Abstract
The role of adaptive immunity in contributing to post-traumatic neuroinflammation and neuropathology after head injury remains largely unexplored. The present study was designed to investigate the pathophysiological sequelae of closed head injury in Rag1−/− mice devoid of mature B and T lymphocytes. C57BL/6 wild-type and Rag1−/− mice were subjected to experimental closed head injury, using a standardized weight-drop device. Outcome parameters consisted of neurological scoring, quantification of blood–brain barrier (BBB) function, measurement of inflammatory markers and mediators of apoptosis in serum and brain tissue, and assessment of neuronal cell death, astrogliosis, and tissue destruction. There was no difference between wild-type and Rag1−/− mice with regard to injury severity and neurological impairment for up to 7 days after head injury. The extent of BBB dysfunction was in a similar range for both groups. Quantification of complement activation fragments in serum revealed significantly attenuated C3a levels in Rag1−/− mice compared to wild-type animals. In contrast, the levels of pro- and anti-inflammatory cytokines and pro-apoptotic and anti-apoptotic mediators remained in a similar range for both groups, and the histological analysis of brain sections did not reveal a difference in reactive astrogliosis, tissue destruction, and neuronal cell death in Rag1−/− compared to wild-type mice. These findings suggest that adaptive immunity is not of crucial importance for initiating and sustaining the inflammatory neuropathology after closed head injury. The attenuated extent of post-traumatic complement activation seen in Rag1−/− mice implies a cross-talk between innate and adaptive immune responses, which requires further investigation in future studies.
Key words: adaptive immunity, closed head injury, complement system, natural antibodies, Rag1
Introduction
Traumatic brain injury (TBI) has a complex underlying pathophysiology, characterized by early neuroinflammation, which appears to be largely driven by an over-activation of the innate immune response (Cederberg and Siesjo, 2010; Elward and Gasque, 2003; Francis et al., 2003). The complement system has been identified as an important effector arm of the innate immune system which mediates the hyperinflammatory response after severe head injury, leading to a breakdown of the blood–brain barrier (BBB), development of cerebral edema, and induction of delayed neuronal cell death (Bellander et al., 2010,2011; Gasque, 2004; Stahel and Barnum, 2006; Stahel et al., 1998,2001; van Beek et al., 2003). Historically, the classic dogma of “immune privilege” in the brain sustained the long-standing notion that adaptive immune responses are not involved in the pathophysiology of TBI, mainly due to the absence of B and T lymphocytes in the central nervous system (CNS; Galea et al., 2007a; Hazlett and Hendricks, 2010; Perry, 1998; Rhodes, 2011). This outdated view has been challenged by more recent data showing (1) the presence of low numbers of T cells in the healthy brain (Hendrix and Nitsch, 2007; Hickey 1999); (2) an apparent role of activated CD4+ and CD8+ T cells infiltrating the injured brain and contributing to secondary brain damage (Bellander et al., 2010; Clausen et al., 2007; Czigner et al., 2007; Fee et al., 2003), and (3) by the discussion of the rationale of a therapeutic vaccination for TBI (Kipnis et al., 2003; Schwartz and Kipnis, 2001). The BBB represents the main barrier keeping peripheral blood T cells from infiltrating the intracranial compartment (Bechmann et al., 2007; Engelhardt and Coisne, 2011; Pachter et al., 2003). Twenty years ago, Hickey described the presence of CD4+ and CD8+ T cells in the CNS, and postulated a “random fashion” of infiltration secondary to antigenic stimulation (Hickey, 1991). The current notion is that T cells provide immunological surveillance within the CNS, and are able to cross the BBB through distinct pathways (Farkas et al., 2003; Finsen and Owens, 2011; Galea et al., 2007b; Gong et al., 2011; Hendrix and Nitsch, 2007; Sayed et al., 2011). The complement system was recently found to provide a “bridge” between innate and adaptive immune responses (e.g., by regulating B- and T-cell responses through C3d ligand binding on complement receptor type 2 [CR2] expressed on lymphocytes; Carroll, 2004; Morgan et al., 2005; Toapanta and Ross, 2006; Volanakis, 2002). Interestingly, CR2-targeted complement inhibition was recently described as a promising new neuroprotective strategy in CNS trauma (Qiao et al., 2006). In addition, CR2−/− mice lacking the CR2 receptor expressed on B cells were shown to be protected from ischemia/reperfusion injury, an inflammatory condition which is largely mediated by complement activation (Fleming et al., 2004). One of the potential mechanisms responsible for the attenuated inflammatory pathology in CR2−/− mice has been the lack of a pathogenic natural antibody repertoire (Austen et al., 2004; Fleming et al., 2002; Holers, 2005; Holers and Kulik, 2007; Reid et al., 2002; Zhang et al., 2006). In addition, Rag1−/− mice deficient in mature B and T cells, which lack pathogenic natural antibodies, were also shown to be resistant to ischemia/reperfusion injury, an effect which was reversible by reconstitution of specific subsets of natural antibodies (Kulik et al., 2009).
Until now, the exact role of B and T cells, and of pathogenic natural antibodies, in the pathophysiology of complement-mediated neuroinflammation remains poorly investigated and far from fully understood (Ankeny et al., 2009; Fee et al., 2003; Griffiths et al., 2010; Hendrix and Nitsch, 2007; Liesz et al., 2009; Qiao et al., 2006). The current study was designed to investigate the role of the adaptive immune response in contributing to the neuropathological sequelae after TBI, based on a standardized model of experimental closed head injury in mice (Chen et al., 1996; Flierl et al., 2009). We hypothesized that mice deficient in the Rag1 gene, which lack mature B and T lymphocytes and pathogenic natural antibodies (Mombaerts et al., 1992), will show signs of improved histological and neurological outcomes after closed head injury, compared to brain-injured wild-type mice.
Methods
Animals
The generation and characterization of Rag1−/− mice was previously described (Mombaerts 1995; Mombaerts et al., 1992). These mice were found to have small lymphoid organs lacking mature B and T lymphocytes. The phenotype characterization of Rag1−/− mice has not been linked to any neuroanatomical, neurological, or behavioral abnormalities (Mombaerts et al., 1992). Adult male Rag1−/− mice (n=100) and wild-type mice (n=137) on a C57BL/6 background (Jackson Laboratory, Bar Harbor, ME), 10–12 weeks of age, weighing 25–30 g, were used in all experiments. For each set of experiments, 6–10 mice were used per time point, for each condition tested in each group. The mice were housed in single cages, and bred in a selective pathogen-free (SPF) environment under standardized conditions of temperature (21°C), humidity (60%), light and dark cycles (12-h:12-h), with food and water provided ad libitum. All mice were acclimatized to the new environment for at least 7 days after shipping, before being subjected to the experimental procedures. All procedures were performed in accordance with the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals. The study was approved by the Institutional Animal Care Committee from the University of Colorado.
