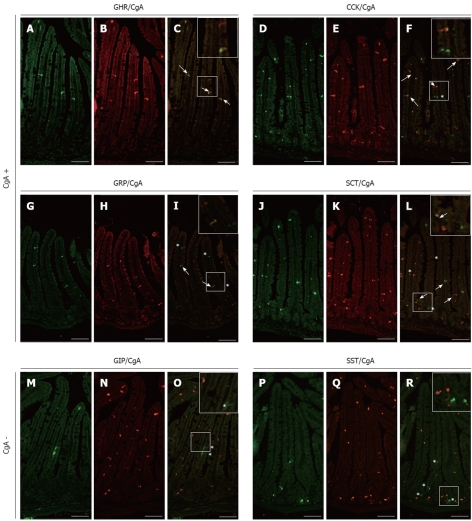
Mice homozygous for the floxed exon 5 of the Pten gene[23] were bred to the villin-Cre transgenic line, which directs expression of the transgene in all epithelial cells of the small intestine and colon, including stem cells, but not in the mesenchymal compartment[20]. Conditional knockout mice for Pten (Pten∆IEC) were born at the expected Mendelian ratios, survived for more than 1 year, and grew normally without obvious gross physical abnormalities[10]. In a previous study with these mice, we reported an overall decrease in the number of enteroendocrine cells using a CgA antibody[10]. Over the years, there has been a lingering controversy where a number of studies showed that all endocrine cell subpopulations express CgA[18,24,25] while others reported that some cell subpopulations do not express CgA[17,26]. Therefore, individual analysis of various intestinal endocrine subpopulations was first performed for their co-expression with CgA in the mouse small intestine. Double-labelling with CgA (Figure 1B, E, H and K) and specific antibodies directed against ghrelin (Figure 1A), CCK (Figure 1D), gastrin (Figure 1G) and secretin (Figure 1J) confirmed co-expression of CgA with ghrelin (Figure 1C) as well as with CCK- (Figure 1F), gastrin- (Figure 1I) and secretin- (Figure 1L) producing enteroendocrine cells in the mouse small intestine. On the other hand, double-labelling with CgA antibody (Figure 1N and Q) and specific antibodies directed against GIP (Figure 1M) and SST (Figure 1P) supported the exclusion of co-expression between GIP (Figure 1O), SST (Figure 1R) and CgA in the mouse small intestine. The specificity of our CgA antibodies was confirmed with the use of two different CgA antibodies from two different commercial sources, in which the exact same cells were labeled in consecutive sections from a same specimen with both antibodies.
Figure 1.
Analysis of chromogranin A co-expression in mouse small intestinal endocrine subpopulations. Small intestine sections of adult control mice were co-immunostained with antibodies directed against ghrelin (GHR) (A), cholecystokinin (CCK) (D), gastrin-releasing peptide (GRP) (G), secretin (SCT) (J) glucose-dependent insulinotropic peptide (GIP) (M) or somatostatin (SST) (P) and against chromogranin A (CgA) (respectively B, E, H, K, N and Q). The arrows in images C, F, I and L show co-expression of CgA respectively with GHR, CCK, GRP and SCT while asterisks in images F, I, L, O and R point to CgA-negative enteroendocrine cells. The number of arrows and asterisks within the crypt-villus axis represents the average proportion of labelled cells per units. Scale bar: 50 μm.
