Abstract
AIM: To investigate the mechanisms of the biological roles of Dickkopf-3 (Dkk-3) in cell invasion, survival and apoptosis in colon cancer cells.
METHODS: Three human colon cancer cell lines, i.e., HT-29, LoVo and SW480, were used. Overexpression of Dkk-3 induced by pEGFP-N1-Dkk-3-GFP plasmid in LoVo cells was performed using Lipofectamine 2000 reagent. Reverse transcription polymerase chain reaction and Western blotting were performed to determine the mRNA and protein expression levels of Dkk-3, respectively. Cell proliferation assay, cell cycle analysis, hoechst 33258 assay and Matrigel invasion assay were performed on Dkk-3 overexpressing transfectants.
RESULTS: The mRNA and protein expressions of Dkk-3 in HT-29 (mRNA: 0.06 ± 0.02, protein: 0.06 ± 0.01) and LoVo (mRNA: 0.07 ± 0.02, protein: 0.07 ± 0.02) cells were significantly lower than that in SW480 cells (mRNA: 0.92 ± 0.04, protein: 0.69 ± 0.13; all P < 0.05), and the greatest levels of invasiveness was in LoVo cells. Dkk-3 overexpression inhibited the proliferation and invasion of LoVo cells and induced cell cycle arrest at G0/G1 phase and subsequent apoptosis, as indicated by increased chromatin condensation and fragments, upregulated Bax and cytochrome c protein, downregulated survivin and Bcl-2 protein, and the activation of caspase-3 and caspase-9. Furthermore, Dkk-3 overexpression reduced the accumulation of cytosolic fraction of β-catenin.
CONCLUSION: Dkk-3 overexpression induced apoptosis in human colon cancer possibly through the mitochondrial pathway. Dkk-3 may be involved in the Wnt/β-catenin signaling pathways in colon cancer.
Keywords: Dickkopf-3, Overexpression, Invasion, Apotosis, Colon cancer, Mitochondria
INTRODUCTION
The prevalence of colorectal cancer is increasing in Asia. Many Asian countries, including China, Japan, South Korea, and Singapore, have experienced a 2-4 folds increase in the incidence of colorectal cancer (CRC) during the past few decades[1]. Even in the United States, colorectal cancer is the third most commonly diagnosed cancer and the second leading cause of cancer deaths among cancers that affect both men and women[2,3]. However, the cellular mechanisms involved in CRC are not fully described. Recent studies have shown that the Wnt signaling pathway, which is composed of canonical Wnt signaling via Wnt/β-catenin and noncanonical Wnt signaling via the Wnt/Ca2+ pathway and Wnt/c-Jun N-terminal kinase (JNK) (planar cell polarity), regulates proliferation, fate specification, polarity and migration of cells[4,5]. The Wnt signaling pathway can be blocked by two functional classes of Wnt antagonists: the secreted frizzled-related proteins (sFRP) and the Dickkopf (Dkk)[6].
Dkk-3, also known as reduced expression in immortalized cells, is a member of a recently identified gene family encoding secreted proteins that control cell fate during embryonic development[7-9]. Deletion at Dkk-3 locus has been found in many cancers, such as lung cancer[10], gastric cancer[11] and ovarian cancer[12]. In acute lymphoblastic leukaemia[13], prostate cancer[14], bladder cancer[15,16] and renal cell carcinoma[16], Dkk-3 expression is reduced or silenced. Interestingly, Dkk-3 is strongly expressed at the base of the crypts in human colon, which is known to contain proliferating epithelial precursor cells[17]. Therefore, Dkk-3 may be an important component of the gastrointestinal proliferative regulatory net work[17].
However, the relationship between Dkk-3 and colon cancer remains unclear. We hypothesized that: (1) Dkk-3 expression may be inhibited epigenetically in colon cancer cells; (2) Dkk-3 may be a tumor suppressor and plays an important role in mitochondria-mediated apoptosis; and (3) Dkk-3 may be involved in the Wnt/β-catenin signaling pathways in colon cancer cells. In the present study, we investigated the mechanisms of the biological roles of Dkk-3 in cell invasion, survival and apoptosis of human colon cancer cells.
MATERIALS AND METHODS
Construction of expressing plasmids
The pEGFP-N1-Dkk-3-GFP plasmid constructed to target Dkk-3 (RefSeq ID: BC007660) was obtained from Genechem Co., Ltd. (Shanghai, China). pEGFP-N1 plasmid (Genechem Co., Ltd.) was cut with XhoI/KpnIand ligated by T4 DNA ligase with gene encoding Dkk-3, making Dkk-3-pEGFP construct. The plasmid construct was confirmed by DNA sequencing.
Cell culture and transfection conditions
The human colon cancer cell lines HT-29, LoVo and SW480 were obtained from the Cell Collection Center of Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (GibcoBRL, Grand Island, NY, United States) supplemented with 10% fetal bovine serum and were maintained in a humidified incubator at 37 °C with a supply of 5% CO2/95% air atmosphere. Transfections were performed using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. After 48 h of transfection, cells were used for cell cycle analysis, hoechst 33258 assay, Matrigel invasion assay, reverse transcription polymerase chain reaction (RT-PCR) analysis and Western blotting analysis. The transient expression of green fluorescent protein (GFP) was detected under a fluorescence microscope (Olympus; Shinjuku-ku, Tokyo, Japan) at an excitation wavelength of 460-490 nm.
RT-PCR
After 48 h of transfection, total cellular RNA was isolated by Trizol (Invitrogen, Carlsbad, CA) and 2 μg of RNA was treated with DNase and used as a template for the reverse transcription reaction following the manufacturer’s instructions (Fermentas, United States). The resultant cDNA was then used in PCRs and analyzed by gel electrophoresis. The following primers were used: Dkk-3 sense 5’-GGGAGACGAAGAAGGCAGAAGG-3’ and Dkk-3 antisense 5’-CCAGGTGATGAGGTCCAGAAGC-3’; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense, 5’-AGGTGAAGGTCGGAGTCAAC-3’, and GAPDH anti-sense, 5’-CGCTCCTGGAAGATGGTGAT-3’. The PCR conditions were as follows: 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 1 min. The final extension was at 72 °C for 5 min. PCR products were analyzed on 1.2% agarose gels containing 0.5 g/mL ethidium bromide and were visualized under ultraviolet light. Band density was analyzed and quantified using Genetools software (Syngene, Cambridge, United Kingdom).
