Abstract
A prospective case-control study was conducted in a tertiary care pediatric intensive care unit (PICU) to evaluate the use of near infrared spectroscopy (NIRS) for the detection of intracranial hemorrhage (ICH) in children. Subjects 0–14 years of age who had a computed tomography (CT) scan of the head performed as part of clinical care were eligible for enrollment. The children were stratified into two groups based on whether the CT was normal or abnormal. Children in the abnormal imaging cohort were further divided into those with ICH and those with other abnormalities of the brain parenchyma (contusions, diffuse axonal injury [DAI], or cerebral edema) or fractures. NIRS measurements were performed on all subjects within 24 h of head CT. The NIRS operator was blinded to the presence or absence of ICH. NIRS measurements were performed in eight different scalp locations (four bilaterally). A total of 103 measurements were made. The optical density (OD) was automatically calculated by comparing the reflected and diffused optical signal. A ΔOD>0.2 between hemispheres in any scalp location was considered abnormal. NIRS was performed in a total of 28 subjects: 7 had normal imaging and 21 had abnormal imaging. Of those with abnormal imaging, 12 had ICH. The sensitivity and specificity of NIRS at detecting ICH was 1.0 and 0.8, respectively. The positive and negative predictive values were 0.8 and 1.0, respectively. In conclusion, NIRS correctly identified all cases of ICH in this pilot study. Our preliminary results suggest that NIRS may be beneficial in the evaluation of a child with possible ICH.
Key words: abusive head trauma, children, intracranial hemorrhage, near infrared spectroscopy, traumatic brain injury
Introduction
Head injuries are a common reason for emergency department (ED) visits and hospital admissions in children. In the United States, childhood blunt head trauma accounts for ∼650,000 ED visits, 50,000 hospitalizations, and 3000 deaths annually (Centers for Disease Control and Prevention, 2000,2002). Traumatic brain injury (TBI) from these insults, either accidental or inflicted, accounts for more than 70% of fatal childhood injuries. Evaluation and assessment of children in the ED can be difficult, and brain injuries that are subtle or without external signs of trauma can go undetected. Rapid triage and assessment of victims of suspected TBI is crucial to determine if underlying brain injury exists, and to further prevent secondary neurologic injury that could increase overall morbidity and mortality.
For infant/children victims of closed head injury (blunt head trauma or abusive head trauma [AHT]), there are compelling reasons to develop a sensitive tool to detect intracranial hemorrhage (ICH). In this population, the history provided by the caretaker may be unreliable and the patients are generally non-verbal. The neurological examination is limited and the Glasgow Coma Scale (GCS) score (the most widely used objective measure of mental status in TBI) has not been validated. No single physical sign reliably indicates the presence of an ICH, and focal neurological findings are found in only a fraction of patients with surgical hematomas (Greenes and Schutzman, 1999; Schutzman et al, 2001). Typical presentations of infants and young children with ICH are non-specific and include fussiness, vomiting, seizures, lethargy, and poor feeding. A unilateral dilated pupil is the most reliable lateralizing sign when it occurs in a child with a hematoma. However, the presence of this finding does not clearly identify the presence of a surgical hematoma and is often a late finding signaling herniation (Chestnut et al., 1994). Computed tomography (CT) is the gold standard for identification and localization of intracranial pathology in trauma victims, and ICH is a common CT finding in this setting. Early detection of ICH can improve triage and allow earlier institution of central nervous system (CNS)-directed therapies that can minimize secondary brain injury and improve outcome. Moreover, missed diagnoses of ICH can have catastrophic consequences. Unfortunately, CT scans represent an imaging modality that is not without risk.
The frequency and use of CT has increased dramatically in the past decade. Coren and associates (1998) reported a 63% increase in requests for pediatric CT scans from 1991–1994 in the U.S., and in the decade from 1995–2005 CT use more than doubled (Kupperman et al., 2009). Brenner and colleagues (2001) reported that roughly 600,000 abdomen and head CT studies are performed annually in children<15 years of age in the U.S., and that of these individuals, ∼500 will die from a radiation-related malignancy. In order to minimize radiation exposure while not missing cases of ICH, Kupperman and co-workers (2009) validated a prediction rule to help identify children at low risk of clinically-important TBI.
