Abstract
Dementia pugilistica (DP) is associated with chronic traumatic brain injury (CTBI), and leads to a “punch drunk” syndrome characterized by impairments in memory and executive function, behavioral changes, and motor signs. Microscopic features include the accumulation of neurofibrillary tangles (NFTs), beta-amyloid (Aβ), and TAR DNA binding protein 43 (TDP-43) pathology. Here we describe detailed clinical and neuropathological data about a 55-year-old retired boxer (ApoE3/4), who presented with executive dysfunction and behavioral impairments. At autopsy, significant Aβ pathology was seen, primarily in the form of diffuse plaques. Tau pathology was extensive and was determined to be of Braak and Braak stage VI. Frontal white matter showed evidence of glial tau inclusions (astrocytes and oligodendroglia). Cerebrovascular pathology was minimal with patchy amyloid angiopathy. Inflammation was another key feature, including microglial activation and significant C1q labeling of neurons, along with NFTs. TDP-43-positive pathology was also observed. Inflammation may be a key inciting as well as propagating feature of DP neuropathology.
Key words: beta-amyloid, C1q, chronic traumatic encephalopathy, tauopathy, TDP-43
Introduction
Dementia pugilistica (DP) is associated with repeated head trauma, resulting in a progressive disorder first described as “punch-drunk syndrome” in 1928 by Martland (Martland, 1928). Approximately 17% of retired professional boxers exhibit signs of cognitive dysfunction associated with chronic traumatic brain injury (CTBI), and more recently chronic traumatic encephalopathy (CTE), with severe dementia occurring in about 6% of subjects (Heilbronner et al., 2009; Roberts, 1969). DP is characterized by memory deficits, disorientation, confusion, and frontal signs such as loss of insight and inappropriate behaviors (Corsellis et al., 1973; Forstl et al., 2010; Gavett et al., 2011; Roberts, 1969; Spillane, 1962). Additional behavioral changes, such as irritability, aggression, disinhibition, euphoria, and impaired insight, have also been reported (Gavett et al., 2011; Mendez, 1995). Motor abnormalities such as ataxia, spasticity, impaired coordination, and parkinsonism (pugilistica parkinsonism) are also consistent features of the syndrome (Forstl et al., 2010; Gavett et al., 2011; Mendez, 1995). Interestingly, the clinical signs and progression of dementia in boxers may share features with other frontotemporal dementias (FTDs; Scully et al., 1999), although exceptions have been noted (Areza-Fegyveres et al., 2007).
The neuropathological features of DP have several consistent patterns across case reports (Corsellis, 1989; Gavett et al., 2011; Jordan, 2000; McKee et al., 2009; Nowak et al., 2009). These include an accumulation of neurofibrillary tangles (NFTs), and a lesser degree of beta-amyloid (Aβ) pathology (Allsop et al., 1990; Hof et al., 1992; Jordan et al., 1995; McKee et al., 2009; McKenzie et al., 1996; Nowak et al., 2009; Roberts, 1988; Scully et al., 1999; Tokuda et al., 1991). Another relatively consistent feature of DP is the presence of extracellular NFTs, with some studies showing Aβ-positive extracellular NFTs (Allsop et al., 1990; Hof et al., 1992; Schmidt et al., 2001; Tokuda et al., 1991). Recently, TAR DNA binding protein 43 (TDP-43) pathology has also been observed in DP (King et al., 2010), which is also a feature of other dementias (Wilson et al., 2011). Septal abnormalities (fenestration), widening of the lateral ventricles, and thinning of the fornix have both been observed at autopsy (Corsellis et al., 1973; Spillane, 1962), and in in vivo studies (Moseley, 2000; Scully et al., 1999; Spillane, 1962; Zhang et al., 2003,2006). Often there is scarring of the cerebellum and pronounced Purkinje cell loss (Corsellis et al., 1973). Significant loss of pigmented neurons in the substantia nigra may cause extrapyramidal motor signs in affected subjects (Corsellis et al., 1973; Forstl et al., 2010). Basal forebrain cholinergic neurons are also affected (Uhl et al., 1982).
The purpose of the current study was to report on an interesting DP patient who was thoroughly evaluated clinically and neuropathologically. Although serious concussions can cause permanent neurologic damage, in boxing the acute neurologic injury is more often transient (Forstl et al., 2010; Gavett et al., 2011; Heilbronner et al., 2009). On the other hand, repetitive concussive and subconcussive blows to the brain over many years have been identified as the primary cause of neurologic symptoms, and can occur many years and even decades after the individual has retired from boxing. While the exact mechanisms involved in the significant long-term delayed sequelae associated with sports-related CTE are poorly understood (DeKosky et al., 2010), the clinical presentation is consistent with a slowly progressive tauopathy (McKee et al., 2009). In the current study we focused on the frontal cortex, given the clinical signs, and comparisons were made with patients of similar age who had clinical features suggestive of FTD, but who proved pathologically to have Alzheimer's disease (AD; n=2), with patients who had AD (n=2), and with non-demented controls (n=3). We describe extracellular NFT-associated proteins, inflammation, and signs of white matter and vascular changes in the frontal cortex of this DP case.
Methods
Human tissue samples
Autopsy samples were acquired from a DP patient who died at the age of 55 years. Comparison cases were selected from the University of California Irvine Alzheimer Disease Research Center (UCI-ADRC) Tissue Repository (Table 1), two with clinical signs consistent with FTD, but later proven neuropathologically to be AD (FTD-AD), and two with AD (AD-AD) who died at ∼61 years of age. Finally, three non-demented control cases who died at an age similar to that of the DP patient, two from the UCI Tissue Repository and one from the Maryland Developmental Disorders Brain Bank, were examined.
Table 1.
Case Demographic Data
| Case | Age (y) | Sex | PMI (h) | Clinical diagnosis | Neuropathology diagnosis | Cause of death | Brain weight (g) | B&B tangle stage |
|---|---|---|---|---|---|---|---|---|
| 1 | 55 | M | 5.8 | Possible AD, FTD, dementia pugilistica | AD | Dementia | 1115.9 | VI, A |
| 2 | 53 | F | 5 | Possible FTD | AD | Unknown | 950 | VI, C |
| 3 | 56 | F | 3.9 | Possible FTD | AD | Dementia | 1004.3 | VI, C |
| 4 | 59 | M | 3.3 | Possible AD | AD | Dementia | 1211.6 | VI, C |
| 5 | 61 | M | 5.6 | Possible AD | AD | Pneumonia | 994.8 | VI, C |
| 6 | 61 | M | 14 | Nondemented | Control | Pneumonia | 1500 | N/A |
| 7 | 64 | F | 8 | Nondemented | Control | Myocardial infarction | 1182 | N/A |
| 8 | 54 | F | 5 | Nondemented | Control | Pulmonary thrombo-embolus | N/A | N/A |
AD, Alzheimer's disease; FTD, frontotemporal dementia; PMI, post mortem interval; B&B, Braak & Braak; N/A, not applicable.