Loss of intestinal epithelial Pten impairs the specification of CgA expressing enteroendocrine cells
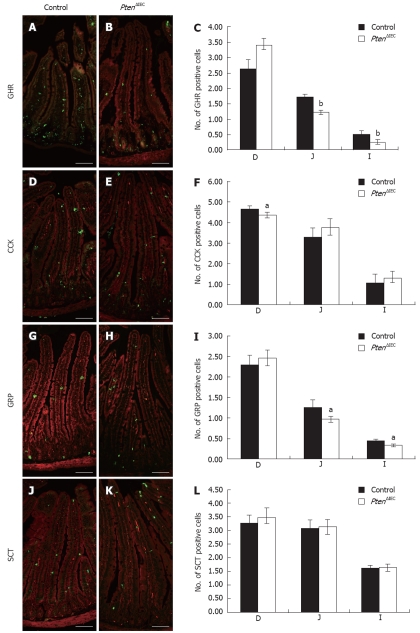
Enteroendocrine subtype specification appears to be regulated by distinct mechanisms[17,26]. Since our previous study only investigated CgA-expressing cells, the impact of Pten loss of expression on the specification of the various enteroendocrine cell subpopulations in the small intestine was further analyzed. We first analyzed how the loss of epithelial Pten alters specification of CgA-expressing enteroendocrine cells along the various sections of the small intestine (duodenum, jejunum and ileum). Although some enteroendocrine cells are restricted to specific regions of the small intestine, each region was analyzed in order to verify the possible delocalization of subpopulations along the rostro-caudal axis of the gut. The intestinal mucosa of Pten∆IEC and control mice was stained with specific markers for each enteroendocrine cell subtype and positive cells were counted (Figure 2). A significant decrease of 29% in the jejunum (1.2 positive cells per crypt-villus axis vs 1.7) and 51% in the ileum (0.25 cell vs 0.52 cell) was observed (Figure 2C) in the ratio of positive ghrelin cells in Pten mutant mice (Figure 2B) compared to control littermates (Figure 2A). A modest but significant decrease of 10% (Figure 2F) was also observed in the ratio of positive CCK cells in the duodenum of the mutant mice (4.4 cell vs 4.7 cell) (Figure 2E). There was also a significant 23% decrease in gastrin-positive cells in the jejunum (1 cell vs 1.3 cell) and a decrease of 29% in the ileum (0.34 cell vs 0.44 cell) in Pten∆IEC (Figure 2H and I) when compared to control mice (Figure 2G and I). Finally, secretin immunostaining showed no modulation in the number of secretin-positive cells in Pten∆IEC (Figure 2K and L) vs control mice (Figure 2J and L). Taken together, these results suggest that Pten positively influences production of CgA-expressing enteroendocrine cell subpopulations.
Figure 2.
Epithelial Pten positively regulates commitment of chromogranin A-positive enteroendocrine subpopulations in the small intestine. Duodenum, jejunum and ileum of adult control and Pten∆IEC mice were immunostained with antibodies against ghrelin (GHR) (A and B), cholecystokinin (CCK) (D and E), gastrin-releasing peptide (GRP) (G and H) and secretin (SCT) (J and K). Positive cells were counted from intestinal sections of controls (n = 6) and mutants (n = 5). Statistical analysis (C, F, I, L) represents the average number of positive cells per crypt-villus axis in each section of the intestine. Error bars represent SE. Scale bar: 50 μm. D: Duodenum; J: Jejunum; I: Ileum. aP < 0.05, bP < 0.001.
Loss of epithelial intestinal Pten positively influences the specification of CgA negative enteroendocrine cells
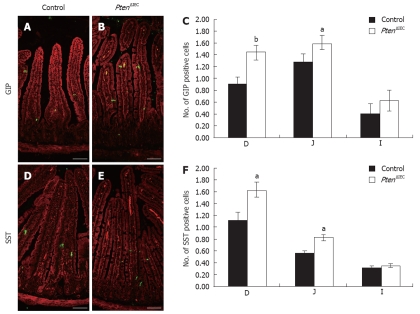
We next examined if the specification of CgA-negative cells was affected following the loss of epithelial Pten. As illustrated in Figure 3, there was a marked 61% (duodenum) and 25% (jejunum) increase in GIP-positive cells in Pten∆IEC (Figure 3B and C) when compared to control mice (Figure 3A and C) (respectively 1.45 positive cells vs 0.9 in the duodenum and 1.6 cells vs 1.28 in the jejunum). SST immunostaining in both duodenum and jejunum revealed an increase of 45% in the number of SST-positive cells in Pten∆IEC (Figure 3E and F) vs control mice (Figure 3D and F) (respectively 1.65 cells vs 1.15 cell and 0.85 cell vs 0.6 cell). Hence, these data suggest that Pten signalling negatively controls specification of CgA-negative cells in the intestinal epithelium.
Figure 3.
Epithelial Pten negatively regulates commitment of chromogranin A-negative enteroendocrine subpopulations in the small intestine. Duodenum, jejunum and ileum of adult control and Pten∆IEC mice were immunostained with antibodies against glucose-dependent insulinotropic peptide (GIP) (A and B) and somatostatin (SST) (D and E). Positive cells were counted from intestinal sections of controls (n = 6) and mutants (n = 5). Statistical analysis (C and F) represents the average number of positive cells per crypt-villus axis in each section of the intestine. Error bars represent SE. Scale bar: 50 μm. D: Duodenum; J: Jejunum; I: Ileum. aP < 0.05, bP < 0.01.
