Abstract
A number of studies have established a deleterious role for inflammatory molecules and reactive oxygen species (ROS) in the pathology of traumatic brain injury (TBI). Caffeic acid phenethyl ester (CAPE) has been shown to exert both antioxidant and anti-inflammatory effects. The primary objective of the present study was to examine if CAPE could be used to reduce some of the pathological consequences of TBI using rodent models. Male Sprague-Dawley rats and C57BL/6 mice were subjected to controlled cortical impact (CCI) injury. Blood–brain barrier (BBB) integrity was assessed by examining claudin-5 expression and the extravasation of Evans blue dye. The effect of post-injury CAPE administration on neurobehavioral function was assessed using vestibulomotor, motor, and two hippocampus-dependent learning and memory tasks. We report that post-TBI administration of CAPE reduces Evans blue extravasation both in rats and mice. This improvement was associated with preservation of the levels of the tight junction protein claudin-5. CAPE treatment did not improve performance in either vestibulomotor/motor function (tested using beam balance and foot-fault tests), or in learning and memory function (tested using the Morris water maze and associative fear memory tasks). However, animals treated with CAPE were found to have significantly less cortical tissue loss than vehicle-treated controls. These findings suggest that CAPE may provide benefit in the treatment of vascular compromise following central nervous system injury.
Key words: claudin-5, neurovascular function, reactive oxygen species, tight junction, vascular permeability
Introduction
Traumatic brain injury (TBI) results in tissue damage from both primary (mechanical) and secondary (neurochemical) processes. The primary event is brief and triggers secondary processes, many of which remain active for hours to days, and contribute to a number of brain pathologies, including neurovascular dysfunction, tissue loss, and neurocognitive impairments (Floyd and Lyeth, 2007; Giri et al., 2000; Hicks et al., 2010; Meaney and Smith, 2011; Ray et al., 2002; Smith et al., 2010; Stoica and Faden, 2010; Werner and Engelhard, 2007; Yakovlev and Faden, 2001). A number of molecular mechanisms that underlie these secondary processes have been identified in rodent models and clinical studies, and represent potential targets for therapeutic intervention (Beauchamp et al., 2008; Fedor et al., 2010; Hayes et al., 2009; Huh and Raghupathi, 2009; Ottens et al., 2010; Saatman et al., 2010; Shellington et al., 2011; Sullivan et al., 2011). These include oxidative damage resulting from increased production of reactive oxygen species (ROS) and reduced glutathione levels, inflammation resulting from local and infiltrating cells, mitochondrial dysfunction, and activation of apoptotic cell death cascades (Babikian et al., 2010; Fan et al., 2003; Hall et al., 2010; Hayes and Dixon, 1994; Igarashi et al., 2001; Kelley et al., 2007; Lifshitz et al., 2003). The combined actions of these and other mechanisms ultimately determine injury progression and outcome. Based on these and other observations, there is a general consensus that either a drug combination, or a single drug that acts on multiple targets, is likely to be effective in attenuating TBI pathology (Loane and Faden, 2010; Margulies and Hicks, 2009; Sayeed and Stein, 2009; Vink and Bullock, 2010).
Caffeic acid (3,4-dihydroxycinnamic acid) is a phenolic compound found in fruits and vegetables (Sondheimer, 1958) that has been shown to block the production of ROS, increase glutathione levels, and reduce inflammatory cytokine levels following exposure to various toxic agents (Kono et al., 1997; Stewart et al., 2001; Uz et al., 1998). CAPE is a naturally-occurring derivative of caffeic acid that is highly cell permeable due to the presence of an ester linkage. Once inside the cell, the ester bond is cleaved by intracellular esterases to release caffeic acid, the active moiety. Similarly to caffeic acid, CAPE has also been shown to decrease inflammatory processes, brain lipid peroxidation (Irmak et al., 2003), and free radical damage. It has been reported that these effects of CAPE are due to its influences on xanthine/xanthine oxidase, nuclear factor-κB (NF-κB), cyclooxygenase-2 (COX-2), 5-lipoxygenase (5-LOX), the production of inflammatory cytokines, and the release of cytochrome c from mitochondria (Chan et al., 1995; Koshihara et al., 1984; Michaluart et al., 1999; Mirzoeva and Calder, 1996; Natarajan et al., 1996; Sud'ina et al., 1993; Uz et al., 1998).
Since TBI triggers many of the pathological cascades targeted by CAPE, we hypothesized that CAPE treatment would lead to decreased brain pathology and improved neurological outcomes. In the present study, we examined if post-injury CAPE administration can reduce TBI-induced enhanced blood–brain barrier (BBB) permeability, tissue loss, and neurological dysfunction in animals with TBI. Our results show that while post-injury CAPE administration reduced BBB permeability and cortical tissue loss, it failed to offer significant improvements in either motor or memory tasks.
Methods
Materials
Male Sprague-Dawley rats (250–275 g) and C57BL/6 mice (23–25 g) were purchased from Harlan Laboratories (Indianapolis, IN). CAPE was purchased from Biomol International, LP (Plymouth Meeting, PA). Antibodies for von Willebrand factor (vWF) and claudin-5 were purchased from AbD Serotec (Raleigh, NC) and Invitrogen (Carlsbad, CA), respectively. Antibodies for neuronal nuclei (NeuN) and glial fibrillary acidic protein (GFAP) were purchased from Millipore (Billerica, MA).
Rat controlled cortical impact injury
All experimental procedures were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals. A controlled cortical impact (CCI) model was used to cause brain trauma as previously described (Dixon et al., 1991). Animals were initially anesthetized using 5% isoflurane with a 1:1 N2O:O2 mixture, mounted on a stereotaxic frame, and then maintained with 2.5% isoflurane with 1:1 N2O:O2 mixture via a face mask. For BBB studies, a midline incision was made and a 6-mm-diameter craniotomy was performed on the right side midway between the bregma and the lambda, with the medial edge of the craniotomy 1.0 mm lateral to the midline. An electromagnetic impact device (Custom Design and Fabrication, Richmond, VA) was used to deliver an impact at an angle of 10° from the vertical plane at 6.0 m/sec with 3.3 mm deformation using an impactor tip 5 mm in diameter. Based on our observations, this deformation caused an injury comparable to that seen using a depth of 1.8 mm in the pneumatic CCI device. For behavioral studies, the aforementioned craniotomy was made on both sides of the skull and the contusion was delivered only to the right parietal cortex at a velocity of 4.0 m/sec and a deformation of 2.7 mm (equivalent to 1.5 mm in the pneumatic CCI device). After injury, the incision was closed with wound clips. Core body temperature was maintained at 37°C during the surgery using a rectal thermometer coupled to a heating pad. All animals were allowed to completely recover from the anesthesia in a warm chamber before being returned to their home cages. Sham-operated animals received all the aforementioned surgical procedures except for the craniotomy and the impact.