Thus, research has focused on non-radiation-based methods of detection of ICH, including ultrasound (Veyrac et al., 1990), biomarkers (Hergenroeder et al., 2008; Kochanek et al., 2008), clinical decision rules (Kupperman et al., 2009; Osmond et al., 2010), and near infrared spectroscopy (NIRS). Ultrasound is a useful modality to diagnose intraventricular hemorrhage (IVH) in infants when the anterior fontanelle is still open, but it loses its utility in older infants and children. Biomarkers such as S100β and neuron-specific enolase (NSE) have been identified, and are increased in the serum and cerebrospinal fluid (CSF) of pediatric patients after TBI (Berger et al., 2002,2009; Haqqani et al., 2007; Shore et al., 2007), but these biomarkers have not made it into the clinical arena yet. NIRS is a technique that affords the clinician the opportunity to rapidly assess a patient for the presence of ICH. Of potential relevance, NIRS technology, if shown to be useful, represents a painless, portable, and radiation-free tool.
The principle used in identifying ICH with NIRS is that extravascular blood absorbs NIR light more than intravascular blood, since there is a greater (usually 10-fold) concentration of heme-based proteins such as hemoglobin in the acute hematoma than in the brain tissue, where blood is contained within vessels. Acute hematomas or hemorrhages thus lead to changes in the optical density (OD) of infrared light absorption versus normal brain in the contralateral hemisphere. Consequently, ICH can then be detected by the difference in optical density, ΔOD.
In adults, NIRS technology can diagnose ICH on presentation, as well as late hematomas that develop (Francis et al., 2005; Gopinath et al., 1993,1995; Kahraman et al., 2006; Robertson et al., 1995,1997; Zang et al., 2000). Although the type of hematoma (subdural [SDH] versus epidural [EDH]) cannot be determined, the presence of any hematoma would be useful to triage a patient for CT scanning.
The prior adult data (Francis et al., 2005; Gopinath et al., 1993,1995; Kahraman et al., 2006; Robertson et al., 1995,1997; Zang et al., 2000) suggest that this goal is feasible with NIRS. In initial studies, the sensitivity for identification of traumatic extracerebral (EDH and SDH), and intracerebral hematomas, was 100% and 98%, respectively (Gopinath et al., 1993). Another study showed that the ΔOD was highly predictive of findings on head CT, specifically for SDH, EDH, and intracerebral hematomas, and that the ΔOD occurred prior to increases in intracranial pressure (ICP) or changes in the neurologic examination (Robertson et al., 1997). Robertson and associates (2010) also reported a sensitivity of 68.7% and a specificity of 90.7% in detecting ICH, and supported the potential utility of NIRS in TBI triage.
This is the first study to evaluate the feasibility of using NIRS in the pediatric population, and to describe the sensitivity and specificity of NIRS for identifying ICH exclusively in children. We hypothesized that NIRS would be successful in discriminating between children with and without ICH as diagnosed on head CT.
Methods
This study was approved by the University of Pittsburgh Institutional Review Board (IRB), and consent was obtained from the parents of all children enrolled, and assent was also obtained from capable study subjects. Children 0–14 years of age who were admitted to the pediatric intensive care unit (PICU) and had received a head CT scan as part of their routine clinical care were eligible for inclusion in this study. Exclusion criteria included known pre-existing abnormalities on previous imaging studies, presence of internalized CSF diversion devices that might unblind the investigator to abnormalities (e.g., ventriculo-peritoneal shunt), or skin abnormalities that would limit the ability of the NIRS device to contact the skin (e.g., open lacerations).
Demographic data were collected, including age, sex, race, hair color, past medical history, reason for head CT scan, mechanism of injury if any (e.g., fall or motor vehicle accident), physical examination findings, and results of head CT scan. Information about hair color was collected since darker hair color may be associated with alterations in light absorption and may affect NIRS results. Prior to NIRS determinations, children were stratified into two groups based on CT findings: normal and abnormal. Since the IRB required different consent forms for children with normal and abnormal neuroimaging, and the person obtaining consent was the same as the person performing the NIRS, the operator of the NIRS device knew whether the subject had a normal or abnormal head CT, but was blinded to the nature of the abnormality identified on CT. Children with abnormal imaging were further divided into two groups: (1) those with abnormalities within the brain parenchyma (contusions, diffuse axonal injury, or cerebral edema) or skull fractures; and (2) those with extra-axial collections of blood, defined as blood within the skull but outside the brain. For this study, ICH was defined as hemorrhage within the epidural (EDH), subdural (SDH), or subarachnoid (SAH) space exclusively. Children with punctate hemorrhages (more consistent with DAI) were not considered to have ICH for this study. All CT scan results were obtained from the dictated report from the attending pediatric neuroradiologist uninvolved with this study.