Loss of epithelial Pten signalling leads to deregulation of circulating GIP and SST levels
In light of these observations, we next investigated whether deregulation in the number of enteroendocrine cells in the intestinal epithelium of the Pten∆IEC mice has an impact on their circulating levels. We chose to focus on the enteroendocrine subpopulations where the deregulation was more considerable. Circulating ghrelin, GIP and SST levels were analysed by ELISA assay. A 1.5-fold and 1.3-fold increase in GIP (Figure 4B) and SST (Figure 4C) levels, respectively, were observed in Pten∆IEC mice when compared to control littermates. No significant difference in ghrelin levels was observed between Pten∆IEC mice and control mice (Figure 4A).
Figure 4.
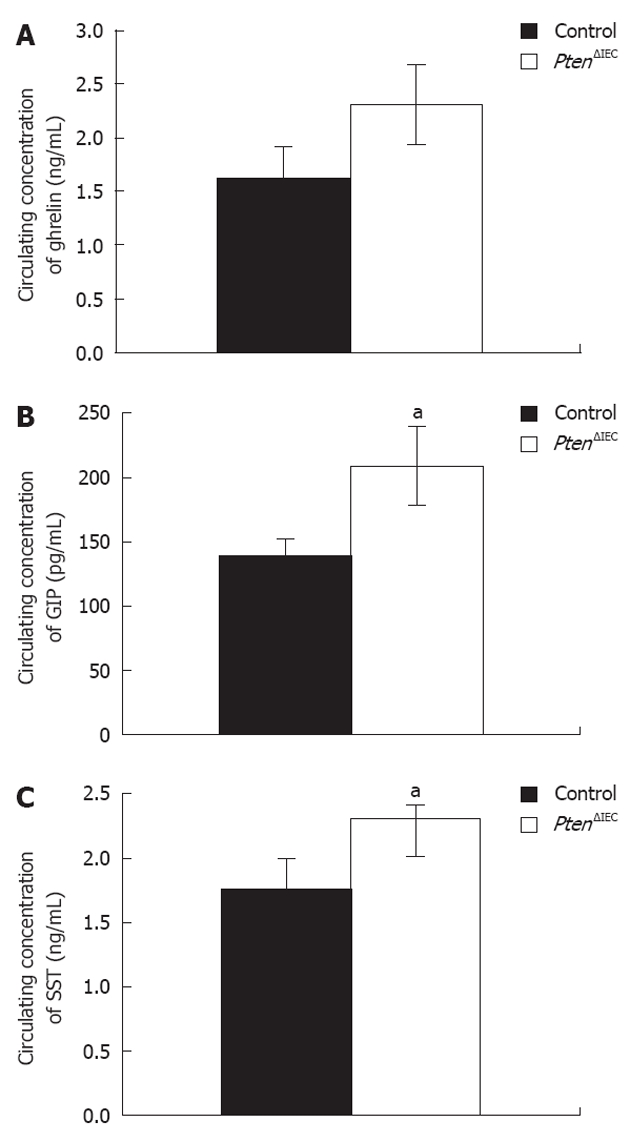
Loss of epithelial Pten signalling modulates circulating levels of glucose-dependent insulinotropic peptide and somatostatin. A: Analysis of circulating ghrelin level revealed no significant modulation between adult Pten∆IEC mice (n = 10) and control littermates (n = 10); B: Analysis of circulating glucose-dependent insulinotropic peptide (GIP) level revealed a 1.5-fold increase in adult Pten∆IEC mice (n = 10) when compared to control littermates (n = 10); C: Analysis of circulating somatostatin (SST) level revealed a 1.3-fold increase in adult Pten∆IEC mice (n = 10) when compared to control littermates (n = 10). Error bars represent SE. aP < 0.05.
