Abstract
The orexigenic neuropeptide ghrelin is an endogeneous ligand for the growth hormone secretagogue receptor (GHS-R). This orexigen is expressed in both the periphery and in the central system, including portions of mesolimbic dopaminergic circuitry that play a role in affective behaviors. Here we examined pharmacological antagonism of GHS-R in motivational incentive learning, as reflected in Pavlovian-to-instrumental transfer (PIT). Furthermore it is currently unclear whether the previous effects of ghrelin on food intake are mediated by pre- and/or post-ingestive influences on ingestive behavior. Thus, we also conducted detailed analyses of the temporal dynamics of sucrose licking. Mice received low (50 nmol), moderate (100 nmol) and high (200 nmol) intraperitoneal injections of the GHS-R antagonist GHRP-6 [D-Lys3] prior to subsequent transfer and sucrose consumption tests. Low and moderate doses led to an augmentation of PIT, while high dose injections led to generalized performance deficits. In addition, moderate and high doses of the antagonist resulted in reductions in sucrose intake by reducing palatability of the sucrose. These results suggest dissociable functions of GHS-R in its influence over motivational learning and ingestive behavior.
Keywords: Pavlovian to instrumental transfer, ghrelin, licking microstructure, palatability, mice
Introduction
The discovery that ghrelin—the endogenous agonist for the growth hormone secretagogue receptor (GHS-R)—acts as an orexigen, has led to an wealth of research characterizing the central mechanisms involved in ghrelin-driven ingestive behavior (Kojima et al., 1999; Wren et al., 2001). Ghrelin is synthesized both in the periphery and centrally, and crosses the blood-brain barrier in a bidirectional manner (Banks et al., 2002; Diano et al., 2006). In the central nervous system a major target for ghrelin is the arcuate nucleus, from which two groups of neurons project to the paraventricular nucleus of the hypothalamus: (1) those that express the appetite-enhancing orexigenic neuropeptides, neuropeptide Y (NPY) and agouti-related protein (AGRP), (2) those that contain the appetite-suppressing anorexigenic neuropeptides proopiomelanacortin (POMC) and cocaine-and amphetamine-regulated transcript (CART) (Schwartz et al., 2000; Tschop et al., 2000). NPY/AGRP-containing neurons terminate on POMC/CART-containing neurons and release GABA when activated by ghrelin. Thus, ghrelin directly stimulates NPY/AGRP neurons and indirectly inhibits the anorexigenic actions of POMC/CART-containing neurons (Cowley et al., 2003; Holst & Schwartz et al., 2004).
Although it is apparent that ghrelin regulates energy intake via modulation of CNS feeding circuitry, the GHS-R ligand is also expressed outside of the hypothalamus (Guan et al., 1997; Zigman et al., 2006), including in dopaminergic and GABAergic neuronal subpopulations of the ventral tegmental area (VTA). Ghrelin is known to increase firing rate of VTA dopamine (DA) neurons, and DA turnover in the ventral striatal nucleus accumbens (Abizaid et al., 2006; Guan et al., 1997; Jerlhag et al., 2007). In further support of its stimulation of mesolimbic dopaminergic circuitry, peripheral administration of ghrelin can augment cocaine-enhanced locomotor activity and induce cocaine conditioned place preference at sub-threshold doses (Davis et al., 2007; Wellman et al., 2005). Moreover, GHS-R antagonists directed at the VTA block the ghrelin-mediated feeding response, while administration of ghrelin in this region stimulates both locomotor activity and feeding responses (Abizaid et al., 2006; Jerlhag et al., 2006). This mesolimbic circuitry has long been implicated in reinforcement of both natural rewards and addictive drugs (Everitt & Wolf, 2002; Wise 1981), and thus suggests the mechanism of ghrelin-mediated hyperphagia is broader than that currently ascribed (Olszewski et al., 2008).
Here we examined whether pharmacological antagonism of GHS-R affects motivational incentive learning, as reflected in Pavlovian-to-instrumental transfer (PIT), in which Pavlovian conditioned stimuli (CSs) modulate the performance of ongoing instrumental behavior (Rescorla & Solomon, 1967). PIT depends on the intact function of the mesolimbic system, where GHS-R is expressed (El-Amamy & Holland, 2007; Murschall & Hauber, 2006; Wyvell & Berridge, 2000), and thus might be sensitive to GHS-R activity. In addition, while manipulations of ghrelin have been shown to lead to changes in ingestive behavior (Asakawa et al., 2003; Wren et al., 2000) it remains unclear whether these effects result from changes in gustatory stimulation, orosensory positive feedback, conditioned/unconditioned negative feedback, or some combination thereof (Davis & Smith, 1992; Smith, 2001; Spector et al., 1998). Thus, we performed microstructural analysis of licking responses for liquid sucrose, to establish which component(s) of ingestive behavior are influenced by GHS-R inactivation.