Near-infrared spectroscopy data processing and data collection
A commercially-available NIRS device was used based on the manufacturer's instructions (InfraScan, Philadelphia, PA). This device included a sensor and mobile computing platform for data collection and processing. The sensor included a safe Class I near-infrared diode laser, optically coupled to the patient's head through two disposable light guides. This allows for adequate sensor contact in areas with and without hair. The sensors were placed 4 cm apart with one acting as a light source and the other as a detector of absorbance. The light source uses a 760-nm wavelength, and the detector was covered by a band-pass filter around this wavelength to minimize background light interference. Acquired signals from the detector were digitized and transmitted by a wireless link to the mobile computing platform.
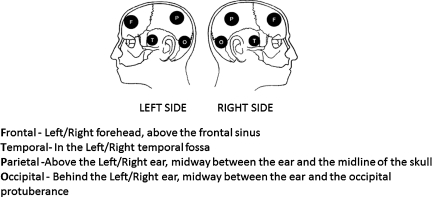
For this study, four regions of the brain were examined bilaterally (frontal, temporal, parietal, and occipital; Fig. 1). Absorbance of light was measured and the device determined the OD within the various regions, and ΔOD of the various regions was electronically calculated using the following formula:
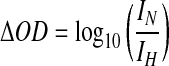 |
FIG. 1.
Scalp locations for near infrared spectroscope placement (F, frontal; P, parietal; T, temporal; O, occipital).
In this formula, IN is the intensity of reflected light on the presumed normal side, and IH is the intensity of reflected light on the presumed abnormal side. Based on previous studies (Robertson et al., 1997), a ΔOD>0.2 was considered abnormal for this study. Negative ΔOD values were reported with right-sided abnormalities; positive ΔOD values represented left-sided abnormalities.
NIRS measurements were performed on all children in the eight previously described locations within 24 h of imaging, and abnormal ΔOD values were confirmed a second time. In subjects with abnormal CT scans, data were collected on the results of the imaging study (type of abnormality, presence or absence of ICH, size of ICH, depth of ICH from scalp, type of hemorrhage [SDH, EDH, or SAH], or intraparenchymal hemorrhage), the location of the hemorrhages, and the presence or absence of scalp swelling.
Statistical analysis
The sensitivity (proportion of ICH correctly identified as such), specificity (proportion of non-ICH correctly identified), positive predictive value (PPV, the probability that a positive test is a true positive), and negative predictive values (NPV, probability that a negative test is a true negative) of NIRS for predicting ICH as detected on head CT was calculated using SPSS version 15.0 (SPSS Inc,. Chicago, IL). All data are presented as mean±standard error of the mean (SEM) unless otherwise specified.
Results
Patient enrollment and demographics
Demographics and diagnoses of the subjects are summarized in Table 1. A total of 29 subjects were originally enrolled in the study; 1 child was excluded when the parents refused NIRS testing, leaving a study population of 28 children. Within this population, 15 children received CT scans for non-traumatic medical conditions, and 13 underwent head CT scans for concerns about traumatic intracranial injury.
Table 1.