Pten expression impacts differently on various pro-enteroendocrine specification factors
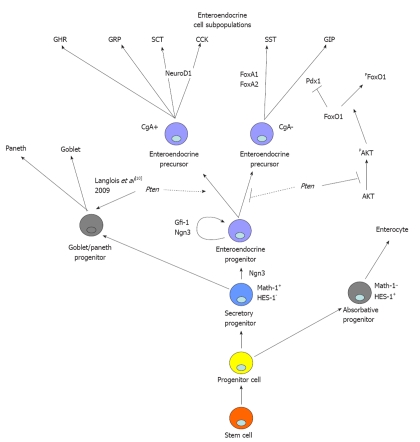
Comparative analysis of secretory lineage and specific pro-enteroendocrine determination factors was next investigated by quantitative PCR to clarify the role of Pten during enteroendocrine subtype specification. The Notch pathway, and more specifically the transcription factors Hairy enhancer of Split (Hes-1) and Math1, is crucial in the determination of the intestinal progenitor cell to absorptive or secretory cell fate (Figure 5)[27,28]. We also investigated if loss of epithelial Pten could deregulate the production of secretory precursors. Quantitative PCR analysis of mutant vs wild-type littermates revealed no modulation of Math1 or Hes-1 mRNA levels in the mutant animals (Table 1). Modifications downstream of the Notch pathway during enteroendocrine cell determination were also subsequently assessed. The proendocrine bHLH transcription factor Ngn3 has been shown to contribute to the maintenance and specification of enteroendocrine precursors (Figure 5)[29]. Our analysis revealed that the Ngn3 mRNA expression was significantly reduced by 2.07-fold in the mutant animals (Table 1). BETA2/NeuroD1, Pancreatic and duodenal homeobox 1 gene (Pdx1), the winged helix Foxa1 and the forkhead box-containing (FoxO1) transcription factors have also been shown to control the determination of specific enteroendocrine subpopulations[16,30-33]. The gene transcript level of BETA2/NeuroD1, linked to specification of secretin and CCK producing cells[34], was reduced by 1.40-fold in mutant animals (Table 1). Pdx1, which regulates serotonin and GIP producing cells (Figure 5)[31,32], was found to be significantly increased by 2.15-fold at the gene transcript level in mutant mice (Table 1). FoxO1 factors are downstream targets of the PI3K/AKT pathway[35] and affect the subcellular localization of Pdx1 in the pancreas and, hence, its transcriptional activity[36]. FoxO1 gene transcript level was found to be reduced by 1.95-fold in the Pten∆IEC
Figure 5.
Proposed model for mode of action of epithelial Pten signalling in intestinal epithelial determination and specification of enteroendocrine progenitor cell fate. Epithelial Pten signalling is not essential for maintenance or determination of the secretory precursor. Pten represses specification of glucose-dependent insulinotropic peptide (GIP)-expressing cells by maintaining FoxO1 in the nucleus. GHR: Ghrelin; GRP: Gastrin-releasing peptide; SCT: Secretin; CCK: Cholecystokinin; SST: Somatostatin; GIP: Glucose-dependent insulinotropic peptide; CgA: Chromogranin A.
Table 1.
Gene expression changes in the small intestine of Pten∆IEC mice
| Gene description | Gene symbol | Fold | P value |
| Hairy and enhancer of split 1 | Hes1 | -1.08 | NS |
| Atonal homolog 1 | Math 1 | -2.05 | NS |
| Neurogenin 3 | Ngn3 | -2.07 | 0.033 |
| Growth factor independent 1 | Gfi1 | 1.07 | NS |
| Neurogenic differentiation 1 | NeuroD1 | -1.40 | 0.002 |
| Pancreatic and duodenal homeobox 1 | Pdx1 | 2.15 | 0.048 |
| Forkhead box O1 | FoxO1 | -1.95 | 0.017 |
| Forkhead box a1 | Foxa1 | 2.64 | 0.017 |
| Forkhead box a2 | Foxa2 | -1.25 | NS |
Target expression was quantified relatively to PDGB expression. Fold changes represent the ratio of mean expression values (control/mutant). Negative values indicate reduction in Pten∆IEC intestines. NS: Non significant fold change (Mann-Whitney test).
mice (Table 1). Finally, the winged helix transcription factors Foxa1 is essential for the differentiation of SST-, GLP-1- and PYY-expressing endocrine cells (Figure 5)[33]. Accordingly, we found an increase of 2.64-fold in Foxa1 gene transcript expression in Pten∆IEC mice (Table 1).
