Abstract
Enteropathogenic E. coli (EPEC) are a leading cause of infantile diarrhea in developing countries, resulting in millions of deaths each year. EPEC secrete virulence factors, also called effectors, directly into host intestinal epithelial cells via type three secretion systems. Secreted effectors then affect host signaling pathways to induce several phenotypes, which ultimately lead to disease. Among the over 20 secreted effectors is E. coli secreted protein F (EspF), a 206 amino acid protein believed to be central to EPEC pathogenesis, as it disrupts tight junction structure and function. Although the mechanism by which this occurs is unknown, EspF has recently been found to contain several protein–protein interaction domains that may be involved. We have shown EspF to interact with the endocytic regulators sorting nexin 9 (SNX9) and N-WASP via non-exclusive binding sites. These interactions induce actin polymerization in vitro, and interaction with SNX9 alters its endocytic activity, as EspF induces the formation of tubular vesicles in a manner dependent upon its interaction with SNX9. EspF, therefore, appears to hijack endocytic regulation via SNX9 and possibly N-WASP interaction, to affect an as yet unidentified pathogenic phenotype.
Keywords: EPEC, EspF, SNX9, tight junction
Introduction
Diarrheal disease due to bacterial infection is a significant cause of morbidity and mortality in developing countries, particularly among infant populations. Major contributors to this disease burden are enteropathogenic E. coli (EPEC), as they are responsible for millions of deaths annually.1 EPEC belong to a family of closely related pathogens, including enterohemmorhagic E. coli (EHEC), rabbit EPEC and Citrobacter rodentium, which collectively are known as attaching and effacing (A/E) pathogens due to the characteristic lesions they elicit upon adherence to intestinal epithelial cells (IECs).1 A typical A/E lesion features tight contact between the bacterial outer membrane and the intestinal cell plasma membrane and effacement of the adjacent brush border microvilli. EPEC differ from commensal E. coli largely due to the presence of a 35 kilo-base pathogenicity island located within the chromosome called the Locus of Enterocyte Effacement (LEE). Although EPEC express non-LEE–encoded virulence factors, most of the known virulence genes exist within the LEE, including genes encoding for the A/E phenotype, type three secretion system (T3SS), effector proteins and chaperones.2 Many species of gram-negative bacteria express type three secretion systems, which are essentially molecular syringes that span both bacterial membranes and the host cell plasma membrane, thus allowing injection of bacterial effector proteins directly into the host cell cytoplasm. Effectors delivered in this way by EPEC induce A/E lesions and disrupt normal intestinal epithelial physiology, ultimately leading to diarrhea.1,2
EPEC Alters Tight Junctions
It is essential that the intestinal epithelium maintain a tight barrier between cells in order to separate the lumenal contents of the digestive tract from the submucosa and regulate the passage of only specific nutrients between these two compartments. Tight junctions (TJs) ensure integrity of transcellular transport by maintaining cell polarity and separation of the apical and basolateral membranes (fence function).3 Normal IECs feature continuous strands of TJ proteins, consisting of occludin, claudins, junctional adhesion molecule, and the coxsackie-adenovirus receptor, all of which encircle the most apical aspect of the lateral plasma membrane. These transmembrane proteins form homotypic and, in the case of certain claudin isoforms, heterotypic contacts between adjacent cells, thus sealing the paracellular space and preventing unregulated movement of water, ions, solutes, dietary antigens, and microbes between the lumen of the intestine and submucosa.4 During certain disease states, including EPEC infection, the ability of the intestinal epithelium to regulate absorption and secretion is compromised, resulting in diarrhea.