Western blotting analysis
Equal amounts of proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Millipore, Bedford, MA, United States) by wet transfer system (Bio-Rad, Hercules, CA, United States). Membranes were blocked with 10% non-fat dry milk in Tris-buffered saline Tween-20 and incubated first with primary antibodies at 4 °C overnight and then with horseradish peroxidase-conjugated anti-mouse, anti-rabbit or anti-goat secondary antibody for 2 h at room temperature. The following antibodies were used: Dkk-3 (R and D Systems Inc., Minneapolis, MN, United States) 1.5 μg/mL, β-catenin (Abcam, Cambridge, United Kingdom) 1:5000, survivin, Bax, Bcl-2 and Cyt-c (Santa Cruz Biotechnology, Santa Cruz, CA, United States) 1:1000, Caspase-9 (Abcam, United States) 1:1000, Caspase-3 (Abcam, United States) 1:250 and Actin (Santa Cruz Biotechnology, Santa Cruz, CA, United States) 1:2000. Specific proteins were visualized using an enhanced chemiluminescence system (Millipore, Bedford, MA, United States) and then exposed with Kodak X-ray film. Protein band intensities were determined densitometrically using the video-imaging CMIASWIN system (Bio-Rad, Hercules, CA, United States).
Cell proliferation assay
Cell proliferation was determined by the WST-8 tetrazolium salt assay (Cell Counting Kit-8, Beyotime Inst Biotech, China), which quantifies the amount of formazan dye formed when tetrazolium salt is cleaved by cellular mitochondrial dehydrogenase present in viable cells. Cells were seeded in 96-well plates at a density of 2 × 103/well in 0.1 mL of culture medium. Viability of cells 0, 12, 24, 36, 48, 60 and 72 h after transfection was evaluated. Two hours before the end of the specified incubation period, 10 μL WST-8 reagent was added to the cells. At the end of the incubation, cell density was estimated by measuring the absorbance of the colored formazan reaction product at 450 nm using an iMark Microplate Absorbance Reader (Bio-Rad, United States).
Cell cycle analysis
Cell cycle status was determined by measuring cellular DNA content after staining with propidium iodide by flow cytometry. After 48 h transfection, cells were centrifuged, washed twice with ice-cold phosphate buffer saline (PBS), and fixed in 70% ethanol at 4 °C for 24 h. Cells were then centrifuged at 1000 r/min for 5 min, and the supernatant was discarded. The pellets were then washed twice with 4 mL PBS and then stained with 0.5 mL RNase A (2 mg/mL) and 0.5 mL propidium iodide (0.1% in 0.6% Triton-X in PBS) for 30 min in the dark. Samples were then analyzed on a FACSCalibur flow cytometer (Beckman Coulter, Inc. Fullerton, CA).
Hoechst 33258 assay for apoptosis
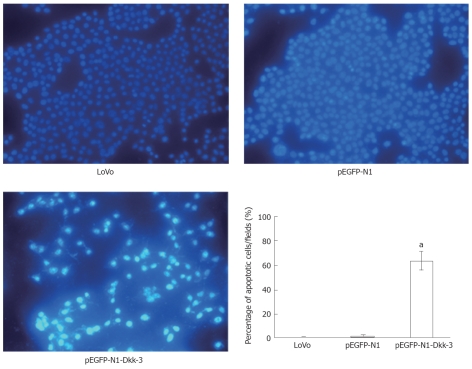
Apoptotic cells were detected by Hoechst 33258 staining following the manufacturer’s protocol (Apoptosis Hoechst staining kit, Beyotime Biotechnology, Jiangsu, China) after 48 h transfection. Briefly, cells were first fixed in 0.5 mL methanol for 30 min and then rinsed with PBS twice; 1 mg/mL Hoechst 33258 reagent was used to stain the apoptotic cells in dark at room temperature for 5 min, after which the cells were again washed with PBS twice. The stained cells were examined and immediately photographed under a fluorescence microscope (Olympus; Shinjuku-ku, Tokyo, Japan) at an excitation wavelength of 330-380 nm. Apoptotic cells were identified on the basis of morphologic changes in their nuclear assembly by observing chromatin condensation and fragment staining by Hoechst 33258. In each group, ten microscopic fields were selected randomly and counted.
Invasion assay
Transwell chambers (Corning, New York, NY, United States) were used to examine the ability of cells to invade through a Matrigel-coated filter following the manufacturer’s instructions. DMEM was added to the upper chambers and allowed to hydrate for 2 h at 37 °C with 5% CO2. Next, 1 × 105 LoVo cells transfected with various plasmids were added to the upper chamber and grown in serum-free medium on 8.0 μm porous polycarbonate membranes, which were coated with diluted Matrigel basement membrane matrix. The lower chambers were filled with DMEM medium containing 10% fetal bovine serum. After 24 h incubation, the cells remaining on the upper surface of the filter were removed using cotton tips, and the cells that migrated to the underside of the membrane were fixed with 4% paraformal dehyde and stained with Giemsa (Sigma). Cells in 10 random fields of view at ×400 magnifications were counted and expressed as the average number of cells/field of view.
Colony formation assay
Cells from the colon cancer cell line LoVo (2 × 105 cells per well) were transfected with 0.5 μg Dkk-3-expressing or empty vector (pEGFP-N1) using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). Transfected cells were selected with antibiotic G-418 Sulfate (0.4 mg/mL) (Merck, Darmstadt, Germany) for 2 wk. Colonies were fixed with methanol/acetone (1:1), stained with Giemsa, and counted. All experiments were performed in triplicate.
Statistical analysis
All continuous values were expressed as mean ± SD. One-way analysis of variance was used for comparisons among groups. Student’s t test was used for comparison of the values between two groups. SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, United States) was used for statistical analysis. Statistical significance was defined as P < 0.05.