DISCUSSION
Endocrine cells found scattered in the gastrointestinal epithelium represent the major endocrine organ of the body[15,16]. The various hormones produced by these endocrine cells control numerous physiological functions[16,18,19]. Recently, by using conditional tissue-specific disruption of Pten in the epithelium of the gut, we revealed a key role for epithelial Pten in intestinal morphogenesis, in the maintenance of crypt-villus axis architecture, in cell proliferation and in secretory cell commitment[10]. We had also reported an overall decrease in the number of enteroendocrine cells using a CgA antibody. However, the choice of CgA as a pan marker for all enteroendocrine cells has been challenged. Commonly used as a biomarker for endocrine granules, CgA plays a role in the biogenesis of mobile secretory granules and the release of hormones through the regulated secretory pathway[37]. Over the years, there has been a lingering controversy in which some studies showed that all endocrine cell subpopulations express CgA[18,24,25] while others reported that enteroendocrine cell subpopulations producing GIP, GLP-1 or SST do not express CgA[17,26]. Fixation artefacts and different CgA antibodies may account for this controversy. Also, it has been demonstrated that CgA expression in enteroendocrine subpopulations varies from one species to another as well as in pathologies such as colorectal cancer and inflammatory bowel diseases[38-42]. Herein, our analysis of the various intestinal endocrine subpopulations with CgA antibodies confirmed the absence of co-expression between GIP and SST with CgA. Therefore, the important role of enteroendocrine cells in whole body homeostasis prompted us to further analyze the effect of intestinal epithelial deletion of Pten on the specification of the various enteroendocrine subpopulations. Since all enteroendocrine subtype cells are still detectable in the mutant mice, our results suggest that Pten is not a direct and indispensable regulator of enteroendocrine cell determination. Nevertheless, our data revealed a dual role for Pten signalling in enteroendocrine cell specification. Indeed, our results indicate that Pten signalling facilitates the specification of CgA-expressing enteroendocrine cell subpopulations while it negatively controls specification of CgA-negative cells in the intestinal epithelium. Furthermore, our results showed that the number of GIP and SST cells as well as their associated circulating hormone levels was increased in mutant mice. Although the number of ghrelin cells was decreased, no significant modulation in ghrelin serum level was observed in the Pten∆IEC mice. This may be explained by the fact that ghrelin endocrine cells found in the stomach epithelium are strong contributors for total circulating ghrelin levels[43,44], and are not likely affected by the loss of epithelial Pten in the intestine. Nevertheless, the lack of modulation in circulating levels of ghrelin does not imply that the reduction observed in the cell number in the intestine has no local consequences in this tissue. Indeed, such a reduction could influence specific physiological intestinal functions, such as motility, digestion and epithelial renewal[16,18,19]. Finally, analyses of each enteroendocrine cell subtypes along the rostro-caudal axis of the small intestine confirmed that the loss of Pten does not influence normal distribution of these endocrine cell subpopulations.
Our data also indicate that Pten affects the expression of key regulators for cell lineages and/or proenteroendocrine determination. Since the Notch/Hes-1 path is required for the specification of progenitor cells into the absorptive lineage and since Math1 is required for specification into the secretory lineage[27,28], we therefore analyzed whether the loss of epithelial Pten could alter their expression. Lack of modulation in Math1 and Hes-1 gene transcripts suggest that Pten is not involved in the initial decision steps for lineage determination. Once the initial decision is made between secretory and absorptive cell lineages, the fate of enteroendocrine progenitor cells is defined by proendocrine bHLH transcription factors such as Ngn3 and BETA2/NeuroD1. Ngn3 acts downstream of Math1[27,29] and has been shown to contribute to the maintenance of the enteroendocrine precursors and to the differentiation of all enteroendocrine subpopulations in mice[29,45,46]. Unlike Ngn3, expression of BETA2/NeuroD1 is restricted to a subset of enteroendocrine cells[34]. BETA2/NeuroD1 controls terminal differentiation of secretin and CCK producing cells in the intestine as revealed by the absence of these subpopulations in BETA2/NeuroD1 null mice[34]. In addition, BETA2/NeuroD1 acts downstream of Ngn3[45]. Our analysis revealed that the expression of both bHLH transcription factors was reduced in absence of epithelial Pten, thereby impacting on the production of specific enteroendocrine subpopulations (Figure 5). Over the years, other factors have been shown to be important in the differentiation/specification of several enteroendocrine cell subpopulations[16,30-33]. Such is the case for the winged helix transcription factor Foxa1, previously shown to be essential for the differentiation of SST, GLP-1 and PYY expressing cells[33]. Foxa1 expression was found to be increased in Pten∆IEC mice, hence correlating with the increased production of SST-expressing cells in these mice (Figure 5). The same logic can be applied to Pdx-1. Indeed, studies from Pdx1-null mice revealed an increase in the number of serotonin cells and a decrease in the GIP-expressing cell population[31,32]. Herein, Pdx-1 gene transcript was found to be significantly increased in absence of epithelial Pten, thereby matching the deregulation seen in GIP cell specification (Figure 5). In addition, FoxO1 gene transcript was found to be significantly reduced in the absence of epithelial Pten. FoxO1 competes with FoxA2 for binding to the Pdx1 promoter, resulting in inhibition of Pdx1 transcription[36] (Figure 5). Aside from these observations, one could speculate that phosphorylation of FoxO1 affects its subcellular localisation leading to its exclusion from the nucleus. This nuclear/cytoplasm shuttling phosphorylation of FoxO1 ultimately decreases its transactivation potential[36,47]. Furthermore, PI3K/Akt is a major upstream signalling pathway leading to the phosphorylation of FoxO1 and its exclusion from the nucleus[35]. In a previous study with Pten∆IEC mice, we reported that loss of Pten resulted in increased phosphorylation levels of Akt[10]. Thus, it is tempting to extrapolate that following the loss of intestinal epithelial Pten and activation of Akt, targeted FoxO1 protein would become more phosphorylated and exported to the cytoplasm allowing expression of Pdx1 and specification of GIP-expressing cells.