Materials and Methods
Subjects
Behavioral testing was conducted using male C57BL6/J strain mice (n=24), purchased from Jackson Laboratories, and transferred to the Neurogenetics and Behavior Center (NBC), Johns Hopkins University, Baltimore, MD, at 6–8 weeks of age. On arrival mice weighed ≈ 30 grams, and were housed 3–4 per cage and kept under a 12 hr light/dark cycle (lights on 7:00 – 19:00). All handling, training and testing occurred within the light cycle between 9:00 and 17:00. Mice were food deprived to 85% of their ad-libitum weight 3 d prior to the start of training, by restricting food access to a single daily meal. The experiment was conducted in two replications, under the auspices of the Institutional Animal Care and Use Committee.
Apparatus
Behavioral training took place in eight identical chambers, which consisted of aluminum front and back walls, clear polycarbonate sides and ceiling, and floor comprised of parallel, stainless-steel rods, all housed in sound-attenuating shells (Med Associates, St. Albans, VT). Chambers were outfitted with a food cup into which 0.1 ml of liquid reward could be delivered. Food cups were connected to programmable vacuums, which could suction off reward when desired. Infrared photocells installed in the food cup monitored the time spent and number of entries into the cup. The food cups also contained lickometers (Schoenbaum et al., 2001), which used fiber optics to introduce a light beam through the fluid-air interface of a fluid bolus. This system allowed for the detection and accurate time-stamping of licks, detected as disturbances in the amplified light surface within the interface when the sucrose fluid was contacted. These data were subsequently analysed for licking microstructure (Test Phase 2). Within each chamber, retractable ultra-sensitive mouse levers (Med Associates) were available to the right and left sides of the food cup. Each chamber also contained a speaker that delivered a 3 kHz tone (amplitude set at ≈ 80 dB), and a heavy-duty 10 Hz clicker module mounted outside of the chamber on the opposite side from the food cup. Ambient light was supplied by a 28V, 100mA house light mounted inside the sound attenuating shell. For test phase 2, an additional set of 8 chambers, identical to the training chambers, were used. An IBM-compatible computer, with Med PC Software, controlled the apparatus and recorded data.
Drugs
Prior to the test phases, the GHRP-6 [D-Lys3] ghrelin receptor antagonist (Phoneix Peptides) was dissolved in sterile sodium chloride solution (0.9%), and administered 250 µl intraperitoneally (IP) at a concentration of 50, 100, or 200 nmol, depending on the group allocation for each mouse. For the control condition, matched volumes of saline were administered IP. These doses of the antagonist were chosen as the higher doses (200 nmol) had been previously shown to suppress food intake in mice (Asakawa et al., 2003).
Behavioral Training Procedures
Initially mice received a single food cup training session for two days, where 60 deliveries of sucrose occurred (0.1 ml of 10% w/v for 10 s) on a random time (RT) 30 s schedule.
Following food cup training, mice received single daily Pavlovian training sessions for three days. Each session consisted of 10 pseudorandomly presented 2 min cues: 5 reward-paired (CS+; either tone or clicker) and 5 nonreward-paired (CS−). For half the mice the tone served as CS+ and the clicker as CS−, while the remaining mice received the opposite contingencies. Sucrose was delivered on an RT 30 s schedule during the CS+, but not during the CS− trials. Any remaining sucrose at the end of a CS+ trial was removed via the vacuum. Trials were separated by a 2 min variable intertrial interval (ITI).
Next, mice were trained to perform a lever-press response for the sucrose reward in three 30-min sessions. At this time both levers were available; presses on the lever designated active resulted in sucrose delivery, while presses on the other, inactive lever did not. Active and inactive lever locations (i.e., left and right-side) were fully counterbalanced across mice. To facilitate lever responding on the first day, a small amount of sweetened condensed milk solution was placed on the active lever. Each response on the active lever resulted in sucrose delivery (i.e. a continuous reinforcement schedule).
Subsequently mice received Pavlovian and instrumental training sessions on alternate days. Pavlovian sessions continued as previously described, while the response-reinforcement schedule associated with active lever instrumental training increased to a variable interval (VI) 30 s then, after 3 more instrumental sessions to a VI 60 s, and for the final 3 sessions mice were trained on a VI 90 s schedule. Training was complete when mice received 12 separate sessions of both Pavlovian and instrumental training.
Test Phase 1: Pavlovian to Instrumental Transfer
Twenty-four hrs following the final training session, mice were divided equally (i.e., n=8) into groups (50 nmol, 100 nmol and 200 nmol) counterbalanced according to previous Pavlovian and instrumental conditioning histories. Twenty-minutes prior to the PIT test, half the mice from each group were injected with the ghrelin antagonist, at the dose relevant to their group assignment, while the remaining mice were injected with saline. In the test, mice had access to both levers but no rewards were delivered at any time. Thus the test was conducted in extinction. After a 6-min initial stimulus-free period intended to reduce and stabilize lever press response rates, each cue (CS+, CS−) was presented 5 times in a pseudorandom order, separated by a 2-min fixed interval, with the criterion that the same cue would not be repeated more than twice consecutively. We expected Pavlovian-instrumental transfer to be expressed as an augmentation of the rate of ongoing lever-pressing by CS+, but not CS−.