Closed head injury model
Mice were subjected to experimental closed head injury using a standardized weight-drop device, as previously described (Chen et al., 1996; Flierl et al., 2009). In brief, after induction of isoflurane anesthesia, the skull was exposed by a longitudinal midline scalp incision. The head was fixed and a 333-g weight was dropped on the skull from a height of 3 cm, inducing a focal blunt injury to the left hemisphere. After trauma, the surgical incision was closed using monofilament suture. All mice received supporting oxygenation with 100% O2 until fully awake. Analgesia was provided by repeated injections of fentanyl (0.05 mg/kg) IP every 12 h. Sham-operated mice underwent anesthesia, analgesia, scalp incision, and suturing of the surgical wound, but not head trauma.
Neurological Severity Score (NSS and ΔNSS)
A standardized 10-point Neurological Severity Score (NSS) was used for quantification of post-traumatic neurological impairment, as described elsewhere in detail (Beni-Adani et al., 2001; Flierl et al., 2009; Stahel et al., 2000). The NSS was assessed at 1 h, 4 h, 24 h, 48 h, 72 h, and 7 days after trauma. The score comprises 10 individual parameters, including tasks on motor function, alertness, and physiological behavior, whereby one point is given for failure of the task and no point for succeeding. The baseline NSS at 1 h reflects the initial severity of injury. A maximum NSS of 10 points indicates severe neurological dysfunction, with failure of all tasks. Spontaneous recovery over time, for up to 4 weeks after trauma, has been observed in this model system (Flierl et al., 2009; Rancan et al., 2003). Evaluation of task performance was performed in a blinded fashion with regard to the allocated treatment groups.
The ΔNSS, calculated as the difference between the NSS at 1 h and the NSS at any later time point, represents a parameter reflecting the degree of spontaneous recovery after TBI, as previously described (Stahel et al., 2000).
Blood–brain barrier (BBB) function
Quantitative analysis of the BBB function was performed at 4 h after trauma by measuring the amount of Evans blue extravasation into the injured brain, as previously described (Rancan et al., 2003; Stahel et al., 2000). The time-point of 4 h corresponds to the peak extent of post-traumatic BBB dysfunction in this model system (Chen et al., 1996; Flierl et al., 2009; Rancan et al., 2003). In brief, 125 μL of a 2% solution of Evans blue in 0.9% saline was injected into the penis vein 3 h after trauma, and was allowed to circulate for 60 min before tissue harvest. Injected Evans blue has a high affinity for serum albumin (Lindner and Heinle, 1982), and therefore represents an ideal colorimetric marker for albumin extravasation across the BBB. Subsequently, the chest wall was surgically opened under anesthesia and the intravascular dye was removed by 0.9% saline perfusion (40 mL) through the left heart ventricle. The brain was then removed and the cerebellum was cut off, split into left and right hemispheres, weighed, and kept on ice before homogenization. To process each hemisphere, 500 μL of 50% trichloroacetic acid was added and the tissue was homogenized using an Omni Tissue Master 125 (Omni International, Kennesaw, GA), followed by centrifugation for 20 min at 10,000 rpm and 4°C. The supernatants were then aliquoted and diluted 1:4 in 100% EtOH, followed by a 1:3 dilution in a 50% trichloracetic acid/100% EtOH solution. Final reads were performed using a fluorospectrophotometer (Glomax Mulit JR; Promega, Madison, WI), and the preinstalled red fluorescence optical kit at an excitation wavelength of 620 nm and an emission wavelength of 680 nm. As a standard, a twofold dilution series using 500 ng/mL Evans blue stock solution and 50% trichloroacetic acid/100% ethanol as the diluent was created, yielding a 7-point linear standard curve.
Inflammatory mediators in serum and brain tissue
Whole blood taken via cardiac puncture was transferred to sterile serum microtubes (Sarstedt, Nümbrecht, Germany), immediately placed on ice, and centrifuged at 14,000 rpm for 10 min (4°C), aliquotted, and stored at −80°C until analysis.
The animals were euthanized and harvested 4 h, 24 h, and 7 days after injury. The harvested brains were split into left and right hemispheres, snap-frozen in liquid nitrogen, and stored at −80°C until analysis. Brain hemispheres were then homogenized in 500 μL of RIPA (0.5 M Tris-HCl [pH 7.4], 1.5 M NaCl, 2.5% deoxycholic acid, 10% NP-40, and 10 mM EDTA) lysis buffer (Roche, Indianapolis, IN), followed by mechanical homogenization using a Omni Tissue Master 125. The samples were placed on ice on a shaker for 60 min, and then centrifuged for 15 min at 14,000 rpm (4°C). The supernatants were then aliquotted and stored at −80°C until analysis. Samples from serum and brain homogenates were analyzed by commercially-available enzyme-linked immunosorbent assay (ELISA) specific for mouse TNF-α, IL-6, and IL-10, according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). Optical density was read at a wavelength of 450 nm using a plate reader (Anthos 2010; Biochrome, Cambridge, U.K.). As a standard curve a 4-parameter fit line of the sevenfold standard was used according to the manufacturer's instructions. C3a serum values were also assessed using ELISA technique. A 96-well microplate was incubated overnight at 4°C with 50 μL of capture antibody purified rat anti-mouse C3a (BD Pharmingen, San Diego, CA). The plate was then washed three times, blocked with reagent diluent for 1 h at 37°C, washed again, and the standards applied. For standards purified mouse C3a protein (BD Pharmingen) was used, creating a 7-point linear standard curve. Biotin rat anti-mouse C3a (BD Pharmingen) was used for detection (incubation of 100 μL/well for 1 h at 37°C). After the final washing step, 100 μL substrate solution (R&D Systems) was added, and the reaction was stopped after 20–30 min with 2 N sulfuric acid. The plate was read at 450 nm wavelength using the above-described plate reader.