RESULTS
Correlation between Dickkopf-3 expression levels and invasion ability in human colon cancer cell lines
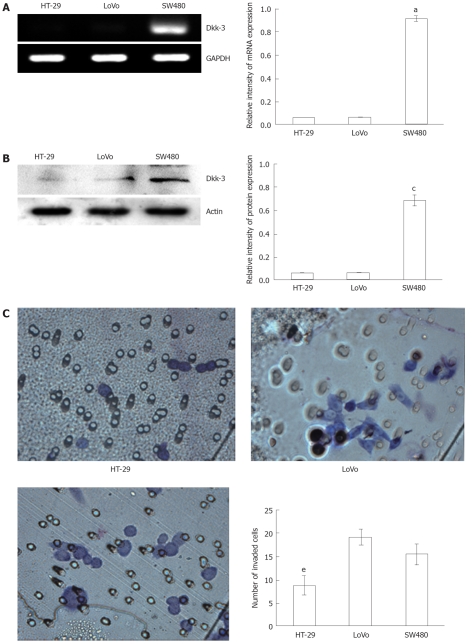
To determine the endogenous expression of Dkk-3, we compared the Dkk-3 level in three human colon cancer cell lines (HT-29, LoVo and SW480). As shown in Figure 1A and B, Dkk-3 expression was significantly higher in SW480 cells (mRNA: 0.92 ± 0.04, protein: 0.69 ± 0.13; all P < 0.05) as compared with HT-29 (mRNA: 0.06 ± 0.02, protein: 0.06 ± 0.01) and LoVo cells (mRNA: 0.07 ± 0.02, protein: 0.07 ± 0.02). We also examined the ability of these cells to invade Matrigel, which is a well-established in vitro model for assessing tumor invasiveness. The result showed that the greatest levels of invasiveness was in LoVo cells (19.25 ± 1.65), which was followed by the SW480 (15.50 ± 2.12) and HT-29 (8.75 ± 2.10, P < 0.05 vs LoVo or SW480), an order consistent with their known metastatic potentials (Figure 1C). These preliminary findings provoked us to track the question of whether modulation of Dkk-3 could affect colon cancer progression.
Figure 1.
Levels of Dickkopf-3 mRNA and protein expression correlate with invasive potential of human colon cancer cell lines. A: Semi-quantitative reverse transcription polymerase chain reaction of RNA extracted from colon cancer cell lines, HT-29, LoVo and SW480, respectively, and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was amplified as a control. aP < 0.05 vs HT-29 or LoVo using Student’s t test; B: Endogenous Dickkopf-3 (Dkk-3) protein expression was examined by immunoblot analysis of total cellular protein isolated from three colon cancer cell lines: HT-29, LoVo and SW480, and actin was utilized as a loading control. cP < 0.05 vs HT-29 or LoVo using Student’s t test; C: Human colon cancer cells, HT-29, LoVo and SW480 invading through the Matrigel were counted under a microscope in ten random fields at × 400 magnification. eP < 0.05 vs LoVo or SW480 using Student’s t test.
Overexpression of Dickkopf-3 by pEGFP-N1-Dkk-3-GFP plasmid in human colon cancer LoVo cells
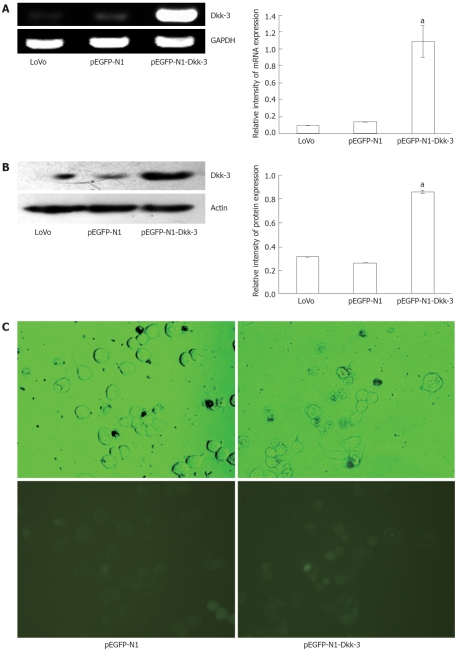
To study the biological role of Dkk-3 in colon cancer progression, we used pEGFP-N1-Dkk-3-GFP plasmid coding for full-length human Dkk-3 to enhance the Dkk-3 gene expression in the human colon cancer LoVo cells. The expression of the recombinant human Dkk-3 was analyzed by RT-PCR and Western blotting analysis. As shown in Figure 2A, analysis of the transfected cells (1.09 ± 0.11, P < 0.05) for Dkk-3 expression via semi-quantitative RT-PCR demonstrated a specific increase in mRNA levels for each gene relative to the pEGFP-N1 plasmid-transfected cells (0.14 ± 0.02) or untreated LoVo cells (0.10 ± 0.02). Immunoblot analysis of cell extracts was carried out to determine whether increased mRNA expression, as observed, correlated with increased translation of the gene product. Figure 2B shows that the protein expression level of Dkk-3 was significantly increased in pEGFP-N1-Dkk-3 group (0.86 ± 0.12, P < 0.05) compared with the pEGFP-N1 group (0.26 ± 0.04) or untreated LoVo cells (0.31 ± 0.04). A similar trend was observed by immunoblot analysis with the result of RT-PCR. The transient expression of GFP was observed under a fluorescence microscope after 48 h transfection (Figure 2C). Figure 2C indicates that the efficient transduction of pEGFP-N1-Dkk-3-GFP plasmid was approximately 70% after 48 h transfection.
Figure 2.
pEGFP-N1-Dkk-3-GFP plasmid induces overexpression of Dickkopf-3 in human colon cancer LoVo cells. A: Semi-quantitative reverse transcription polymerase chain reaction of RNA extracted from pEGFP-N1-Dkk-3-GFP plasmid transfected LoVo cells and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was amplified as a control; B: Immunoblotting of total protein lysates extracted from pEGFP-N1-Dkk-3-GFP plasmid transfected LoVo cells, and actin was included as a loading control; C: Green fluorescent protein (GFP) was also detected under a fluorescence microscope in pEGFP-N1-Dkk-3-GFP plasmid transfected LoVo cells (× 400). aP < 0.05 vs LoVo or pEGFP-N1 using Student’s t test.