Demographic and Clinical Characteristics of the Study Population
| Subject | Age (years) | Gender | Hair color | CT indication | CT | ICH |
|---|---|---|---|---|---|---|
| 1 | 14 | M | Black | DKA; mental status | Abnormal | N |
| 2 | 1.4 | M | Black | Ataxia/ fever | Abnormal | N |
| 3 | 11 | M | Brown | Trauma | Abnormal | Y |
| 4 | 8 | M | Black | Trauma | Normal | N |
| 5 | 0.4 | F | Blonde | Trauma | Abnormal | Y |
| 6 | 4 | F | Blonde | New seizure | Normal | N |
| 7 | 13 | M | Brown | Trauma | Abnormal | N |
| 8 | 0.04 | F | Scant | Fever; r/o meningitis | Normal | N |
| 9 | 0.1 | M | Black | Infection | Normal | N |
| 10 | 0.3 | M | Blonde | New seizures | Abnormal | N |
| 11 | 2 | M | Brown | MVA | Abnormal | Y |
| 12 | 0.1 | M | Black | r/o meningitis | Abnormal | N |
| 13 | 0.003 | M | Blonde | Fall from bed | Abnormal | N |
| 14 | 1.3 | M | Black | Trauma | Abnormal | N |
| 15 | 0.1 | F | Blonde | Infection | Normal | N |
| 16 | 1.2 | M | Blonde | Seizure | Normal | N |
| 17 | 0.6 | F | Black | Drowning | Normal | N |
| 18 | Excluded | |||||
| 19 | 1.9 | F | Brown | AHT; lethargy | Abnormal | N |
| 20 | 0.2 | M | Black | Seizures; vomiting | Abnormal | Y |
| 21 | 0.1 | M | Scant | Trauma | Abnormal | Y |
| 22 | 0.1 | M | Brown | HDN | Abnormal | Y |
| 23 | 0.2 | M | Blonde | Irritability; L arm fracture | Abnormal | Y |
| 24 | 0.1 | M | Brown | Trauma | Abnormal | Y |
| 25 | 2 | F | Brown | Severe closed head injury | Abnormal | Y |
| 26 | 0.2 | M | Scant | Found unresponsive | Abnormal | Y |
| 27 | 14 | M | Brown | Infection | Abnormal | N |
| 28 | 0.3 | M | Black | Found limp/apneic | Abnormal | Y |
| 29 | 0.3 | F | Blonde | BB gun injury to head | Abnormal | Y |
DKA, diabetic ketoacidosis; MVA, motor vehicle accident; AHT, abusive head trauma; HDN, hemorrhagic disease of the newborn; L, left; Y, yes; N, no; r/o, rule out.
The mean age was 2.6±0.86 years, with a range of 1 day to 14 years. The CT scan was abnormal in 21 children (75%) and normal in 7 children (25%). Twelve of the 21 children with an abnormal head CT scan had an ICH, while 16 did not (Table 2). Of the 12 children with ICH, 4 for had isolated acute SDH, 3 had acute and chronic SDH, 1 had an EDH, and 1 had an SAH (Table 3). An additional 9 subjects had abnormal CT findings without ICH (agenesis of the corpus callosum, bilateral chronic fluid collections, posterior fossa cyst, cerebral edema, bilateral frontal contusions, empyema, fracture, cerebral infarction, and subgaleal hematoma; Table 4).
Table 2.
Demographics of the Study Population
| ICH (n=12) | No ICH (n=16) | |
|---|---|---|
| Age range (years) | 0.08–13 | 0.003–14 |
| (median) | (0.25) | (1.16) |
| (Interquartile range) | (0.153,2) | (0.12,3.475) |
| Gender (% male) | 77 | 63 |
| Race (% Caucasian) | 85 | 63 |
| Hair color (%) | Black 17 | Black 47 |
| Brown 41 | Brown 18 | |
| Blonde 25 | Blonde 29 | |
| Scant 17 | Scant 6 |
ICH, intracranial hemorrhage.
Table 3.
Type of Intracranial Hemorrhage (ICH), Location, and Corresponding ΔOD in Subjects with ICH
| Subject | Age (years) | Gender | Type of ICH | Location | ΔOD |
|---|---|---|---|---|---|
| 3 | 11 | M | EDH | L temporal | 0.25 (T) |
| 5 | 0.4 | F | SDH, acute | R | 0.22 (T) |
| 11 | 2 | M | SDH, acute | Along falx | −0.73 (T) −1.37 (O) |
| 20 | 0.2 | M | SDH, acute/chronic | R temporoparietal | −0.23 (T) −1.68 (P) |
| 21 | 0.1 | M | SAH | L frontotemporal | 0.44 (F) 0.45 (T) |
| 22 | 0.1 | M | SDH, acute | R frontal | −0.75 (F) |
| 23 | 0.2 | M | SAH | L frontoparietal | 0.34 (F) 0.84 (P) |
| 24 | 0.1 | M | SDH/SAH | R temporoparietal | −0.6 (P) −0.23 (O) |
| 25 | 2 | F | SDH, acute | L frontal, R diffuse | −0.96 (T) |
| 26 | 0.2 | M | SDH, acute/chronic, SAH | L frontotemporoparietal | 0.67 (F) 1.66 (T) 1.01 (P) |
| 28 | 0.3 | M | SDH, acute/chronic | R posterior, along falx | 0.6 (P) |
| 29 | 0.3 | F | SDH, acute | L temporal | 0.24 (T) |
Negative value denotes right-sided lesions.