Western blot analysis of brain homogenates
Protein levels of pro- and anti-apoptotic mediators were assessed in homogenized mouse brains by Western blot analysis, as previously described (Leinhase et al., 2006a, 2006b). The brains were harvested and processed as described above. The protein concentrations of the individual samples were determined using a commercially available colorimetric assay (BCA Protein Assay; Pierce/Perbio Science, Bonn, Germany). In brief, a 50-μg sample of total protein was denatured in loading buffer (Laemmli sample buffer +5% mercaptoethanol), and separated under reducing conditions on 10% (Fas), 12,5% (Fas-L and β-actin), or 15% (Bax and Bcl-2) polyacrylamide gels (Bio-Rad Laboratories, Munich, Germany). Proteins were transferred using “Iblot” gel transfer stacks by dry electroblotting (Invitrogen, Carlsbad, CA). The blots were blocked for 1 h in 5% milk and then incubated with either polyclonal rabbit anti-mouse Fas-L and anti-mouse Fas (each diluted 1:300), or monoclonal anti-mouse Bax (1:600) and monoclonal anti-mouse Bcl-2 (1:300) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). An anti-β-actin antibody (1:1000) was used for internal control of equal loading. After a 60-min incubation with alkaline phosphatase (AP)-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) diluted 1:5000, antibody binding was visualized by AP detection using NBT/BCIP stock solution dissolved in a diluent buffer consisting of 0.1 M Tris, 0.1 M NaCl, and 0.05 M MgCl2. Western blots were quantified using Alpha Innotech Fluorchem SP software. Single bands on each blot were scanned and quantified as the ratio of the individual marker divided by the β-actin intensity. Scans of multiple blots from different brains with identical conditions (strain, procedure, and time point) were pooled and data are shown as medians±standard deviation (SD).
TUNEL and immunohistochemistry
All brain tissue sections were routinely stained with hematoxylin and eosin (H&E) for determining neuronal cell layer anatomy and the extent of post-traumatic tissue damage. In addition, neuronal cell death was determined in brain tissue cryosections 10-μm thick, using the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) method (Fluorescein In Situ Cell Death Detection Kit; Roche, Mannheim, Germany), as previously described (Harhausen et al., 2010; Stahel et al., 2009). Briefly, the cerebellum of the frozen brains was cut off, the brain was mounted on a removable chuck on the cryostat, and the brain was covered with OCT (Tissue Tek; Sakura Finetek, Torrance, CA). The temperature of the cryostat was set to −22°C, and 10-μm coronal brain sections were cut and mounted on slides, then stored at −80°C until analysis. For analysis the slides were dried for 2 h at 37°C, followed by immersion fixation using 2% paraformaldehyde. After washing, the sections were incubated in 25 μL permeabilization solution for 2 min at 4°C. The slides were then incubated with the TdT-enzyme for 60 min at 37°C in a humidified chamber. Negative control was performed using only reaction buffer without enzyme. Then 50 μL of converter AP was added to each section and incubated for 30 min at 37°C. After a washing step, 100 μL of substrate solution was added and incubated for 10 min at room temperature in the dark, and the sections were counter-stained and examined using an Olympus BX 41 microscope. Pictures were taken using the Altra 20 soft imaging system (Olympus, Philadelphia, PA).
Immunohistochemical staining of brain sections from serial coronal 10-μm cryosections was performed using a commercially available kit, according to the manufacturer's instructions (ImmunoCruz Mouse LSAB; Santa Cruz Biotechnology). In brief, cryosections were produced as described above. The frozen slides were dried for 2 hours at 37°C, and fixed with pure chilled acetone (−20°C) for 10 min, followed by washing.
The following primary antibodies were used as cell-specific markers: (1) monoclonal anti-NeuN antibody (MAB 377, clone A60; Millipore, Billerica, MA), as a cell marker for neurons (1:2000); and (2) monoclonal anti-GFAP antibody (sc-2050; Santa Cruz Biotechnology), as a cell marker for astrocytes (1:1000). After 2 h of incubation at room temperature, the slides were washed and incubated with HRP-streptavidin complex for 30 min. HRP substrate was added according to the manufacturer's instructions. Diaminobenzidine tetrachloride was used as chromogen. The detection takes approximately 5 min, and the sections were examined using an Olympus BX 41 microscope and an Altra 20 soft imaging camera system.
Statistical analysis
Statistical analysis was performed using commercially available software (SPSS 9.0 for Windows). Differences in serum and brain tissue mediator levels (ELISA), and Evans blue concentrations between the groups, were determined by the unpaired Student's t-test. The one-way analysis of variance (ANOVA) with Bonferroni correction for multiple measures was used for assessing differences in neurological scores (NSS and ΔNSS). A p value <0.05 was considered statistically significant.
Results
Neurological outcome
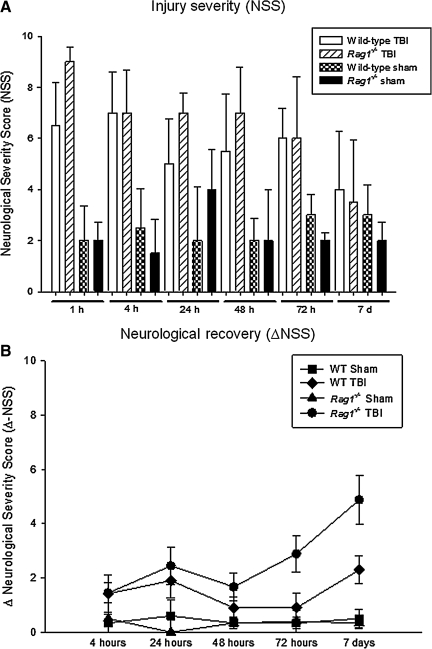
The neurological outcome after closed head injury in the different animal groups, based on the 10-point NSS, is shown in Figure 1A. The median NSS after closed head injury was highest in both groups at 1 h, reflecting the initial severity of injury (Rag1−/− 9.0±1.2 points; wild-type 6.5±2.1 points; medians±SD). The NSS decreased over time until 7 days, as a sign of spontaneous neurological recovery (Rag1−/− 3.5±2.4; and WT 4.0±2.5). No statistically significant difference was noted in the mean NSS at any time point between head-injured Rag1−/− mice and wild-type animals (p>0.05 by one-way ANOVA with Bonferroni correction). In contrast to the lack of differences between injury severity, there appeared to be faster recovery of the Rag1−/− mice, compared to head-injured wild-type mice, as determined by a higher ΔNSS (5.0±2.6 versus 2.0±1.6; medians±SD) at 7 days (Fig. 1B). The post-traumatic mortality was in the same range (15%) as previously reported (Flierl et al., 2009), and there was no difference in short-term (<24 h) or long-term mortality (7 days) between the two groups (data not shown).
FIG. 1.
Assessment of injury severity, neurological impairment, and recovery in C57BL/6 wild-type (n=97) and Rag1−/− mice (n=87) subjected to closed head injury or sham surgery, using a standardized 10-point Neurological Severity Score (NSS; A). A maximal score of 10 points corresponds to severe neurological impairment, whereas a low score of 0–3 points reflects normal physiological behavior. The ΔNSS (B), calculated as the difference between the NSS at 1 h and the NSS at any later time point, represents a parameter reflecting the degree of spontaneous recovery after TBI, as previously described. All data are presented as medians±standard deviation. No statistically significant differences were seen in NSS between head-injured wild-type and Rag1−/− mice at any time point assessed. In contrast, head-injured Rag1−/− mice showed improved recovery by 7 days, as reflected by a significantly increased ΔNSS at 7 days, compared to wild-type animals (p<0.01; TBI, traumatic brain injury).