Effect of Dickkopf-3 overexpression on invasion in human colon cancer LoVo cells
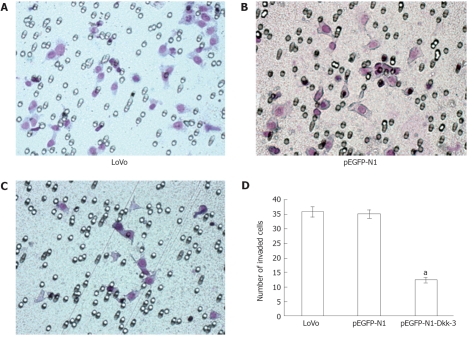
To evaluate the impact of Dkk-3 overexpression on invasion of human colon cancer LoVo cells, a Matrigel invasion assay was performed. When compared with normal LoVo cells (36.00 ± 1.85) or cells transfected with pEGFP-N1 plasmid (36.25 ± 1.49), pEGFP-N1-Dkk-3-GFP plasmid-transfected cells (12.50 ± 0.96, P < 0.05) showed a substantial reduction in invasive ability. Invasion of LoVo cells was reduced to about 70% of the controls by pEGFP-N1-Dkk-3-GFP plasmid (Figure 3). Thus, LoVo cell invasion into Matrigel was substantially regulated by Dkk-3 function. Dkk-3 expression was required for colon cancer cell invasion leading to tumor metastasis.
Figure 3.
Overexpression of Dickkopf-3 inhibits invasion in human colon cancer LoVo cells. Representative number of invading cells through the Matrigel was counted under microscope in ten random fields at × 400 magnification. Each bar represented the mean ± SD. aP < 0.05 vs LoVo or pEGFP-N1 using Student’s t test. The results are representative of three separate experiments.
Effect of Dickkopf-3 overexpression on proliferation in human colon cancer LoVo cells
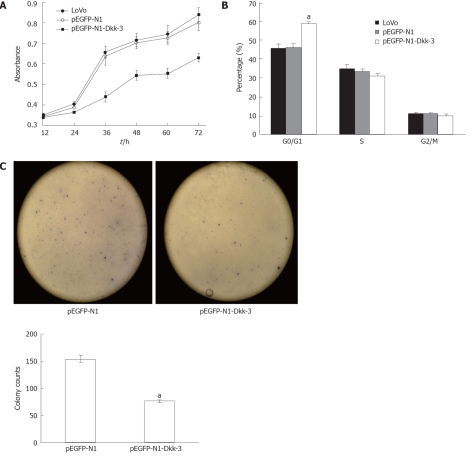
To assess the potential effects of Dkk-3 overexpression on proliferation in human colon cancer LoVo cells, we investigated cell growth in vitro. Using the tetrazolium salt (WST-8) cell viability assay (see “Materials and Methods”), we generated a time-response curve by incubating cultures of transfected LoVo cells for 12, 24, 36, 48, 60 and 72 h, which showed a time-dependent inhibition of cell viability (Figure 4A). pEGFP-N1 transfection had no effect on the proliferative ability of LoVo cells, whereas pEGFP-N1-Dkk-3-GFP plasmid transfection caused a dramatic reduction in the proliferation of LoVo cells (P < 0.05). On the other hand, we performed colony formation assays using LoVo cells transfected with a Dkk-3 gene construct (pEGFP-N1-Dkk-3-GFP) or with an empty vector (pEGFP-N1). The number of colonies formed was counted after 2 wk culture. When compared with the pEGFP-N1 plasmid-transfected cells (154.67 ± 5.86), we observed that Dkk-3 overexpression (77.00 ± 2.65, P < 0.05) decreased markedly the number of colonies (Figure 4C).
Figure 4.
Overexpression of Dickkopf-3 inhibits proliferation and induces G0/G1 arrest in human colon cancer LoVo cells. A: Dickkopf-3 (Dkk-3) inhibits proliferation in human colon cancer LoVo cells; B: Forty-eight hours after transfection, LoVo was used for cell cycle analysis using a FACSCalibur flow cytometer; C: The dickkopf homolog 3 gene Dkk-3 inhibited tumor cell colony formation. LoVo cells were transfected with pEGFP-N1-Dkk-3-GFP plasmid or with pEGFP-N1 and were maintained in the presence of G418 sulfate for 2 wk. Quantitative analysis of colony numbers are shown as the mean ± SD. aP < 0.05 vs pEGFP-N1 using Student’s t test.
Effect of Dickkopf-3 overexpression on cell cycle in human colon cancer LoVo cells
To investigate the precise mechanisms of the decreased cell viability observed in LoVo transient Dkk-3 transfectants, we analyzed the cell cycle distribution profile by flow cytometry with propidium iodide. After 48 h transfection, the cells were fixed and stained with the DNA intercalating fluorescent dye propidium iodide. As shown in Figure 4B, untreated LoVo cells had normal cell cycle profiles with approximately 45% of cells in G0/G1 phase containing 2N DNA content and 12% of cells in G2/M phase containing 4N DNA content. The percentage of cells in the G0/G1 phase of the cell cycle was significantly higher in Dkk-3 transfected LoVo cells (0.59 ± 0.01, P < 0.05).
Effect of Dickkopf-3 overexpression on apoptosis in human colon cancer LoVo cells
The morphological changes of the apoptotic cells were detected by Hoechst 33258 staining (Figure 5). After 48 h transfection, cells were fixed and stained with Hoechst 33258 at room temperature. In the untreated LoVo cells (0.67 ± 0.52) and pEGFP-N1 group (1.33 ± 1.21), the nuclei were stained weak homogeneous blue, while in the group transfected with pEGFP-N1-Dkk-3-GFP plasmid (63.67 ± 7.71, P < 0.05), bright chromatin condensation and nuclear fragmentation were found.
Figure 5.