Brain tissue procedures
The right hemisphere, brainstem, and cerebellum were fixed in 4% paraformaldehyde for 2 weeks and preselected blocks were embedded in paraffin and sectioned at 8 μm. Additionally, the fixed frontal cortex and hippocampus were blocked and sectioned at 50 μm using a vibratome and stored in phosphate-buffered saline (pH 7.5) containing 0.02% sodium azide for additional studies.
Histological and immunohistochemical procedures
As part of the standard UCI-ADRC Neuropathology Core protocol, modified Bielschowsky, hematoxylin and eosin, and Klüver-Barrera stains, and immunostains for tau, α-synuclein, ubiquitin, glial fibrillary acidic protein (GFAP), and CD68 were applied (Table 2). In thicker 50-μm-thick sections, additional proteins of interest were visualized and are listed in Table 2. Standard immunohistochemistry methods were used (Head et al., 2006). Prussian blue histochemistry was by previously published methods (Petrushina et al., 2007).
Table 2.
Antibodies Used in the Study
| Antibody | Marker | Host | Source |
|---|---|---|---|
| C1q | Classical complement pathway | Polyclonal | Dako, Carpinteria, CA |
| CD3 | T lymphocytes | Monoclonal | Novocastra Laboratories, Ltd., Newcastle upon Tyne, U.K. |
| CD4 | T lymphocytes | Monoclonal | Novocastra Laboratories, Ltd. |
| CD8 | T lymphocytes | Monoclonal | Novocastra Laboratories, Ltd. |
| CD40 | B cells | Monoclonal | Novocastra Laboratories, Ltd. |
| GFAP | Astrocytosis | Polyclonal | Dako |
| LN-3 | Microglial activation | Monoclonal | ICN Biomedicals, Aurora, OH |
| CD68 | Microglial activation | Monoclonal | Dako, Temcula, CA |
| 6E10 | Senile Plaques | Monoclonal | Signet Laboratories, Dedham, MA |
| Anti-Aβ42 | Senile plaques | Polyclonal | Biosource International, Camarillo, CA |
| Anti-Aβ40 | Senile plaques | Polyclonal | Biosource International |
| 22C11 | APP N-terminus | Monoclonal | Chemicon International, Temecula, CA |
| AT8 | Neurofibrillary tangles | Monoclonal | Pierce Biotechnology, Rockford, IL |
| MC-1 | Early neurofibrillary tangles | Monoclonal | Peter Davies, Einstein, NY |
| PHF-1 | Late-stage neurofibrillary tangles | Monoclonal | Peter Davies |
| HT7 | Phosphorylated tau | Monoclonal | Pierce Biotechnology |
| Ubiquitin | Lewy bodies | Polyclonal | Dako |
| α-Synuclein | Lewy bodies | Polyclonal | Chemicon International |
| Tau-CCP | Caspase-cleaved fragments | Polyclonal | Troy Rohn, Boise State University, Boise, ID |
| TDP-43 | TAR DNA binding protein-43 | Polyclonal | ProSci Incorporated, Poway, CA |
| ApoE | Apolipoprotein E | Polyclonal | Chemicon International |
Results
DP patient clinical characteristics and neurological examination
On presentation, the patient was a right-handed, 47-year-old Caucasian male with 12 years of education with complaints of memory problems and personality changes. He was followed annually at the UCI-ADRC for a total of seven visits. He had been a professional lightweight to heavyweight boxer for 13 years. During his boxing career he was knocked out twice, once with amnesia lasting 3 days. According to his wife, memory problems were first observed when he was 32 years of age, approximately 1 year after he retired from boxing. Problems with increased forgetfulness were evidenced by difficulty completing tasks, an inability to follow instructions, and trouble holding a job. Problems with recent memory became progressively more noticeable over time, along with difficulties in attention, speed of thinking, and a variety of fronto-executive behaviors, including abstraction, judgment, planning, and organization. At his initial visit, personality changes, including frequent outbursts of anger, poor judgment, inability to tolerate frustration, and marked disinhibition, were the wife's primary concerns. For example, he would make insensitive and inappropriate comments to strangers on the street. His affect was described as fluctuating between euphoric and flat or apathetic, but not depressed. Although there was no history of alcohol abuse or tobacco use, the patient had used cocaine and marijuana occasionally for an unknown period ending in his late 20s. His family history was significant for dementia in both his father, with age of onset of 75 years, and an older brother, also a professional boxer (heavyweight), who died at age 53 years with severe impairment. No neuropathological data were available for these individuals. During the entire 7-year period the patient was being followed clinically, none of his surviving family members, including his mother and 6 siblings, exhibited dementia per his wife, although all reportedly had difficulties with depression. The initial neurological examination was within normal limits, with the exception of bilateral slowing in fine sequential finger movements, and mild difficulties with tandem gait. Additional impairments in gait, including short steps with mild shuffling and a bilateral lack of arm swing, were observed during the last two evaluations, as well as bilateral paramyotonia and bradykinesia, plus a snout reflex.
Neuroimaging
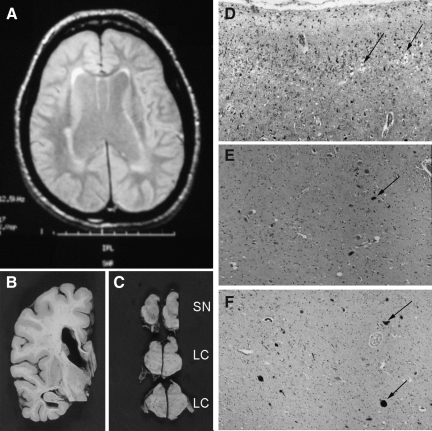
Computed tomography (CT) of the head performed 6 years prior to his initial visit revealed a mild degree of central atrophy, slight prominence of the third and lateral ventricles, and a large cavum septum pellucidum. A magnetic resonance imaging (MRI) scan performed during his first visit confirmed the presence of mild diffuse cerebral atrophy, along with nonspecific bilateral periventricular white matter changes. His final MRI scan, performed during his fifth visit, revealed moderate central atrophy and mild generalized cortical atrophy abnormal for his age (Fig. 1A). This MRI also showed thinning of the corpus callosum, mild-to-moderate hippocampal atrophy with the right side being more affected than the left, and mild-to-moderate white matter changes without focal lesions.
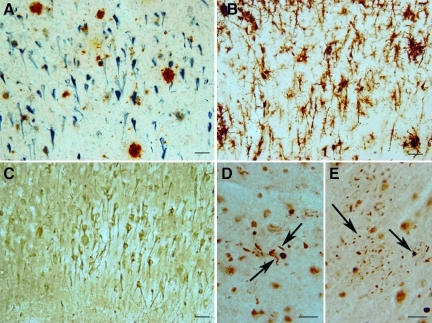
FIG. 1.
General characteristics of the dementia pugilistica (DP) patient. (A) Magnetic resonance image scan from the patient's fifth visit showed widened ventricles, and a mild-to-moderate degree of white matter change without any evidence of specific focal lesions. (B) A coronal section of the brain showed ventricular enlargement, absence of the septum pellucidum, a thinned corpus callosum, and minimal cortical atrophy. (C) There was a moderately severe reduction of neuromelanin pigmentation in the substantia nigra (SN) and locus ceruleus (LC). (D) Rarefaction within cortical layer 2 was noted in the superior temporal gyrus (arrow). (E) This higher-magnification image of the substantia nigra shows a loss of neuromelanin-bearing neurons (arrows). (F) Neurofibrillary tangle pathology (arrows) was present within the locus ceruleus. The photographs in D–F were taken with a 10×objective.
