Abstract
AIM: To investigate the association between hepatitis C infection and type 2 diabetes mellitus.
METHODS: Observational studies assessing the relationship between hepatitis C infection and type 2 diabetes mellitus were identified via electronic and hand searches. Studies published between 1988 to March 2011 were screened, according to the inclusion criteria set for the present analysis. Authors performed separate analyses for the comparisons between hepatitis C virus (HCV) infected and not infected, and HCV infected and hepatitis B virus infected. The included studies were further subgrouped according to the study design. Heterogenity was assessed using I2 statistics. The summary odds ratios with their corresponding 95% CIs were calculated based on a random-effects model. The included studies were subgrouped according to the study design. To assess any factor that could potentially affect the outcome, results were further stratified by age group (proportion of ≥ 40 years), gender (proportion of male gender), body mass index (BMI) (proportion of BMI ≥ 27), and family history of diabetes (i.e., self reported). For stability of results, a sensitivity analysis was conducted including only prospective studies.
RESULTS: Combining the electronic database and hand searches, a total of 35 observational studies (in 31 articles) were identified for the final analysis. Based on random-effects model, 17 studies (n = 286 084) compared hepatitis C-infected patients with those who were uninfected [summary odds ratio (OR): 1.68, 95% CI: 1.15-2.45]. Of these 17 studies, 7 were both a cross-sectional design (41.2%) and cohort design (41.2%), while 3 were case-control studies (17.6%). Nineteen studies (n = 51 156) compared hepatitis C-infected participants with hepatitis B-infected (summary OR: 1.92, 95% CI: 1.41-2.62). Of these 19 studies, 4 (21.1%), 6 (31.6%) and 9 (47.4%) were cross-sectional, cohort and case-control studies, respectively. A sensitivity analysis with 3 prospective studies indicated that hepatitis C-infected patients had a higher risk of developing type 2 diabetes compared with uninfected controls (summary odds ratio: 1.41, 95% CI: 1.17-1.7; I2 = 0%). Among hepatitis C-infected patients, male patients (OR: 1.26, 95% CI: 1.03-1.54) with age over 40 years (summary OR: 7.39, 95% CI: 3.82-9.38) had an increased frequency of type 2 diabetes. Some caution must be taken in the interpretation of these results because there may be unmeasured confounding factors which may introduce bias.
CONCLUSION: The findings support the association between hepatitis C infection and type 2 diabetes mellitus. The direction of association remains to be determined, however. Prospective studies with adequate sample sizes are recommended.
Keywords: Hepatitis C, Type 2 diabetes mellitus, Observational studies, Meta-analysis
INTRODUCTION
Hepatitis C virus (HCV) infections has been identified as one of the leading causes of chronic liver disease with serious sequelae such as end-stage cirrhosis and liver cancer[1]. Moreover, chronic HCV infection has been associated with several extrahepatic complications[2-4]. The suggestion that HCV may be associated with type 2 diabetes mellitus (type 2 DM) was first made by Allison in 1994. Since then, scores of observational studies assessing the association between HCV and type 2 DM have been published. However, these studies have provided inconclusive results, with some studies supporting the excess type 2 DM risk with HCV infection compared to non-HCV infected controls[3,5], and some studies showed differently[6-8]. There are narrative reviews which have assessed the association between HCV infections and type 2 DM[9-13]. In 2008, a meta-analysis of observational studies reported an excess type 2 DM risk with HCV infection[14]. After these reviews were published, new observational studies in which prevalence of type 2 DM in patients with HCV infection was assessed have been carried out in endemic countries. As the epidemiology of HCV is complex and heterogeneous, information from studies across geographic regions is important. Moreover, the current review also assesses the traditional risk factors.
The objectives were (1) to investigate the available evidence on the association between HCV infections and type 2 DM; and (2) to assess the effect of study design and traditional risk factors on the association.
MATERIALS AND METHODS
Data sources and search strategy
Published studies that assess the association between HCV and type 2 DM were searched in MEDLINE, EMBASE and PubMed databases covering the period from 1980 to March 2011. Literature search was carried out using the combination of terms “diabetes”, “diabetes mellitus”, “type II diabetes mellitus”, “type 2 diabetes mellitus”, “type II diabetes”, “T2D”, “T2DM”, “type 2 DM”, “non-insulin dependent diabetes”, or “NIDDM”and “hepatitis”, “hepatitis C”, “hepatitis C virus”, “ HCV”, “HVC”, or “chronic hepatitis” and “risk”, “risk factor”, “case-control”, “cohort”, “clinical trial”, “cross-sectional”, “epidemiology”, “observational”, “meta-analysis”, “systematic review”, or “review”. In addition, we searched Cochrane Database of Systematic Reviews, Cochrane Central Database of Controlled Trials, Database of Abstracts of Reviews of Effects, Google Scholar, European Association for the Study of the Liver, Eurosurveillance (http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=695), and GlaxoSmithKline (http://www.gsk.com/reportsandpublications.htm). We also searched the reference lists of the retrieved articles and reviews of this field[9,10,13,14]. Our search was limited to human studies and English publications. We also contacted the corresponding authors for any missing data or clarification.