Test Phase 2: sucrose consumption test
Immediately following the completion of the first testing phase (approximately 60 min post injection), mice were transferred to a new testing chamber and given a 30 min consumption test. During this test session, the photo-beam lickometers were used. Sucrose was available at the start of the session, and additional reward was delivered every 25 licks, to allow free sucrose access for the duration of the test. Due to a computer malfunction licking microstructure data for 4 animals in 100 and 200 nmol were lost for one test day.
Retraining and test
On completion of test phases 1 and 2, mice received three additional Pavlovian and instrumental training sessions, prior to a final PIT and consumption test. These tests were identical to the previous Test Phases, with the exception that drug contingencies (i.e., GHRP-6 [D-Lys3] ghrelin receptor antagonist, or saline) were reversed for each mouse.
Data Analysis: lick microstructure
Microstructure data were analysed according to previously established parameters (Davis & Smith, 1992; Spector et al., 1998). Total consumption was measured as the total number of licks made by mice during test phase 2. Inter-lick intervals (ILIs) were analysed in several ways. The majority of ILIs have been shown to fall < 250 ms, and are normally distributed below this interval (Smith, 2001). A second distribution of ILIs 251 ms-500 ms, that reflect brief interruptions in licking intake have been identified. The third region ILIs >500 ms describes longer interruptions of licking and unlike the previous two, this final distribution is skewed.
To measure licking responses associated with the dynamics of ingestive behavior, we defined two measures: size of licking bursts and number of licking bursts. A licking burst was defined as two or more consecutive licks with no ILI exceeding 1 s (Spector et al., 1998). The size of the burst was calculated by the total number of licks in all bursts in the meal, while the number of these events reflected the number of licking bursts. The size of licking bursts reflects pre-ingestive influences that typically maintain ingestive behavior via signals that include excitation of gustatory, olfactory and trigeminal receptors, whereas the number of licking bursts is associated with post-ingestive effects and typically declines as these influences become more apparent.
Statistical analysis
The training data (rates of food cup entry and lever pressing) were analyzed with two-way ANOVA with group (50 nmol, 100 nmol, 200 nmol) as a between-subjects variable and either cue (CS+, CS−) or lever (active, inactive) as a within-subjects variable. The PIT test lever press data were analyzed with three-way ANOVA, with the between-subject variable of group (50 nmol, 100 nmol, 200 nmol) and within-subject variables of period (CS+, CS−, ITI) and drug state (antagonist- or saline-treated). The consumption test licking data were analyzed with three-way ANOVA, with the between-subject variable of group (50 nmol, 100 nmol, 200 nmol) and within-subject variables of time bin (5 min periods) and drug state (antagonist- or saline-treated). The three-way ANOVAs were followed by individual two-way ANOVAs or t-tests, as appropriate.
Results
Pavlovian and Instrumental training
For the mean of the final two Pavlovian training sessions, all mice showed similar rates of entry into the food cup during CS+ (Entries per min ± SEM; 50 nmol = 6.48 ± 0.93; 100 nmol = 4.78 ± 1.73; 200 nmol = 4.64 ± 0.64) compared to CS− (50 nmol = 0.88 ± 0.15; 100 nmol = 1.07 ± 0.21; 200 nmol = 0.61 ± 0.16). All data from one subject in the 100 nmol condition were removed from this and all subsequent analyses because of excessive entries in the food cup during the CS+ (25 entries per minute, well beyond two SDs above the mean for this group). Two-way ANOVA with group (50 nmol, 100 nmol, 200 nmol) and cue (CS+, CS−) as variables, revealed a main effect of cue (F(1,20) = 52.84, p<0.00001), but no effect of group or group X cue interaction (F’s<1; p’s>0.49).
Similarly, by the end of instrumental training lever response rates were significantly greater for the active (50 nmol = 8.75 ± 0.93; 100 nmol = 8.47 ± 0.99; 200 nmol = 9.64 ± 0.87) compared to the inactive lever (50 nmol = 0.59 ± 0.11; 100 nmol = 0.45 ± 0.05; 200 nmol = 0.52 ± 0.14). Group X response (active, inactive) ANOVA confirmed a main effect of response only (F(1,20) = 258.84, p<0.00001), with no effects of group or interaction (F’s<1, p’s>0.64). Thus, prior to test and administration of the D-Lys3-GHRP-6 antagonist, all mice displayed similar levels of Pavlovian and instrumental responding.