Neuropsychological evaluations
Scores on 18 tests in the UCI-ADRC neuropsychological battery are reported in Table 3 for each of the patient's seven visits. At the time of his initial visit, his score of 23/30 points on the Mini-Mental State Examination (MMSE) suggested a mild dementia. Scores on the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Word List, a test of recent verbal memory, were below average, with impaired performance on measures of delayed free recall, recognition, and the use of organizational strategies. Immediate attention span, as measured by the Wechsler Adult Intelligence Scale-III (WAIS-III) Digit Span test, was also reduced. He was slower than 95% of his peers at processing information in working memory, with severely impaired scores on the Symbol Digit Modality Test, Part A of the Trail-Making Test, and the Kendrick Digit Copy tests. Given the personality changes reported by his wife, it is not surprising that he showed significant deficits on multiple measures of executive functioning, including everyday problem solving (e.g., Social Judgment), abstract reasoning (e.g., WAIS-III Similarities), and mental flexibility (e.g., Part B of the Trail-Making Test). Although verbal comprehension and repetition were unaffected, he showed mild problems on tests of confrontational object naming (e.g., Boston Naming Test), and verbal fluency (e.g., Category and Letter Fluency). Basic constructional abilities, such as copying pictures of different geometric shapes on the CERAD Drawing task, remained preserved except when the task required the use of executive skills such as reasoning, planning, and organization (e.g., WAIS-III Block Design). Over the course of his illness, a slow but progressive deterioration in his scores was observed on almost all neuropsychological measures except for basic drawing ability. At his last visit 7 years following his entry into the UCI-ADRC, and approximately 11 months prior to his death, he was severely demented and unable to perform any of the cognitive tests in the battery. His scores on the MMSE and Severe Impairment Battery (SIB) at that time were 10/30 and 74/100 points, respectively.
Table 3.
Scores on Neuropsychological Tests Across Multiple Visits
| Test | Visit 7 | Visit 6 | Visit 5 | Visit 4 | Visit 3 | Visit 2 | Visit 1 | Norms mean±SD | Min-max score |
|---|---|---|---|---|---|---|---|---|---|
| Mental status | |||||||||
| MMSE | 10 | 15 | 21 | 21 | 26 | 24 | 23 | 28.9±1.2 | 0-30 |
| Recent memory: CERAD Word List | |||||||||
| Total Recall: All 3 trials | FC | 11 | 13 | 10 | 15 | 13 | 10 | 21±2.7 | 0-30 |
| 5-min Delayed Recall | FC | 1 | 3 | 3 | 2 | 2 | 3 | 7.2±1.8 | 0-10 |
| 30-min Delayed Recall | FC | 0 | 0 | 2 | 0 | 1 | 0 | 7.5±1.9 | 0-10 |
| 5-min Recognition | FC | 15 | 19 | 20 | 16 | 19 | 15 | 19.8±0.7 | 0-20 |
| 30-min Recognition | FC | 15 | 14 | 16 | 16 | 17 | 19 | 19.6±0.5 | 0-20 |
| Attention/concentration | |||||||||
| WAIS-III Digit Span | FC | 7 | 9 | 5 | 7 | 8 | 6 | 10±3 | 1-19 |
| Symbol Digit Modality Test | FC | FC | 7 | 7 | 7 | 16 | 17 | 52±8 | 0-120 |
| Language | |||||||||
| Boston Naming Test | FC | 10 | 10 | 19 | 14 | 16 | 19 | 27±2.9 | 0-30 |
| Letter Fluency | FC | 2 | 3 | 6 | 6 | 6 | 8 | 10±3 | N/A |
| Category Fluency | FC | 5 | 7 | 11 | 8 | 14 | 15 | 20±4 | N/A |
| Visual-spatial abilities | |||||||||
| CERAD Drawing | FC | 10 | 8 | 10 | 10 | 10 | 10 | 10.1±1.3 | 0-11 |
| WAIS-III Block Design | FC | 4 | 5 | 6 | 6 | 8 | 5 | 10±3 | 19-Jan |
| Executive functioning | |||||||||
| WAIS-III Similarities | FC | 2 | 4 | 6 | 5 | 4 | 4 | 10±3 | 19-Jan |
| Social Judgment | FC | 1 | 5 | 5 | 6 | 4 | 2 | 5.3 | 0-6 |
| Trail-Making Test (A) | FC | 2 | 2 | 2 | 2 | 2 | 2 | 10±3 | 18-Feb |
| Trail-Making Test (B) | FC | FC | FC | 2 | 2 | 2 | 2 | 10±3 | 18-Feb |
| Psychomotor speed | |||||||||
| Kendrick Digit Copy (sec) | FC | FC | 242 | 236 | 141 | 102 | 173 | 52±13 | N/A |
FC, failed to complete; N/A, not available; MMSE, Mini-Mental State Examination; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; SD, standard deviation; WAIS-III, Wechsler Adult Intelligence Scale-III.
Neuropsychiatric changes
Table 4 chronicles the patient's behavioral symptoms as reported annually by his wife on the Neuropsychiatric Inventory (NPI; Cummings et al., 1994). On the NPI, the informant is asked to report the presence or absence of 12 behavioral symptoms during the preceding month. When present, the informant is asked to rate how frequently the behavior occurred on a 4-point scale (i.e., occasionally, often, frequently, or very frequently), as well as its severity using a 3-point scale (i.e., mild, moderate, or severe). Scores reported in Table 4 range from 0–12, and represent the sum of the frequency by severity ratings for each behavior. Overall, the NPI shows a clinical profile suggestive of a frontal syndrome, with the highest scores seen on measures of apathy, depression, disinhibition, agitation, and irritability. Additional symptoms included inappropriate elation, delusions of grandeur, changes in appetite and eating behavior, and repetitive motor activities. More specifically, he showed a childish sense of humor and would laugh at things other people did not find funny, as well as make grandiose comments about himself and his boxing career. In fact, for 5 of the 7 years he was followed, he believed that he was still boxing, and could easily perform the jobs of other professionals (e.g., doctors and lawyers) without any training. He also showed an increased appetite for sweets, resulting in weight gain, and would occasionally put too much food into his mouth. On most visits, his wife also reported that he had problems with excessive fidgeting and an inability to stop a task once started. For example, he would repetitively engage in household activities, such as stacking coins, sweeping the floor, and watering plants. Prior to death, the DP patient suffered recurrent febrile episodes, with the last recorded temperature being 102.2°F. ApoE genotyping was conducted and the DP patient was ApoE3/4.
Table 4.