Several events related to the loss of intestinal epithelial homeostasis have been observed as a consequence of EPEC infection, including decreased Na+ absorption via downregulation of NHE3 activity, increased interleukin-8 secretion leading to PMN transmigration, and disruption of TJs and intestinal barrier function2 (Fig. 1). The latter phenotype is considered to be central to EPEC pathogenesis because TJs are solely responsible for sealing the space between adjacent cells and regulating paracellular transport. EPEC-induced TJ disruption has been demonstrated in several model systems in vitro and in vivo.5–7 Whereas the transmembrane TJ proteins occludin and claudin-1 normally associate with the cytosolic plaque protein ZO-1, which links the TJ to cortical F-actin, Muza-Moons et al. demonstrated by co-immunoprecipitation experiments that these interactions are lost in response to EPEC infection.8 In addition, they showed by confocal microscopy that occludin and claudin-1 diffuse along the lateral plasma membrane of IECs during EPEC infection. Also, as a result of EPEC-induced TJ disruption, the basolateral transmembrane protein β1-integrin appeared on the apical plasma membrane, indicating a breakdown of TJ fence function.9 Interestingly, the EPEC surface protein intimin, which normally binds to the translocated intimin receptor (Tir) after it is secreted into the host cell by the T3SS, can also bind to β1-integrin, providing another downstream effect of EPEC-induced TJ disruption that may promote pathogenesis.2
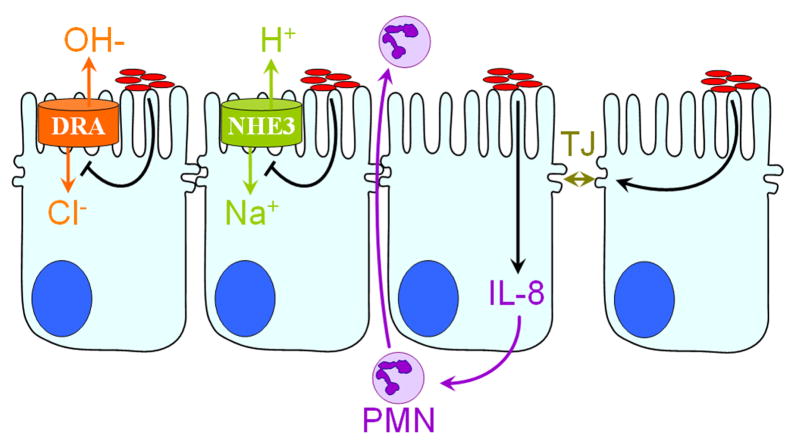
Figure 1.
EPEC alter intestinal epithelial physiology. After intimate attachment to IECs, and injection of effector proteins into host cells, EPEC (red ovals) induce several phenotypes detrimental to intestinal homeostasis. A net increase in lumenal sodium and chloride concentration occurs by the inhibition of chloride and sodium absorption via NHE3 and DRA. Secretion of the inflammatory cytokine IL-8 is increased and transmigration of PMNs from the sub-mucosa to the lumen is induced. EPEC also cause disruption of barrier function by perturbing tight junctions (TJs).
A hallmark of intact barrier function is maintenance of transepithelial electrical resistance (TER) across epithelial monolayers, which has been observed to decrease over time due to EPEC-induced barrier disruption.5–7 Occludin rearrangement and TER loss also occur in mice following EPEC infection, suggesting this to be an important facet of EPEC pathogenesis.6 Interestingly, E. coli–secreted protein F (EspF), a 206 amino-acid effector protein that is injected into IECs via the T3SS during EPEC infection, has been implicated as being largely responsible for TJ breakdown.8,10,11
Effect of EspF on Host Cell Function
EspF is one of the better-characterized proteins among the over 20 effectors secreted by EPEC. Strains deficient in EspF resemble wild-type EPEC with regard to growth rate, attachment to IECs, phosphorylation of host proteins, and A/E lesion formation.12 Several groups have shown EspF deletion to significantly attenuate the effect of EPEC on TER (Fig. 2). This is coupled with preservation of occludin localization.6,11 However, MDCK cells stably transfected with EGFP-EspF exhibited normal TJ morphology, suggesting that EspF does not affect TJ formation.13 EspF has also been demonstrated to be required for barrier function disruption during infection with the related pathogens EHEC and Citrobacter rodentium, suggesting that EspF-mediated TJ disruption is an evolutionarily conserved phenotype among A/E pathogens.10,14 Despite the extensive evidence implicating EspF in the disruption of TJ barrier structure and function, a detailed molecular mechanism has yet to be elucidated. Recently, however, we and others have begun to unravel details within the secondary structure of EspF that may help define this mechanism. The C-terminus of EspF is comprised of three nearly identical repeats of approximately 45 amino acids in length; they are rich in proline residues and exhibit putative interacting sites for several mammalian proteins, including profilin, actin, N-WASP and SNX9. Indeed, EspF has been shown to interact with N-WASP and SNX9 by separate, non-exclusive binding sites13,15 (Fig. 3). N-WASP is a well-characterized activator of ARP2/3, which induces branching and elongation of actin filaments. SNX9, which also interacts with N-WASP, dynamin, clathrin and AP-2, regulates clathrin-mediated endocytosis (CME) by way of its ability to couple actin rearrangement (via N-WASP) to membrane remodeling.16,17 To determine the effects of EspF on SNX9 regulation of CME, we utilized total internal-reflection fluorescence microscopy (TIRF), which allows imaging of fluorescently tagged proteins within 100 nm of the plasma membrane and is thus ideal for observing endocytic events. Clathrin light chain (CLC) labeled with DsRed, for example, is visible when concentrated at the plasma membrane during the formation of clathrin-coated pits (CCP), yet, shortly after CCP budding from the membrane, the signal disappears from the TIRF field of view. Interestingly, when co-expressed, GFP-EspF and CLC-DsRed co-localized during CCP formation, and disappeared from the field of view almost simultaneously, suggesting that EspF is targeted to sites of CME (Fig. 4). When GFP-EspF was co-expressed with mCherry-SNX9, these proteins co-localized in tubule-like structures that were determined to be membrane-enclosed vesicles coated with EspF as determined by electron microscopy. Taken together, these observations suggest that EspF alters SNX9-mediated regulation of CME.