Study selection
Inclusion criteria for studies were: (1) An epidemiologic study design to conduct a primary or secondary data analysis; (2) At least 1 comparison group without HCV; (3) Provision of sufficient data to calculate odds ratio (OR) or relative risk (RR) comparing type 2 DM in HCV infected patients to non-HCV infected patients; (4) Controlled for at least age and gender in the study design or analysis; and (5) Conducted with not less than 20 HCV-infected patients. HCV was confirmed with the detection of anti-HCV (tested with ELIZA) or HCV RNA (detected by reverse transcriptase polymerase chain reaction). Type 2 DM was confirmed with one of the following criteria; (1) Self-reported type 2 DM (i.e., physician diagnosed); (2) Self-reported diabetes with no history of insulin medication; (3) If fasting plasma glucose exceeding 7.0 mmol/L (126 mg/dL) on two separate occasions; or (4) Impaired fasting glycaemia was between 6.1 mmol/L and 7.0 mmol/L with no insulin medication. Where available, hepatitis B virus (HBV is confirmed with positive hepatitis B surface antigen and/or detectable serum HBV DNA. Definition of covariates such as family history of diabetes was taken directly from included studies. Studies with patients having other causes of chronic liver disease such as cirrhosis, autoimmune hepatitis, steatohepatitis, primary biliary cirrhosis, primary cholangitis, and hepatocellular carcinoma were excluded. One author (Mak JW) first screened titles and abstracts of publications using eligibility criteria.
Two authors (Naing C, Ahmed SI) independently recorded the detailed information from each primary study using piloted forms that include relevant items: author, year of publication, country, confirmation of type 2 DM, confirmation of HCV, confirmation of HBV (if presented), study design, number of controls and of cases, genotype of HCV (if provided), distribution of age and gender, family history of diabetes. Any discrepancy between these two investigators was resolved by discussion, and by consultation with another author (Maung M).
Statistical analysis
The degree of heterogeneity between studies was assessed using chi-square and I2 test. An I2 value greater than > 50% is considered substantial heterogeneity[15]. We used the assumptions that OR from a case control study approximates the RR in a cohort study. The summary OR with their corresponding 95% CI was calculated based on a random-effects model. We performed separate analysis for the comparisons between (1) HCV infected and not infected and (2) HCV infected and HBV infected. The included studies were subgrouped by the study design. In order to assess any factor that could potentially affect the outcome, results were stratified by age group (proportion of ≥ 40 years), gender (proportion of male gender), body mass index (BMI) (proportion of BMI ≥ 27), and family history of diabetes (i.e. self reported), where there was enough data. We also examined the funnel plots for potential publication bias among the included studies. A sensitivity analysis was conducted including only prospective studies Data entry and analysis was performed using RevMan 5.1[16]. The methods and findings of the present review have been reported based on the preferred reporting items for systematic reviews and meta-analysis checklist (PRISMA) (Table 1)[17].
Table 1.
Preferred reporting items for systematic reviews and meta-analysis reporting
| Section/topic | No. | Checklist item | Reported on page |
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | Title: Meta-analysis |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | Abstract |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | Introduction |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes and study design | Introduction |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | NA |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | Methods: Search strategy and eligibility of relevant studies |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | Methods: Search strategy and eligibility of relevant studies |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Search strategy |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | Methods: Eligibility of relevant studies; PRISMA flowchart provided |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | Methods: Data extraction and outcome measures |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | Methods: Statistical analysis |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | |
PICOS: Participants, interventions, comparisons, outcomes and study design; PRISMA: Preferred reporting items for systematic reviews and meta-analysis; NA: Not available.
RESULTS
Study selection
Figure 1 provides a flowchart of the present review. We retrieved 57 full articles. Of these, 26 publications were excluded because: (1) They were reviews[10-12]; (2) They did not adequately distinguish type 2 DM from diabetes[18,19]; (3) They were conducted in a special population such as transplant patients[20,21], patients with Human immunodeficiency virus[22], with cryoglobulinemia[23], or with thalassemia[24]; (4) They did not include or provide data on patients without HCV infection[25-31]; (5) They were conducted in patients with known chronic liver disease[32-35]; (6) It had less than 20 HCV infected patients[36-38]; and (7) It had duplicate data[39]. The remaining 31 publications of 35 independent studies[5,7,8,40-67] were eligible for inclusion in the present meta-analysis. Four publications[8,55,56,66] assessed both HCV positive vs negative and HCV positive vs HBV positive.
Figure 1.
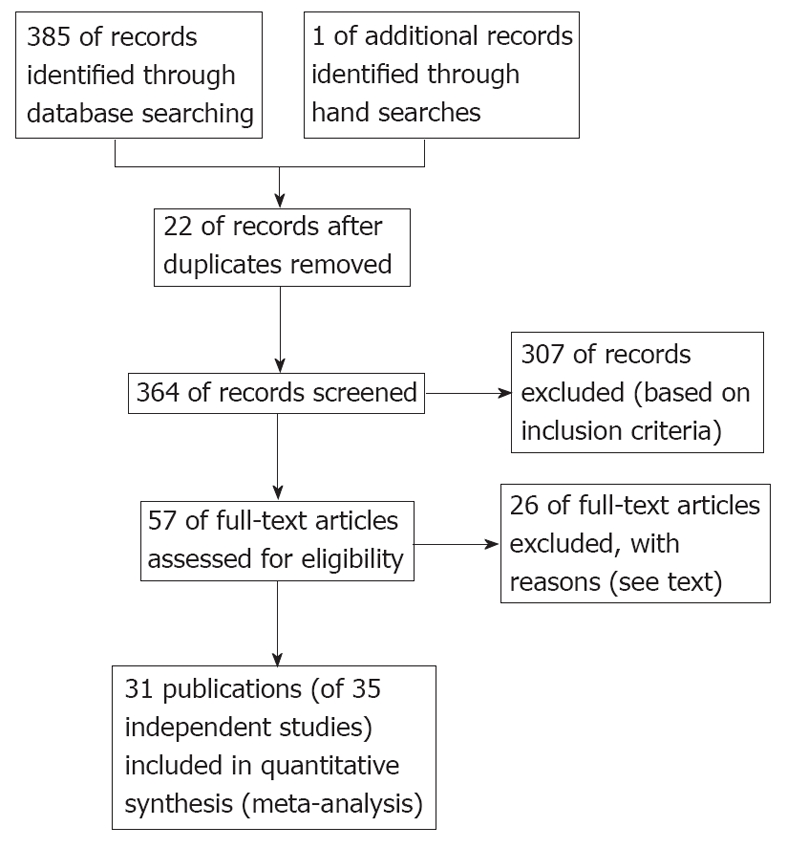
Flowchart of studies identified for the present review.