Scores on the Neuropsychiatric Inventory Across Multiple Visits
| Behavioral symptoms | Visit 7 | Visit 6 | Visit 5 | Visit 4 | Visit 3 | Visit 2 | Visit 1 |
|---|---|---|---|---|---|---|---|
| Delusions | 0 | 8 | 4 | 6 | 1 | 3 | 0 |
| Hallucinations | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Agitation/aggression | 2 | 8 | 8 | 8 | 3 | 8 | 6 |
| Depression | 0 | 0 | 0 | 8 | 6 | 12 | 3 |
| Anxiety | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Euphoria/elation | 2 | 0 | 0 | 0 | 8 | 8 | 0 |
| Apathy/indifference | 9 | 8 | 4 | 8 | 8 | 8 | 4 |
| Disinhibition | 4 | 12 | 8 | 12 | 4 | 8 | 8 |
| Irritability/lability | 0 | 6 | 0 | 8 | 4 | 12 | 2 |
| Aberrant motor | 6 | 6 | 6 | 0 | 6 | 3 | 2 |
| Sleep/nighttime | 2 | 0 | 0 | 0 | 4 | 6 | 3 |
| Appetite/eating | 2 | 3 | 8 | 0 | 4 | 4 | 2 |
| Total | 27 | 51 | 38 | 50 | 49 | 72 | 30 |
Gross pathology and final diagnosis
At autopsy, the brain weighed 1115.9 g. The cerebral arteries at the base of the brain were free of atherosclerosis and the leptomeningeal vessels appeared normal. In coronal section there was minimal cortical atrophy in the sylvian region (Fig. 1B). Before the brain was hemisected, the septum pellucidum was absent, and there was moderately severe ventricular enlargement with thinning of the corpus callosum. An irregular 0.4-cm infarct was observed within the medial pallidal segment. There was moderate depigmentation in the substantia nigra (Fig. 1C and E) and the locus ceruleus (Fig. 1C). In the cerebral cortex there was rarefaction that was prominent in layer 2 of the temporal cortex (Fig. 1D). Lewy bodies were not observed. As the pathological criteria for other FTDs were not met, and in consideration of the clinical details, the final diagnosis was dementia pugilistica.
Grey matter Aβ pathology
Diffuse Aβ plaques visualized by silver staining were widespread, yet mild-to-moderate neuritic plaques were distributed in the middle frontal and rostral and caudal cingulate cortices. Neuritic plaques were minimal within the superior temporal, inferior parietal, and calcarine/pericalcarine cortices, and within the hippocampal CA1, subiculum, entorhinal-transentorhinal region, and the amygdala.
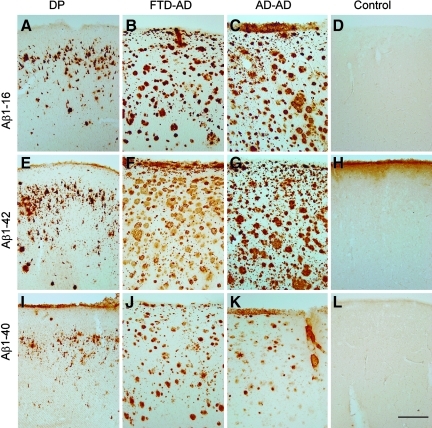
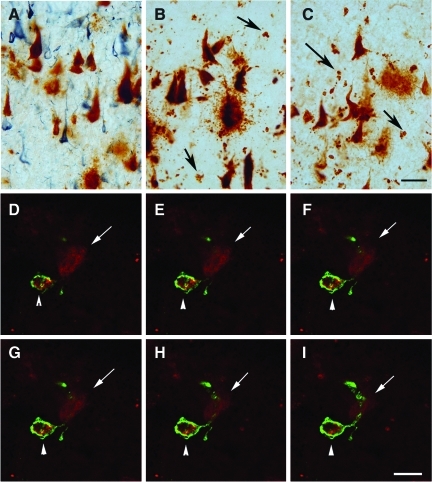
By immunohistochemistry, the DP patient showed primarily Aβ1-16 in diffuse plaques and on extracellular NFTs, compared to the extensive plaque labeling seen in the FTD-AD patient, and in a typical AD case (Fig. 2A–D). Aβ1-42 immunostaining in the DP case, the FTD-AD case, the typical AD case, and the control (H) were similar to that observed with immunolabeling for Aβ1-16 (Fig. 2E–H). Less Aβ1-40 immunolabeling was observed in the DP case, with deposits being seen primarily within diffuse plaques and on extracellular NFTs (Fig. 2I–L). In comparison, Aβ1-40 was observed in plaques in the FTD-AD case, primarily within neuritic plaque cores and associated with blood vessels in the typical AD case (Fig. 2K). Numerous extracelluler tangles were positive for Aβ (Fig. 3A–C). In addition, particularly for Aβ1-40, there were small punctuate deposits in the vicinity of Aβ-positive NFTs (Fig. 3C), that were subsequently determined to be localized within microglial cells that were positive for HLA-DR (Fig. 3D–I).
FIG. 2.
Frontal cortical beta-amyloid (Aβ) neuropathology in the dementia pugilistica (DP) case compared to the Alzheimer's disease (AD) and nondemented control cases. Aβ1-16 immunostaining illustrates primarily diffuse plaque and extracellular neurofibrillary tangle (NFT) labeling in the DP case (A) as compared to the extensive plaque labeling seen in the frontotemporal dementia (FTD)-AD case (B), and the typical AD case (C), but is absent in the control case (D). Aβ1-42 immunostaining in the DP case (E), the FTD-AD case (F), the typical AD case (G), and the control (H), was similar to that observed with immunolabeling for Aβ1-16. Less Aβ1-40 immunolabeling was observed in the DP case (I), with deposits being primarily seen within diffuse plaques and on extracellular NFTs. In comparison, Aβ1-40 was observed in plaques in the FTD-AD case (J), and primarily within neuritic plaque cores and associated with blood vessels in the typical AD case (K), and was absent in the control case (L; scale bar=500 μm). Color image is available online at www.liebertonline.com/neu
FIG. 3.