EDH, epidural hematoma; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; R, right; L, left; F, frontal; T, temporal; P, parietal; O, occipital; OD, optical density.
Table 4.
Type of Non-Intracranial Hemorrhage (ICH) Head CT Abnormalities, Corresponding Locations, and ΔOD
| Subject | Age (years) | Gender | Abnormal CT finding | Location | ΔOD |
|---|---|---|---|---|---|
| 1 | 14 | M | Cerebral edema | Diffuse | All ≤ 0.17 |
| 2 | 1.4 | M | Posterior fossa cyst | Bilateral occipital | All ≤ 0.15 |
| 7 | 13 | M | Bilateral contusions | Frontal | All ≤ 0.07 |
| 10 | 0.3 | M | Chronic subdural collections | Bilateral frontal | All ≤ 0.07 |
| 12 | 0.1 | M | Agenesis of corpus callosum | N/A | All ≤ 0.11 |
| 13 | 0.003 | M | Skull fracture | R occipital | All ≤ 0.15 |
| 14 | 1.3 | M | Skull fracture; subgaleal hematoma | Parietal; frontal | −0.28 (RF) O: omitted |
| 19 | 1.9 | F | Skull fracture; infarct | R occipital; Large, left infarct | −0.47 (RF) −0.49 (RT) −0.34 (RP) 0.09 |
| 27 | 14 | M | Subdural empyema | L frontal | All ≤ 0.13 |
ΔOD≥0.2 is abnormal.
Negative values denote right-sided abnormalities.
All=frontal, parietal, temporal, and occipital; R, right; L, left; F, frontal; T, temporal; P, parietal; O, occipital; N/A, not applicable.
Near-infrared spectroscopy measurements and relationship to intracranial hemorrhage
The time needed to complete all NIRS measurements in the accessible scalp locations was no more than 15 min for any subject. All eight NIRS regions were successfully assessed in 22 of 28 (79%) subjects. In the remaining subjects, the occipital (n=6), and/or parietal (n=2) regions were unable to be assessed due to the presence of cervical collars or wound dressings. A plurality of subjects (11/28, 39%) were mechanically ventilated and sedated at the time of NIRS testing. Of those children not requiring mechanical ventilation, infants were assessed either at the bedside with the child asleep in bed or while a caregiver/nurse held the child. Older children (>4 years) were able to tolerate the performance of measurements after simple explanation and instruction and did not require any additional interventions.
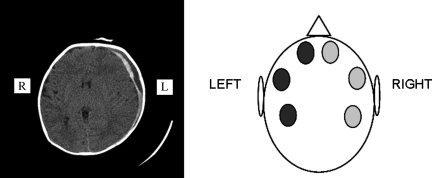
Of the 12 children with ICH, all subjects demonstrated at least 1 abnormal NIRS measurement at the time of testing (1.7 sites±0.8 site; Table 3). Figure 2 illustrates an example of one of these children. In the 9 children with an abnormal CT scan but without ICH, 2 had at least 1 abnormal NIRS measurement at the time of testing; these were considered false-positives. In each case the abnormal NIRS measurement was at the site of the abnormality: a subgaleal hematoma in one case and a cerebral infarction in the other. In the 7 children with a normal CT scan, 6 (86%) had normal NIRS measurements at all sites; 1 had abnormal NIRS measurements, and this was most likely due to technical difficulty in performing the measurements due to patient movement, or other confounding effects resulting in a false-positive result.
FIG. 2.
Head computed tomography image of a child with large left subdural hematoma and corresponding near infrared spectroscopy measurements. Occipital measurements could not be performed in this subject (light gray=normal; dark gray=abnormal).
The sensitivity, specificity, and positive and negative predictive values of the NIRS device relative to ICH detection were calculated. The sensitivity and specificity of NIRS were 1.0 and 0.8, respectively. The positive and negative predictive values were 0.8 and 1.0, respectively.
Discussion
This is the first study to evaluate the use of NIRS to detect ICH in children. The results of this exploratory study suggest that this technique is feasible and has potential utility as a screening tool in a clinical setting to detect ICH without imposing any additional risks to the patient.