Study characteristics
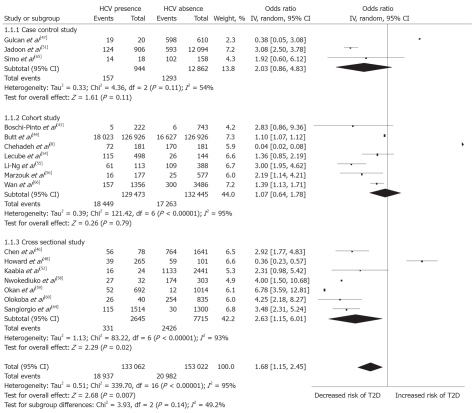
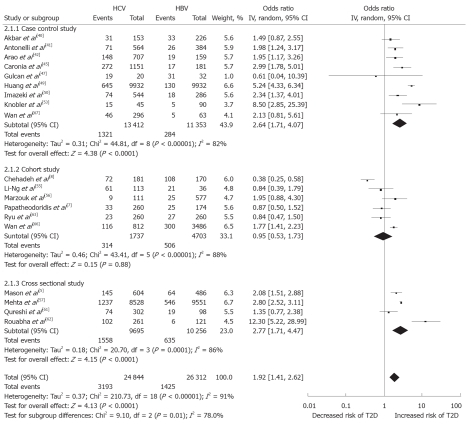
A summary of study characteristics in the present analysis is presented in Table 2. Five studies were carried out in United States[5,44,48,55,57], and three each in Italy[41,45,64], Japan[42,43,50] and Taiwan[46,49,66], among others. Notably, 4 studies[51,60,62,67] identified for the present analysis were published between 2010 and March 2011. Of the included studies, 17 studies (n = 286 084) compared HCV-infected participants with those uninfected; 7 were both a cross-sectional design (41.2%) and cohort design (41.2%), while 3 (17.6%) were case-control studies (Figure 2). Nineteen studies (n = 51 156) compared HCV-infected participants with HBV-infected; 4 (21.1%), 6 (31.58%) and 9 (47.4%) were cross-sectional, cohort and case-control, respectively (Figure 3). The sample size of the included studies widely varied from 135[52] to 126 926 participants[43].
Table 2.
Characteristics of the included studies
| Study | Country | Type of study | Age, yr (mean ± SD) | Confirmation of HCV | Confirmation of T2D |
| Akbar et al[40] | Saudi Arabia | CC | 94% had > 402 | Anti-HCV | FBS |
| Antonelli et al[41] | Italy | CC | 65 ± 10 | Anti-HCV, HCV RNA | FPG |
| Arao et al[42] | Japan | CC | Anti-HCV, HCV RNA | Random, FBG | |
| Boschi-Pinto et al[43] | Japan | Cohort | 65% had > 542 | Anti-HCV | Nil |
| Butt et al[44] | United States | Cohort | 50.82 | ICD-9 | |
| Caronia et al[45] | Italy | CC | 57.5 ± 8 | Anti-HCV | FPG |
| Chehadeh et al[8] | Kuwait | Cohort | 51 (23-73)3 | HCV RNA | FPG |
| Chen et al[46] | Taiwan | CS | Anti-HCV | ||
| Gulcan et al[47] | Turkey | CC | 56.89 ± 11.9 | Anti-HCV, HCV RNA | Guideline5 |
| Howard et al[48] | United States | CS | 51 (37-75)3 | Anti-HCV, HCV RNA | Patient reported, FPG |
| Huang et al[49] | Taiwan | CC | 52.7 ± 0.73 | Anti-HCV, HCV RNA | FPG |
| Imazeki et al[50] | Japan | CC | 45 ± 16.5 | Anti-HCV, HCV RNA | FBS |
| Jadoon et al[51] | Nigeria | Cohort | 48.19 ± 10.32 | Anti-HCV | Clinic diagnosed |
| Kaabia et al[52] | Tunisia | CS | 55.62 | Anti-HCV, HCV RNA | Patient reported |
| Knobler et al[53] | Israel | CC | 54 ± 14 | HCV RNA | FPG |
| Lecube et al[54] | Spain | Cohort | 52.9 ± 14.1 | Anti-HCV, HCV RNA | FPG |
| Li-Ng et al[55] | United States | Cohort | 30-794 | HbsAg1 | ICD-9 |
| Mason et al[5] | United States | CS | 72% had > 37 | Anti-HCV | FPG, Random |
| Marzouk et al[56] | Egypt | Cohort | > 252 | Anti-HCV, HCV RNA | FBS |
| Mehta et al[57] | United States | CS | > 202 | Anti-HCV | FPG |
| Nwokediuko et al[58] | Nigeria | CS | 55.8 ± 11.84 | Anti-HCV | FPG |
| Okan et al[59] | Turkey | CS | 51.92 | Anti-HCV, HCV RNA | Nil |
| Olokoba et al[60] | Nigeria | CS | 51.5 ± 12 | Anti-HCV | FBS |
| Papatheodoridis et al[7] | Greece | Cohort | 48.1 ± 15.3 | Anti-HCV, HCV RNA | FPG |
| Qureshi et al[61] | Pakistan | CS | 42 ± 13 | Anti-HCV | Random |
| Rouabhia et al[62] | Pakistan | CS | 55 ± 9 | Anti-HCV, HCV RNA | FPG |
| Ryu et al[63] | Korea | Cohort | 44 ± 14 | Anti-HCV | FPG |
| Sangiorgio et al[64] | Italy | CS | Anti-HCV | ||
| Simó et al[65] | Spain | CC | 46.4 ± 21.2 | Anti-HCV | WHO |
| Wang et al[66] | Taiwan | Cohort | 50.9 ± 14.2 | Anti-HCV | FPG |
| Wang et al[67] | China | CC | 50.9 ± 14.2 | HCV RNA | FBS |
For HBV infection;
Mean;
Mean and range;
Range only;
American Diabetes Association Guideline. CC: Case-control study; CS: Cross-sectional study; FPG: Fasting plasma glucose; FBS: Fasting blood sugar; IFG: Impaired fasting glycaemic; HCV: Hepatitis C virus; T2D: Type 2 diabetes mellitus; HbsAg: Hepatitis B surface antigen; ICD-9: International Classification of Diseases, Ninth Revision; WHO: World Health Organization.