In summary, our results reveal a distinctive role for Pten in specification/differentiation of enteroendocrine cell subpopulations. Pten signalling negatively regulates the enteroendocrine subtype specification of non-expressing CgA cells such as GIP and SST expressing cells. In contrast, Pten signalling positively affects CgA-expressing cells such as ghrelin, gastrin and CCK cells. Many of these enteroendocrine cell subtypes are known to play critical roles in whole body physiological functions. Incretin hormones such as GLP-1 and GIP have been shown to potentiate glucose-stimulated insulin secretion[48], while double-mutant mice for GIP and GLP-1 exhibit glucose intolerance[49]. Likewise, the importance of enteroendocrine cells in lipid absorption has recently been shown with the generation of intestinal-conditional Ngn3 null mice[46]. A study with Gip-receptor null mice revealed a crucial role for GIP in promoting the efficient storage of ingested fat suggesting that inhibition of the GIP signal could represent a therapeutic approach against obesity[50]. Further analysis will be needed to better evaluate the impact and possible networking of small intestinal endocrine cell deregulation following the loss of Pten signalling on overall metabolism in the mouse.
ACKNOWLEDGMENTS
The authors thank Garand MP and Lamarre S for help with statistical analysis, Morisset JA for the use of reagents and Dr. Gumucio DL for providing the 12.4kbVilCre transgenic line used in the study.
COMMENTS
Background
The phosphatase and tensin homolog (PTEN) tumour suppressor gene is a lipid and protein phosphatase frequently mutated/deleted in various human cancers. Its best-known substrate, the phosphatidylinositol 3,4,5-trisphosphate, is a lipid second messenger mainly produced by class IA phosphatidylinositol 3-kinases (PI3Ks). PI3Ks have been implicated in many signalling pathways that regulate cell survival, growth, proliferation, migration, phagocytosis, and metabolism. In previous study, authors reported that Pten is important for intestinal homeostasis as well as in the commitment of enteroendocrine cells. The important role of enteroendocrine cells in whole body homeostasis prompted people to further analyze the effect of intestinal epithelial deletion of Pten on the specification of the various enteroendocrine subpopulations.
Research frontiers
Enteroendocrine cells located in the gut epithelium are the largest and least understood population of hormone-producing cells in the body. The various hormones and peptides produced by these endocrine cells control important physiological functions, such as gastrointestinal motility, glycaemia, exocrine pancreatic secretion, biliary secretion, digestion, gut epithelial renewal and appetite. In recent years, studies have placed the regulation of these gut hormones as potential targets for novel treatments of metabolic diseases such as type 2 diabetes and obesity.
Innovations and breakthroughs
In the current study, the authors report a distinctive role for Pten in specification/differentiation of enteroendocrine cell subpopulations. Pten signalling negatively regulates the enteroendocrine subtype specification of non-expressing chromogranin A (CgA) cells such as glucose-dependent insulinotropic peptide and somatostatin expressing cells. In contrast, Pten signalling affects positively CgA-expressing cells such as ghrelin, gastrin and cholecystokinin cells.
Applications
Many of these enteroendocrine cell subtypes are known to play critical roles in whole body homeostasis. These experimental data can be used in further studies to better evaluate the impact on general metabolism and possible networking of small intestinal endocrine cell deregulation following the loss of Pten signalling.
Peer review
This is a high quality descriptive study in which authors analyze the impact of the PTEN intestinal knockdown in the specification of intestinal enteroendocrine subpopulations.