Detection of apoptosis by Hoechst 33285. The apoptotic feature was assessed by observing chromatin condensation and fragment staining. aP < 0.05 vs control cells (untreated LoVo cells or pEGFP-N1-transfected LoVo cells).
Effect of Dickkopf-3 overexpression on cytoplasmic β-catenin accumulation in human colon cancer LoVo cells
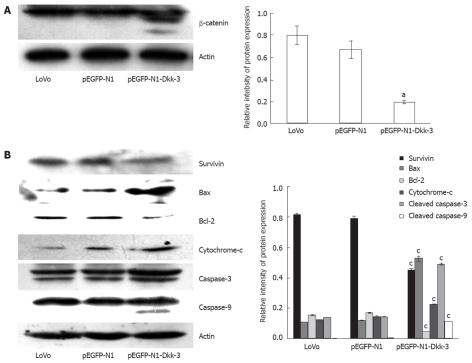
Dkk-3 has been reported to induce changes in β-catenin localization consistent with an increase in cell-cell adhesion[9]. To address this question in colon cancer, we examined the β-catenin expression level in mock and Dkk-3 transfectants. As shown in Figure 6A, transient transfection of Dkk-3 affected Wnt signaling by reducing the accumulation of cytosolic fraction of β-catenin. β-catenin often yields doublets on Western blot analysis, perhaps as a result of being phosphorylated (most often tyrosine or serine phosphorylation)[9].
Figure 6.
Overexpression of Dickkopf-3 inhibits downstream signaling and induces apoptosis in LoVo cells through mitochondrial pathway. A: Dickkopf-3 (Dkk-3) reduces cytoplasmic accumulation of β-catenin; B: Western blotting analysis of survivin, Bcl-2, Bax, cytochrome c, caspase-3 and caspase-9 protein after 48 h transfection with pEGFP-N1-Dkk-3-GFP plasmid in LoVo cells. Actin was used as a loading control. aP < 0.05 vs LoVo or pEGFP-N1 using Student’s t test.
Overexpression of Dickkopf-3 induces apoptosis through activation of mitochondrial pathway in LoVo cells
To further investigate the detailed apoptotic mechanism, we examined the effect of Dkk-3 overexpression on mitochondrial pathway. As shown in Figure 6B, Dkk-3 overexpression caused a decline in survivin levels, a member of the inhibitors of apoptosis proteins family, which is known to block apoptosis by inhibiting caspases and mitochondria-mediated apoptosis[18]. It has been proposed that one of the main regulatory steps of programmed cell death is controlled by the ratio of antiapoptotic and proapoptotic members of the Bcl-2 family of proteins[19,20]. The role of mitochondrial damage in apoptosis was suggested to be mediated by the release of cytochrome c[21]. Upon cleavage by upstream proteases in an intracellular cascade, the activation of caspase-3 is considered as a hallmark of the apoptotic process[22]. Dkk-3 overexpression also induced an increase in Bax protein levels and a decrease in Bcl-2 levels in LoVo cells, which led to a decrease in the antiapoptotic/proapoptotic (Bcl-2/Bax) ratio (Figure 6B). In addition, the expression levels of the cytosolic cytochrome c which was suggested to be involved in mitochondrial damage, the activated caspase-3 and the activated caspase-9 were significantly increased with Dkk-3 overexpression.
DISCUSSION
The Wnt signalling pathway has long been known to direct growth and patterning during embryonic development[23,24]. Recent evidence also implicates that Wnt signalling pathway is involved in the postembryonic regulation of stem-cell number in epithelia, such as those of the skin and intestine, which undergo constant renewal[24]. The pathway is composed of canonical Wnt signaling via Wnt/β-catenin and noncanonical Wnt signaling via the Wnt/Ca2+ pathway and Wnt/c-JNK (planar cell polarity)[4,5]. Wnt signaling pathway is often activated in many cancers and the expression of Wnt antagonists is often downregulated epigenetically[4,7,24-29]. The extracellular antagonists of the Wnt signalling pathway can be divided into two broad classes. Both classes of molecule prevent ligand-receptor interactions, but by different mechanisms: members of the first class, including the sFRP family, Wnt inhibitory factor-1 and Cerberus, primarily binds to Wnt proteins; the second class comprising certain members of the Dkk family, binds to one subunit of the Wnt receptor complex[6].
Human Dkk-related genes are composed of Dkk-1, Dkk-2, Dkk-3, and Dkk-4, together with a unique Dkk-3 related protein termed Soggy (Sgy)[7]. Dkk-3, the most divergent family member, is proposed to function as a secreted tumor suppressor since it is downregulated in a number of cancer cells[28]. Dkk-3 has been reported to be silenced or down-regulated in 12 (70.6%) of 17 gastric cancer cell lines and in 3 (33.3%) of 9 colon cancer cell lines, and the loss of gene expression was associated with promoter methylation, which could be restored by demethylating agents[30]. Tissue microarrays have shown that the number of microvessels in Dkk-3-positive CRC samples was significantly higher than that in Dkk-3-negative samples (P = 0.001), and Dkk-3 expression was also increased with rising numbers of microvessels (P < 0.0001)[29]. In addition, Dkk-3 has been revealed to inhibit cancer proliferation and induce apoptosis in several cancers[25,26,31-36]. Overexpression of Dkk-3 in normal fibroblasts suppresses tumor growth via induction of interleukin-7[32]. In malignant glioma[37], Dkk-3 transfection led to apoptosis due to the activation of phosphorylated jun proto-oncogene, caspase-9, and caspase-3 and the reduction of β-catenin. In renal cell carcinoma[25], prostate cancer[26,31,35], testicular cancer[38], bladder cancer[34] and breast cancer[33], overexpression of Dkk-3 was found to lead to apoptotic cell death in a c-JNK phosphorylation-dependent manner and/or endoplasmic reticulum stress. In osteosarcoma[9], transfection of Dkk-3 and dominant-negative LRP5 significantly lowers the cell invasion capacity and cell motility, and also induces changes in β-catenin localization consistent with an increase in cell-cell adhesion. However, Dkk-3 functional analysis and the regulation mechanism have not been reported in colorectal cancer.