Figure 2.
Forest plot of comparison: Hepatitis C virus-infected patients vs hepatitis C virus-noninfected patients, outcome is type 2 diabetes mellitus. HCV: Hepatitis C virus; IV: Inverse variance; T2D: Type 2 diabetes mellitus.
Figure 3.
Forest plot of comparison: Hepatitis C virus-infected patients vs hepatitis B virus-infected patients, outcome is type 2 diabetes mellitus. HCV: Hepatitis C virus; HBV: Hepatitis B virus; IV: Inverse variance; T2D: Type 2 diabetes mellitus.
Main results
Of the included studies, 17 studies (n = 286 084) compared HCV-infected participants with those uninfected and the pooled OR was 1.68 (95% CI: 1.15-2.45). There was, however, substantial heterogeneity among studies (I2 = 95%, heterogeneity P < 0.001). Nineteen studies (n = 51 156) compared HCV- infected participants with HBV-infected and the pooled OR was 1.92 (95% CI: 1.41-2.62). There was evidence of considerable heterogeneity among studies (I2 = 91%, heterogeneity P < 0.001). Among HCV-infected patients, based on available data, male patients (summary OR: 1.26, 95% CI: 1.03-1.54) with age over 40 years (summary OR: 7.39, 95% CI: 5.82-9.38) had significantly increased type 2 DM prevalence (Table 3). Funnel plots of the associations between HCV and type 2 DM were investigated, providing little evidence of publication bias (Figure not shown).
Table 3.
Stratified analysis of type 2 diabetes mellitus in hepatitis C virus-infected participants
| Description | Cases | OR | 95% CI |
| Age (k = 3; n = 599) | 455 vs 144 | 7.39 | 5.82-9.38 |
| ≥ 40 yr | |||
| < 40 yr | |||
| BMI (k = 3; n = 190) | 65 vs 190 | 0.87 | 0.08-9.19 |
| ≥ 27 | |||
| < 27 | |||
| Gender (k = 8; n = 757) | 401 vs 356 | 1.26 | 1.03-1.54 |
| Male | |||
| Female | |||
| Family history of diabetes (k = 3; n = 580) | 420 vs 164 | 4.64 | 0.57-38.04 |
| Yes | |||
| No |
OR: Odds ratio; BMI: Body mass index; k: Number of primary studies; n: Number of participants.
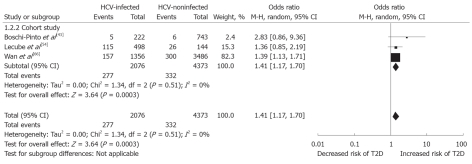
For better stability of the results, sensitivity analysis with three prospective studies (n = 6449)[43,54,66] provided the pooled OR: 1.41 (95% CI: 1.17-1.7, I2 = 0%), supporting the increased frequency of type 2 DM in HCV (Figure 4).
Figure 4.
Sensitivity analysis with prospective studies: Hepatitis C virus-infected patients vs hepatitis C virus-non-infected patients, outcome is type 2 diabetes mellitus. HCV: Hepatitis C virus; M-H: Mantel-Haenszel; T2D: Type 2 diabetes mellitus.
DISCUSSION
This review indicates that patients with HCV infections were at higher risk of developing type 2 DM compared with patients with HBV infection. Findings of this review are comparable with a previous review[14], and a large sample-individual study[56]. In the 2008 review[14] an excess risk of type 2 DM in HCV-infected cases was observed in comparison to HBV-infected controls (summary OR: 1.63, 95% CI: 1.11-2.39). In the present analysis, this is also encountered (summary OR: 1.92, 95% CI: 1.41-2.62) and the association becomes stronger over time.
As both these viruses can replicate in extra-hepatic sites they can produce β-cell damage resulting in diabetes[10,61]. The lower risk in HBV infection could be explained by two factors: (1) Hepatitis B has been controlled in most developed countries, with active HBV vaccination programme; the occurrence of chronic HBV and its complications in these countries is very low; and (2) The disease progression is rather fast in HBV infection and therefore very few patients reach the level of cirrhosis and thus diabetes frequency is lower in this population[61].
An excess risk of type 2 DM in HCV infected cases was also observed in comparison to non-HCV infected controls in the present analysis (summary OR: 1.63, 95% CI: 1.11-2.39). The evidence of heterogeneity in these studies could be explained by variation in definition of case and control subjects and in the sample size of the primary studies.
Available studies had suggested that an expression of the HCV core protein induces hepatic insulin resistance through alterations in signaling in the insulin receptor substrate-1 pathway. This, along with other factors such as diet and obesity, can result in expression of the diabetic phenotype[38,61,68-70]. When insulin resistance reaches extents no longer compensated by the β-cell, insulin secretion declines and hyperglycemia emerges[10,68,70]. The complex interaction of chronic HCV infection with the host hepatic glucose and lipid metabolism has not been fully understood[10,65,67,70] and it remains to be determined.