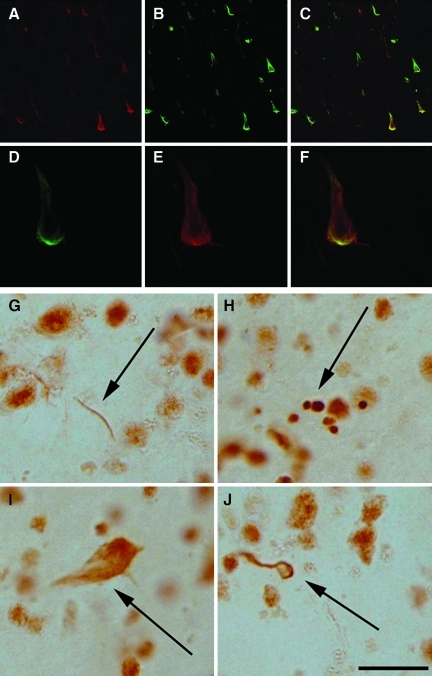
Tau and beta-amyloid (Aβ) in a case with dementia pugilistica (DP). (A). Neuronal Aβ1-16 immunolabeling was associated with larger neurons without clearly visible plasma membranes, suggesting extracellular tangles (brown) as compared to smaller, well-defined intracellular neurofibrillary tangles (NFTs) identified with PHF-1 (blue). Extracellular tangles were positive for (B) Aβ1-42, and (C) Aβ1-40 (note the extracellular punctuate positive deposits indicated by the arrows). (D–I) Extracellular punctuate deposits of Aβ1-40 (red) were localized within HLA-DR-positive microglial cells (green), and could be distinguished from extracellular NFT labeling (arrow; scale bars=20 μm). Color image is available online at www.liebertonline.com/neu
Grey matter tau pathology in the frontal cortex
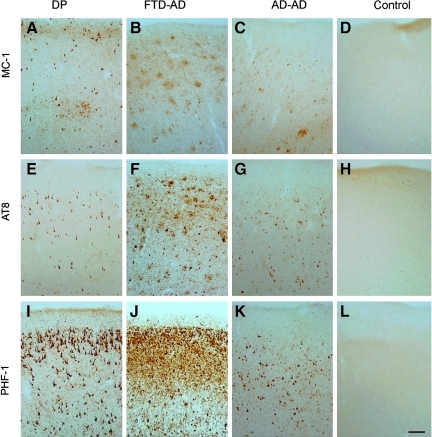
NFTs were extensive within the frontal, temporal, and parietal neocortices, and within the CA1, subiculum, and the entorhinal-transentorhinal region. NFTs were also observed within the midbrain tegmentum, substantia nigra, pontine tegmentum, locus ceruleus (Fig. 1F), and medullary tegmentum, and in scattered distribution within the striatum, globus pallidus, and thalamus. Extracellular NFTs were prominent, particularly where intracellular NFT formation was heavy. Using a battery of markers representing different phosphorylation states of tau that may indicate disease progression (Augustinack et al., 2002), we observed differences in the distribution and extent of different phosphorylated tau species compared to the other cases (Fig. 4). First, MC-1 labeling specific for a folded conformation of tau (Jicha et al., 1997), which is also thought to represent early NFT formation (Uboga and Price, 2000) appears in scattered neurons, whereas in the AD cases, it is also distributed within dystrophic neurites associated with plaques (Fig. 4A–D). Interestingly, AT8-positive neurons containing tau phosphorylated at S199/S202/T205, also thought to be an early event in NFT formation (Su et al., 1994), were similar in extent and distribution in the DP case compared to the AD cases. However, AD patients with clinical signs of frontal lobe dysfunction showed more extensive AT8 labeling (Fig. 4E–H). Lastly, there was a differential distribution of PHF-1, reflecting mature NFT formation and abnormal phosphorylation at Ser396/Ser404 (Otvos et al., 1994), with immunostaining prominent in layer 2 in the DP case, compared to the AD cases (Newman et al., 2005; Fig. 4I–L). This pattern in the DP case was similar to that previously reported (Hof et al., 1992). For all antibodies against phosphorylated tau, we observed neuropil threads in all cases examined, but not in controls.
FIG. 4.
Patterns of tau immunostaining in the frontal cortex of the patient with dementia pugilistica (DP), compared to Alzheimer's disease (AD) and nondemented cases. MC-1 immunolabeling of phosphorylated tau revealed neuronal labeling in the DP case (A), plaque-associated neuritic labeling in the frontotemporal dementia (FTD)-AD case (B), a mix of neurofibrillary tangles (NFTs) and dystrophic neurites in the typical AD case (C), but absent in the control case (D). AT8 immunoreactivity also was limited to intracellular NFTs in the DP case (E), and within NFTs and plaque-associated dystrophic neurites in the FTD-AD case (F) and the typical AD case (G), but were not present in the control case (H). PHF-1 immunostaining was the most extensive of the three pathological tau markers, and showed significant intracellular and extracellular NFT labeling in the DP case (I), and within NFTs and plaque-associated dystrophic neurites in the FTD-AD case (J), but was less extensive and limited to NFTs in the typical AD case (K). No PHF-1-positive tangles were observed in the control case (L; scale bar=100 μm). Color image is available online at www.liebertonline.com/neu
We next determined whether caspase activation had occurred in NFT-bearing neurons, as has been reported in AD, which may be an early event contributing to NFT formation (Newman et al., 2005; Rohn and Head, 2008). We observed a significant presence of tau-caspase cleavage product (tau-CCP) in NFT-bearing neurons in the DP case, in association with extracellular NFTs (Fig. 5A–F). As in the AD cases, neuropil threads in the DP case also contained tau-CCP. The only distinguishing feature in the DP case was the lack of plaque-associated tau-CCP-positive dystrophic neurites.
FIG. 5.
Caspase activation and TDP-43 in dementia pugilistica (DP). (A–F) Tau-caspase cleavage product (green) was present within a subset of PHF-1 positive tangles (red). TDP-43 was observed within dystrophic neurites (G), intracellular inclusions (H), extracellular neurofibrillary tangles (NFTs) (I), and within oligodendroglial cells (J; scale bar=10 μm). Color image is available online at www.liebertonline.com/neu
Grey matter TDP-43 pathology in the frontal cortex
Immunostaining for TDP-43 in the frontal cortex of our DP case revealed fibrils, dense granules, NFT labeling, and coiled body-like comma-shaped TDP-43-positive oligodendrocytes (Brandmeir et al., 2008; Fig. 5G–J).
Grey matter glial cell activation, complement, ApoE, and APP in the frontal cortex
In acute brain injury there is evidence that inflammation may be a priming event for the future development of AD (Griffin et al., 1994). We used several markers to detect inflammation in our DP case, for purposes of comparison with the AD cases (Fig. 6). Despite the small numbers of neuritic Aβ deposits, we observed significant GFAP protein labeling in the DP case, with morphology consistent with hypertrophied astrocytes (Fig. 6A–D), although the extent and intensity was less than that seen in the AD cases. HLA-DR-positive microglial cells were also detected, although less extensively than those observed in the AD cases (Fig. 6E–H). The intensity of astrocytosis and of microglial cell labeling with HLA-DR was higher in all AD cases and lowest or absent in controls. We have previously shown that complement activation on neurons may contribute to neuron loss in the hippocampus in a patient with relapsing polychondritis (Head et al., 2006). We hypothesized that complement may also be upregulated in DP, despite the limited numbers of neuritic plaques observed. Complement proteins were observed in the frontal cortex, at intensities similar to those seen in the AD cases (Fig. 6I–L). However, in the AD cases C1q was associated with Aβ deposition and NFT-bearing neurons, whereas in the DP case C1q labeling was limited to neurons (Fig. 7A). Furthermore, in our DP case, non-NFT-bearing neurons as well as extracellular NFTs contained C1q (Fig. 7A). Apolipoprotein E (ApoE) is also thought to mediate the neuropathology of repetitive head trauma (Jordan et al., 1995), and of traumatic brain injury (DeKosky et al., 2007), and we observed ApoE labeling within extracellular NFTs in the DP case (Fig. 7B). In the AD cases, ApoE was found within intracellular NFTs and in association with plaques, as reported previously (Thal et al., 2005).
FIG. 6.