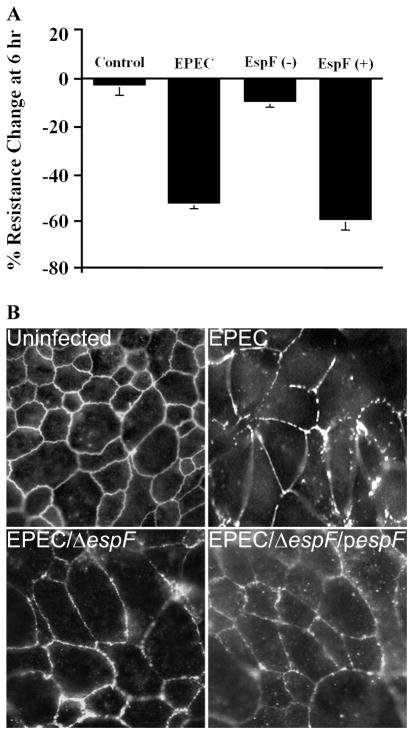
Figure 2.
EspF is required for EPEC-induced TER loss and tight junction disruption. (A) T84 intestinal epithelial cells grown on permeable membrane supports prevent the flow of electrical current between the apical and basolateral media, representing an intact epithelial barrier, which is indicated by constant TER measurements over time (Control). When these cells are infected with EPEC for 6 hours, however, TER decreases by approximately 50% (EPEC). This is greatly attenuated by deletion of espF (EspF(−)) while complementation of the espF deletion strain with plasmid encoded espF (EspF(+)) restores the wild-type phenotype, demonstrating the requirement for EspF in EPEC-induced barrier disruption. (B) Un-infected T84 cells exhibit intact tight junctions as indicated by the characteristic chicken wire pattern observed with occludin immunostaining. After EPEC infection, junctional occludin is disrupted and the staining pattern becomes perforated. This is alleviated by EspF knockout and restored by EspF complementation.
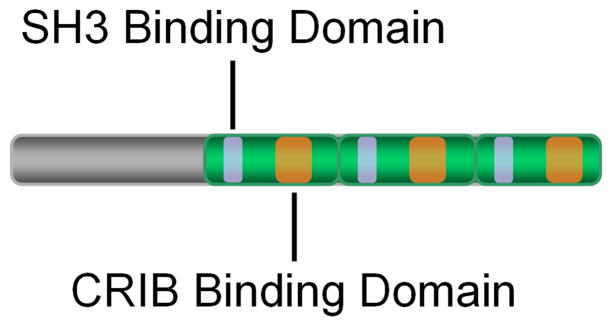
Figure 3.
EspF contains several domains for interaction with host proteins. The C-terminus of EspF consists of three proline-rich repeats (green segments), each of which contains an SH3 binding domain (purple segments) and a putative CRIB binding domain (orange segments). The former has been shown to permit interaction with the SH3 domain of SNX9, and the latter is proposed to mediate N-WASP binding.
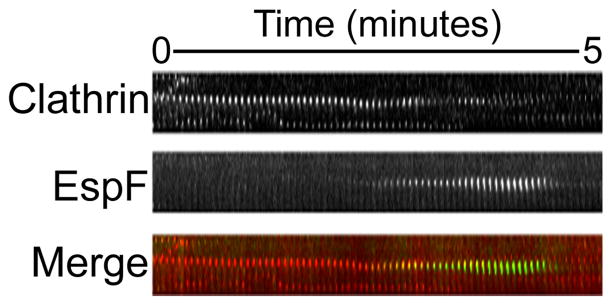
Figure 4.