Test Phase 1: Pavlovian to Instrumental transfer
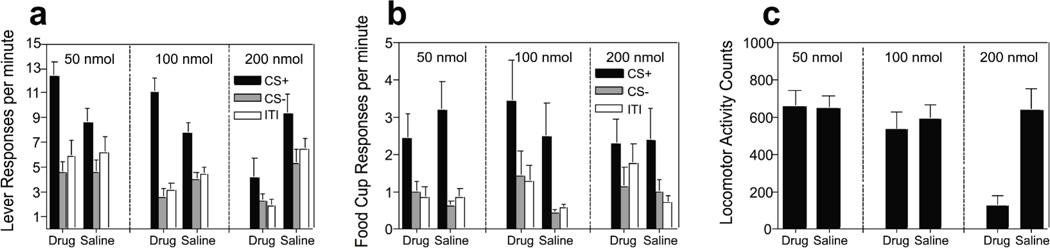
The data of primary interest, those from the Pavlovian-to-instrumental test stage, are depicted in Figure 1a. All groups of mice displayed a similar PIT effect when under saline-treated control conditions as evidenced by an increase in active lever responding during the CS+, compared to responding during the CS− or ITI (right set of bars in each panel of Figure 1a). Interestingly, the effects of the antagonist on PIT (left set of bars in each panel) varied as a function of dose, such that mice injected with 50 nmol or 100 nmol showed an elevation of PIT relative to their saline-treated control condition. By contrast, mice in the 200 nmol group showed a reduction in responding to both cues relative to their saline control condition. A three-way, group (50 nmol, 100 nmol, 200 nmol) X period (CS+, CS−, ITI) X drug state (antagonist- or saline-treated) ANOVA indicated no effect of group (F(2,20) = 2.19, p=0.14) or state (F(1,20) = 3.63, p=0.07), but a main effect of period (F(1,20) = 60.76, p<0.00001), and importantly, a significant three-way interaction (F(4,,20) = 6.96, p<0.001). To interpret the nature of this interaction, separate period X drug state within-subject ANOVAs were conducted for each group. For the 50 nmol group this analysis revealed no main effect of state (F(1,7) = 0.19, p=0.19), a main effect of period (F(2,14) = 22.69, p<0.0001) and a significant interaction between the two variables (F(1,7) = 20.85, p<0.0001), with CS+ (F(1,7) = 10.97, p=0.01) but not CS− (F(1,7) = 0.03, p=0.86) or ITI responses (F(1,7) = 0.25, p=0.63) differing as a function of drug state. Similarly for the 100 nmol group the analysis revealed a main effect of period (F(2,12) = 60.26, p<0.0001), no effect of drug state (F(1,6) = 0.11, p=0.74) and a significant interaction between the two variables (F(2,12) = 12.14, p<0.01), with CS+ responses (F(1,6) = 6.06, p<0.05), but not CS− (F(1,6) = 4.2, p=0.09) or ITI responses (F(1,6) = 2.20, p=0.18) differing between antagonist and saline-treated conditions. Thus, the drug affected only responding to CS+ in these groups. By contrast, the analysis for mice in the 200 nmol condition revealed a main effect of period (F(2,14) = 6.41, p=0.01), a main effect of state (F(1,7) = 15.97, p<0.01), but no interaction between the two variables (F(2,14) = 1.43, p=0.27). In addition, CS+ (F(1,7) = 11.67, p=0.01), CS− (F(1,7) = 6.04, p<0.05) and ITI active lever responses (F(1,7) = 18.52, p<0.01) under the antagonist were significantly reduced compared to the control condition.
Figure 1. Test Phase 1: Pavlovian to instrumental transfer test stage results.
(a) Active lever response rates per minute during presentations of CS+ (black bars), CS− (grey bars) or ITI (open bars) for mice injected with low (50 nmol), moderate (100 nmol) or high (200 nmol) doses of GHS-R antagonist, or saline. (b) Food cup entries per minute during presentation of CS+ (closed bars), CS− (grey bars) or ITI (open bars) following, saline, low, medium, or high antagonist injections. (c) Locomotor activity levels during period of no stimulus presentation (ITI) following, saline, low, medium, or high antagonist injections. Error bars indicate standard error of the mean (SEM).
Despite these differences in PIT, the antagonist failed to influence entries into the food cup, with all mice displaying greater food cup entries during CS+ compared to CS− or ITI (Figure 1b). Thus, the antagonist affected only motivational learning manifest in PIT, and not simple Pavlovian approach responses. Three-way ANOVA revealed a main effect of period (F(1,20) = 18.12, p<0.00001), with no effect of group or state and no interactions among any of the variables (largest F-value; group X state X period interaction, F(4,40)=1.68, p=0.17).
Finally, the antagonist was found to have a dramatic effect on baseline locomotor activity scores when administered at a dose of 200 nmol (Figure 1c). Two-way ANOVAs with group as a between-subject variable and drug state as a within-subject variable revealed a main effect of group (F(2.20) = 6.16, p<0.01), drug state (F(1.20) = 32.27, p<0.001) and interaction between the two variables (F(2,20) = 7.43, p<0.01), with activity counts under the 200 nmol drug condition differing significantly from all other scores (p’s <0.001).