In the present study, we focused on proliferation and apoptosis of colon cancer cells. We examined the anti-proliferation ability of Dkk-3 overexpression by pEGFP-N1-Dkk-3-GFP plasmid in human colon cancer LoVo cells, and measured the extent of cell proliferation by the WST-8 assay (Figure 4A). Interestingly, overexpression of Dkk-3 effectively suppressed cellular proliferation of colon cancer cells in a time-dependent fashion (Figure 4A). On the other hand, overexpression of Dkk-3 inhibited tumor cell colony formation in LoVo cells (Figure 4C).
To investigate the precise mechanisms responsible for the Dkk-3 overexpression-mediated abortive cell divisions, we sought to examine the cell cycle distribution profile of Dkk-3 transfectants. The percentage of cells arrested in G0/G1 phase of the cell cycle was also increased in Dkk-3 transfectants. It was also revealed that Dkk-3 overexpression resulted in apoptosis (Hoechst 33258) in Dkk-3 transfectants (Figure 5). The morphological features in LoVo cells of apoptotic vs necrotic cell death can be distinguished under microscopy[39]. Apoptotic LoVo cells were identified by observing chromatin condensation and fragment staining by Hoechst 33258. It suggests that if early apoptotic cells are not ingested by phagocytes in time, secondary necrosis would proceed[40].
One of the main regulatory steps of programmed cell death is controlled by the ratio of antiapoptotic and proapoptotic members of the Bcl-2 family of proteins[19,20]. Overexpression of antiapoptotic Bcl-2 family members can tip the delicate balance in favor of survival, thereby conferring drug resistance, at least in some cellular tumor model systems[41-43]. On the other hand, overexpression of proapoptotic Bax or Bak is sufficient to increase the sensitivity of malignant cancer cells to apoptosis and to overcome drug resistance[43-45]. Bcl-2 in the unphosphorylated form complexes with Bax, and thus its phosphorylation releases Bax from the Bcl-2-Bax complex[22,46-48]. Unbound Bax translocates from cytosol to the mitochondrial membrane to signal triggering of the downstream apoptotic cascade, such as release of cytochrome c and activation of executionery caspases[22,44-46]. The activation of caspase-3, upon its cleavage by upstream proteases, is considered as a hallmark of the apoptotic process[41]. In agreement with the hypothesis, activated caspase-3 and -9 were detected in Dkk-3 transfectants. Our results showed that overexpression of Dkk-3 decreased Bcl-2/Bax ratio, caused the release of cytochrome c, and the activation of caspase-3 and caspase-9 in LoVo cells (Figure 6B). Further studies are required to determine the exact mechanism whether Dkk-3 enhances apoptosis-inducing effects on human colon cancer cells, which is cross-talking activation of death receptors pathway of apoptosis.
In conclusion, Dkk-3 is anti-proliferative and proapoptotic in colon cancer LoVo cells. Overexpression of Dkk-3 caused 2N DNA accumulation in LoVo cells, suggesting that the Dkk-3 transfectants arrest in G0/G1 phase preceding cell death. These abnormal cells probably trigger activation of programmed cell death that is mitochondrially-driven and executed through the activated caspase by the cleavage of downstream targets. The LoVo cell death program is also mediated through downregulation of survivin. Therefore, Dkk-3 functions as a tumor suppressor in colon cancer cells and its downregulation may be involved in colon cancer progression. Moreover, Dkk-3 may be involved in the Wnt/β-catenin signaling pathways in colon cancer cells.
COMMENTS
Background
The Wnt signal transduction pathway is activated in many cancers and the expression of Wnt antagonists are often downregulated epigenetically. Wnt antagonists can be divided into two functional classes, the secreted frizzled related proteins and the Dickkopf (Dkk). The Dkk gene family of secretory modulators of canonical Wnt/β-catenin signaling is involved in the control of proliferation, polarity and migration, cell fate specification and differentiation. Dkk-3, also known as reduced expression in immortalized cells, is the most divergent family member and proposed to function as a secreted tumor suppressor since it is downregulated in a number of cancer cells.
Research frontiers
Recently, Wnt antagonists have received increasing and specific attention due to their potential role in carcinogenesis. Dkk-3 has been revealed to inhibit cancer proliferation and induce apoptosis in malignant glioma, breast cancer, osteosarcoma, renal cell carcinoma, prostate cancer, testicular cancer and bladder cancer.
Innovations and breakthroughs
Few studies have described the correlation between Wnt antagonists and the development of colon cancer. The results of this study suggest that Dkk-3 may act as negative regulators of Wnts and may be involved in the Wnt/β-catenin signaling pathways in colon cancer cells. Dkk-3 may be a crucial Wnt signaling regulator in colon cancer and an important component of the gastrointestinal proliferative regulatory net work.
Applications
In this study, the mRNA and protein expressions of Dkk-3 were investigated in colon cancer cells and that the aberrant expression of Wnt antagonists may play an important role in carcinogenesis of colon cancer. This finding may help improve early diagnosis and new therapies by blocking this pathway in the treatment of colon cancer.
Terminology
The Wnt signaling pathway is one of evolutionarily-conserved signal transduction pathways to direct growth and patterning during embryonic development, from Hydra to humans. Wnt signals regulate many aspects of development which include the proliferation, fate specification, polarity, and migration of cells. Moreover, overactivation of Wnt signaling by mutation is an important factor in oncogenesis in the human colon and other tissues. The pathway is composed of canonical Wnt signaling via Wnt/β-catenin and noncanonical Wnt signaling or pathways that are β-catenin independent.
Peer review
The authors investigated the mechanisms of the biological roles of Dkk-3 in colon cancer. It revealed that Dkk-3 played an important role in mitochondria-mediated apoptosis and Dkk-3 may be involved in the Wnt/β-catenin signaling pathways in colon cancer cells. The article is a good attempt to work on the hypothesis and the results may represent a molecular mechanism of colon carcinogenesis.