CAPE administration
CAPE was prepared in 50% dimethylsulfoxide (DMSO) in saline and was administered by intraperitoneal (IP) injection with a dose of 10 mg/kg at designed time points following brain injury. The total injection volume for each animal was 1 mL for rat studies and 500 μL for mouse studies. Vehicle-treated animals received an identical volume of 50% DMSO by IP injection at corresponding time points. CAPE was administered 30 min following injury for immunohistochemistry assays and Evans blue studies, and was administered 30 min following injury, then daily for the next 4 days, for the behavioral studies.
Immunohistochemistry and fluorescence intensity quantification
Twenty-four hours after injury, the rats were decapitated and the brains were removed and quickly frozen in −80°C isopentane. Twenty-micron-thick coronal cryosections were prepared and mounted directly on slides. After air drying, the sections were fixed with 100% methanol for 20 min at −20°C, and then rinsed in Tris-buffered saline, pH 8.0 (TBS). The sections were blocked in 5% goat serum in TBS with 0.25% Triton X-100 (TBST) at room temperature for 1 h, followed by incubation with primary antibodies (1 μg/mL) in 2.5% goat serum in TBST at 4°C for 48 h. Following extensive washing in TBST, species-specific secondary antibodies conjugated to Alexa-Fluor (in 2.5% goat serum in TBST) were added and allowed to incubate for 3 h at room temperature. Images of immunofluorescence were captured using a Zeiss Axiovert S100 microscope and a MicroFire (Optronics, Goleta, CA) camera. The parameters used for image acquisition were preset to minimize the background and optimize the signal using a tissue section from an injured animal. These parameters were kept constant across all subsequent groups. Two non-overlapping regions in the ipsilateral cortex from each section and two sections from each animal were used for imaging. The fluorescence intensities, measured using Image J software (from the National Institutes of Health [NIH]), were averaged for each section, and the two sections were averaged for each animal.
Measurement of BBB permeability
At the indicated time points following injury, 3% Evans blue (EB) dye in saline was injected slowly through the jugular vein (4 mL/kg) and allowed to circulate for 1.5 h for both rats and mice. Following the circulation time, the animals were transcardially perfused with 1× phosphate-buffered saline (PBS). The brains were removed, and the cerebral hemispheres were separated and homogenized in 50% trichloroacetic acid (TCA). The homogenate was incubated at 4°C for 1 h and then centrifuged at 20,000g for 30 min. The supernatant solution was collected and 2× volume of ethanol was added to each sample followed by thorough vortexing. The optical density of the resultant solution was measured at 620 nm and used to determine the relative amount of EB dye in each sample.
Assessment of motor function
All behavioral tests were conducted by an experimenter blind to the treatment groups. A vestibulomotor (beam balance) and a motor skill task (paw placement) were used to determine the animals' motor performance following injury on days 1–4 and day 7 post-CCI. For the beam balance, three daily trials were given during which the length of time spent on a narrow wooden beam (1.5 cm wide) was recorded for up to 60 sec. Paw placement was evaluated by placing the rat on a wire grid (opening size of 2×2 cm) and counting the number of foot faults out of a total of 50 steps. A foot fault was defined as when a front paw misses and appears below the plane of the grid. Paw placement was repeated three times to give an average daily score.
Assessment of cognitive function
The rats were tested for their cognitive performance using the standard hidden platform version of the Morris water maze (MWM; Dash et al., 1995,2002; Hamm et al., 1992; Royo et al., 2007). All animals had recovered from the TBI-associated motor dysfunction prior to performing the cognitive testing. The animals were given 4 consecutive training trials per day for 9 days, with an inter-trial interval (iti) of 4 min (Hamm et al., 1992). If the animal failed to locate the platform within 60 sec on any given trial, it was led there by the experimenter. Movement within the maze was monitored using a video camera linked to tracking software (Ethovision; Noldus Information Technology, Leesbury, VA). The time to locate the platform during training was used as a measure of learning and memory. A probe trial in which the platform was removed from the water maze was given 24 h after training to assess measures of platform localization. The rats were allowed to search for the hidden platform for a period of 60 sec, during which the latency to first platform crossing and the number of platform crossings were recorded.
Contusion volume measurement
Following the completion of the behavioral studies, the animals were deeply anesthetized with sodium pentobarbital (100 mg/kg) and transcardially perfused with PBS followed by 4% paraformaldehyde. The brains were removed, post-fixed overnight in perfusant, then cryoprotected in a 30% sucrose solution. Cortical tissue loss was estimated by experimenters kept blind with respect to the treatment groups. In brief, cryosections (40-μm thick) spanning the rostral-caudal extent of the injured cortex were selected and stained with cresyl violet by an experimenter given only the animal's identifier code. Images of the resultant slides were then used for tissue loss measurement by a second experimenter. The area of cortical tissue loss for each section was carefully outlined and quantified by Image J software. Contusion volume was calculated using the equation A1(0.5X1)+A2(0.5X1+0.5X2)+An−1(0.5Xn−1+0.5Xn)+An(0.5Xn), where A is the area (mm2) of the contusion for each slice, and X is the distance (mm) between two sequential slices. Once the contusion volume had been calculated for each animal, the blind code was broken and group differences were assessed.
Statistical analysis
For evaluation of EB extravasation, immunofluorescence intensity, and contusion volume, two-tailed Student's t-tests for unpaired variables were used to determine differences between the vehicle-treated and CAPE-treated groups. For evaluation of behavioral data, a two-way repeated-measures analysis of variance (ANOVA) was utilized to determine group main effects or interactions. A Holm-Sidak method for multiple comparisons post-hoc test was used to determine the data points with significant differences. All data were tested for normality using a Shapiro-Wilk normality test. Any data found not to have a normal distribution or equal variance were analyzed using the appropriate non-parametric test. Data were considered significant at p<0.05 and presented as mean±standard error of the mean (SEM).