Posttraumatic BBB dysfunction
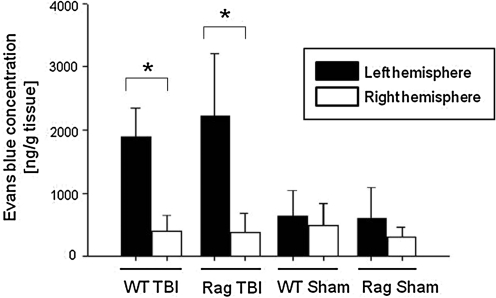
The amount of Evans blue dye extravasation into both hemispheres at 4 h after TBI, as a correlate of damaged BBB integrity, is shown in Figure 2. The focal trauma to the left hemisphere induced a significant increase in Evans blue extravasation in the injured (left) hemisphere compared with the non-injured contralateral (right) hemisphere (Rag1−/− left hemisphere: 2224.0±877.9 ng/g tissue, versus right hemisphere: 372.0±280.0 ng/g tissue; wild-type left hemisphere: 1889.8±421.4 ng/g tissue, versus right hemisphere: 401.8±231.1 ng/g tissue; p<0.05 for both strains). In addition, the Evans blue concentrations in the injured left hemispheres were significantly increased in the trauma group of both strains, compared to the respective sham-operated control group (p<0.05). However, no statistically significant difference in Evans blue concentrations was detected in the injured hemispheres of Rag1−/− compared to wild-type mice, implying that the extent of post-traumatic BBB dysfunction is not altered in the immunodeficient Rag1−/− mice (Fig. 2).
FIG. 2.
Quantification of post-traumatic blood–brain barrier dysfunction in C57BL/6 wild-type (n=11) and Rag1−/− mice (n=14) at 4 h after closed head injury or sham surgery. Evans blue extravasation into the injured (left) and non-injured (right) brain hemispheres was quantified by fluorospectrophotometry, as outlined in the methods section. Data are presented as medians±standard deviation; (*p<0.05 for left versus right hemisphere in head-injured mice, and for left hemisphere in head-injured versus sham-operated mice in both groups; TBI, traumatic brain injury).
Mediators of inflammation and apoptosis
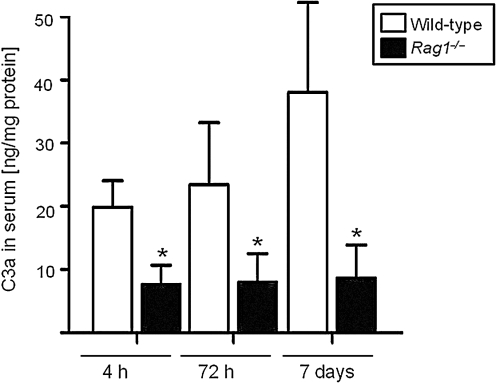
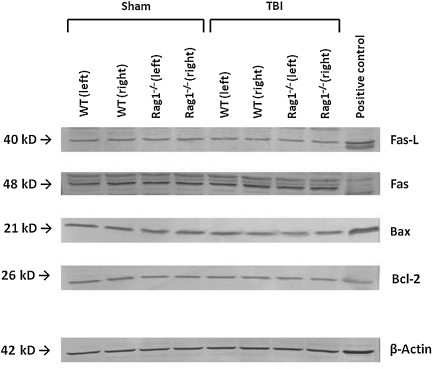
The quantification of complement anaphylatoxin C3a concentrations in serum samples of head-injured mice by ELISA revealed significantly attenuated C3a levels in Rag1−/− mice, compared to wild-type animals, from 4 h to 7 days after trauma (Fig. 3). In contrast, ELISA measurements of proinflammatory (TNF-α and IL-6) and anti-inflammatory cytokines (IL-10) in serum and brain tissue homogenates did not reveal any difference in mediator concentrations between head-injured wild-type and Rag1−/− mice at any time point assessed up to 7 days (data not shown). In addition, Western blot analysis of brain homogenates revealed similar levels of pro-apoptotic (Fas, FasL, and Bax) and anti-apoptotic mediators (Bcl-2) in both groups at all time points tested, as shown by the representative blot at 4 h after trauma illustrated in Figure 4.
FIG. 3.
Complement anaphylatoxin C3a serum concentrations in C57BL/6 wild-type (n=22) and Rag1−/− mice (n=23) at 4 h, 72 h, and 7 days after closed head injury. C3a levels were determined by a mouse-specific ELISA and normalized by total protein concentration, as described in the methods section. Data are shown as medians±standard deviation (*p<0.05 for wild-type versus Rag1−/− mice at all time points; ELISA, enzyme-linked immunosorbent assay).
FIG. 4.
Representative Western blot analysis of brain homogenates from injured (left) and uninjured (right) hemispheres of C57BL/6 wild-type (WT) and Rag1−/− mice at 4 h after sham surgery or closed head injury. Equal concentrations of protein (50 μg per lane) were loaded on SDS-Page membranes, and consistent loading was confirmed by β-actin control blotting. Mouse-specific primary antibodies against Fas, FasL, Bax, and Bcl-2, were used on nitrocellulose membranes and visualized by a colorimetric assay using alkaline phosphatase, as described in the methods section.
Neuronal cell death, astrogliosis, and tissue injury
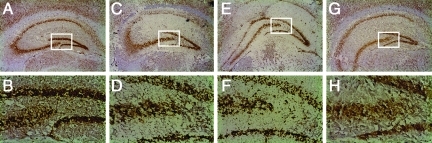
All brain tissue sections were analyzed by H&E staining, immunohistochemistry, and TUNEL histochemistry, to determine the extent of tissue damage and neuronal cell death. No difference in the size of the traumatic contusion in the injured left hemisphere, and in the pattern and extent of neuronal cell death, was detected at any time point after trauma between wild-type and Rag1−/− mice. Figure 5 shows representative coronal sections through the hippocampus of sham-operated and head-injured wild-type and Rag1−/− mice at 24 h, stained by immunohistochemistry using an anti-NeuN antibody as a neuron-specific cell marker. No difference in the extent of neuronal morphology and microarchitecture was seen in the hippocampal CA3/CA4 regions, which represent the most vulnerable neuronal cell layers susceptible to early neuronal cell death after trauma (Fig. 5). Similarly, no difference in pattern and extent of neuronal cell death was detected in head-injured wild-type and Rag1−/− mice at any time point, as determined by TUNEL histochemistry (data not shown).
FIG. 5.