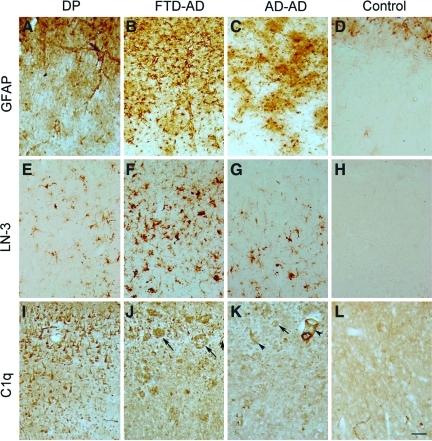
Astrocyte, microglial, and complement labeling in the prefrontal cortex of a case with dementia pugilistica (DP). Astrocytosis visualized with anti-glial fibrillary acidic protein (GFAP) immunolabeling was present in the DP case, despite a lack of neuritic plaques (A). More extensive GFAP labeling was observed in the frontotemporal dementia (FTD)-Alzheimer's disease (AD) cases (B), but the typical AD cases showed clusters of astrocytes (C). Gliosis in the control case was limited to layer 1 (D). HLA-DR labeling using the LN-3 antibody showed a microglial reaction that was scattered in the DP case (E), was more extensive in the FTD-AD case (F), was present as clusters in the typical AD case (G), and was rare in the control case (H). C1q was primarily observed on extracellular neurofibrillary tangles (NFTs) and neurons (I), whereas in the AD cases (J and K) it was seen in association with plaques (arrows), neurons, and blood vessels (arrowhead). Light diffuse C1q was observed in the control case (L; scale bar=100 μm). Color image is available online at www.liebertonline.com/neu
FIG. 7.
Neuronal Ciq and apolipoprotein E (ApoE) labeling in the frontal cortex of the dementia pugilistica (DP) case. (A) C1q-positive neurons were observed on extracellular neurofibrillary tangles (NFTs), as was (B) ApoE labeling (scale bar=20 μm). Color image is available online at www.liebertonline.com/neu
White matter pathology in the frontal cortex
DP has been associated with white matter lesions and loss of brain volume, and it has been suggested that blood–brain barrier compromise may potentiate neurodegeneration. There was a significant number of microglial cells positive for HLA-DR in the white matter, and clusters of intense reactivity where microglial cells had adopted a spherical shape (Fig. 8A). ApoE immunolabeling also revealed positive blood vessels, but most appeared to be morphologically normal. C1q was found in association with a subset of blood vessels and within glial cells in proximity to blood vessels (Fig. 8B). In other areas of the frontal white matter, there appeared to be extravasation of C1q in clouds in the vicinity of unlabeled blood vessels in our DP case.
FIG. 8.
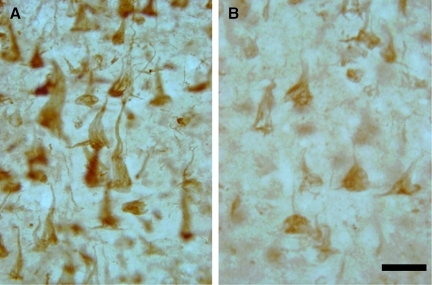
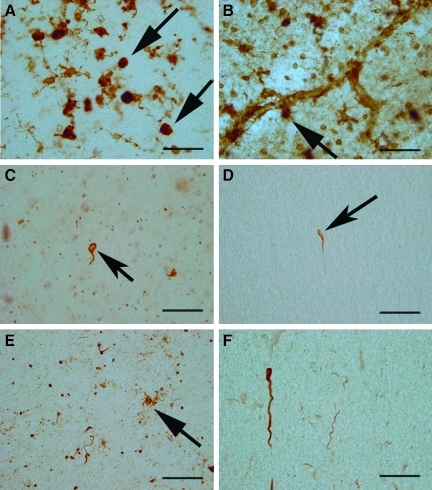
Frontal white matter pathology in dementia pugilistica (DP). (A) HLA-DR-positive microglial cells and macrophages (arrows) were observed in clusters. (B) C1q labeled a subset of blood vessels in addition to adjacent glial cells (arrow). MC-1 positive punctate white matter deposits were observed, as well as rare oligodendroglial positive inclusions (arrow in C), and fiber labeling (arrow in D). HT7 labeling, indicating total tau, was increased in the DP case, and associated with glial tangles (arrow in E), and fibers (F; scale bar=20 μm). Color image is available online at www.liebertonline.com/neu
Markers for NFT pathology in the white matter also revealed some interesting differences between DP and AD. Using MC-1, our patient showed punctate white matter deposits that included scattered coiled bodies, representing oligodendrocyte tau inclusions (Fig. 8C) and fibers (Fig. 8D). Interestingly, AT8 labeling was negative in the white matter of our patient, but presented as threads or fibers in the AD cases. Lastly, HT7 labeling, which recognizes tau independently of phosphorylation state, showed dark and intense punctuate labeling in the white matter of the DP case (Fig. 8E), and was associated with fibers (Fig. 8F). Thus in our patient there was evidence for glial tangles and coiled bodies in the white matter that were positive for both phosphorylated tau and nonphosphorylated tau, consistent with other reports of autopsy cases with FTLD (Cairns et al., 2007a; McKhann et al., 2001). However, overall these tau-positive deposits were relatively rare in the DP case.
Hippocampal pathology
As in the frontal cortex, Aβ plaques were primarily of the diffuse type (Fig. 9A) in DP, whereas a mixture of diffuse, mature, and neuritic plaques was seen in the AD cases (data not shown). Extensive HLA-DR-positive microglial cells were seen in all subfields of the hippocampus in the DP case (Fig. 9B). Neuronal and extracellular NFT labeling for C1q was observed in the hippocampus (Fig. 9C), whereas the distribution in AD was seen predominantly in association with plaques. Neurons within the DP hippocampus were positive for tau-CCP (data not shown). TDP-43 immunolabeling revealed the presence of dystrophic neurites (Fig. 9D), and of clusters of shorter dystrophic neurites with swollen nerve terminals (Fig. 9E). Thus, as in the frontal cortex, DP was associated with diffuse plaques, extracellular NFTs, and extensive intracellular NFT formation, along with evidence of neuroinflammation.
FIG. 9.
Hippocampal pathology in a case with dementia pugilistica (DP). Neurofibrillary tangles (NFTs, blue), and beta-amyloid (Aβ) depositions (brown), in area CA1 show that plaques were primarily diffuse or primitive (A). Extensive microglial involvement (B) and C1q deposition (C) were observed in area CA1 of the hippocampus. The images in D and E show TAR DNA binding protein 43 mislocalization to dystrophic neurites and swollen neuron terminals, respectively (arrows; scale bars in A–C=50 μm, in D and E=10 μm). Color image is available online at www.liebertonline.com/neu
Discussion
We describe the clinical and neuropathological features of a patient with DP who presented with clinical signs predominantly localizable to the frontal lobe. At autopsy, we observed neuropathology that overlaps with both AD and with FTLD. Aβ deposition was primarily seen within diffuse plaques and associated with extracellular NFTs. In contrast, the distribution of NFTs within subcortical nuclei and within white matter glial inclusions was similar to some FTLD cases. Further, tau protein accumulating within neurons of the DP brain had undergone caspase cleavage. Elevation in neuroinflammation was a major component of the neuropathology of DP, along with an accumulation of APP on white matter blood vessels, that has not been reported previously.