Results
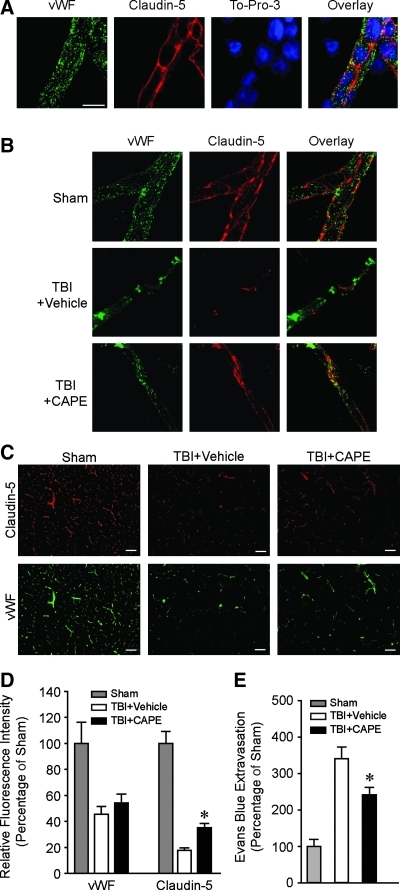
Post-injury CAPE administration reduces TBI-induced loss of claudin-5
TBI disrupts cerebral autoregulation and causes breakdown of the BBB, allowing infiltration of circulating cells, blood products, and fluid into the brain (Baldwin et al., 1996; Golding et al., 1999; Lotocki et al., 2009). This can cause further brain damage by enhancing inflammation, edema, free radical production, and neurovascular dysfunction. Capillary endothelial cells and tight junctions are two major components of the BBB. We therefore examined if CAPE can reduce endothelial cell loss, preserve tight junction protein levels, or both after TBI. To address these possibilities, brain sections were prepared 24 h post-injury from rats injected IP with either CAPE (10 mg/kg; n=4) or vehicle (n=3) for the evaluation of vWF (a marker of endothelial cells), and claudin-5 (a marker of tight junctions) immunoreactivity. A group of sham-operated animals (n=4) were used as uninjured controls. This dose of CAPE was chosen based on its reported effectiveness in reducing infarct size in a rodent model of ischemia (Khan et al., 2007).
Figure 1A shows representative photographs of a blood vessel labeled with vWF and claudin-5, counterstained with the nuclear stain To-Pro-3. Consistent with it being a secreted endothelial cell protein, vWF immunoreactivity appears punctate and defines the location of the vessel. Claudin-5 localizes to the plasma membrane and can be seen as a continuous strip-like staining pattern along the vascular tube. The overlay image demonstrates the localization of vWF and claudin-5 relative to the nuclei of the endothelial cells. As To-Pro-3 is not selective for endothelial cells, nuclei belonging to presumed pericytes (overlaying the vessel) and neurons (distal to the vessel) can be observed. As we and others have previously described, TBI causes the loss of both vWF and claudin-5 immunoreactivity, although regions of vessels immunopositive for vWF but claudin-5 negative can be seen (TBI+vehicle; Fig. 1B; Khan et al., 2009; Zhao et al., 2007). CAPE treatment appears to protect both the levels and cellular localization of claudin-5 (TBI+CAPE; Fig. 1B), suggesting a preservation of vascular integrity. To quantify these changes, low-magnification images of vWF and claudin-5 immunoreactivity in the ipsilateral cerebral cortex from sham, TBI+vehicle, and TBI+CAPE animals (Fig. 1C) were used for fluorescence intensity measurements. Although the loss of vWF immunoreactivity was not influenced by post-injury CAPE treatment (TBI+vehicle=45.37±6.05%; TBI+CAPE=54.31±6.62%; p=0.382; Fig. 1D), a significant increase in claudin-5 levels was detected, suggesting preservation of tight junctions in the surviving endothelial cells (TBI+vehicle=17.65±1.11%; TBI+CAPE=35.06±3.47%; p=0.009; Fig. 1D).
FIG. 1.
Post-injury caffeic acid phenethyl ester (CAPE) administration reduces the loss of claudin-5 and improves vascular integrity following traumatic brain injury (TBI). (A) Representative photomicrographs of a cortical vessel immunostained for the endothelial cell marker von Willibrand Factor (vWF), and the tight junction protein claudin-5, counterstained with To-Pro-3. (B) High-magnification images showing that TBI causes loss of both vWF and claudin-5, and that CAPE treatment (10 mg/kg) appears to partially preserve claudin-5 immunoreactivity. (C) Low-magnification images demonstrating the extent of vWF and claudin-5 loss after TBI. An apparent increase in claudin-5 levels can be seen in the cortex of animals treated with CAPE. (D) Summary data showing that post-injury CAPE significantly increases immunoreactivity for claudin-5 compared to the levels detected in injured animals treated with vehicle. (E) Summary data showing that administration of CAPE 30 min post-injury (n=10/group) significantly decreases the extravasation of Evans Blue. Data are presented as the mean±standard error of the mean; *p<0.05; scale bar in A=25 μm and representing A and B; scale bar in C=100 μm).
Post-injury CAPE administration reduces TBI-associated vascular dysfunction
Several studies have shown that CCI injury causes an increase in vascular permeability (Baldwin et al., 1996; Baskaya et al., 1997; DeWitt and Prough, 2003; Whalen et al., 1998). Based on the preservation of claudin-5 levels we observed, we questioned if this was associated with improved BBB integrity. Rats were injured, then treated with CAPE (10 mg/kg IP; n=10) or vehicle (n=10) 30 min following injury. Permeability was assessed 24 h later by measuring the extravasation of EB into the brain following its intravenous administration. The results presented in Figure 1E show that brain injury increases EB extravasation compared to sham-operated controls, and that post-injury administration of CAPE significantly reduced these levels compared to those seen in vehicle-treated, injured controls (TBI+vehicle: 340.96±32.34%; TBI+CAPE: 242.07±20.02%; p=0.018). This protective effect of CAPE on BBB integrity following TBI was confirmed using a second species, C57BL/6 mice (Supplementary Fig. 1; see online supplementary material at http://www.liebertonline.com).
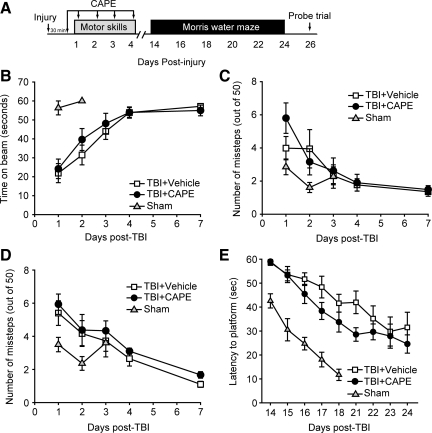
CAPE administration does not improve motor or cognitive function following TBI
Encouraged by the protection offered by CAPE on vascular permeability, we tested if CAPE can improve motor and cognitive functions in brain-injured animals. Rats (n=10/group) were injured, injected with either 10 mg/kg CAPE or an equal volume of vehicle 30 min later, and then daily for the first 4 days post-injury. Vestibulomotor and motor performance was assessed using the beam balance and paw placement tasks, respectively, on days 1–4 and day 7 post-injury (Fig. 2A). A separate group of animals received sham surgery and were tested on the beam balance and paw placement tasks to demonstrate normal performance in these tasks. Figure 2B–D show that CAPE treatment did not improve performance in either the beam balance (repeated measures two-way ANOVA: F(1,18)=0.310, p=0.584; Fig. 2B), nor the paw placement task (repeated measures two-way ANOVA: ipsilateral F(1,18)=0.410, p=0.530, Fig. 2C; contralateral F(1,18)=0.991, p=0.332; Fig. 2D) compared to vehicle-treated animals.