Clinical studies have recognized the importance of early identification of causes of brain injury (i.e., delayed hematomas, hypoxia, and ischemia) in an effort to prevent secondary brain injury and improve neurological outcome. Moreover, successfully detecting occult ICH in victims of child abuse with a device that constitutes no risk to the subject is an extremely attractive addition to the armamentarium of the bedside clinician, not to be used as a diagnostic tool, but rather as a device that might detect brain injury as a cause of presenting symptoms. With such a device, children with non-specific symptoms or signs and a normal GCS could subsequently be screened in the ED setting for any suggestion of ICH. Then appropriate secondary testing with CT scans or other imaging modalities could confirm the initial findings.
NIRS technology offers many benefits to the bedside clinician. It is a portable painless bedside tool that can be gently applied to the scalp to quickly perform measurements. The device itself can perform readings on all eight scalp locations in 3–5 min under ideal circumstances. A more realistic time requirement when performing measurements on infants and children is 8–10 min, accounting for the varying levels of cooperation of children due to their short attention span, associated movements, and the need to repeat measurements.
Another attractive feature of the NIRS device is its applicability to two different clinical settings: the ED with a clinically stable patient or in the PICU with a critically ill patient. Measurements on subjects in our study were frequently performed on sleeping infants and children while lying in their parents' arms or in a crib/bed with minimal disruption to their sleep. Measurements were also carefully and successfully obtained by a trained operator on critically ill subjects in the PICU who were mechanically ventilated and sedated, with different obstacles to overcome with regard to NIRS placement, such as electroencephalogram (EEG) leads present on the scalp, and intracranial monitoring devices present, including external ventricular drains, used to both measure ICP and drain CSF when needed. Another potential benefit of utilizing NIRS in the PICU population might be the early detection of a new bleed, although that was not the aim of this study.
Although this study provides promising initial data regarding the use of NIRS in children, there are several limitations to the study and to the NIRS device itself. First, the presence of bilateral ICH would limit the utility of NIRS, since the technique relies on comparison of light absorption between the hemispheres. In AHT, interhemispheric SDH occurs more commonly (Ewing-Cobbs et al., 2000), and that might limit its application in this patient population. The purpose of utilizing NIRS technology is not to use it in isolation for the detection of occult head injuries, but rather to incorporate it into a battery of tools used in the detection of brain injury, including biomarkers, clinical decision rules, and other imaging modalities. Second, the depth of the ICH may be a limiting factor. Gopinath and colleagues (1993) demonstrated that intracranial hematomas absorb light less intensely than extracranial hematomas. Our data set is too small to know if this represents an important limitation. Third, the utility of NIRS in detecting subacute or chronic ICH could not be tested given our limited sample size and narrow time window to perform NIRS measurements (24 h). NIRS may be less effective when hemoglobin is undergoing degradation in the subacute or chronic setting, a strong possibility in the context of AHT. One subject in our study was diagnosed with bilateral chronic subdural collections on head CT. These were subsequently identified as chronic subdural hemorrhages on MRI. NIRS was negative in this case; this suggests that NIRS may have one of the same limitations as head CT: the inability to identify chronic hemorrhage. Fourth, scalp hematomas can result in false-positive NIRS measurements, as occurred in our single subject with a subgaleal hematoma. NIRS data must be interpreted with caution in patients with more superficial injuries (i.e., scalp hematomas), and must be validated with the gold standard, head CT, for the detection of ICH. Fifth, approximately 20% of our patient population had scalp locations that could not be examined with the NIRS device due to the presence of cervical collars limiting manipulation of the head, which is needed to obtain the measurements. In no case, however, did this result in a false-negative result. We had technical difficulty in performing NIRS on one subject, and this may have been related to patient movement or other possible confounding effects resulting in a false-positive result. This limitation may be more important in the ED setting with children who are less critically ill and therefore more likely to be moving. Lastly, NIRS technology is designed for the detection of acute ICH in eight scalp locations based on ΔOD values. It does not provide any further information that would direct medical versus surgical management of ICH (i.e., presence or absence of midline shift, impending herniation, or size of the ICH). Normal NIRS measurements would also not eliminate the possibility of severe brain injury without ICH (i.e., DAI).
Our study was limited by the small sample size, by the fact that all NIRS measurements were performed by a single operator in a single institution, and by the fact that the NIRS operator was not completely blinded to CT results. Performing NIRS measurements prior to head CT would eliminate any bias in obtaining NIRS measurements.