The patient's neuropsychological and behavioral symptoms support a diagnosis of FTD, although many of the clinical features may be present in other forms of dementia. Neary and associates identified the core diagnostic features of FTD as early (1) decline in social interpersonal conduct, (2) impairment in the regulation of personal conduct, (3) emotional blunting, and (4) loss of insight (Neary et al., 1998). At the time of his initial evaluation, our patient exhibited all four of these symptoms. The most noticeable early cognitive deficits in FTD occur in executive functioning rather than recent memory. Although delayed recall and recognition, as measured by the CERAD Word List, were significantly impaired, our patient's scores were higher than those typically seen in AD patients. The fact that our patient's scores on tests of executive functioning, such as insight, reasoning (e.g., Social Judgment and Similarities), mental flexibility (e.g., Trail-Making Test), and concentration (e.g., Digit Span and Symbol Digit Modality Test), were disproportionately impaired was more similar to FTD.
The most striking neuropathological feature in this case was extensive NFT pathology, affecting cortical regions and brainstem nuclei as reported previously (Areza-Fegyveres et al., 2007). The presence of motor dysfunction in DP may reflect NFT pathology compromising frontal circuits and brainstem motor systems. Further, the distribution of NFT pathology in our patient, relative to other AD cases and to frontal AD cases, is consistent with a previous report in a 58-year-old boxer (Hof et al., 1992), in whom NFTs were more frequently found in superficial layers of the cortex associated with corticocortical pathways. Thus, although there is significant NFT pathology in DP, the distribution of tangles can distinguish these cases from AD (i.e., superficial cortical layer involvement, brainstem NFTS, and white matter glial tangles; Hof et al., 1992).
White matter degeneration has been reported previously in DP, including a loss of myelin and gliosis (Corsellis et al., 1973). Distorted end swellings and reduction of axons in white matter have also been noted (Lampert and Hardman, 1984). There are few reports of glial tangles in DP. Ikeda and colleagues observed thorn-shaped astrocytes, but not tuft-shaped astrocytes or coiled bodies (Ikeda et al., 1998). Schmidt and colleagues observed glial (astrocyte) NFTs in neocortical white and gray matter, as well as in the brainstem and spinal cord (Schmidt et al., 2001). In the current case study, we observed glial NFTs and coiled bodies, which are frequently found in corticobasal degeneration and progressive supranuclear palsy (Ikeda et al., 1998). Interestingly, these two diseases are characterized by extrapyramidal motor signs and subcortical tangles (Belfor et al., 2006; Kumar-Singh and Van Broeckhoven, 2007). Motor signs in our patient may thus be associated with white matter glial NFTs and subcortical NFTs, as reported in other case studies (Corsellis et al., 1973).
The extent of Aβ deposition in previous case reports is variable and may reflect differences in detection techniques (e.g., silver stains versus immunohistochemistry), and in the ages of the patients at autopsy. The presence of diffuse plaques is consistent with several previous reports (Areza-Fegyveres et al., 2007; Roberts, 1988; Schmidt et al., 2001; Tokuda et al., 1991), although other studies report no plaque pathology (Geddes et al., 1999; Hof et al., 1992). Typically, Aβ deposition in diffuse plaques has not been reported in young boxers despite the presence of tau accumulation (Geddes et al., 1999). In a transgenic mouse model of AD (Tg2576), repetitive mild brain injury in 9-month-old animals (pre-Aβ deposition) accelerated Aβ plaque pathology and induced cognitive impairments (Uryu et al., 2002). Interestingly, no motor impairments were observed, which may be due to a lack of NFT pathology, which contrasts with a report in a tauopathy mouse model (Yoshiyama et al., 2005). The most distinguishing feature of the Aβ pathology in this case was that of extracellular NFTs, as reported previously (Allsop et al., 1990; Hof et al., 1992; Tokuda et al., 1991). We further characterized extracellular NFT pathology and showed that both Aβ1-40 and Aβ1-42 are involved. The source of extracellular NFT-associated Aβ is difficult to determine, but morphologically appears to be derived from disrupted neuronal membranes. Also, the presence of an ApoE4 allele (our patient was ApoE3/4), may also have contributed to the level of Aβ pathology (Jellinger, 2004; Jordan et al., 1995,1997).
We tested whether significant cerebrovascular damage would be present in our patient given previous reports in younger boxers and in TBI patients (Jordan, 2000). Interestingly, in a report of one 23-year-old boxer, NFTs were distributed in a perivascular pattern, suggesting a possible link between vascular compromise and neurodegeneration (Geddes et al., 1999). We observed little cerebral amyloid angiopathy in the current case, which is consistent with a previous study (Tokuda et al., 1991). However, we did find increased amyloid precursor protein (APP) immunolabeling in large blood vessels within the frontal cortex white matter that was substantially different from the AD and control cases. Increased vascular APP may be consistent with reports of neuronal overexpression of APP and associated increases in IL-1α-positive activated microglial cells in head injury cases (Griffin et al., 1994). Targeting IL-1 has been proposed as a potential therapy for TBI, and the IL-1 receptor antagonist (IL-1ra) that can attenuate IL-1 signaling is well tolerated by rheumatoid arthritis patients (Lucas et al., 2006). Intraventricular delivery of antibodies against IL-1α or IL-1β significantly attenuated TBI-induced neuronal loss in Sprague-Dawley rats (Lu et al., 2005). Whether increased vascular APP reflects a compensatory response or an injury response in our patient is difficult to determine.
Additional observations we provide in the current case study include evidence for neuroinflammation, caspase activation, the presence of aberrant cellular localization of TDP-43 (also recently reported by King et al., 2010), and further evidence of white matter pathology. Many of these features appear to be more similar to those observed in FTLD, which may account for the primarily frontal syndrome observed in the clinic. We observed large numbers of HLA-DR-positive microglial cells within the frontal cortex and hippocampus, despite the lack of significant neuritic plaque accumulation. Similar microglial activation has been described in FTLD, both by in vivo imaging (Cagnin et al., 2004), and by immunohistochemistry (Arnold et al., 2000; Schofield et al., 2003). Microglial cell activation may result in the release of proinflammatory cytokines and chemokines and exacerbate neuronal dysfunction (Lucas et al., 2006; Nguyen et al., 2002).
As a second marker for upregulation of the inflammatory cascade, we immunostained the brain of the DP case with anti-C1q antibodies. The complement (C′) system is critically involved with humoral and cellular immunity and inflammatory responses, and has been implicated in several neurodegenerative diseases (van Beek et al., 2003). C1q was primarily neuronal in our case and seen on extracellular NFTs, in contrast to both neuronal- and plaque-associated C1q in the AD cases (Afagh et al., 1996; Fonseca et al., 2004). This may suggest that C1q may be increased in DP in response to the disruption of NFT-bearing neuronal membranes that may stimulate an immune response. A role for Aβ accumulation on extracellular NFTs as another C1q activator is also possible, because in AD, fibrillary forms of Aβ can bind and activate C1, the first component of the classical C′ pathway (Jiang, 1994; McGeer and Rogers, 1992; Webster, 1997). Complement-mediated generation of proinflammatory factors can initiate or induce the recruitment and activation of glial cells, which in turn may contribute to neuronal degeneration. Consistent with this hypothesis is the observation of HLA-DR-positive microglial cells containing Aβ in the vicinity of Aβ-positive extracellular NFTs.