FIG. 2.
Caffeic acid phenethyl ester (CAPE) does not improve neurobehavioral outcome in injured rats. (A) Time line of drug administration and behavioral assessments. Rats (n=10/group) were injured then injected with either CAPE (10 mg/kg) or an equal volume of vehicle 30 min post-injury. Rats received daily injections for the first 5 days post-injury. (B–D) When tested for their motor function, rats treated with CAPE did not perform significantly differently from vehicle-treated rats as tested using a (B) beam balance task, or in the number of (C) ipsilateral or (D) contralateral foot faults. (E) When tested for spatial learning and memory on days 14–18 and 21–24 post-injury, rats treated with CAPE had learning curves that were not significantly different than those treated with vehicle. Data are presented as the mean±standard error of the mean.
Spatial learning and memory was tested using the hidden platform version of the MWM task (Dixon et al., 1999; Hamm et al., 1992). Training in the task was carried out on days 14–18 post-injury, then following a 2-day rest period, the animals were given 4 more days of training. A group of sham animals were identically trained and used to demonstrate baseline performance. Figure 2E shows that when the latencies to find the hidden platform were analyzed, no significant difference was observed between vehicle and CAPE-treated animals (repeated measures two-way ANOVA: F(1,18)=1.870, p=0.188). When latency and number of platform crossings were assessed during a probe trial given 24 h following the completion of training, no differences were detected between the two groups (data not shown). In addition, when tested using a contextual fear memory task, no difference in memory retention was observed between injured animals treated with CAPE and those receiving vehicle (Supplementary Fig. 2; see online supplementary material at http://www.liebertonline.com).
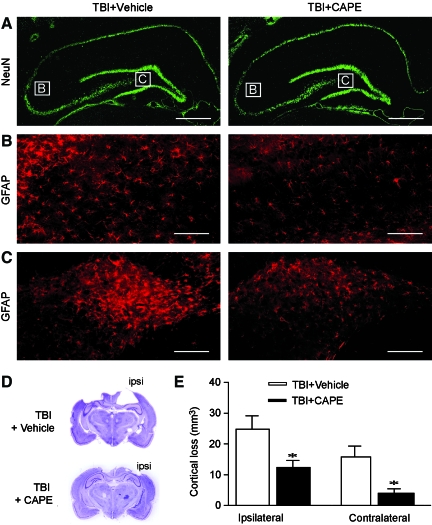
CAPE administration reduces cortical contusion volume
After completion of the behavioral tests, the animals were perfused with paraformaldehyde and the brains were processed for immunohistochemistry using antibodies against NeuN (a neuron-specific marker) and GFAP (a glial-specific marker). Representative images of ipsilateral hippocampi immunostained for NeuN and GFAP from a sham, a vehicle-treated, and a CAPE-treated animal are shown in Figure 3A–C. The boxes in Figure 3A indicate the relative positions of the GFAP images represented in Figure 3B and C. Visual inspection of NeuN-immunostained sections revealed a moderate degree of cell layer thinning in the CA1 and CA3 subfields of the hippocampus after TBI. However, no overt difference in hippocampal cell loss was observed between vehicle-treated and CAPE-treated animals (Fig. 3A). Consistent with previous reports, TBI increased GFAP immunoreactivity in the CA3 (Fig. 3B) and dentate gyrus (Fig. 3C) subfields, an effect that was modestly reduced in CAPE-treated animals.
FIG. 3.
Administration of caffeic acid phenethyl ester (CAPE) after traumatic brain injury (TBI) reduces cortical tissue loss. (A) Representative immunofluorescence images of hippocampi immunostained with stain for neuronal nuclei (NeuN). Boxes indicate the positions of the photomicrographs for glial fibrillary acidic protein (GFAP) shown in B and C. (B) High-magnification images of GFAP immunoreactivity in the CA3 subfield of the ipsilateral hippocampus from a vehicle-treated and a 10-mg/kg CAPE-treated animal. (C) High-magnification images of GFAP immunoreactivity in the dentate gyrus subfield of the ipsilateral hippocampus from a vehicle-treated and a CAPE-treated animal. (D) Representative cresyl violet-stained images of brain sections from a rat receiving vehicle and one treated with CAPE. (E) Summary data showing that post-injury administration of CAPE significantly reduced both ipsilateral and contralateral cortical contusion volume compared to injured rats treated with vehicle (scale bar=750 μm in A; scale bar=100 μm in B and C). Data are presented as the mean±standard error of the mean (*p<0.05). Color image is available online at www.liebertonline.com/neu
As we observed that CAPE reduced claudin-5 loss and improved vascular integrity in the cerebral cortex, we questioned if these observations were associated with a reduction in cortical tissue loss. Figure 3D shows representative images of cresyl violet-stained tissue sections from a vehicle-treated and a CAPE-treated injured rat. Quantification of cortical tissue loss throughout the rostral-caudal extent of the injury revealed that CAPE significantly reduced both ipsilateral (TBI+vehicle: 24.82±4.27 mm3; TBI+CAPE: 12.32±2.28 mm3; p=0.017), and contralateral (TBI+vehicle: 15.74±3.59 mm3; TBI+CAPE: 3.93±1.46 mm3; p=0.006) contusion volumes compared to injured animals receiving vehicle (Fig. 3E).
Discussion
A number of studies have shown that TBI pathophysiology is complex and results from the combined action of multiple cellular and neurochemical mechanisms (Bramlett and Dietrich, 2007; Giza et al., 2002; Mechoulam and Shohami, 2007; Smith et al., 2010). Thus, it is thought that drugs that selectively target a single mechanism may not be as effective as drugs that attenuate more than one mechanism (Loane and Faden, 2010; Margulies and Hicks, 2009; Sayeed and Stein, 2009; Stoica and Faden, 2010). CAPE has been shown to reduce damage arising both from oxidative stress and inflammation, two important mechanisms in the progression of TBI pathology (Irmak et al., 2003). The present study revealed three major findings: (1) post-injury administration of CAPE reduces TBI-induced vascular permeability and (2) cortical tissue loss, but (3) had no effect on post-injury motor or cognitive performance.