In the studies identified, anti-HCV antibody was assessed only at the entry point of the study. Anti-HCV is considered as time-varying[43]. As such, there may be a likelihood of changes in serological status of anti-HCV over the study period. Liver disease and endocrine disorders, both common in the general population, have a bidirectional and complex relationship[69]. In addition, it is conceivable that patients with an earlier stage of chronic HCV infection have β-cell dysfunction but that diabetes does not become established until cirrhosis has supervened. Thus, a combination of β-cell dysfunction and insulin resistance is required for overt expression of diabetes mellitus[2,10,45]. Patients in some of the primary studies were not confirmed for the absence of cirrhosis by liver biopsy which is the best predictor of disease progression[2]. As such, we were unable to rigorously exclude cirrhosis individuals from the present analysis, and including these patients in the analysis may have exaggerated the association estimated. Of interest, it has been postulated that HCV has a permissive rather than a direct effect on the development of diabetes and acts in concert with other determinants to lead to diabetes[70]. Recent animal model evidence suggests a more direct effect of HCV infection on insulin resistance in the liver[38] indicating the role of hepatic tumor necrosis factor-α in affecting insulin signaling in this animal model of HCV infection[71]. In the present review, as cross-sectional and longitudinal prospective studies both show the same evidence, an excess type 2 DM risk in HCV-infected persons suggests a direct role of HCV in inducing derangement of glucose metabolism[9,10,45]. Further, there may be other factors influencing the development of type 2 DM in HCV infected patients which is not possible to address in the present analysis.
There are limitations to the present study. Most, if not all, observational studies have the potential for ascertainment bias[10,70] particularly for the studies in which diabetes status was defined by self report. Thus, there may be biased estimates of association. Moreover, recall bias is a factor in case-control studies. Although confounding factors were addressed in many of these observational studies, it is likely that there may be unmeasured confounding factors which may introduce bias into our findings. Further, as patient level data were not available for each study, we could not make further adjustments for important factors such as genotype that were not included in most of the primary studies.
Biological plausibility
Findings of those prospective studies[42,53,66] which have measured HCV prior to diagnosis of type 2 DM support evidence for a temporal relationship between exposure and outcome. In a study[43], a significant link between viral load and diabetes was found and it supported the diabetogenic role of HCV infection. The influence of viral load on the progression rate of type 2 DM was not examined in most of the studies. More research is needed to assess a dose-response association. It is also recommended that surveillance of HCV could indicate whether trends in its incidence continue to reflect changes in the prevalence of type 2 DM in the defined group.
Public health implications
If the associations do support temporality, the early detection and provision of aggressive antiviral treatment for HCV could prevent the development of type 2 DM, particularly in patients at high risk of HCV.
Nevertheless, the findings of the current analysis, to a certain extent, represent the HCV endemic countries. The present study has significant strengths in two ways: (1) It is comprehensive, including most recent studies; and (2) It addresses traditional risk factors (age, gender, BMI, family history of diabetes) which could potentially affect the outcome. As the prevalence of obese patients obtained in the group of HCV-positive patients with type 2 DM was significantly lower than that in diabetic HCV-negative patients found in an independent study[8] and also in the present meta-analysis, it is suggesting the pathogenesis of diabetes in HCV infection could be different from that in the general population.
ACKNOWLEDGMENTS
We are grateful to the participants and the researchers of the primary studies identified for this analysis. We wish to thank the International Medical University (IMU), Malaysia for giving an opportunity to conduct this study. We extend our thanks to Cik Zuhanariah Mohd Nordin and Farhana Abdul Ghafar (IMU library) for their help in literature collection.
COMMENTS
Background
Several observational studies assessing the association between hepatitis C virus (HCV) infection and type 2 diabetes mellitus (type 2 DM) have been published. However, these studies have provided inconclusive results, with some studies supporting the excess type 2 DM with HCV infection compared to non-infected controls, and some studies showing differently. The authors, thus, performed a meta-analysis to synthesise the available evidence on the association between HCV infections and type 2 DM.
Research frontiers
Based on the available evidence, the present study aimed to investigate the association between HCV infection and type 2 DM, and also the effect of study design and traditional risk factors on the association
Innovations and breakthroughs
Combining the electronic database and hand searches, a total of 35 observational studies (in 31 articles) were identified for the final analysis, based on the inclusion criteria set for the present analysis. The findings support the association between HCV infection and type 2 DM. However, the direction of association remained to be determined.
Applications
The results support an association between HCV infection and type 2 DM. Findings of this review are comparable with previous reviews, and a large sample-individual study. An early detection and provision of aggressive antiviral treatment for HCV could prevent the development of type 2 DM.
Peer review
The authors show the review of the association between HCV infection and diabetes by meta-analysis. This paper is an interesting and instructive manuscript.