EspF localized to sites of clathrin-coated pit formation. Swiss 3T3 cells co-transfected with dsRed-clathrin light chain (Clathrin) and EGFP-EspF (EspF) were observed by live-cell total internal reflection fluorescence microscopy. Shown is a kymograph of a single clathrin-coated vesicle (CCV) budding from the plasma membrane. As the dsRed flu-orescent signal diminishes, representing pinching off of the CCV, EGFP signal temporarily co-localizes with the dsRed and subsequently disappears, suggesting the arrival of EspF at the site of CCV formation at the later stage of vesicle budding. This mimics the spa-tiotemporal association of SNX9 with CCVs, suggesting that EspF may be coordinating with SNX9 to affect clathrin-mediated endocytosis.
By introducing scanning aspartate mutations within the putative SNX9 interacting regions of EspF, a single arginine residue was identified to be required for SNX9 interaction (Fig. 5). Full-length EspF constructs containing R-D point mutations at the corresponding residues were created and used to transfect mammalian cells or expressed in an EspF-deficient EPEC strain, allowing assessment of the role of EspF-SNX9 interaction in EPEC infection. Interestingly, infection of IECs with EPEC induced the formation of vesicular membrane tubules that contain both SNX9 and EspF18 (Fig. 6). Similar tubule structures were observed in Swiss 3T3 cells transfected with EGFP-EspF, but not EGFP-EspFD3, con-firming that EspF-SNX9 interaction modulates SNX9-dependent endocytosis. This type of membrane tubulation has been observed in studies of endocytic regulation by SNX9 as well as by creation of Salmonella-containing vacuoles involving SNX1.19 We have proposed that these unusual membrane vesicles form due to EspF stimulation of SNX9 membrane tubulating and endocytic regulation. Whereas tubular vesicle formation is evident after GFP-EspF and mCherry-SNX9 co-transfection and infection with wild-type EPEC, transfection with GFP-EspFD3 or infection with EspF-deficient EPEC, or EPEC expressing EspFD3 fails to induce membrane tubulation. Therefore, EspF-SNX9 interaction is specifically required for this phenotype, although the pathogenic significance thereof is not known at this time.
Figure 5.

Point mutations within the SH3 binding region of EspF prevent interaction with SNX9. Short peptides homologous to the SH3 binding region of EspF were synthesized such that sequential residues were point-mutated to aspartate. These mutated peptides were affixed to nitrocellulose along with a wild-type peptide, overlaid with radio-labeled SNX9 and radioactive signal due to bound SNX9 visualized by autoradiogram. These studies define specific amino acids that are required for EspF-SNX9 interaction.
Figure 6.
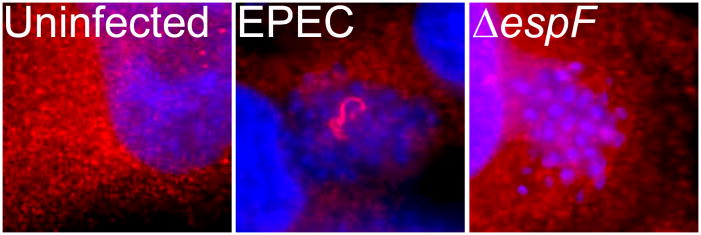
EspF-SNX9 interaction leads to the formation of aberrant tubular vesicles during EPEC infection. HeLa cells were infected with wild-type EPEC or an espF-deficient strain (ΔespF) and stained with SNX9 antibodies. As compared to uninfected cells, EPEC-infected cells displayed tubular structures, which were not apparent after infection with ΔespF. These tubular vesicles were found to be membrane-enclosed, suggesting that EspF alters SNX9 regulation of endocytosis during EPEC infection.
To determine the relevance of EspF-SNX9 interaction with respect to EPEC pathogenesis, we compared EspF-deficient EPEC expressing EspFD3 to strains expressing wild-type EspF with respect to their ability to decrease TER of IEC monolayers. EspF-SNX9 interaction was found to be dispensable for EPEC-induced TER loss as EspF and EspFD3 reduced TER to equal levels.