CAPE has been reported to have anti-inflammatory, antioxidant, neuroprotective, antiviral, antitumoral, antiatherosclerotic, and immunomodulatory properties in a variety of systems (Fesen et al., 1993; Hishikawa et al., 2005; Ilhan et al., 2004; Liao et al., 2003; Orban et al., 2000; Park and Kahng, 1999; Wei et al., 2004). These diverse effects of CAPE may be attributed to its ability to readily permeate cells, where it inhibits enzymes such as lipoxygenases, cyclooxygenases, glutathione S-transferase, and xanthine oxidase (Chan et al., 1995; Koshihara et al., 1984; Michaluart et al., 1999; Mirzoeva and Calder, 1996; Orsolic et al., 2005). In addition, CAPE has been reported to be a potent inhibitor of NF-κB activation (Natarajan et al., 1996). Consistent with this, we found that CAPE reduces both the basal and tumor necrosis factor-α–induced expression of a NF-κB reporter gene in HT22 mouse hippocampal cells (unpublished data).
BBB breakdown is one of the early pathological events triggered by TBI that contributes to the infiltration of circulating cells and molecules, and facilitates the entry of fluid into the brain, all of which can exacerbate brain damage and tissue loss. A number of studies have reported that ROS and inflammatory molecules can compromise BBB integrity following TBI (Adelson et al., 1998). Consistent with this, it has been reported that either exogenous administration of free radical scavengers, or exogenous activation of endogenous antioxidant pathways, reduces loss of tight junction proteins and improves BBB integrity (Povlishock and Kontos, 1992; Smith et al., 1994; Zhao et al., 2007). We found that post-injury administration of CAPE effectively reduces BBB permeability, an effect seen in both rat and mouse models of TBI. Although the mechanisms by which CAPE reduces BBB permeability are not known at present, we observed that this improvement was associated with an increase in the levels of the tight junction protein claudin-5. Unlike endothelial barriers in other organs, the expression of high levels of claudin-5 in brain microvessels has been shown to give rise to the high trans-endothelial electrical resistance that is critical for maintaining brain homeostasis (Honda et al., 2006). Our finding that CAPE may improve BBB integrity by preserving claudin-5 levels is consistent with other studies that reported that activation of endogenous antioxidant proteins restores claudin-5 levels and markedly protects against TBI-induced enhanced permeability (Zhao et al., 2007).
A number of in vitro and in vivo studies have demonstrated CAPE to be neuroprotective. For example, CAPE/caffeic acid has been reported to protect the brain against induced diabetes, quinolinic acid-induced brain damage, aluminum-induced toxicity, neonatal hypoxia, and transient focal ischemia (Celik and Erdogan, 2008; Kalonia et al., 2009; Khan et al., 2007; Wei et al., 2004; Yang et al., 2008; Zhou et al., 2006). Caffeic acid has also been reported to reduce lesion area and glial scar formation following cryoinjury in mice (Zhang et al., 2007). Our finding that CAPE reduces cortical tissue loss is consistent with these studies. As TBI has been shown to cause oxidative damage, apoptosis, and necrosis of cortical cells (Colicos et al., 1996; Hall, 1993; Raghupathi, 2004; Stoica and Faden, 2010), the inhibition of one or more of these pathways may have contributed to the reduction of cortical contusion volume we observed.
Based on CAPE's effectiveness in reducing vascular permeability after injury, we anticipated that it would improve the function of the injured brain. Although we observed that CAPE reduced parietal cortex tissue loss, this was not accompanied by a reduction in motor dysfunction. The lack of motor skill improvement is likely due to the fact that the contusion caused by the CCI injury employed in the present study does not encompass the entire motor cortex (Supplementary Fig. 3; see online supplementary material at http://www.liebertonline.com), giving rise to motor dysfunction that not only resolves over time, but is unlikely to be affected by preservation of cortical tissue. Similarly, the cognitive tasks employed, the MWM and contextual fear conditioning tasks, are critically dependent on the integrity of the hippocampus and the entorhinal cortex, but not the parietal cortex. For example, in a series of elegant experiments, Kolb and colleagues demonstrated that lesioning of the parietal cortex does not cause significant deficits in the acquisition of the MWM task, nor in the memory of the platform location (Kolb et al., 1983). In contrast, a reduction in hippocampal cell loss would be anticipated to result in improved spatial learning and memory (Shear et al., 2010). Unfortunately, CAPE did not appear to reduce cell loss in the hippocampus, nor was spatial memory improved. This suggests that the mechanisms underlying hippocampal neuron loss/dysfunction may not have been effectively attenuated by CAPE. A similar effect was previously observed when caffeinol was administered after injury, in that cortical protection was seen that was not associated with behavioral improvements (Dash et al., 2004). The reason for the differential neuroprotection offered by CAPE in the cortex versus the hippocampus is not known, but it may be due to the degree of inflammation seen within the cortex relative to the hippocampus in this model of TBI. Thus, CAPE in combination with agents that reduce hippocampal cell loss and/or improve hippocampal plasticity (Kochanek et al., 2006; Lyeth et al., 1990; Phillips and Reeves, 2001; Reeves et al., 2000) may be an effective strategy to improve neurovascular function and outcome after TBI.
Supplementary Material
Acknowledgments
The authors thank Cameron Jeter for her critical reading of the manuscript. The studies were made possible by funds from the NIH (NS049160).
Author Disclosure Statement
No competing financial interests exist.