Test Phase 2: Sucrose consumption tests, licking microstructure
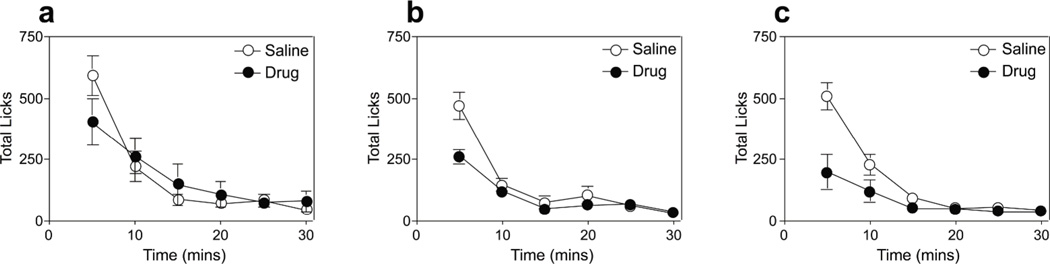
All mice in the saline-treated control condition displayed a high rate of initial intake followed by a steep slope of decline in ingestion rate (Figure 2). This initial avid consumption was inhibited following 50 nmol injections of the D-Lys3-GHRP-6 antagonist (Figure 2a) and was further reduced in 100 nmol and 200 nmol conditions (Figures 2b & 2c). Three-way ANOVA with variables of group, time bin and drug state revealed no main effect of group (F(2,13) = 1.81, p=0.2), a main effect of state (F(1,13) = 26.69, p<0.0001) and time bin (F(5,65) = 64.02, p<0.00001), and a significant group X drug state interaction (F(2,13) = 7.47, p<0.01). Post-hoc comparisons of the significant interaction revealed significant differences in consumption for 100 and 200 nmol groups when tested under the antagonist, compared to all other test combinations (p’s <0.01).
Figure 2. Test Phase 2: Sucrose consumption test.
Sucrose intake (licks/ 5-min) under both antagonist (closed circles) and saline-treated control conditions (open circles) for (a) 50 nmol (b) 100 nmol, and (c) 200 nmol groups. Error bars indicate SEM.
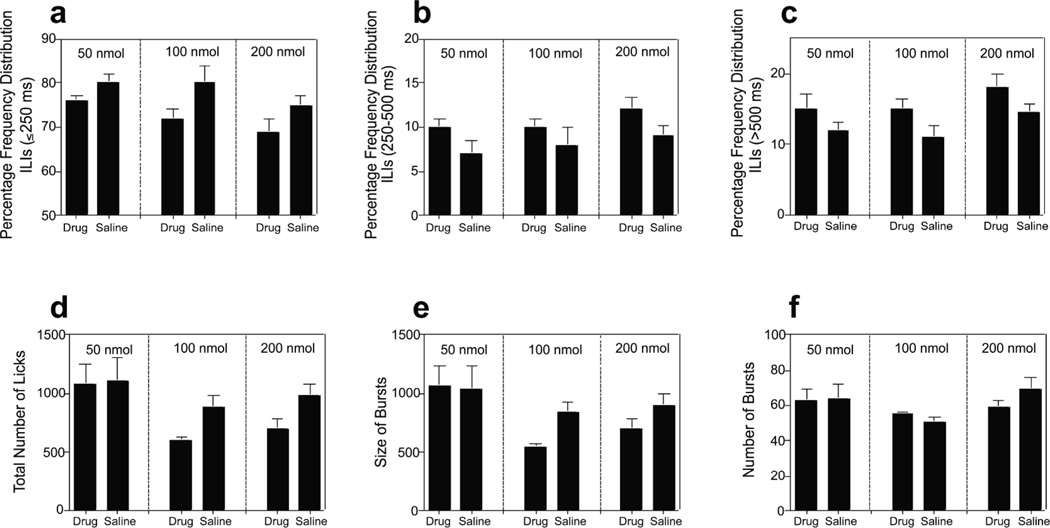
The inter-lick-intervals (ILIs) were analysed based on three different temporal regions. The majority of ILIs fall ≤ 250 ms; a second region (251ms-500 ms) reflects brief interruptions of licking; the final region (>500 ms) describes longer interruptions of licking. Analysis of the frequency distribution of ILIs showed that the D-Lys3-GHRP-6 antagonist resulted in a significant decrease in the proportion of ILIs < 250ms (F(1,13) = 23.94, p<0.001); and a subsequent increase in ILIs 251ms-500 ms (F(1,13) = 12.72, p<0.01) and >500 ms (F(1,13) = 26.41, p<0.001) (Figures 3a–c).
Figure 3. Test Phase 2: Licking microstructure for 50, 100 and 200 nmol groups.
Frequency distribution of ILI’s (a) <250 ms (b) 250–500 ms (c) >500 ms. (d) Total number of licks (e) size of licking bursts (f) number of licking bursts for the sucrose consumption tests. Error bars indicate SEM.