Footnotes
Peer reviewer: Yasuji Arase, MD, Department of Gastro-enterology, Toranomon Hospital, 2-2-2 Toranomonminato-ku, Tokyo 105-8470, Japan
S- Editor Shi ZF L- Editor O’Neill M E- Editor Li JY
References
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Mauss S, Berg T, Rockstroh J, Sarrazin C, Wedemeyer H, editors . Hepatology - A Clinical Textbook. 2nd ed. Dusseldorf: Flying publisher; 2010. pp. 19–27. [Google Scholar]
- 3.Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135–1139. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 4.Purcell R. The hepatitis C virus: overview. Hepatology. 1997;26:11S–14S. doi: 10.1002/hep.510260702. [DOI] [PubMed] [Google Scholar]
- 5.Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, Guo L, Jacob S, Regenstein FG, Zimmerman R, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 6.Mangia A, Schiavone G, Lezzi G, Marmo R, Bruno F, Villani MR, Cascavilla I, Fantasia L, Andriulli A. HCV and diabetes mellitus: evidence for a negative association. Am J Gastroenterol. 1998;93:2363–2367. doi: 10.1111/j.1572-0241.1998.00688.x. [DOI] [PubMed] [Google Scholar]
- 7.Papatheodoridis GV, Chrysanthos N, Savvas S, Sevastianos V, Kafiri G, Petraki K, Manesis EK. Diabetes mellitus in chronic hepatitis B and C: prevalence and potential association with the extent of liver fibrosis. J Viral Hepat. 2006;13:303–310. doi: 10.1111/j.1365-2893.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- 8.Chehadeh W, Abdella N, Ben-Nakhi A, Al-Arouj M, Al-Nakib W. Risk factors for the development of diabetes mellitus in chronic hepatitis C virus genotype 4 infection. J Gastroenterol Hepatol. 2009;24:42–48. doi: 10.1111/j.1440-1746.2008.05503.x. [DOI] [PubMed] [Google Scholar]
- 9.Negro F. Insulin resistance and HCV: will new knowledge modify clinical management? J Hepatol. 2006;45:514–519. doi: 10.1016/j.jhep.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15:1537–1547. doi: 10.3748/wjg.15.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serfaty L, Capeau J. Hepatitis C, insulin resistance and diabetes: clinical and pathogenic data. Liver Int. 2009;29 Suppl 2:13–25. doi: 10.1111/j.1478-3231.2008.01952.x. [DOI] [PubMed] [Google Scholar]
- 12.Azócar J. Adult Onset Diabetes Mellitus in Hepatitis C Virus Infection. Hepatitis C Support Project. 2003. Available from: http: //www.hcvadvocate.org/hcsp/articles/Azocar-1.html. Accessed 25 April 2011. [Google Scholar]
- 13.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 14.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831–844. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. Available from: www.cochrane-handbook.org. [Google Scholar]
- 16.Review Manager (RevMan) [Computer program] Version 5. 1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2011. [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 18.Hourigan LF, Macdonald GA, Purdie D, Whitehall VH, Shorthouse C, Clouston A, Powell EE. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology. 1999;29:1215–1219. doi: 10.1002/hep.510290401. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CS, Liu CH, Liu CJ, Wang CC, Chen CL, Lai MY, Chen PJ, Chen DS, Kao JH. Association of lipid profiles with hepatitis C viral load in chronic hepatitis C patients with genotype 1 or 2 infection. Am J Gastroenterol. 2009;104:598–604. doi: 10.1038/ajg.2008.125. [DOI] [PubMed] [Google Scholar]
- 20.AlDosary AA, Ramji AS, Elliott TG, Sirrs SM, Thompson DM, Erb SR, Steinbrecher UP, Yoshida EM. Post-liver transplantation diabetes mellitus: an association with hepatitis C. Liver Transpl. 2002;8:356–361. doi: 10.1053/jlts.2002.31745. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Yan WW. Chronic hepatitis C virus infection and post-liver transplantation diabetes mellitus. World J Gastroenterol. 2005;11:6085–6089. doi: 10.3748/wjg.v11.i39.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain MK, Aragaki C, Fischbach L, Gibson S, Arora R, May L, Vardhineni K, Lee WM. Hepatitis C is associated with type 2 diabetes mellitus in HIV-infected persons without traditional risk factors. HIV Med. 2007;8:491–497. doi: 10.1111/j.1468-1293.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- 23.Antonelli A, Ferri C, Fallahi P, Sebastiani M, Nesti C, Barani L, Barale R, Ferrannini E. Type 2 diabetes in hepatitis C-related mixed cryoglobulinaemia patients. Rheumatology (Oxford) 2004;43:238–240. doi: 10.1093/rheumatology/keh011. [DOI] [PubMed] [Google Scholar]
- 24.Labropoulou-Karatza C, Goritsas C, Fragopanagou H, Repandi M, Matsouka P, Alexandrides T. High prevalence of diabetes mellitus among adult beta-thalassaemic patients with chronic hepatitis C. Eur J Gastroenterol Hepatol. 1999;11:1033–1036. doi: 10.1097/00042737-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Chuang LM, Tsai ST, Huang BY, Tai TY. The status of diabetes control in Asia--a cross-sectional survey of 24 317 patients with diabetes mellitus in 1998. Diabet Med. 2002;19:978–985. doi: 10.1046/j.1464-5491.2002.00833.x. [DOI] [PubMed] [Google Scholar]
- 26.Ponce-De-Leon A, Garcia-Garcia Md Mde L, Garcia-Sancho MC, Gomez-Perez FJ, Valdespino-Gomez JL, Olaiz-Fernandez G, Rojas R, Ferreyra-Reyes L, Cano-Arellano B, Bobadilla M, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27:1584–1590. doi: 10.2337/diacare.27.7.1584. [DOI] [PubMed] [Google Scholar]