Conclusion
In conclusion, we have revealed a novel activity of EspF that is likely to be involved in its pathogenic function: the ability to bind membrane- and actin-remodeling host factors, namely SNX9 and N-WASP, and alter their activity. EspF modulation of endocytic function is intriguing as a mechanism for barrier disruption, as the TJ is regulated by actin as well as endocytosis.20 In addition, endocytic pathways have previously been shown to induce TER loss in other disease models, such as inflammatory bowel disease.21 EspF appears to induce redistribution of occludin to the cytosol, possibly by modulating endocytic events at the TJ by way of SNX9 and N-WASP. Investigating the role of specific domains of EspF by the introduction of point mutations has been used previously to elicit the function of EspF with respect to mitochondrial localization and apoptosis. Nagai et al. showed that a single point mutation at leucine 16 prevented EspF-induced apoptosis.22 We have utilized a similar strategy to investigate other domains within EspF, which will provide a powerful tool for investigating the specific mechanisms of EspF function.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaper JB, NJ, Mobley HLT. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 3.Schneeberger EE, et al. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 4.Denker BM, et al. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 5.Spitz J, et al. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G374–G379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- 6.Shifflett DE, et al. Enteropathogenic E. coli disrupts tight junction barrier function and structure in vivo. Lab Invest. 2005;85:1308–1324. doi: 10.1038/labinvest.3700330. [DOI] [PubMed] [Google Scholar]
- 7.Canil C, et al. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun. 1993;61:2755–2762. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muza-Moons MM, et al. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol. 2004;6:783–793. doi: 10.1111/j.1462-5822.2004.00404.x. [DOI] [PubMed] [Google Scholar]
- 9.Muza-Moons MM, et al. Disruption of cell polarity by enteropathogenic Escherichia coli enables basolateral membrane proteins to migrate apically and to potentiate physiological consequences. Infect Immun. 2003;71:7068–7078. doi: 10.1128/IAI.71.12.7069-7078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viswanathan VK, et al. Comparative analysis of EspF from enteropathogenic and enterohemorrhagic Escherichia coli in alteration of epithelial barrier function. Infect Immun. 2004;72:3218–3227. doi: 10.1128/IAI.72.6.3218-3227.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNamara BP, et al. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Invest. 2001;107:621–629. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNamara BP, et al. A novel proline-rich protein, EspF, is secreted from enteropathogenic Escherichia coli via the type III export pathway. FEMS Microbiol Lett. 1998;166:71–78. doi: 10.1111/j.1574-6968.1998.tb13185.x. [DOI] [PubMed] [Google Scholar]
- 13.Alto NM, et al. The type III effector EspF coordinates membrane trafficking by the spatiotemporal activation of two eukaryotic signaling pathways. J Cell Biol. 2007;178:1265–1278. doi: 10.1083/jcb.200705021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guttman JA, et al. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell Microbiol. 2006;8:634–645. doi: 10.1111/j.1462-5822.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- 15.Marches O, et al. EspF of enteropathogenic Escherichia coli binds sorting nexin 9. J Bacteriol. 2006;188:3110–3115. doi: 10.1128/JB.188.8.3110-3115.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin N, et al. SNX9 regulates tubular invagination of the plasma membrane through interaction with actin cytoskeleton and dynamin 2. J Cell Sci. 2008;121:1252–1263. doi: 10.1242/jcs.016709. [DOI] [PubMed] [Google Scholar]
- 17.Shin N, et al. Sorting nexin 9 interacts with dynamin 1 and N-WASP and coordinates synaptic vesicle endocytosis. J Biol Chem. 2007;282:28939–28950. doi: 10.1074/jbc.M700283200. [DOI] [PubMed] [Google Scholar]
- 18.Alto NM, et al. The type III effector EspF coordinates membrane trafficking by the spatiotemporal activation of two eukaryotic signaling pathways. J Cell Biol. 2007;178:1265–1278. doi: 10.1083/jcb.200705021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bujny MV, et al. Sorting nexin-1 defines an early phase of Salmonella-containing vacuole-remodeling during Salmonella infection. J Cell Sci. 2008;121:2027–2036. doi: 10.1242/jcs.018432. [DOI] [PubMed] [Google Scholar]
- 20.Shen L, Turner J. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruewer M, et al. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 22.Nagai T, et al. Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J Biol Chem. 2005;280:2998–3011. doi: 10.1074/jbc.M411550200. [DOI] [PubMed] [Google Scholar]