Neuronal morphology and microarchitecture in the hippocampus of sham-operated (A–D) and head-injured (E–H) wild-type and Rag1−/− mice, as determined by immunohistochemistry in 10-μm-thick coronal brain cryosections. A monoclonal anti-NeuN antibody was used as a neuron-specific marker. Adult male C57BL/6 and Rag1−/− mice (n=3 per group) were euthanized at 24 h after the surgical procedure. The lower panels represent fivefold magnifications of the boxed areas in their respective upper panels (100×and 20×original magnifications), depicting the hippocampal CA3/CA4 cell layers. Sham-operated wild-type (A and B) and Rag1−/− mice (C and D) showed a similar hippocampal structure and neuronal morphology. In contrast, head injury induced cellular disruption and changes in neuronal morphology in the CA3/CA4 cell layers; however, this did not differ in extent between wild-type (E and F) and Rag1−/− mice (G and H).
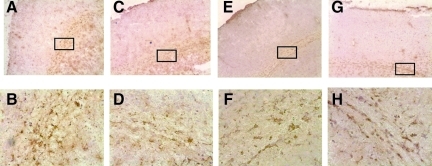
The astroglial reaction in injured mouse brains after closed head injury was assessed by immunohistochemistry using glial fibrillary acidic protein (GFAP) as primary antibody, a specific cell marker for astrocytes (Fig. 6). While sham-operated mice showed a similar baseline morphology of GFAP expression in both mouse strains, closed head injury induced reactive astrocytosis in the injured cortex by 7 days. However, the qualitative assessment of astrocyte morphology did not reveal any differences between head-injured wild-type and Rag1−/− mice (Fig. 6).
FIG. 6.
Astroglial reaction in injured mouse brains at 7 days after closed head injury. Immunohistochemical staining of 10-μm-thick coronal brain was done using glial fibrillary acidic protein (GFAP) as a specific cell marker for astrocytes. Sham-operated wild-type (A and B), and Rag1−/− mice (C and D), showed a similar baseline morphology of GFAP expression, with the classic feature of non-reactive astrocytes. In contrast, closed head injury induced reactive astrocytosis in the injured cortex of wild-type (E and F) and Rag1−/− mice (G and H), with identical changes in morphology in both mouse strains by 7 days after trauma. The lower panels represent fivefold magnifications of the boxed areas of their respective upper panels (100×and 20×original magnifications).
Discussion
The present study was designed to evaluate the role of adaptive immunity in mediating neuroinflammation and neuropathology in a standardized experimental model of closed head injury in mice. We hypothesized that Rag1−/− mice, devoid in mature B cells and T cells, would display a reduced extent of inflammation and neurodegeneration, and improved neurological recovery after TBI. Interestingly, the present study revealed no difference between head-injured Rag1−/− mice and wild-type animals, with regard to injury severity, extent of neurological impairment, BBB dysfunction, inflammation, astrogliosis, tissue damage, and neuronal cell death, for up to 7 days after trauma. These findings imply that the adaptive immunity mediated by B and T cells is not crucial in mounting and perpetuating the immunological response in our model of closed head injury in mice. These findings confirm the historic notion that lymphocytes do not represent important effector cells of the immunological response to TBI, in contrast to neutrophils and monocytes/macrophages, and activated microglia (Bellander et al., 2010; Biagas et al., 1992; Ramlackhansingh et al., 2011; Rhodes, 2011; Szmydynger-Chodobska et al., 2011). In fact, we have shown that neutrophils infiltrate the injured brain within 24 h in the present model of closed head injury in mice, and that cellular infiltration is largely regulated by chemokines and chemokine receptors expressed by neutrophils, macrophages, and resident cells of the CNS (Otto et al., 2001; Stahel et al., 2000). The only relevant differences in outcome parameters analyzed in this study consisted of significantly attenuated serum levels of the complement activation fragment C3a (Fig. 3), and improved early neurological recovery of head-injured Rag1−/− mice, as reflected by a higher NSS at 7 days after trauma (Fig. 1B).
In support of our current findings, experimental studies on Rag1−/− and Rag2−/− mice, using a facial motor nucleus axotomy model, did not reveal any difference in the extent of neuronal loss at 14 days after injury (Ha et al., 2006). In contrast, Fee and colleagues reported an attenuated extent of neuropathology in Rag1−/− mice after a focal cortical stab-wound freeze injury (Fee et al., 2003). In their study, immunodeficient Rag1−/− mice showed a decreased area of injury, a decreased number of apoptotic cells, and decreased density of infiltrating neutrophils within 24 h of cerebral freeze injury, compared to C57BL/6 wild-type mice (Fee et al., 2003). Impressively, the adoptive transfer of CD4+ T cells into Rag1−/− mice 24 h before injury restored the severity of brain injury to an extent similar to that seen in wild-type mice. The authors concluded that activated CD4+ T cells are involved in mediating exacerbated brain damage in their cortical freeze-injury model (Fee et al., 2003). The discrepancy between those findings and the data from our current study may in part be explained by the distinct experimental setting. For example, the study by Fee and colleagues was performed exclusively in mice of female gender (Fee et al., 2003), while our current model relied on using male animals exclusively (Flierl et al., 2009), in order to avoid the potential neuroprotective effect of female reproductive hormones (Hu et al., 2009; Wise, 2002). In addition, the study by Fee and associates was performed in a distinct experimental model system, which is likely characterized by a distinct underlying pathophysiology. While the search for the perfect experimental model of TBI remains elusive (Morganti-Kossmann et al., 2010), one could unequivocally argue that our current model of closed head injury using a standardized weight-drop device (Flierl et al., 2009) is more likely to be representative of the injury pattern seen in humans, compared to a cortical stab-wound freeze injury applied by a liquid nitrogen probe (Fee et al., 2003).
The lack of differences seen in neurological outcome and neuropathology between head-injured Rag1−/− mice and wild-type animals on the same genetic C57BL/6 background remains speculative. One argument consists of the notion that the complexity of the pathophysiology of TBI, which is reflected by the lack of any pharmacological treatment (Beauchamp et al., 2008; Loane and Faden, 2010), is associated with multiple redundant and complementary pathways that may extinguish each other with regard to morphologically or physiologically meaningful outcomes. This notion has been historically exemplified as the “yin-yang” or the “double-edged sword” of neuroprotection and neurotoxicity mediated by post-traumatic neuroinflammation (Alexander et al., 2008; Morganti-Kossmann et al., 2002; van Beek et al., 2003).