An analysis of the overall consumption, as reflected by the total number of ILIs revealed significant reduction in consumption for 100 nmol (t(3) = 2.91, p=0.05) and 200 nmol groups (t(7) = 2.38 p=0.04), but not the 50 nmol group (t(3) = 0.59, p=0.59) relative to their saline-treated control condition (Figure 3d). This difference was related to a reduction in the size of the licking bursts following administration of the antagonist for both 100 nmol (t(3) = 3.09, p=0.05) and 200 nmol groups (t(7) = 2.44, p=0.04), but not for the number of licking burst episodes for each group: (t(3) =2.56, p=0.09) and (t(7) =1.39, p=0.2), respectively. These two measures are thought to reflect differential properties that influence meal intake. The size of licking bursts increases as a function of sucrose concentration (Spector et al., 1998) and is thought to reflect measures of palatability independent of post-ingestive influence, whereas the number of bursts is believed to reflect the potency of post-ingestive negative feedback (Smith, 2001). These data suggest that the antagonist served to suppress food intake for 100 and 200 nmol groups by reducing palatability of the sucrose.
Discussion
This study demonstrates that peripheral administration of a GHS-R antagonist has dissociable effects on aspects of ingestive behavior and reward learning in mice. Consistent with previous data, mice receiving 100 or 200 nmol doses of GHRP-6 [D-Lys3] antagonist showed a general reduction in sucrose consumption, which our licking microstructure analysis suggests may reflect influences on tastant palatability associated with GHS-R inactivation. By contrast, 50 and 100 nmol injections of the antagonist led to a significant increase in PIT test active lever responding during the CS+, but not CS− or ITI, compared to their saline-treated control conditions. This outcome suggests that in the absence of GHS-R mediated activity, the motivational significance of the Pavlovian excitor is enhanced. Importantly, this enhancement was not simply due to motoric actions of drug administration because responding to CS− after these doses did not differ from CS− responding in saline-treated control conditions. Furthermore, there were no changes in other response measures that might be expected to be sensitive to motor deficits, including locomotor activity and food cup entries. Finally, 200 nmol injections of GHRP-6 [D-Lys3] antagonist led to a reduction in responding to both CS+ and CS−, and a substantial reduction in locomotor activity, indicating non-specific performance deficits associated with this dose.
Although most studies have focused on the role of ghrelin in food intake, previous reports indicate it plays a role in aspects of reward learning as well. For example, central injections of growth hormone-releasing factor lead to increases in progressive ratio breakpoint responding for food (Feifel & Vaccarino, 1990), and peripheral administration of ghrelin elevates cocaine-enhanced locomotor activity and induces cocaine conditioned place preference at sub-threshold doses (Davis et al., 2007; Wellman et al., 2005). These effects may be linked to GHS-R activity in classical reward pathways. GHS-R is expressed in mesolimbic dopaminergic circuitry, particularly the ventral tegmental area (VTA) and substantia nigra pars compacta (Zigman et al., 2006), where its ligand promotes generation of action potentials and synapse formation in VTA. Notably, targeted injections of the GHS-R antagonist BIM28163 in this region block the orexigenic actions of peripherally administered ghrelin (Abizaid et al., 2006). Since transient inactivation of the VTA reduces the display of PIT (Murschall & Hauber, 2006), our observation that GHS-R inactivation augmented transfer was unexpected. However, other data suggest that VTA’s influence on PIT within broader neural systems may be more complex. For example, El-Amamy and Holland (2007) found that functional disconnection of VTA from the amygdala central nucleus (CeA), another brain region critical to PIT, rescued the deficit in PIT produced by unilateral damage to VTA. This result suggests that VTA and CeA may normally interact in an inhibitory fashion in PIT, providing a basis for the enhancement of PIT after GHS-R inactivation in VTA.
We have noted other dissociations between PIT and ingestive behavior in our laboratory as well. For example, in addition to facilitating operant responding, Pavlovian cues associated with food can potentiate ingestive behaviors in food-sated animals, resulting in consumption of a large amount of food in a relatively short period of time (Holland et al., 2002; Petrovich et al., 2002, 2007; Weingarten, 1983). Whereas this cue-potentiated feeding is absent in rats with lesions of the basolateral amygdala (BLA) and unaffected by lesions of the CeA, PIT was eliminated by CeA lesions but not by BLA lesions (Holland & Gallagher, 2003). Thus, the different effects of GHS-R antagonist on PIT and the ingestion of sucrose may reflect different consequences of GHS-R inactivation within distinct brain circuits that mediate those activities.