We conclude that NIRS measurements predicted the presence of ICH in the majority of patients with CT images identifying ICH. These exploratory results suggest that NIRS may be a beneficial adjunct in the rapid evaluation of an infant or child with suspected TBI in an emergent setting when CT imaging may not be imminently available, or as a screening tool to guide further clinical management. Normal NIRS measurements, however, cannot rule out the possibility of TBI from AHT or other causes, since diffuse brain injury and other forms of TBI without a contusion or hemorrhage are well described. The importance of developing alternate methods to head CT to rapidly assess infants and children with suspected intracranial pathology in the clinical setting and to guide clinical decision-making is of utmost importance, and needs to be a focus of further pediatric research.
Acknowledgments
This work was supported by grants from the National Institutes of Health 5T32HD040686-09 (to R.S. and P.M.K.), NS30318 (to P.M.K.), and the National Heart, Lung, and Blood Institute T32HL007820 (to R.S. and M.R.P.).
Author Disclosure Statement
No competing financial interests exist.
References
- Berger R. Pierce M. Wisniewski S. Adelson P. Clark R. Ruppel R. Kochanek P. Neuron specific enolase and S100B in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatrics. 2002;109:1–6. doi: 10.1542/peds.109.2.e31. [DOI] [PubMed] [Google Scholar]
- Berger R. Ta'Asan S. Rand A. Lokshin A. Kochanek P. Multiplex assessment of serum biomarker concentrations in well-appearing children with inflicted traumatic brain injury. Pediatr. Res. 2009;65:97–102. doi: 10.1203/PDR.0b013e31818c7e27. [DOI] [PubMed] [Google Scholar]
- Brenner D. Elliston C. Hall E. Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR. 2001;176:289–296. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Center for Health Statistics, Notional Hospital Ambulatory Medical Care Survey, Emergency Department File 2002.
- Centers for Disease Control and Prevention. National Center for Injury Prevention and Control Traumatic Brain Injury in the United States: assessing outcomes in children 2000.
- Chesnut R.M. Gautille T. Blunt B. Klauber M. Marshall L. The localizing value of asymmetry in pupillary size in severe head injury: relation to lesion type and location. Neurosurgery. 1994;34:840–846. doi: 10.1227/00006123-199405000-00008. [DOI] [PubMed] [Google Scholar]
- Coren M. Ng V. Rubens M. Rosenthal M. Bush A. The value of ultrafast computed tomography in the investigation of pediatric chest disease. Pediatr. Pulmonol. 1998;26:389–395. doi: 10.1002/(sici)1099-0496(199812)26:6<389::aid-ppul3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L. Prasad M. Kramer L. Louis P. Baumgartner J. Fletcher J. Alpert B. acute neuroradiologic findings in young children with inflicted or non-inflicted traumatic brain injury. Child's Nerv. Syst. 2000;16:25–34. doi: 10.1007/s003810050006. [DOI] [PubMed] [Google Scholar]
- Francis S. Ravindran G. Visvanathan K. Ganapathy K. Screening for unilateral intracranial abnormalities using near infrared spectroscopy: a preliminary report. J. Clinical Neurosci. 2005;12:291–295. doi: 10.1016/j.jocn.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Gopinath S. Robertson C. Contant C. Narayan R. Grossman R. Chance B. Early detection of delayed traumatic intracranial hematomas using near infrared spectroscopy. J. Neurosurg. 1995;83:438–444. doi: 10.3171/jns.1995.83.3.0438. [DOI] [PubMed] [Google Scholar]
- Gopinath S. Robertson C. Grossman R. Chance B. Near infrared spectroscopic localization of intracranial hematomas. J. Neurosurg. 1993;79:43–47. doi: 10.3171/jns.1993.79.1.0043. [DOI] [PubMed] [Google Scholar]
- Greenes D. Schutzman S. Clinical indicators of intracranial injury in head injured infants. Pediatrics. 1999;104:861–867. doi: 10.1542/peds.104.4.861. [DOI] [PubMed] [Google Scholar]