References
- Adelson P.D. Whalen M.J. Kochanek P.M. Robichaud P. Carlos T.M. Blood-brain barrier permeability and acute inflammation in two models of traumatic brain injury in the immature rat: a preliminary report. Acta Neurochir. Suppl. 1998;71:104–106. doi: 10.1007/978-3-7091-6475-4_31. [DOI] [PubMed] [Google Scholar]
- Babikian T. Prins M.L. Cai Y. Barkhoudarian G. Hartonian I. Hovda D.A. Giza C.C. Molecular and physiological responses to juvenile traumatic brain injury: focus on growth and metabolism. Dev. Neurosci. 2010;32:431–441. doi: 10.1159/000320667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin S.A. Fugaccia I. Brown D.R. Brown L.V. Scheff S.W. Blood-brain barrier breach following cortical contusion in the rat. J. Neurosurg. 1996;85:476–481. doi: 10.3171/jns.1996.85.3.0476. [DOI] [PubMed] [Google Scholar]
- Baskaya M.K. Rao A.M. Dogan A. Donaldson D. Dempsey R.J. The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci. Lett. 1997;226:33–36. doi: 10.1016/s0304-3940(97)00239-5. [DOI] [PubMed] [Google Scholar]
- Beauchamp K. Mutlak H. Smith W.R. Shohami E. Stahel P.F. Pharmacology of traumatic brain injury: where is the “golden bullet”? Mol. Med. 2008;14:731–740. doi: 10.2119/2008-00050.Beauchamp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlett H.M. Dietrich W.D. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog. Brain Res. 2007;161:125–141. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- Celik S. Erdogan S. Caffeic acid phenethyl ester (CAPE) protects brain against oxidative stress and inflammation induced by diabetes in rats. Mol. Cell Biochem. 2008;312:39–46. doi: 10.1007/s11010-008-9719-3. [DOI] [PubMed] [Google Scholar]
- Chan W.S. Wen P.C. Chiang H.C. Structure-activity relationship of caffeic acid analogues on xanthine oxidase inhibition. Anticancer Res. 1995;15:703–707. [PubMed] [Google Scholar]
- Colicos M.A. Dixon C.E. Dash P.K. Delayed, selective neuronal death following experimental cortical impact injury in rats: possible role in memory deficits. Brain Res. 1996;739:111–119. doi: 10.1016/s0006-8993(96)00819-0. [DOI] [PubMed] [Google Scholar]
- Dash P.K. Mach S.A. Moore A.N. The role of extracellular signal-regulated kinase in cognitive and motor deficits following experimental traumatic brain injury. Neuroscience. 2002;114:755–767. doi: 10.1016/s0306-4522(02)00277-4. [DOI] [PubMed] [Google Scholar]
- Dash P.K. Moore A.N. Dixon C.E. Spatial memory deficits, increased phosphorylation of the transcription factor CREB, and induction of the AP-1 complex following experimental brain injury. J. Neurosci. 1995;15:2030–2039. doi: 10.1523/JNEUROSCI.15-03-02030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P.K. Moore A.N. Moody M.R. Treadwell R. Felix J.L. Clifton G.L. Post-trauma administration of caffeine plus ethanol reduces contusion volume and improves working memory in rats. J. Neurotrauma. 2004;21:1573–1583. doi: 10.1089/neu.2004.21.1573. [DOI] [PubMed] [Google Scholar]
- DeWitt D.S. Prough D.S. Traumatic cerebral vascular injury: the effects of concussive brain injury on the cerebral vasculature. J. Neurotrauma. 2003;20:795–825. doi: 10.1089/089771503322385755. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Clifton G.L. Lighthall J.W. Yaghmai A.A. Hayes R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Kochanek P.M. Yan H.Q. Schiding J.K. Griffith R.G. Baum E. Marion D.W. DeKosky S.T. One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J. Neurotrauma. 1999;16:109–122. doi: 10.1089/neu.1999.16.109. [DOI] [PubMed] [Google Scholar]
- Fan P. Yamauchi T. Noble L.J. Ferriero D.M. Age-dependent differences in glutathione peroxidase activity after traumatic brain injury. J. Neurotrauma. 2003;20:437–445. doi: 10.1089/089771503765355513. [DOI] [PubMed] [Google Scholar]
- Fedor M. Berman R.F. Muizelaar J.P. Lyeth B.G. Hippocampal theta dysfunction after lateral fluid percussion injury. J. Neurotrauma. 2010;27:1605–1615. doi: 10.1089/neu.2010.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesen M.R. Kohn K.W. Leteurtre F. Pommier Y. Inhibitors of human immunodeficiency virus integrase. Proc. Natl. Acad. Sci. USA. 1993;90:2399–2403. doi: 10.1073/pnas.90.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd C.L. Lyeth B.G. Astroglia: important mediators of traumatic brain injury. Prog. Brain Res. 2007;161:61–79. doi: 10.1016/S0079-6123(06)61005-4. [DOI] [PubMed] [Google Scholar]
- Giri B.K. Krishnappa I.K. Bryan R.M., Jr. Robertson C. Watson J. Regional cerebral blood flow after cortical impact injury complicated by a secondary insult in rats. Stroke. 2000;31:961–967. doi: 10.1161/01.str.31.4.961. [DOI] [PubMed] [Google Scholar]
- Giza C.C. Prins M.L. Hovda D.A. Herschman H.R. Feldman J.D. Genes preferentially induced by depolarization after concussive brain injury: effects of age and injury severity. J. Neurotrauma. 2002;19:387–402. doi: 10.1089/08977150252932352. [DOI] [PubMed] [Google Scholar]
- Golding E.M. Robertson C.S. Bryan R.M., Jr. The consequences of traumatic brain injury on cerebral blood flow and autoregulation: a review. Clin. Exp. Hypertens. 1999;21:299–332. doi: 10.3109/10641969909068668. [DOI] [PubMed] [Google Scholar]
- Hall E.D. The role of oxygen radicals in traumatic injury: clinical implications. J. Emerg. Med. 1993;11(Suppl. 1):31–36. [PubMed] [Google Scholar]
- Hall E.D. Vaishnav R.A. Mustafa A.G. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm R.J. Dixon C.E. Gbadebo D.M. Singha A.K. Jenkins L.W. Lyeth B.G. Hayes R.L. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- Hayes R.L. Dixon C.E. Neurochemical changes in mild head injury. Semin. Neurol. 1994;14:25–31. doi: 10.1055/s-2008-1041055. [DOI] [PubMed] [Google Scholar]
- Hayes R.L. Robinson G. Muller U. Wang K.K. Translation of neurological biomarkers to clinically relevant platforms. Methods Mol. Biol. 2009;566:303–313. doi: 10.1007/978-1-59745-562-6_20. [DOI] [PubMed] [Google Scholar]
- Hicks R.R. Fertig S.J. Desrocher R.E. Koroshetz W.J. Pancrazio J.J. Neurological effects of blast injury. J. Trauma. 2010;68:1257–1263. doi: 10.1097/TA.0b013e3181d8956d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishikawa K. Nakaki T. Fujita T. Oral flavonoid supplementation attenuates atherosclerosis development in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005;25:442–446. doi: 10.1161/01.ATV.0000148404.24271.fc. [DOI] [PubMed] [Google Scholar]