Footnotes
Supported by The Canadian Institutes of Health Research team grant, CTP-82942 to Carrier JC, Boudreau F, Rivard N, Perreault N; Carrier JC, Boudreau F and Perreault N are scholars from the Fonds de la Recherche en Santé du Québec; Rivard N is a recipient of a Canadian Research Chair in Signaling and Digestive Physiopathology; Rivard N, Perreault N, Carrier JC and Boudreau F are members of the FRSQ-funded “Centre de Recherche Clinique Étienne Lebel”
Peer reviewer: Silvana Zanlungo, Professor, Department of Gastroenterology, Pontificia Universidad Católica de Chile, Marcoleta 367, Santiago 114-D, Chile
S- Editor Gou SX L- Editor A E- Editor Zheng XM
References
- 1.Chow LM, Baker SJ. PTEN function in normal and neoplastic growth. Cancer Lett. 2006;241:184–196. doi: 10.1016/j.canlet.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 2.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 3.Yoo LI, Liu DW, Le Vu S, Bronson RT, Wu H, Yuan J. Pten deficiency activates distinct downstream signaling pathways in a tissue-specific manner. Cancer Res. 2006;66:1929–1939. doi: 10.1158/0008-5472.CAN-05-1986. [DOI] [PubMed] [Google Scholar]
- 4.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L, Mansukhani M, Murty VV, Gaciong Z, Meek SE, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7:193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 7.Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, Geschwind DH, Liu X, Kornblum HI, Wu H. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci USA. 2006;103:111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo W, Lasky JL, Chang CJ, Mosessian S, Lewis X, Xiao Y, Yeh JE, Chen JY, Iruela-Arispe ML, Varella-Garcia M, et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature. 2008;453:529–533. doi: 10.1038/nature06933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, et al. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell. 2003;3:117–130. doi: 10.1016/s1535-6108(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 10.Langlois MJ, Roy SA, Auclair BA, Jones C, Boudreau F, Carrier JC, Rivard N, Perreault N. Epithelial phosphatase and tensin homolog regulates intestinal architecture and secretory cell commitment and acts as a modifier gene in neoplasia. FASEB J. 2009;23:1835–1844. doi: 10.1096/fj.08-123125. [DOI] [PubMed] [Google Scholar]
- 11.White ES, Atrasz RG, Hu B, Phan SH, Stambolic V, Mak TW, Hogaboam CM, Flaherty KR, Martinez FJ, Kontos CD, et al. Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10) Am J Respir Crit Care Med. 2006;173:112–121. doi: 10.1164/rccm.200507-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 14.Knobbe CB, Lapin V, Suzuki A, Mak TW. The roles of PTEN in development, physiology and tumorigenesis in mouse models: a tissue-by-tissue survey. Oncogene. 2008;27:5398–5415. doi: 10.1038/onc.2008.238. [DOI] [PubMed] [Google Scholar]
- 15.Lee CS, Kaestner KH. Clinical endocrinology and metabolism. Development of gut endocrine cells. Best Pract Res Clin Endocrinol Metab. 2004;18:453–462. doi: 10.1016/j.beem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 16.May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. 2010;323:70–75. doi: 10.1016/j.mce.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Liu J, Li L, Wice BM. Individual subtypes of enteroendocrine cells in the mouse small intestine exhibit unique patterns of inositol 1,4,5-trisphosphate receptor expression. J Histochem Cytochem. 2004;52:53–63. doi: 10.1177/002215540405200106. [DOI] [PubMed] [Google Scholar]
- 18.Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann N Y Acad Sci. 2004;1014:1–12. doi: 10.1196/annals.1294.001. [DOI] [PubMed] [Google Scholar]
- 19.Skipper M, Lewis J. Getting to the guts of enteroendocrine differentiation. Nat Genet. 2000;24:3–4. doi: 10.1038/71653. [DOI] [PubMed] [Google Scholar]
- 20.Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 21.Auclair BA, Benoit YD, Rivard N, Mishina Y, Perreault N. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology. 2007;133:887–896. doi: 10.1053/j.gastro.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 22.Rehfeld JF. Accurate measurement of cholecystokinin in plasma. Clin Chem. 1998;44:991–1001. [PubMed] [Google Scholar]
- 23.Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32:148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 24.Buffa R, Gini A, Pelagi M, Siccardi AG, Bisiani C, Zanini A, Solcia E. Immunoreactivity of hormonally-characterized human endocrine cells against three novel anti-human chromogranin B(B11 and B13) and chromogranin A (A11) monoclonal antibodies. Arch Histol Cytol. 1989;52 Suppl:99–105. doi: 10.1679/aohc.52.suppl_99. [DOI] [PubMed] [Google Scholar]