Interestingly, we found a significantly attenuated extent of post-traumatic complement activation in Rag1−/− mice, compared to wild-type mice, as indicated by the decreased levels of complement anaphylatoxin C3a found in serum from 4 h to 7 days after closed head injury (Fig. 3). These findings support the notion that complement is activated after TBI (Bellander et al., 2011; Stahel et al., 2001), and imply a cross-link between innate and adaptive immunity, as it relates to attenuated C3a levels observed in Rag1−/− mice. One of the common links in this network of innate and adaptive immune responses may be represented by pathogenic natural antibodies produced by B cells (Fleming, 2006; Holers, 2005; Holers and Kulik, 2007). Natural antibodies are polyreactive with multiple host-derived self antigens, and recognize neo-epitopes in injured and ischemic tissues. Target epitopes include apoptotic cells, lipoproteins, phospholipids, and nuclear and cytoplasmic components released from injured cells (Fleming, 2006; Holers, 2005; Holers and Kulik, 2007). Rag1−/− mice are devoid of pathogenic natural antibodies secondary to the lack of mature B cells. These immunodeficient mice were recently shown to be resistant to intestinal ischemia/reperfusion injury, while the pathology was reversed by the reconstitution of pathogenic natural antibodies to annexin IV (Kulik et al., 2009). Since pathogenic natural antibodies target phospholipids, which are released and exposed in the injured brain, this pathway may play a role in activating the complement cascade. A recent study in an experimental model of arterial wall injury in Rag1−/− mice revealed an intricate interplay between complement and natural antibodies (Dimayuga et al., 2009). This notion is supported by our current finding of decreased C3a serum levels in head-injured Rag1−/− mice. The exact role of pathogenic natural antibodies in contributing to complement activation after TBI, and to the associated downstream pathophysiological events, remains to be further elucidated in future studies.
Acknowledgments
This study was supported by a project grant from the Colorado TBI Trust Fund (COTBITF) to P.F.S. and M.A.F. S.W. was supported by a research fellowship from the German Research Foundation (DFG). V.M.H. was supported by an R01 grant from the NIH (no. AR051749).
Author Disclosure Statement
No competing financial interests exist.
References
- Alexander J.J. Anderson A.J. Barnum S.R. Stevens B. Tenner A.J. The complement cascade: Yin-Yang in neuroinflammation, neuroprotection, and degeneration. J. Neurochem. 2008;107:1169–1187. doi: 10.1111/j.1471-4159.2008.05668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny D.P. Guan Z. Popovich P.G. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austen W.G., Jr. Zhang M. Chan R. Friend D. Hechtman H.B. Carroll M.C. Moore F.D., Jr. Murine hindlimb reperfusion injury can be initiated by a self-reactive monoclonal IgM. Surgery. 2004;136:401–406. doi: 10.1016/j.surg.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Beauchamp K. Mutlak H. Smith W.R. Shohami E. Stahel P.F. Pharmacology of traumatic brain injury: where is the “golden bullet”? Mol. Med. 2008;14:731–740. doi: 10.2119/2008-00050.Beauchamp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann I. Galea I. Perry V.H. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Bellander B.M. Lidman O. Ohlsson M. Meijer B. Piehl F. Svensson M. Genetic regulation of microglia activation, complement expression, and neurodegeneration in a rat model of traumatic brain injury. Exp. Brain Res. 2010;205:103–114. doi: 10.1007/s00221-010-2342-z. [DOI] [PubMed] [Google Scholar]
- Bellander B.M. Olafsson I.H. Ghatan P.H. Bro Skejo H.P. Hansson L.O. Wanecek M. Svensson M.A. Secondary insults following traumatic brain injury enhance complement activation in the human brain and release of the tissue damage marker S100B. Acta Neurochir. (Wien.) 2011;153:90–100. doi: 10.1007/s00701-010-0737-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beni-Adani L. Gozes I. Cohen Y. Assaf Y. Steingart R.A. Brenneman D.E. Eizenberg O. Trembolver V. Shohami E. A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J. Pharmacol. Exp. Ther. 2001;296:57–63. [PubMed] [Google Scholar]
- Biagas K.V. Uhl M.W. Schiding J.K. Nemoto E.M. Kochanek P.M. Assessment of posttraumatic polymorphonuclear leukocyte accumulation in rat brain using tissue myeloperoxidase assay and vinblastine treatment. J. Neurotrauma. 1992;9:363–371. doi: 10.1089/neu.1992.9.363. [DOI] [PubMed] [Google Scholar]
- Carroll M.C. The complement system in regulation of adaptive immunity. Nat. Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- Cederberg D. Siesjo P. What has inflammation to do with traumatic brain injury? Childs Nerv. Syst. 2010;26:221–226. doi: 10.1007/s00381-009-1029-x. [DOI] [PubMed] [Google Scholar]
- Chen Y. Constantini S. Trembovler V. Weinstock M. Shohami E. An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma. 1996;13:557–568. doi: 10.1089/neu.1996.13.557. [DOI] [PubMed] [Google Scholar]
- Clausen F. Lorant T. Lewen A. Hillered L. T lymphocyte trafficking: a novel target for neuroprotection in traumatic brain injury. J. Neurotrauma. 2007;24:1295–1307. doi: 10.1089/neu.2006.0258. [DOI] [PubMed] [Google Scholar]
- Czigner A. Mihaly A. Farkas O. Buki A. Krisztin-Peva B. Dobo E. Barzo P. Kinetics of the cellular immune response following closed head injury. Acta Neurochir. (Wien.) 2007;149:281–289. doi: 10.1007/s00701-006-1095-8. [DOI] [PubMed] [Google Scholar]
- Dimayuga P.C. Cesena F.H. Chyu K.Y. Yano J. Amorn A. Fishbein M.C. Shah P.K. Cercek B. Natural antibodies and complement modulate intimal thickening after arterial injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R1593–R1600. doi: 10.1152/ajpregu.00114.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elward K. Gasque P. “Eat me” and “don't eat me” signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Mol. Immunol. 2003;40:85–94. doi: 10.1016/s0161-5890(03)00109-3. [DOI] [PubMed] [Google Scholar]
- Engelhardt B. Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS. 2011;8:4. doi: 10.1186/2045-8118-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas I.G. Czigner A. Farkas E. Dobo E. Soos K. Penke B. Endresz V. Mihaly A. Beta-amyloid peptide-induced blood-brain barrier disruption facilitates T-cell entry into the rat brain. Acta Histochem. 2003;105:115–125. doi: 10.1078/0065-1281-00696. [DOI] [PubMed] [Google Scholar]