- Honda M. Nakagawa S. Hayashi K. Kitagawa N. Tsutsumi K. Nagata I. Niwa M. Adrenomedullin improves the blood-brain barrier function through the expression of claudin-5. Cell Mol. Neurobiol. 2006;26:109–118. doi: 10.1007/s10571-006-9028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J.W. Raghupathi R. New concepts in treatment of pediatric traumatic brain injury. Anesthesiol. Clin. 2009;27:213–240. doi: 10.1016/j.anclin.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi T. Huang T.T. Noble L.J. Regional vulnerability after traumatic brain injury: gender differences in mice that overexpress human copper, zinc superoxide dismutase. Exp. Neurol. 2001;172:332–341. doi: 10.1006/exnr.2001.7820. [DOI] [PubMed] [Google Scholar]
- Ilhan A. Akyol O. Gurel A. Armutcu F. Iraz M. Oztas E. Protective effects of caffeic acid phenethyl ester against experimental allergic encephalomyelitis-induced oxidative stress in rats. Free Radic. Biol. Med. 2004;37:386–394. doi: 10.1016/j.freeradbiomed.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Irmak M.K. Fadillioglu E. Sogut S. Erdogan H. Gulec M. Ozer M. Yagmurca M. Gozukara M.E. Effects of caffeic acid phenethyl ester and alpha-tocopherol on reperfusion injury in rat brain. Cell Biochem. Funct. 2003;21:283–289. doi: 10.1002/cbf.1024. [DOI] [PubMed] [Google Scholar]
- Kalonia H. Kumar P. Kumar A. Nehru B. Effect of caffeic acid and rofecoxib and their combination against intrastriatal quinolinic acid induced oxidative damage, mitochondrial and histological alterations in rats. Inflammopharmacology. 2009;17:211–219. doi: 10.1007/s10787-009-0012-1. [DOI] [PubMed] [Google Scholar]
- Kelley B.J. Lifshitz J. Povlishock J.T. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J. Neuropathol. Exp. Neurol. 2007;66:989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- Khan M. Elango C. Ansari M.A. Singh I. Singh A.K. Caffeic acid phenethyl ester reduces neurovascular inflammation and protects rat brain following transient focal cerebral ischemia. J. Neurochem. 2007;102:365–377. doi: 10.1111/j.1471-4159.2007.04526.x. [DOI] [PubMed] [Google Scholar]
- Khan M. Im Y.B. Shunmugavel A. Gilg A.G. Dhindsa R.K. Singh A.K. Singh I. Administration of S-nitrosoglutathione after traumatic brain injury protects the neurovascular unit and reduces secondary injury in a rat model of controlled cortical impact. J. Neuroinflammation. 2009;6:32. doi: 10.1186/1742-2094-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek A.R. Kline A.E. Gao W.M. Chadha M. Lai Y. Clark R.S. Dixon C.E. Jenkins L.W. Gel-based hippocampal proteomic analysis 2 weeks following traumatic brain injury to immature rats using controlled cortical impact. Dev. Neurosci. 2006;28:410–419. doi: 10.1159/000094167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B. Sutherland R.J. Whishaw I.Q. A comparison of the contributions of the frontal and parietal association cortex to spatial localization in rats. Behav. Neurosci. 1983;97:13–27. doi: 10.1037//0735-7044.97.1.13. [DOI] [PubMed] [Google Scholar]
- Kono Y. Kobayashi K. Tagawa S. Adachi K. Ueda A. Sawa Y. Shibata H. Antioxidant activity of polyphenolics in diets. Rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochim. Biophys. Acta. 1997;1335:335–342. doi: 10.1016/s0304-4165(96)00151-1. [DOI] [PubMed] [Google Scholar]
- Koshihara Y. Neichi T. Murota S. Lao A. Fujimoto Y. Tatsuno T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochim. Biophys. Acta. 1984;792:92–97. [PubMed] [Google Scholar]
- Liao H.F. Chen Y.Y. Liu J.J. Hsu M.L. Shieh H.J. Liao H.J. Shieh C.J. Shiao M.S. Chen Y.J. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J. Agric. Food Chem. 2003;51:7907–7912. doi: 10.1021/jf034729d. [DOI] [PubMed] [Google Scholar]
- Lifshitz J. Friberg H. Neumar R.W. Raghupathi R. Welsh F.A. Janmey P. Saatman K.E. Wieloch T. Grady M.S. McIntosh T.K. Structural and functional damage sustained by mitochondria after traumatic brain injury in the rat: evidence for differentially sensitive populations in the cortex and hippocampus. J. Cereb. Blood Flow Metab. 2003;23:219–231. doi: 10.1097/01.WCB.0000040581.43808.03. [DOI] [PubMed] [Google Scholar]
- Loane D.J. Faden A.I. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotocki G. Vaccari J.P. Perez E.R. Sanchez-Molano J. Furones-Alonso O. Bramlett H.M. Dietrich W.D. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of post-traumatic hypothermia. J. Neurotrauma. 2009;26:1123–1134. doi: 10.1089/neu.2008.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyeth B.G. Jenkins L.W. Hamm R.J. Dixon C.E. Phillips L.L. Clifton G.L. Young H.F. Hayes R.L. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- Margulies S. Hicks R. Combination therapies for traumatic brain injury: prospective considerations. J. Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney D.F. Smith D.H. Biomechanics of concussion. Clin. Sports Med. 2011;30:19–31. doi: 10.1016/j.csm.2010.08.009. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R. Shohami E. Endocannabinoids and traumatic brain injury. Mol. Neurobiol. 2007;36:68–74. doi: 10.1007/s12035-007-8008-6. [DOI] [PubMed] [Google Scholar]
- Michaluart P. Masferrer J.L. Carothers A.M. Subbaramaiah K. Zweifel B.S. Koboldt C. Mestre J.R. Grunberger D. Sacks P.G. Tanabe T. Dannenberg A.J. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999;59:2347–2352. [PubMed] [Google Scholar]
- Mirzoeva O.K. Calder P.C. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot. Essent. Fatty Acids. 1996;55:441–449. doi: 10.1016/s0952-3278(96)90129-5. [DOI] [PubMed] [Google Scholar]
- Natarajan K. Singh S. Burke T.R., Jr. Grunberger D. Aggarwal B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc. Natl. Acad. Sci. USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban Z. Mitsiades N. Burke T.R., Jr. Tsokos M. Chrousos G.P. Caffeic acid phenethyl ester induces leukocyte apoptosis, modulates nuclear factor-kappa B and suppresses acute inflammation. Neuroimmunomodulation. 2000;7:99–105. doi: 10.1159/000026427. [DOI] [PubMed] [Google Scholar]
- Orsolic N. Terzic S. Mihaljevic Z. Sver L. Basic I. Effects of local administration of propolis and its polyphenolic compounds on tumor formation and growth. Biol. Pharm. Bull. 2005;28:1928–1933. doi: 10.1248/bpb.28.1928. [DOI] [PubMed] [Google Scholar]