- 25.Portela-Gomes GM, Stridsberg M, Johansson H, Grimelius L. Complex co-localization of chromogranins and neurohormones in the human gastrointestinal tract. J Histochem Cytochem. 1997;45:815–822. doi: 10.1177/002215549704500606. [DOI] [PubMed] [Google Scholar]
- 26.Wang SY, Chi MM, Li L, Moley KH, Wice BM. Studies with GIP/Ins cells indicate secretion by gut K cells is KATP channel independent. Am J Physiol Endocrinol Metab. 2003;284:E988–E1000. doi: 10.1152/ajpendo.00398.2002. [DOI] [PubMed] [Google Scholar]
- 27.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 28.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 29.Lee CS, Perreault N, Brestelli JE, Kaestner KH. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–1497. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai S, Loomis Z, Pugh-Bernard A, Schrunk J, Doyle MJ, Minic A, McCoy E, Sussel L. Nkx2.2 regulates cell fate choice in the enteroendocrine cell lineages of the intestine. Dev Biol. 2008;313:58–66. doi: 10.1016/j.ydbio.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujitani Y, Fujitani S, Boyer DF, Gannon M, Kawaguchi Y, Ray M, Shiota M, Stein RW, Magnuson MA, Wright CV. Targeted deletion of a cis-regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation. Genes Dev. 2006;20:253–266. doi: 10.1101/gad.1360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsson LI, Madsen OD, Serup P, Jonsson J, Edlund H. Pancreatic-duodenal homeobox 1 -role in gastric endocrine patterning. Mech Dev. 1996;60:175–184. doi: 10.1016/s0925-4773(96)00609-0. [DOI] [PubMed] [Google Scholar]
- 33.Ye DZ, Kaestner KH. Foxa1 and Foxa2 control the differentiation of goblet and enteroendocrine L- and D-cells in mice. Gastroenterology. 2009;137:2052–2062. doi: 10.1053/j.gastro.2009.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997;11:2323–2334. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glauser DA, Schlegel W. The emerging role of FOXO transcription factors in pancreatic beta cells. J Endocrinol. 2007;193:195–207. doi: 10.1677/JOE-06-0191. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH, Wright CV, White MF, Arden KC, Accili D. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montero-Hadjadje M, Elias S, Chevalier L, Benard M, Tanguy Y, Turquier V, Galas L, Yon L, Malagon MM, Driouich A, et al. Chromogranin A promotes peptide hormone sorting to mobile granules in constitutively and regulated secreting cells: role of conserved N- and C-terminal peptides. J Biol Chem. 2009;284:12420–12431. doi: 10.1074/jbc.M805607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cetin Y, Bargsten G, Grube D. Mutual relationships between chromogranins A and B and gastrin in individual gastrin cells. Proc Natl Acad Sci USA. 1992;89:2912–2916. doi: 10.1073/pnas.89.7.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cetin Y, Grube D. Immunoreactivities for chromogranin A and B, and secretogranin II in the guinea pig entero-endocrine system: cellular distributions and intercellular heterogeneities. Cell Tissue Res. 1991;264:231–241. doi: 10.1007/BF00313960. [DOI] [PubMed] [Google Scholar]
- 40.Cetin Y, Müller-Köppel L, Aunis D, Bader MF, Grube D. Chromogranin A (CgA) in the gastro-entero-pancreatic (GEP) endocrine system. II. CgA in mammalian entero-endocrine cells. Histochemistry. 1989;92:265–275. doi: 10.1007/BF00500540. [DOI] [PubMed] [Google Scholar]
- 41.El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–419. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- 42.Mori M, Mimori K, Kamakura T, Adachi Y, Ikeda Y, Sugimachi K. Chromogranin positive cells in colorectal carcinoma and transitional mucosa. J Clin Pathol. 1995;48:754–758. doi: 10.1136/jcp.48.8.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 44.Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, Kojima M, Kangawa K, Arima T, Matsuo H, et al. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51:124–129. doi: 10.2337/diabetes.51.1.124. [DOI] [PubMed] [Google Scholar]
- 45.Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mellitzer G, Beucher A, Lobstein V, Michel P, Robine S, Kedinger M, Gradwohl G. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J Clin Invest. 2010;120:1708–1721. doi: 10.1172/JCI40794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitamura T, Ido Kitamura Y. Role of FoxO Proteins in Pancreatic beta Cells. Endocr J. 2007;54:507–515. doi: 10.1507/endocrj.kr-109. [DOI] [PubMed] [Google Scholar]
- 48.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, Hansotia T, Drucker DJ, Wollheim C, Burcelin R, et al. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest. 2004;113:635–645. doi: 10.1172/JCI20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8:738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]