- Fee D. Crumbaugh A. Jacques T. Herdrich B. Sewell D. Auerbach D. Piaskowski S. Hart M.N. Sandor M. Fabry Z. Activated/effector CD4+ T cells exacerbate acute damage in the central nervous system following traumatic injury. J. Neuroimmunol. 2003;136:54–66. doi: 10.1016/s0165-5728(03)00008-0. [DOI] [PubMed] [Google Scholar]
- Finsen B. Owens T. Innate immune responses in central nervous system inflammation. FEBS Lett. 2011 doi: 10.1016/j.febslet.2011.05.030. [May 27; Epub ahead of print]). [DOI] [PubMed] [Google Scholar]
- Fleming S.D. Egan R.P. Chai C. Girardi G. Holers V.M. Salmon J. Monestier M. Tsokos G.C. Anti-phospholipid antibodies restore mesenteric ischemia/reperfusion-induced injury in complement receptor 2/complement receptor 1-deficient mice. J. Immunol. 2004;173:7055–7061. doi: 10.4049/jimmunol.173.11.7055. [DOI] [PubMed] [Google Scholar]
- Fleming S.D. Natural antibodies, autoantibodies and complement activation in tissue injury. Autoimmunity. 2006;39:379–386. doi: 10.1080/08916930600739381. [DOI] [PubMed] [Google Scholar]
- Fleming S.D. Shea-Donohue T. Guthridge J.M. Kulik L. Waldschmidt T.J. Gipson M.G. Tsokos G.C. Holers V.M. Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J. Immunol. 2002;169:2126–2133. doi: 10.4049/jimmunol.169.4.2126. [DOI] [PubMed] [Google Scholar]
- Flierl M.A. Stahel P.F. Beauchamp K.M. Morgan S.J. Smith W.R. Shohami E. Mouse closed head injury model induced by a weight-drop device. Nat. Protoc. 2009;4:1328–1337. doi: 10.1038/nprot.2009.148. [DOI] [PubMed] [Google Scholar]
- Francis K. van Beek J. Canova C. Neal J.W. Gasque P. Innate immunity and brain inflammation: the key role of complement. Expert Rev. Mol. Med. 2003;5:1–19. doi: 10.1017/S1462399403006252. [DOI] [PubMed] [Google Scholar]
- Galea I. Bechmann I. Perry V.H. What is immune privilege (not)? Trends Immunol. 2007a;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Galea I. Bernardes-Silva M. Forse P.A. van Rooijen N. Liblau R.S. Perry V.H. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J. Exp. Med. 2007b;204:2023–2030. doi: 10.1084/jem.20070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasque P. Complement: a unique innate immune sensor for danger signals. Mol. Immunol. 2004;41:1089–1098. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Gong N. Liu J. Reynolds A.D. Gorantla S. Mosley R.L. Gendelman H.E. Brain ingress of regulatory T cells in a murine model of HIV-1 encephalitis. J. Neuroimmunol. 2011;230:33–41. doi: 10.1016/j.jneuroim.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths M.R. Gasque P. Neal J.W. The regulation of the CNS innate immune response is vital for the restoration of tissue homeostasis (repair) after acute brain injury: a brief review. Int. J. Inflam. 20102010:151097. doi: 10.4061/2010/151097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha G.K. Hunag Z. Streit W.J. Petitto J.M. Endogenous T lymphocytes and microglial reactivity in the axotomized facial motor nucleus of mice: Effect of genetic background and the RAG2 gene. J. Neuroimmunol. 2006;172:1–8. doi: 10.1016/j.jneuroim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Harhausen D. Khojasteh U. Stahel P.F. Morgan B.P. Nietfeld W. Dirnagl U. Trendelenburg G. Membrane attack complex inhibitor CD59a protects against focal cerebral ischemia in mice. J. Neuroinflammation. 2010;7:15. doi: 10.1186/1742-2094-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett L.D. Hendricks R.L. Reviews for immune privilege in the year 2010: immune privilege and infection. Ocul. Immunol. Inflamm. 2010;18:237–243. doi: 10.3109/09273948.2010.501946. [DOI] [PubMed] [Google Scholar]
- Hendrix S. Nitsch R. The role of T helper cells in neuroprotection and regeneration. J. Neuroimmunol. 2007;184:100–112. doi: 10.1016/j.jneuroim.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Hickey W.F. Leukocyte traffic in the central nervous system: the participants and their roles. Semin. Immunol. 1999;11:125–137. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- Hickey W.F. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991;1:97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Holers V.M. Kulik L. Complement receptor 2, natural antibodies and innate immunity: Inter-relationships in B cell selection and activation. Mol. Immunol. 2007;44:64–72. doi: 10.1016/j.molimm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Holers V.M. Complement receptors and the shaping of the natural antibody repertoire. Springer Semin. Immunopathol. 2005;26:405–423. doi: 10.1007/s00281-004-0186-y. [DOI] [PubMed] [Google Scholar]
- Hu Z. Li Y. Fang M. Wai M.S. Yew D.T. Exogenous progesterone: a potential therapeutic candidate in CNS injury and neurodegeneration. Curr. Med. Chem. 2009;16:1418–1425. doi: 10.2174/092986709787846523. [DOI] [PubMed] [Google Scholar]
- Kipnis J. Nevo U. Panikashvili D. Alexandrovich A. Yoles E. Akselrod S. Shohami E. Schwartz M. Therapeutic vaccination for closed head injury. J. Neurotrauma. 2003;20:559–569. doi: 10.1089/089771503767168483. [DOI] [PubMed] [Google Scholar]
- Kulik L. Fleming S.D. Moratz C. Reuter J.W. Novikov A. Chen K. Andrews K.A. Markaryan A. Quigg R.J. Silverman G.J. Tsokos G.C. Holers V.M. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J. Immunol. 2009;182:5363–5373. doi: 10.4049/jimmunol.0803980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinhase I. Holers V.M. Thurman J.M. Harhausen D. Schmidt O.I. Pietzcker M. Taha M.E. Rittirsch D. Huber-Lang M. Smith W.R. Ward P.A. Stahel P.F. Reduced neuronal cell death after experimental brain injury in mice lacking a functional alternative pathway of complement activation. BMC Neurosci. 2006a;7:55. doi: 10.1186/1471-2202-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinhase I. Schmidt O.I. Thurman J.M. Hossini A.M. Rozanski M. Taha M.E. Scheffler A. John T. Smith W.R. Holers V.M. Stahel P.F. Pharmacological complement inhibition at the C3 convertase level promotes neuronal survival, neuroprotective intracerebral gene expression, and neurological outcome after traumatic brain injury. Exp. Neurol. 2006b;199:454–464. doi: 10.1016/j.expneurol.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Liesz A. Suri-Payer E. Veltkamp C. Doerr H. Sommer C. Rivest S. Giese T. Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Lindner V. Heinle H. Binding properties of circulating Evans blue in rabbits as determined by disc electrohporesis. Atherosclerosis. 1982;43:417–422. doi: 10.1016/0021-9150(82)90040-5. [DOI] [PubMed] [Google Scholar]