The different effects of GHS-R antagonist on PIT and sucrose ingestion indicate that it is simplistic to describe ghrelin’s effects as increasing some unitary motivational state such as “hunger” that drives both procurement and consumption of food. In this regard it is notable that the results of our licking microstructure analysis suggest that the antagonist reduced sucrose consumption at least in part by reducing its palatability— the evaluation of its sensory properties. By contrast, many investigators have suggested that when a single reinforcer is used, PIT may instead reflect a cue-driven shift in general motivational state, unrelated to specific sensory or palatability aspects of the reinforcer (Berridge, 1996; Corbit & Balleine, 2005; Holland, 2004). Our observation that GHS-R inactivation elevated “general PIT” may suggest that food-paired cues interact with ghrelin and enhance food-procurement activities by influencing a general arousal state (Szentirmai et al., 2007). Interestingly, general PIT, as examined in the present study, is often contrasted with a second form of transfer that is obtained when multiple reinforcers are used. This “reinforcer-selective PIT”, which unlike general PIT depends on BLA rather than CeA function (Corbit & Balleine, 2005), reflects the ability of food-paired cues to enhance responding based on the specific sensory features (rather than general motivational significance) of reinforcers (Dickinson and Dawson, 1987). It would be of interest to determine whether ghrelin antagonist might depress PIT after training with multiple reinforcers are used, by reducing the palatability of those reinforcers. While studied mainly in animals, these differential forms of transfer may also be relevant to human behaviors associated with food procurement. For instance, food-related signals (e.g., the golden arches®) may come to direct food procurement by modulating behavior based on the evocation of motivational states such as hunger (i.e., general PIT), while also modulating behavior by evoking thoughts or memories of the specific foods they predict (e.g., a Big-Mac®; reinforcer-specific PIT).
As noted previously, our results suggest that, on contact with food, ghrelin appears to drive ingestive behavior by altering the palatability associated with the tastant. Previous studies have shown peripheral and central injections of ghrelin induce elevated food intake under a variety of conditions (Bomberg et al., 2007; Naleid et al., 2005; Tschop et al., 2000; Wren et al., 2000), while antagonism of its receptor leads to pronounced suppression of ingestive behavior (Asakawa et al., 2003; Esler et al., 2007). These effects on feeding have been limited to analyses of consumption across large time frames, with parameters that usually fail to distinguish between a variety of pre- and post-ingestive factors that influence overall intake. Pre-ingestive factors include measures of palatability, taste evaluation and gustatory stimulation that typically maintain ingestive behavior via signals that include excitation of gustatory, olfactory and trigeminal receptors (Davis & Smith, 1992). On the other hand, post-ingestive factors typically provide negative feedback leading to a suppression of intake mediated through humoral and neural stimuli acting on chemosensors in the small intestine and stomach (Davis & Smith, 1992; Davis et al., 1993; Davis et al., 1999; Smith, 2001; Spector et al., 1998). At the microstructural level it is possible to distinguish between these variables. Pre-ingestive measures are commonly inferred from the rate with which rodents initiate meal consumption and the size of discontinuous licking bursts that occur throughout the feeding session. Both measures systematically increase following increases in tastant concentration and are unaffected by sham-feeding preparations (Davis & Smith, 1992; Spector et al., 1998). By contrast, the number of interruptions in licking bursts increases with sham-feeding preparations and displays an inverted-U-shaped function of concentration with sucrose, indicating sensitivity to post-ingestive feedback.
Interestingly, increases in dose of the antagonist led to reductions in the initial rate of licking, and size, but not number of licking bursts. These results point to a role for ghrelin in mediating tastant intake by influencing measures associated with taste evaluation and palatability (Smith, 2001). Consistent with these findings GHS-R expression has been identified in nucleus of the tractus solitarius (NTS) and the lateral parabrachial nucleus (Guan et al., 1997; Zigman et al., 2006), areas that receive extensive connections from intra-lingual taste nerves (the chorda tympani and the glossopharyngeal nerve; Norgren, 1995; Yamamoto, 2006). However, given that 200 nmol GHS-R led to significant reductions in locomotor activity, it is difficult to interpret whether the effects on food intake noted in the current and past studies (e.g., Asakawa et al., 2003) are due in part to non-specific motoric effects at this dose.
While ghrelin has been shown to elicit voracious feeding under a variety of conditions (Tschop et al., 2000; Wren et al., 2000), recent data suggest this ligand plays a role in learning and memory (Carlini et al., 2007; Diano et al., 2006), reward learning (Davis et al., 2007; Wellman et al., 2005) and plasticity (Abizaid et al., 2006; Diano et al., 2006). We provide additional evidence supporting dissociable roles for ghrelin, where GHS-R inactivation leads to an augmentation of transfer based on the general motivational significance of the Pavlovian excitor, and suppression in consumption due to reductions in taste evaluation and palatability. Future studies assessing GHS-R function in the interaction of homeostatic and non-homeostatic control over food intake (e.g., cue-potentiated feeding) and procurement (e.g., reinforcer-selective PIT) may be particularly informative for elucidating the mechanisms involved in ghrelin-driven behavior.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/bne.