- Ottens A.K. Bustamante L. Golden E.C. Yao C. Hayes R.L. Wang K.K. Tortella F.C. Dave J.R. Neuroproteomics: a biochemical means to discriminate the extent and modality of brain injury. J. Neurotrauma. 2010;27:1837–1852. doi: 10.1089/neu.2010.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E.H. Kahng J.H. Suppressive effects of propolis in rat adjuvant arthritis. Arch. Pharm. Res. 1999;22:554–558. doi: 10.1007/BF02975325. [DOI] [PubMed] [Google Scholar]
- Phillips L.L. Reeves T.M. Interactive pathology following traumatic brain injury modifies hippocampal plasticity. Restor. Neurol. Neurosci. 2001;19:213–235. [PubMed] [Google Scholar]
- Povlishock J.T. Kontos H.A. The role of oxygen radicals in the pathobiology of traumatic brain injury. Hum. Cell. 1992;5:345–353. [PubMed] [Google Scholar]
- Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S.K. Dixon C.E. Banik N.L. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol. Histopathol. 2002;17:1137–1152. doi: 10.14670/HH-17.1137. [DOI] [PubMed] [Google Scholar]
- Reeves T.M. Kao C.Q. Phillips L.L. Bullock M.R. Povlishock J.T. Presynaptic excitability changes following traumatic brain injury in the rat. J. Neurosci. Res. 2000;60:370–379. doi: 10.1002/(SICI)1097-4547(20000501)60:3<370::AID-JNR12>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Royo N.C. LeBold D. Magge S.N. Chen I. Hauspurg A. Cohen A.S. Watson D.J. Neurotrophin-mediated neuroprotection of hippocampal neurons following traumatic brain injury is not associated with acute recovery of hippocampal function. Neuroscience. 2007;148:359–370. doi: 10.1016/j.neuroscience.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman K.E. Creed J. Raghupathi R. Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics. 2010;7:31–42. doi: 10.1016/j.nurt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed I. Stein D.G. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog. Brain Res. 2009;175:219–237. doi: 10.1016/S0079-6123(09)17515-5. [DOI] [PubMed] [Google Scholar]
- Shear D.A. Lu X.C. Bombard M.C. Pedersen R. Chen Z. Davis A. Tortella F.C. Longitudinal characterization of motor and cognitive deficits in a model of penetrating ballistic-like brain injury. J. Neurotrauma. 2010;27:1911–1923. doi: 10.1089/neu.2010.1399. [DOI] [PubMed] [Google Scholar]
- Shellington D.K. Du L. Wu X. Exo J. Vagni V. Ma L. Janesko-Feldman K. Clark R.S. Bayir H. Dixon C.E. Jenkins L.W. Hsia C.J. Kochanek P.M. Polynitroxylated pegylated hemoglobin: A novel neuroprotective hemoglobin for acute volume-limited fluid resuscitation after combined traumatic brain injury and hemorrhagic hypotension in mice. Crit. Care Med. 2011;39:494–505. doi: 10.1097/CCM.0b013e318206b1fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.M. Chen Y. Sullivan M.L. Kochanek P.M. Clark R.S. Autophagy in acute brain injury: feast, famine, or folly? Neurobiol. Dis. 2010 doi: 10.1016/j.nbd.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.L. Andrus P.K. Zhang J.R. Hall E.D. Direct measurement of hydroxyl radicals, lipid peroxidation, and blood-brain barrier disruption following unilateral cortical impact head injury in the rat. J. Neurotrauma. 1994;11:393–404. doi: 10.1089/neu.1994.11.393. [DOI] [PubMed] [Google Scholar]
- Sondheimer E. On the distribution of caffeic acid and the chlorogenic acid isomers in plants. Arch. Biochem. Biophys. 1958;74:131–138. doi: 10.1016/0003-9861(58)90207-8. [DOI] [PubMed] [Google Scholar]
- Stewart L.R. White A.R. Jobling M.F. Needham B.E. Maher F. Thyer J. Beyreuther K. Masters C.L. Collins S.J. Cappai R. Involvement of the 5-lipoxygenase pathway in the neurotoxicity of the prion peptide PrP106-126. J. Neurosci. Res. 2001;65:565–572. doi: 10.1002/jnr.1186. [DOI] [PubMed] [Google Scholar]
- Stoica B.A. Faden A.I. Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics. 2010;7:3–12. doi: 10.1016/j.nurt.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud'ina G.F. Mirzoeva O.K. Pushkareva M.A. Korshunova G.A. Sumbatyan N.V. Varfolomeev S.D. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 1993;329:21–24. doi: 10.1016/0014-5793(93)80184-v. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Sebastian A.H. Hall E.D. Therapeutic window analysis of the neuroprotective effects of cyclosporine a after traumatic brain injury. J. Neurotrauma. 2011;28:311–318. doi: 10.1089/neu.2010.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uz T. Pesold C. Longone P. Manev H. Aging-associated up-regulation of neuronal 5-lipoxygenase expression: putative role in neuronal vulnerability. FASEB J. 1998;12:439–449. doi: 10.1096/fasebj.12.6.439. [DOI] [PubMed] [Google Scholar]
- Vink R. Bullock M.R. Traumatic brain injury: therapeutic challenges and new directions. Neurotherapeutics. 2010;7:1–2. doi: 10.1016/j.nurt.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X. Zhao L. Ma Z. Holtzman D.M. Yan C. Dodel R.C. Hampel H. Oertel W. Farlow M.R. Du Y. Caffeic acid phenethyl ester prevents neonatal hypoxic-ischaemic brain injury. Brain. 2004;127:2629–2635. doi: 10.1093/brain/awh316. [DOI] [PubMed] [Google Scholar]
- Werner C. Engelhard K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- Whalen M.J. Carlos T.M. Kochanek P.M. Heineman S. Blood-brain barrier permeability, neutrophil accumulation and vascular adhesion molecule expression after controlled cortical impact in rats: a preliminary study. Acta Neurochir. Suppl. 1998;71:212–214. doi: 10.1007/978-3-7091-6475-4_61. [DOI] [PubMed] [Google Scholar]
- Yakovlev A.G. Faden A.I. Caspase-dependent apoptotic pathways in CNS injury. Mol. Neurobiol. 2001;24:131–144. doi: 10.1385/MN:24:1-3:131. [DOI] [PubMed] [Google Scholar]
- Yang J.Q. Zhou Q.X. Liu B.Z. He B.C. Protection of mouse brain from aluminum-induced damage by caffeic acid. CNS Neurosci. Ther. 2008;14:10–16. doi: 10.1111/j.1527-3458.2007.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. Zhang W.P. Chen K.D. Qian X.D. Fang S.H. Wei E.Q. Caffeic acid attenuates neuronal damage, astrogliosis and glial scar formation in mouse brain with cryoinjury. Life Sci. 2007;80:530–537. doi: 10.1016/j.lfs.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Zhao J. Moore A.N. Redell J.B. Dash P.K. Enhancing expression of Nrf2-driven genes protects the blood-brain barrier after brain injury. J. Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. Fang S.H. Ye Y.L. Chu L.S. Zhang W.P. Wang M.L. Wei E.Q. Caffeic acid ameliorates early and delayed brain injuries after focal cerebral ischemia in rats. Acta Pharmacol. Sin. 2006;27:1103–1110. doi: 10.1111/j.1745-7254.2006.00406.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.