- 27.Huang ZS, Huang TS, Wu TH, Chen MF, Hsu CS, Kao JH. Asymptomatic chronic hepatitis B virus infection does not increase the risk of diabetes mellitus: a ten-year observation. J Gastroenterol Hepatol. 2010;25:1420–1425. doi: 10.1111/j.1440-1746.2010.06268.x. [DOI] [PubMed] [Google Scholar]
- 28.Khokhar N. Association of chronic hepatitis C virus infection and diabetes mellitus. Pakistan J Med Res. 2002:41. Available from: http: //www.pmrc.org.pk/association.htm. [Google Scholar]
- 29.Samantray J, Zambare S, Seyoum B, Abou-Samra AB. Glucose control and lipid metabolism in African American patients with type 2 diabetes mellitus and chronic hepatitis C viral infection. Endocr Pract. 2011;17:363–368. doi: 10.4158/EP10175.OR. [DOI] [PubMed] [Google Scholar]
- 30.Fartoux L, Poujol-Robert A, Guéchot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003–1008. doi: 10.1136/gut.2004.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picerno I, Di Pietro A, Spataro P, Di Benedetto A, Romano G, Scoglio ME. Is diabetes mellitus a risk factor for HCV infection? Ann Ig. 2002;14:473–477. [PubMed] [Google Scholar]
- 32.Grimbert S, Valensi P, Lévy-Marchal C, Perret G, Richardet JP, Raffoux C, Trinchet JC, Beaugrand M. High prevalence of diabetes mellitus in patients with chronic hepatitis C. A case-control study. Gastroenterol Clin Biol. 1996;20:544–548. [PubMed] [Google Scholar]
- 33.Zein NN, Abdulkarim AS, Wiesner RH, Egan KS, Persing DH. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J Hepatol. 2000;32:209–217. doi: 10.1016/s0168-8278(00)80065-3. [DOI] [PubMed] [Google Scholar]
- 34.Kwon SY, Kim SS, Kwon OS, Kwon KA, Chung MG, Park DK, Kim YS, Koo YS, Kim YK, Choi DJ, et al. Prognostic significance of glycaemic control in patients with HBV and HCV-related cirrhosis and diabetes mellitus. Diabet Med. 2005;22:1530–1535. doi: 10.1111/j.1464-5491.2005.01687.x. [DOI] [PubMed] [Google Scholar]
- 35.Kobashi-Margáin RA, Gutiérrez-Grobe Y, Ponciano-Rodríguez G, Uribe M, Méndez-Sánchez N. Prevalence of type 2 diabetes mellitus and chronic liver disease: a retrospective study of the association of two increasingly common diseases in Mexico. Ann Hepatol. 2010;9:282–288. [PubMed] [Google Scholar]
- 36.Sotiropoulos A, Peppas TA, Skliros E, Apostolou O, Kotsini V, Pappas SI. Low prevalence of hepatitis C virus infection in Greek diabetic patients. Diabet Med. 1999;16:250–252. doi: 10.1046/j.1464-5491.1999.00009.x. [DOI] [PubMed] [Google Scholar]
- 37.Ali SS, Ali IS, Aamir AH, Jadoon Z, Inayatullah S. Frequency of hepatitis C infection in diabetic patients. J Ayub Med Coll Abbottabad. 2007;19:46–49. [PubMed] [Google Scholar]
- 38.Wilson C. Hepatitis C infection and type 2 diabetes in American-Indian women. Diabetes Care. 2004;27:2116–2119. doi: 10.2337/diacare.27.9.2116. [DOI] [PubMed] [Google Scholar]
- 39.Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, Szklo M, Thomas DL. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50–56. doi: 10.1053/jhep.2003.50291. [DOI] [PubMed] [Google Scholar]
- 40.Akbar DH, Siddique AM, Ahmed MM. Prevalence of Type-2 diabetes in patients with hepatitis C and B virus infection in Jeddah, Saudi Arabia. Med Princ Pract. 2002;11:82–85. doi: 10.1159/000058012. [DOI] [PubMed] [Google Scholar]
- 41.Antonelli A, Ferri C, Fallahi P, Pampana A, Ferrari SM, Goglia F, Ferrannini E. Hepatitis C virus infection: evidence for an association with type 2 diabetes. Diabetes Care. 2005;28:2548–2550. doi: 10.2337/diacare.28.10.2548. [DOI] [PubMed] [Google Scholar]
- 42.Arao M, Murase K, Kusakabe A, Yoshioka K, Fukuzawa Y, Ishikawa T, Tagaya T, Yamanouchi K, Ichimiya H, Sameshima Y, et al. Prevalence of diabetes mellitus in Japanese patients infected chronically with hepatitis C virus. J Gastroenterol. 2003;38:355–360. doi: 10.1007/s005350300063. [DOI] [PubMed] [Google Scholar]
- 43.Boschi-Pinto C, Stuver S, Okayama A, Trichopoulos D, Orav EJ, Tsubouchi H, Mueller N. A follow-up study of morbidity and mortality associated with hepatitis C virus infection and its interaction with human T lymphotropic virus type I in Miyazaki, Japan. J Infect Dis. 2000;181:35–41. doi: 10.1086/315177. [DOI] [PubMed] [Google Scholar]
- 44.Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M, Justice AC. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology. 2004;40:115–119. doi: 10.1002/hep.20289. [DOI] [PubMed] [Google Scholar]
- 45.Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, O’Rahilly S, Shore S, Tom BD, Alexander GJ. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059–1063. doi: 10.1002/hep.510300416. [DOI] [PubMed] [Google Scholar]
- 46.Chen HF, Li CY, Chen P, See TT, Lee HY. Seroprevalence of hepatitis B and C in type 2 diabetic patients. J Chin Med Assoc. 2006;69:146–152. doi: 10.1016/S1726-4901(09)70195-9. [DOI] [PubMed] [Google Scholar]
- 47.Gulcan A, Gulcan E, Toker A, Bulut I, Akcan Y. Evaluation of risk factors and seroprevalence of hepatitis B and C in diabetic patients in Kutahya, Turkey. J Investig Med. 2008;56:858–863. doi: 10.2310/JIM.0b013e3181788d28. [DOI] [PubMed] [Google Scholar]