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. Journal of Clincal Investigation. 2006;116(12):3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, Kasuga M. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52(7):947–952. doi: 10.1136/gut.52.7.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. Journal of Pharmacology and Experimental Therapeutics. 2002;302(2):822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: Brain substrates of wanting and liking. Neuroscience and Biobehavioral Reviews. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Bomberg EM, Grace MK, Wirth MM, Levine AS, Olszewski PK. Central ghrelin induces feeding driven by energy needs not by reward. Neuroreport. 2007;18(6):591–595. doi: 10.1097/WNR.0b013e3280b07bb5. [DOI] [PubMed] [Google Scholar]
- Carlini VP, Gaydou RC, Schioth HB, de Barioglio SR. Selective serotonin reuptake inhibitor (fluoxetine) decreases the effects of ghrelin on memory retention and food intake. Regulatory Peptides. 2007;140(1–2):65–73. doi: 10.1016/j.regpep.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. Journal of Neuroscience. 2005;25(4):962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behavioral Neuroscience. 1992;106(1):217–228. [PubMed] [Google Scholar]
- Davis JD, Smith GP, Miesner J. Postpyloric stimuli are necessary for the normal control of meal size in real feeding and sham feeding rats. American Journal of Physiology. 1993;265:R888–R895. doi: 10.1152/ajpregu.1993.265.4.R888. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP, Singh B, McCann DP. Increase in intake with sham feeding experience is concentration dependent. American Journal of Physiology. 1999;277:R585–R571. doi: 10.1152/ajpregu.1999.277.2.R565. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Dawson GR. Pavlovian Processes in the Motivational Control of Instrumental Performance. Quarterly Journal of Experimental Psychology Section B-Comparative and Physiological Psychology. 1987;39(3):201–213. [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nature Neuroscience. 2006;9(3):381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- El-Amamy H, Holland PC. Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation. European Journal of Neuroscience. 2007;25(5):1557–1567. doi: 10.1111/j.1460-9568.2007.05402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler WP, Rudolph J, Claus TH, Tang W, Barucci N, Brown SE, Bullock W, Daly M, Decarr L, Li Y, Milardo L, Molstad D, Zhu J, Gardell SJ, Livingston JN, Sweet LJ. Small-molecule ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology. 2007;148(11):5175–5185. doi: 10.1210/en.2007-0239. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. Journal of Neuroscience. 2002;22(9):3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Vaccarino FJ. Central injections of growth hormone-releasing factor increase operant responding for food reward. Progress in Neuropsychopharmacology and Biological Psychiatry. 1990;14(5):813–820. doi: 10.1016/0278-5846(90)90053-j. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Research Molecular Brain Research. 1997;48(1):23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiology & Behavior. 2002;76(1):117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- Holland PC. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. Journal of Experimental Psychology-Animal Behavior Processes. 2004;30(2):104–117. doi: 10.1037/0097-7403.30.2.104. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothamamic circuit allows learned cues to override satiety and promote eating. Journal of Neuroscience. 2002;22(19):8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Gallagher M, Holland PC. Learned contextual cue potentiates eating in rats. Physiology & Behavior. 2007;90(2–3):362–367. doi: 10.1016/j.physbeh.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst B, Schwartz TW. Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends Pharmacological Science. 2004;25(3):113–117. doi: 10.1016/j.tips.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addiction Biology. 2006;11(1):45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addiction Biology. 2007;12(1):6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Murschall A, Hauber W. Inactivation of the ventral tegmental area abolished the general excitatory influence of Pavlovian cues on instrumental performance. Learning and Memory. 2006;13(2):123–126. doi: 10.1101/lm.127106. [DOI] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26(11):2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Norgren R. Gustatory system. In: Paxinos D, editor. The rat nervous system. San Diego: Academic Press; 1995. pp. 751–771. [Google Scholar]
- Olszewski PK, Schioth HB, Levine AS. Ghrelin in the CNS: from hunger to a rewarding and memorable meal? Brain Research Reviews. 2008;58(1):160–170. doi: 10.1016/j.brainresrev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL. 2-Process Learning Theory - Relationships between Pavlovian Conditioning and Instrumental Learning. Psychological Review. 1967;74(3):151. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Smith GP. John Davis and the meanings of licking. Appetite. 2001;36(1):84–92. doi: 10.1006/appe.2000.0371. [DOI] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behavioral Neuroscience. 1998;112(3):678–694. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- Szentirmai E, Kapas L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. American Journal of Physiology- Regulatory, Integrative and Comparative Physiology. 2007;292:R575–R585. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Weingarten HP. Conditioned cues elicit feeding in sated rats – a role for learning in meal initiation. Science. 1983;220(4595):431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Davis KW, Nation JR. Augmentation of cocaine hyperactivity in rats by systemic ghrelin. Regululatory Peptides. 2005;125(1–3):151–154. doi: 10.1016/j.regpep.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain dopamine and reward. In: Cooper SD, editor. Theory in psychopharmacology. London: Academic; 1981. pp. 102–122. [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50(11):2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141(11):4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward "wanting" without enhanced "liking" or response reinforcement. Journal of Neuroscience. 2000;20(21):8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. Neural substrates for the processing of cognitive and affective aspects of taste in the brain. Archives of Histology Cytology. 2006;29(4):243–255. doi: 10.1679/aohc.69.243. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and mouse brain. Journal of Comparative Neurology. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]