- Loane D.J. Faden A.I. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P. Iacomini J. Johnson R.S. Herrup K. Tonegawa S. Papaioannou V.E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. Lymphocyte development and function in T-cell receptor and RAG-1 mutant mice. Int. Rev. Immunol. 1995;13:43–63. doi: 10.3109/08830189509061737. [DOI] [PubMed] [Google Scholar]
- Morgan B.P. Marchbank K.J. Longhi M.P. Harris C.L. Gallimore A.M. Complement: central to innate immunity and bridging to adaptive responses. Immunol. Lett. 2005;97:171–179. doi: 10.1016/j.imlet.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann M.C. Rancan M. Stahel P.F. Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr. Opin. Crit. Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann M.C. Yan E. Bye N. Animal models of traumatic brain injury: Is there an optimal model to reproduce human brain injury in the laboratory? Injury. 2010;41(Suppl.):S10–S13. doi: 10.1016/j.injury.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Otto V.I. Stahel P.F. Rancan M. Kariya K. Shohami E. Yatsiv I. Eugster H.P. Kossmann T. Trentz O. Morganti-Kossmann M.C. Regulation of chemokines and chemokine receptors after experimental closed head injury. Neuroreport. 2001;12:2059–2064. doi: 10.1097/00001756-200107030-00053. [DOI] [PubMed] [Google Scholar]
- Pachter J.S. de Vries H.E. Fabry Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J. Neuropathol. Exp. Neurol. 2003;62:593–604. doi: 10.1093/jnen/62.6.593. [DOI] [PubMed] [Google Scholar]
- Perry V.H. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J. Neuroimmunol. 1998;90:113–121. doi: 10.1016/s0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- Qiao F. Atkinson C. Song H. Pannu R. Singh I. Tomlinson S. Complement plays an important role in spinal cord injury and represents a therapeutic target for improving recovery following trauma. Am. J. Pathol. 2006;169:1039–1047. doi: 10.2353/ajpath.2006.060248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlackhansingh A.F. Brooks D.J. Greenwood R.J. Bose S.K. Turkheimer F.E. Kinnunen K.M. Gentleman S. Heckemann R.A. Gunanayagam K. Gelosa G. Sharp D.J. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann. Neurol. 2011 doi: 10.1002/ana.22455. [April 18; Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Rancan M. Morganti-Kossmann M.C. Barnum S.R. Saft S. Schmidt O.I. Ertel W. Stahel P.F. Central nervous system-targeted complement inhibition mediates neuroprotection after closed head injury in transgenic mice. J. Cereb. Blood Flow Metab. 2003;23:1070–1074. doi: 10.1097/01.WCB.0000084250.20114.2C. [DOI] [PubMed] [Google Scholar]
- Reid R.R. Woodcock S. Shimabukuro-Vornhagen A. Austen W.G., Jr. Kobzik L. Zhang M. Hechtman H.B. Moore F.D., Jr. Carroll M.C. Functional activity of natural antibody is altered in Cr2-deficient mice. J. Immunol. 2002;169:5433–5440. doi: 10.4049/jimmunol.169.10.5433. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Peripheral immune cells in the pathology of traumatic brain injury? Curr. Opin. Crit. Care. 2011;17:122–130. doi: 10.1097/MCC.0b013e3283447948. [DOI] [PubMed] [Google Scholar]
- Sayed B.A. Christy A.L. Walker M.E. Brown M.A. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J. Immunol. 2011;184:6891–6900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Kipnis J. Protective autoimmunity: regulation and prospects for vaccination after brain and spinal cord injuries. Trends Mol. Med. 2001;7:252–258. doi: 10.1016/s1471-4914(01)01993-1. [DOI] [PubMed] [Google Scholar]
- Stahel P.F. Barnum S.R. The role of the complement system in CNS inflammatory diseases. Expert Rev. Clin. Immunol. 2006;2:445–456. doi: 10.1586/1744666X.2.3.445. [DOI] [PubMed] [Google Scholar]
- Stahel P.F. Flierl M.A. Morgan B.P. Persigehl I. Stoll C. Conrad C. Touban B.M. Smith W.R. Beauchamp K. Schmidt O.I. Ertel W. Leinhase I. Absence of the complement regulatory molecule CD59a leads to exacerbated neuropathology after traumatic brain injury in mice. J. Neuroinflammation. 2009;6:2. doi: 10.1186/1742-2094-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahel P.F. Morganti-Kossmann M.C. Kossmann T. The role of the complement system in traumatic brain injury. Brain Res. Rev. 1998;27:243–256. doi: 10.1016/s0165-0173(98)00015-0. [DOI] [PubMed] [Google Scholar]
- Stahel P.F. Morganti-Kossmann M.C. Perez D. Redaelli C. Gloor B. Trentz O. Kossmann T. Intrathecal levels of complement-derived soluble membrane attack complex (sC5b-9) correlate with blood-brain barrier dysfunction in patients with traumatic brain injury. J. Neurotrauma. 2001;18:773–781. doi: 10.1089/089771501316919139. [DOI] [PubMed] [Google Scholar]
- Stahel P.F. Shohami E. Younis F.M. Kariya K. Otto V.I. Lenzlinger P.M. Grosjean M.B. Eugster H.P. Trentz O. Kossmann T. Morganti-Kossmann M.C. Experimental closed head injury: analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J. Cereb. Blood Flow Metab. 2000;20:369–380. doi: 10.1097/00004647-200002000-00019. [DOI] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J. Strazielle N. Gandy J.R. Keefe T.H. Zink B.J. Ghersi-Egea J.F. Chodobski A. Posttraumatic invasion of monocytes across the blood-cerebrospinal fluid barrier. J. Cereb. Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2011.111. [Aug 10; Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toapanta F.R. Ross T.M. Complement-mediated activation of the adaptive immune responses: role of C3d in linking the innate and adaptive immunity. Immunol. Res. 2006;36:197–210. doi: 10.1385/IR:36:1:197. [DOI] [PubMed] [Google Scholar]
- Van Beek J. Elward K. Gasque P. Activation of complement in the central nervous system: roles in neurodegeneration and neuroprotection. Ann. NY Acad. Sci. 2003;992:56–71. doi: 10.1111/j.1749-6632.2003.tb03138.x. [DOI] [PubMed] [Google Scholar]
- Volanakis J.E. The role of complement in innate and adaptive immunity. Curr. Top. Microbiol. Immunol. 2002;266:41–56. doi: 10.1007/978-3-662-04700-2_4. [DOI] [PubMed] [Google Scholar]
- Wise P.M. Estrogens and neuroprotection. Trends Endocrinol. Metab. 2002;13:229–230. doi: 10.1016/s1043-2760(02)00611-2. [DOI] [PubMed] [Google Scholar]
- Zhang M. Michael L.H. Grosjean S.A. Kelly R.A. Carroll M.C. Entman M.L. The role of natural IgM in myocardial ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2006;41:62–67. doi: 10.1016/j.yjmcc.2006.02.006. [DOI] [PubMed] [Google Scholar]