- 48.Howard AA, Klein RS, Schoenbaum EE. Association of hepatitis C infection and antiretroviral use with diabetes mellitus in drug users. Clin Infect Dis. 2003;36:1318–1323. doi: 10.1086/374838. [DOI] [PubMed] [Google Scholar]
- 49.Huang JF, Dai CY, Hwang SJ, Ho CK, Hsiao PJ, Hsieh MY, Lee LP, Lin ZY, Chen SC, Hsieh MY, et al. Hepatitis C viremia increases the association with type 2 diabetes mellitus in a hepatitis B and C endemic area: an epidemiological link with virological implication. Am J Gastroenterol. 2007;102:1237–1243. doi: 10.1111/j.1572-0241.2007.01181.x. [DOI] [PubMed] [Google Scholar]
- 50.Imazeki F, Yokosuka O, Fukai K, Kanda T, Kojima H, Saisho H. Prevalence of diabetes mellitus and insulin resistance in patients with chronic hepatitis C: comparison with hepatitis B virus-infected and hepatitis C virus-cleared patients. Liver Int. 2008;28:355–362. doi: 10.1111/j.1478-3231.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 51.Jadoon NA, Shahzad MA, Yaqoob R, Hussain M, Ali N. Seroprevalence of hepatitis C in type 2 diabetes: evidence for a positive association. Virol J. 2010;7:304. doi: 10.1186/1743-422X-7-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaabia N, Ben Jazia E, Slim I, Fodha I, Hachfi W, Gaha R, Khalifa M, Hadj Kilani A, Trabelsi H, Abdelaziz A, et al. Association of hepatitis C virus infection and diabetes in central Tunisia. World J Gastroenterol. 2009;15:2778–2781. doi: 10.3748/wjg.15.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knobler H, Schihmanter R, Zifroni A, Fenakel G, Schattner A. Increased risk of type 2 diabetes in noncirrhotic patients with chronic hepatitis C virus infection. Mayo Clin Proc. 2000;75:355–359. doi: 10.4065/75.4.355. [DOI] [PubMed] [Google Scholar]
- 54.Lecube A, Hernández C, Genescà J, Esteban JI, Jardí R, Simó R. High prevalence of glucose abnormalities in patients with hepatitis C virus infection: a multivariate analysis considering the liver injury. Diabetes Care. 2004;27:1171–1175. doi: 10.2337/diacare.27.5.1171. [DOI] [PubMed] [Google Scholar]
- 55.Li-Ng M, Tropp S, Danoff A, Bini EJ. Association between chronic hepatitis B virus infection and diabetes among Asian Americans and Pacific Islanders. Dig Liver Dis. 2007;39:549–556. doi: 10.1016/j.dld.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Marzouk D, Sass J, Bakr I, El Hosseiny M, Abdel-Hamid M, Rekacewicz C, Chaturvedi N, Mohamed MK, Fontanet A. Metabolic and cardiovascular risk profiles and hepatitis C virus infection in rural Egypt. Gut. 2007;56:1105–1110. doi: 10.1136/gut.2006.091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 58.Nwokediuko SC, Oli JM. Hepatitis C virus infection in Nigerians with diabetes mellitus. Niger J Clin Pract. 2008;11:94–99. [PubMed] [Google Scholar]
- 59.Okan V, Araz M, Aktaran S, Karsligil T, Meram I, Bayraktaroglu Z, Demirci F. Increased frequency of HCV but not HBV infection in type 2 diabetic patients in Turkey. Int J Clin Pract. 2002;56:175–177. [PubMed] [Google Scholar]
- 60.Olokoba AB, Badung LH, Abdurrahman MB, Salawu FK, Danburam A, Aderibigbe S, Midala J, Tidi SK. Hepatitis C virus infection in Nigerians with diabetes mellitus. Am J Sci Ind Res. 2010;1:135–138. [Google Scholar]
- 61.Qureshi H, Ahsan T, Mujeeb SA, Jawad F, Mehdi I, Ahmed W, Alam SE. Diabetes mellitus is equally frequent in chronic HCV and HBV infection. J Pak Med Assoc. 2002;52:280–283. [PubMed] [Google Scholar]
- 62.Rouabhia S, Malek R, Bounecer H, Dekaken A, Bendali Amor F, Sadelaoud M, Benouar A. Prevalence of type 2 diabetes in Algerian patients with hepatitis C virus infection. World J Gastroenterol. 2010;16:3427–3431. doi: 10.3748/wjg.v16.i27.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryu JK, Lee SB, Hong SJ, Lee S. Association of chronic hepatitis C virus infection and diabetes mellitus in Korean patients. Korean J Intern Med. 2001;16:18–23. doi: 10.3904/kjim.2001.16.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sangiorgio L, Attardo T, Gangemi R, Rubino C, Barone M, Lunetta M. Increased frequency of HCV and HBV infection in type 2 diabetic patients. Diabetes Res Clin Pract. 2000;48:147–151. doi: 10.1016/s0168-8227(99)00135-7. [DOI] [PubMed] [Google Scholar]
- 65.Simó R, Lecube A, Genescà J, Esteban JI, Hernández C. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care. 2006;29:2462–2466. doi: 10.2337/dc06-0456. [DOI] [PubMed] [Google Scholar]
- 66.Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol. 2007;166:196–203. doi: 10.1093/aje/kwm061. [DOI] [PubMed] [Google Scholar]
- 67.Wang LF, Wu CH, Shan Y, Fan XH, Huo N, Lu HY, Xu XY. Prevalence of abnormal glycometabolism in patients with chronic hepatitis C and related risk factors in China. Chin Med J (Engl) 2011;124:183–188. [PubMed] [Google Scholar]
- 68.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maheshwari A, Thuluvath PJ. Endocrine diseases and the liver. Clin Liver Dis. 2011;15:55–67. doi: 10.1016/j.cld.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Everhart J. A confluence of epidemics: does hepatitis C cause type 2 diabetes? Hepatology. 2001;33:762–763. doi: 10.1002/hep.510330336. [DOI] [PubMed] [Google Scholar]
- 71.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]