Footnotes
Supported by The Fundamental Research Funds for the Central Universities of China, No. 20103020101000197
Peer reviewer: Dr. Sanjay Kumar, Department of Pathology, Post Graduate Institute of Medical Sciences, 4/9J, Medical Enclave, PGIMS, Rohtak 124001, India
S- Editor Shi ZF L- Editor Ma JY E- Editor Li JY
References
- 1.Sung JJ, Lau JY, Goh KL, Leung WK. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871–876. doi: 10.1016/S1470-2045(05)70422-8. [DOI] [PubMed] [Google Scholar]
- 2.Espey DK, Wu XC, Swan J, Wiggins C, Jim MA, Ward E, Wingo PA, Howe HL, Ries LA, Miller BA, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–2152. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 3.Rim SH, Seeff L, Ahmed F, King JB, Coughlin SS. Colorectal cancer incidence in the United States, 1999-2004 : an updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1967–1976. doi: 10.1002/cncr.24216. [DOI] [PubMed] [Google Scholar]
- 4.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 5.Cohen ED, Tian Y, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- 6.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 7.Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- 8.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 9.Hoang BH, Kubo T, Healey JH, Yang R, Nathan SS, Kolb EA, Mazza B, Meyers PA, Gorlick R. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-beta-catenin pathway. Cancer Res. 2004;64:2734–2739. doi: 10.1158/0008-5472.can-03-1952. [DOI] [PubMed] [Google Scholar]
- 10.Girard L, Zöchbauer-Müller S, Virmani AK, Gazdar AF, Minna JD. Genome-wide allelotyping of lung cancer identifies new regions of allelic loss, differences between small cell lung cancer and non-small cell lung cancer, and loci clustering. Cancer Res. 2000;60:4894–4906. [PubMed] [Google Scholar]
- 11.Baffa R, Negrini M, Mandes B, Rugge M, Ranzani GN, Hirohashi S, Croce CM. Loss of heterozygosity for chromosome 11 in adenocarcinoma of the stomach. Cancer Res. 1996;56:268–272. [PubMed] [Google Scholar]
- 12.Lu KH, Weitzel JN, Kodali S, Welch WR, Berkowitz RS, Mok SC. A novel 4-cM minimally deleted region on chromosome 11p15.1 associated with high grade nonmucinous epithelial ovarian carcinomas. Cancer Res. 1997;57:387–390. [PubMed] [Google Scholar]
- 13.Roman-Gomez J, Jimenez-Velasco A, Agirre X, Castillejo JA, Navarro G, Barrios M, Andreu EJ, Prosper F, Heiniger A, Torres A. Transcriptional silencing of the Dickkopfs-3 (Dkk-3) gene by CpG hypermethylation in acute lymphoblastic leukaemia. Br J Cancer. 2004;91:707–713. doi: 10.1038/sj.bjc.6602008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodygin D, Epanchintsev A, Menssen A, Diebold J, Hermeking H. Functional epigenomics identifies genes frequently silenced in prostate cancer. Cancer Res. 2005;65:4218–4227. doi: 10.1158/0008-5472.CAN-04-4407. [DOI] [PubMed] [Google Scholar]
- 15.Urakami S, Shiina H, Enokida H, Kawakami T, Kawamoto K, Hirata H, Tanaka Y, Kikuno N, Nakagawa M, Igawa M, et al. Combination analysis of hypermethylated Wnt-antagonist family genes as a novel epigenetic biomarker panel for bladder cancer detection. Clin Cancer Res. 2006;12:2109–2116. doi: 10.1158/1078-0432.CCR-05-2468. [DOI] [PubMed] [Google Scholar]
- 16.Urakami S, Shiina H, Enokida H, Hirata H, Kawamoto K, Kawakami T, Kikuno N, Tanaka Y, Majid S, Nakagawa M, et al. Wnt antagonist family genes as biomarkers for diagnosis, staging, and prognosis of renal cell carcinoma using tumor and serum DNA. Clin Cancer Res. 2006;12:6989–6997. doi: 10.1158/1078-0432.CCR-06-1194. [DOI] [PubMed] [Google Scholar]
- 17.Byun T, Karimi M, Marsh JL, Milovanovic T, Lin F, Holcombe RF. Expression of secreted Wnt antagonists in gastrointestinal tissues: potential role in stem cell homeostasis. J Clin Pathol. 2005;58:515–519. doi: 10.1136/jcp.2004.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 19.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 20.Daniel PT. Dissecting the pathways to death. Leukemia. 2000;14:2035–2044. doi: 10.1038/sj.leu.2401940. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 22.Karna P, Sharp SM, Yates C, Prakash S, Aneja R. EM011 activates a survivin-dependent apoptotic program in human non-small cell lung cancer cells. Mol Cancer. 2009;8:93. doi: 10.1186/1476-4598-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pennisi E. How a growth control path takes a wrong turn to cancer. Science. 1998;281:1438–1439, 1441. doi: 10.1126/science.281.5382.1438. [DOI] [PubMed] [Google Scholar]
- 24.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 25.Ueno K, Hirata H, Majid S, Chen Y, Zaman MS, Tabatabai ZL, Hinoda Y, Dahiya R. Wnt antagonist DICKKOPF-3 (Dkk-3) induces apoptosis in human renal cell carcinoma. Mol Carcinog. 2011;50:449–457. doi: 10.1002/mc.20729. [DOI] [PubMed] [Google Scholar]
- 26.Abarzua F, Sakaguchi M, Takaishi M, Nasu Y, Kurose K, Ebara S, Miyazaki M, Namba M, Kumon H, Huh NH. Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer Res. 2005;65:9617–9622. doi: 10.1158/0008-5472.CAN-05-0829. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi K, Ouchida M, Tsuji T, Hanafusa H, Miyazaki M, Namba M, Shimizu N, Shimizu K. Reduced expression of the REIC/Dkk-3 gene by promoter-hypermethylation in human tumor cells. Gene. 2002;282:151–158. doi: 10.1016/s0378-1119(01)00838-1. [DOI] [PubMed] [Google Scholar]