- Haqqani A. Hutchison J. Ward R. Stanimirovic D. Protein biomarkers in serum of pediatric patients with severe traumatic brain injury identified by ICAT-LC-MS/MS. J. Neurotrauma. 2007;24:54–74. doi: 10.1089/neu.2006.0079. [DOI] [PubMed] [Google Scholar]
- Hergenroeder G. Redell J. Moore A. Dash P. Biomarkers in the clinical diagnosis and management of traumatic brain injury. Molec. Diagnostic Therapeutics. 2008;12:345–358. doi: 10.1007/BF03256301. [DOI] [PubMed] [Google Scholar]
- Kahraman S. Kayali H. Atabey C. Acar F. Gocmen S. The accuracy of near infrared spectroscopy in detection of subdural and epidural hematomas. J. Trauma. 2006;61:1480–1483. doi: 10.1097/01.ta.0000197616.10279.48. [DOI] [PubMed] [Google Scholar]
- Kochanek P. Berger R. Bayır H. Wagner A. Jenkins L. Clark R. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms and therapeutic decision making. Curr. Opin. Crit. Care. 2008;14:135–141. doi: 10.1097/MCC.0b013e3282f57564. [DOI] [PubMed] [Google Scholar]
- Kupperman N. Holmes J. Dayan P. Hoyle J. Atabaki S. Holubkov R. Nadel F. Monroe D. Stanley R. Borgialli D. Badawy M. Schunk J. Quayle K. Mahajan P. Lichenstein R. Lillis K. Tunik M. Jacobs E. Callahan J. Gorelick M. Glass T. Lee L. Bachman M. Cooper A. Powell E. Gerardi M. Melville K. Muizelaaar J. Wisner D. Zuspan S. Dean J. Wootton-Gorges S. Identification of children at very low risk of clinically-important brain injuries after head trauma: A prospective cohort study. Lancet. 2009;374:1160–1170. doi: 10.1016/S0140-6736(09)61558-0. [DOI] [PubMed] [Google Scholar]
- Osmond M. Klassen T. Wells G. Correll R. Jarvis A. Joubert G. Bailey B. Chauvin-Kimoff L. Pusic M. McConnell D. Nijssen-Jordan C. Silver N. Taylor B. Stiell I. CATCH: A clinical decision rule for the use of computed tomography in children with minor head injury. Canadian Med. Assn. J. 2010;182:341–348. doi: 10.1503/cmaj.091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C. Gopinath S. Chance B. A new application for near infrared spectroscopy: Detection of delayed intracranial hematomas after head injury. J. Neurotrauma. 1995;12:591–600. doi: 10.1089/neu.1995.12.591. [DOI] [PubMed] [Google Scholar]
- Robertson C. Gopinath S. Chance B. Use of near infrared spectroscopy to identify traumatic intracranial hematomas. J. Biomed. Optics. 1997;2:31–41. doi: 10.1117/12.261680. [DOI] [PubMed] [Google Scholar]
- Robertson C. Zager E. Narayan R. Handly N. Sharma A. Hanley D. Garza H. Maloney-Wilensky E. Plaum J. Koenig C. Johnson A. Morgan T. Clinical evaluation of a portable near-infrared device for detection of traumatic intracranial hematomas. J. Neurotrauma. 2010;27:1597–1604. doi: 10.1089/neu.2010.1340. [DOI] [PubMed] [Google Scholar]
- Schutzman S. Barnes P. Duhaime A.C. Greenes D. Homer C. Jaffe D. Lewis R.J. Luerssen T. Schunk J. Evaluation and management of children younger than two year olds with apparently minor head trauma: Proposed guidelines. Pediatrics. 2001;107:983–993. doi: 10.1542/peds.107.5.983. [DOI] [PubMed] [Google Scholar]
- Shore P. Berger R. Varma S. Janesko K. Wisniewski S. Clark R. Adelson P. Thomas N. Lai Y. Bayir H. Kochanek P. Cerebrospinal fluid biomarkers versus Glasgow Coma Scale and glasgow outcome scale in pediatric traumatic brain injury: The role of young age and inflicted injury. J. Neurotrauma. 2007;24:75–86. doi: 10.1089/neu.2006.0062. [DOI] [PubMed] [Google Scholar]
- Veyrac C. Couture A. Baud C. Pericerebral fluid collections and ultrasound. Pediatr. Radiol. 1990;20:236–240. doi: 10.1007/BF02019655. [DOI] [PubMed] [Google Scholar]
- Zang Q. Ma H. Nioka S. Chance B. Study of near infrared technology for intracranial hematoma detection. J. Biomed. Optics. 2000;5:206–213. doi: 10.1117/1.429988. [DOI] [PubMed] [Google Scholar]