- 28.Zenzmaier C, Untergasser G, Hermann M, Dirnhofer S, Sampson N, Berger P. Dysregulation of Dkk-3 expression in benign and malignant prostatic tissue. Prostate. 2008;68:540–547. doi: 10.1002/pros.20711. [DOI] [PubMed] [Google Scholar]
- 29.Zitt M, Untergasser G, Amberger A, Moser P, Stadlmann S, Zitt M, Müller HM, Mühlmann G, Perathoner A, Margreiter R, et al. Dickkopf-3 as a new potential marker for neoangiogenesis in colorectal cancer: expression in cancer tissue and adjacent non-cancerous tissue. Dis Markers. 2008;24:101–109. doi: 10.1155/2008/160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu J, Tao Q, Cheng YY, Lee KY, Ng SS, Cheung KF, Tian L, Rha SY, Neumann U, Röcken C, et al. Promoter methylation of the Wnt/beta-catenin signaling antagonist Dkk-3 is associated with poor survival in gastric cancer. Cancer. 2009;115:49–60. doi: 10.1002/cncr.23989. [DOI] [PubMed] [Google Scholar]
- 31.Kashiwakura Y, Ochiai K, Watanabe M, Abarzua F, Sakaguchi M, Takaoka M, Tanimoto R, Nasu Y, Huh NH, Kumon H. Down-regulation of inhibition of differentiation-1 via activation of activating transcription factor 3 and Smad regulates REIC/Dickkopf-3-induced apoptosis. Cancer Res. 2008;68:8333–8341. doi: 10.1158/0008-5472.CAN-08-0080. [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi M, Kataoka K, Abarzua F, Tanimoto R, Watanabe M, Murata H, Than SS, Kurose K, Kashiwakura Y, Ochiai K, et al. Overexpression of REIC/Dkk-3 in normal fibroblasts suppresses tumor growth via induction of interleukin-7. J Biol Chem. 2009;284:14236–14244. doi: 10.1074/jbc.M808002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawasaki K, Watanabe M, Sakaguchi M, Ogasawara Y, Ochiai K, Nasu Y, Doihara H, Kashiwakura Y, Huh NH, Kumon H, et al. REIC/Dkk-3 overexpression downregulates P-glycoprotein in multidrug-resistant MCF7/ADR cells and induces apoptosis in breast cancer. Cancer Gene Ther. 2009;16:65–72. doi: 10.1038/cgt.2008.58. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi T, Sakaguchi M, Tanimoto R, Abarzua F, Takaishi M, Kaku H, Kataoka K, Saika T, Nasu Y, Miyazaki M, et al. Mechanistic analysis of resistance to REIC/Dkk-3-induced apoptosis in human bladder cancer cells. Acta Med Okayama. 2008;62:393–401. doi: 10.18926/AMO/30945. [DOI] [PubMed] [Google Scholar]
- 35.Abarzua F, Kashiwakura Y, Takaoka M, Watanabe M, Ochiai K, Sakaguchi M, Iwawaki T, Tanimoto R, Nasu Y, Huh NH, et al. An N-terminal 78 amino acid truncation of REIC/Dkk-3 effectively induces apoptosis. Biochem Biophys Res Commun. 2008;375:614–618. doi: 10.1016/j.bbrc.2008.08.079. [DOI] [PubMed] [Google Scholar]
- 36.Abarzua F, Sakaguchi M, Tanimoto R, Sonegawa H, Li DW, Edamura K, Kobayashi T, Watanabe M, Kashiwakura Y, Kaku H, et al. Heat shock proteins play a crucial role in tumor-specific apoptosis by REIC/Dkk-3. Int J Mol Med. 2007;20:37–43. [PubMed] [Google Scholar]
- 37.Mizobuchi Y, Matsuzaki K, Kuwayama K, Kitazato K, Mure H, Kageji T, Nagahiro S. REIC/Dkk-3 induces cell death in human malignant glioma. Neuro Oncol. 2008;10:244–253. doi: 10.1215/15228517-2008-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanimoto R, Abarzua F, Sakaguchi M, Takaishi M, Nasu Y, Kumon H, Huh NH. REIC/Dkk-3 as a potential gene therapeutic agent against human testicular cancer. Int J Mol Med. 2007;19:363–368. [PubMed] [Google Scholar]
- 39.Krysko DV, Vanden Berghe T, D’Herde K, Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44:205–221. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Krysko DV, D’Herde K, Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis. 2006;11:1709–1726. doi: 10.1007/s10495-006-9527-8. [DOI] [PubMed] [Google Scholar]
- 41.Lotem J, Sachs L. Regulation by bcl-2, c-myc, and p53 of susceptibility to induction of apoptosis by heat shock and cancer chemotherapy compounds in differentiation-competent and -defective myeloid leukemic cells. Cell Growth Differ. 1993;4:41–47. [PubMed] [Google Scholar]
- 42.Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151–157. [PubMed] [Google Scholar]
- 43.Aneja R, Zhou J, Zhou B, Chandra R, Joshi HC. Treatment of hormone-refractory breast cancer: apoptosis and regression of human tumors implanted in mice. Mol Cancer Ther. 2006;5:2366–2377. doi: 10.1158/1535-7163.MCT-06-0205. [DOI] [PubMed] [Google Scholar]
- 44.Bargou RC, Wagener C, Bommert K, Mapara MY, Daniel PT, Arnold W, Dietel M, Guski H, Feller A, Royer HD, et al. Overexpression of the death-promoting gene bax-alpha which is downregulated in breast cancer restores sensitivity to different apoptotic stimuli and reduces tumor growth in SCID mice. J Clin Invest. 1996;97:2651–2659. doi: 10.1172/JCI118715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radetzki S, Köhne CH, von Haefen C, Gillissen B, Sturm I, Dörken B, Daniel PT. The apoptosis promoting Bcl-2 homologues Bak and Nbk/Bik overcome drug resistance in Mdr-1-negative and Mdr-1-overexpressing breast cancer cell lines. Oncogene. 2002;21:227–238. doi: 10.1038/sj.onc.1205010. [DOI] [PubMed] [Google Scholar]
- 46.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 47.Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997;57:229–233. [PubMed] [Google Scholar]
- 48.Haldar S, Jena N, Croce CM. Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci USA. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]