Caspase activation detected as caspase-cleaved fragments of tau (tau-CCP) may suggest that activation of apoptosis pathways may mediate cell death in DP, as has been reported for other tauopathies (Gamblin et al., 2003; Newman et al., 2005; Rissman et al., 2004). We extend previous reports of the accumulation of tau-CCP and a possible role in DP-associated neurodegeneration. We observed significant tau-CCP immunostaining in both the frontal cortex and hippocampus of our patient. Tau-CCP was preferentially distributed within NFT-bearing neurons, but was also observed on extracellular NFTs. In contrast to DP, and as reported previously, tau-CCP was present in both NFT-bearing neurons and in dystrophic neurites associated with plaques in AD cases (Gamblin et al., 2003; Rissman et al., 2004). Tau-CCP has been proposed as a possible link between Aβ and tau pathology; caspase activation may be initiated by exposure to Aβ, and lead to the cleavage of tau protein, thus accelerating the production of NFT in AD (de Calignon et al., 2010; Gamblin et al., 2003; Hyman, 2011; Rissman et al., 2004). However, in vivo imaging of both caspase activation and NFT formation in mouse brain suggests that although these two events are linked, there is no evidence for TUNEL labeling or nuclear fragmentation in these NFT-bearing neurons (Spires-Jones et al., 2008), arguing against full engagement of apoptosis. Interestingly, intracerebroventricular injection of a pan-caspase inhibitor in rodents reduced TBI-induced increases in caspase-3 activation, improved histological outcomes, and attenuated Aβ accumulation in the central nervous system (CNS; Abrahamson et al., 2006).
A novel proteinopathy described in FTLD and in amyotrophic lateral sclerosis (ALS) is the mislocalization of TDP-43 (Cairns et al., 2007b; Gitcho et al., 2008; Neumann et al., 2006). In FTLD with ubiquitin-immunostained-positive inclusions, TDP-43 is observed as cytoplasmic inclusions, dystrophic neuritis, and intranuclear inclusions (Armstrong et al., 2010; Neumann et al., 2006). The intensity of normal nuclear TDP-43 is reduced in FTLD with ubiquitin inclusions, suggesting mislocalization and aggregation (Forman et al., 2007). We hypothesized that TDP-43 proteinopathy may also contribute to DP. Immunostaining for TDP-43 in DP showed similar levels of nuclear immunoreactivity to those of controls, but with rare dystrophic neurites. Cytoplasmic aggregates may also be present within fragmented nuclei. There was little evidence of nuclear inclusion formation, which suggests a distribution similar to either a subtype 1 or 2 (Forman et al., 2007; Kwong et al., 2007). A small number of extracellular tangles were positive for TDP-43. Thus TDP-43 pathology was present in the current DP case as reported previously (King et al., 2010), but with far lower frequency than those described previously for FTLD and ALS.
A prime candidate for the spread of tau pathology in CTE is via a transcellular or prion-like mechanism (Aguzzi and Rajendran, 2009; Clavaguera et al., 2009; Frost and Diamond, 2010; Frost et al., 2009; Goedert et al., 2010). Recently there has been considerable emphasis on the transmission or propagation of protein misfolding or proteinopathies in several neurodegenerative diseases, and indications that the mechanism may have similarities to those that underlie prion pathogenesis (Angot et al., 2010; Brundin et al., 2008; Clavaguera et al., 2009; Frost and Diamond, 2010; Goedert et al., 2010; Li et al., 2008; Meyer-Luehmann et al., 2006; Morales et al., 2011; Volpicelli-Daley et al., 2011). Like classical prion disorders, there is some evidence that the source of the nucleating agent and the host can affect the onset and type of neuropathology (Meyer-Luehmann et al., 2006), and that soluble prionoid forms can also induce amyloidogenesis in the CNS (Langer et al., 2011). For example, neuropathological changes in Parkinson's disease (PD) progress slowly and spread according to a characteristic pattern (Kordower et al., 2008,2011; Li et al., 2008). Two of these studies demonstrate that grafted healthy neurons can gradually develop the same pathology as host neurons in the diseased brains (Brundin et al., 2008; Kordower et al., 2008; Li et al., 2008). The latest studies with α-synuclein (Volpicelli-Daley et al., 2011) or Aβ aggregates (Morales et al., 2011) have established that transgenic animal models overexpressing human mutant forms of neuronal proteins are not required to show prion-like behavior, because in these two studies wild-type mice inoculated with misfolded forms of α-synuclein or Aβ that would never develop spontaneous proteinopathies or parenchymal prionoid deposits developed neuropathological changes in the CNS.
Recently, there has been considerable interest in CTBI and CTE in other sports, such as American football, wrestling, hockey, lacrosse, and even soccer (DeKosky et al., 2010; Matser et al., 1999,2001; McKee et al., 2009). While the exact mechanisms involved in the significant long-term delayed sequelae associated with sports-related CTE are poorly understood (DeKosky et al., 2010), the clinical presentation appears to be due to a slowly-progressive tauopathy (McKee et al., 2009). Currently there is an urgent need for better animal models of mild repetitive TBI that replicate the pathogenesis of CTE, so that the cellular and molecular changes that contribute to the propagation of tau pathology can be delineated, and effective therapies can be developed and deployed long before the onset of neurocognitive deficits. Another critical area is the identification of biomarkers that can be used to predict which individuals are at the greatest risk of developing CTE, as well as to provide feedback about the efficacy of future therapeutic interventions.
Acknowledgments
Funding supported by the University of California Irvine Alzheimer' Disease Research Center (National Institutes on Health/National Institutes on Aging Grant #P50 AG16573), National Institutes on Health/National Institutes on Aging Grant #AG21912, and #AG00538. Additional support was received from the University of Kentucky Alzheimer's Disease Center Grant #P30 AG028383. A subset of human tissues was obtained from the National Institute of Child Health and Human Development Brain and Tissue Bank for Developmental Disorders under contracts N01-HD-4-3368 and N01-HD-4-3383. We are grateful to the family and to our patient with DP for their contributions to the study.
Author Disclosure Statement
No competing financial interests exist.
References
- Abrahamson E.E. Ikonomovic M.D. Ciallella J.R. Hope C.E. Paljug W.R. Isanski B.A. Flood D.G. Clark R.S. DeKosky S.T. Caspase inhibition therapy abolishes brain trauma-induced increases in Abeta peptide: implications for clinical outcome. Exp. Neurol. 2006;197:437–450. doi: 10.1016/j.expneurol.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Afagh A. Cummings B.J. Cribbs D.H. Cotman C.W. Tenner A.J. Localization and cell association of C1q in Alzheimer's disease brain. Exp. Neurol. 1996;138:22–32. doi: 10.1006/exnr.1996.0043. [DOI] [PubMed] [Google Scholar]
- Aguzzi A. Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Allsop D. Haga S. Bruton C. Ishii T. Roberts G. W. Neurofibrillary tangles in some cases of dementia pugilistica share antigens with amyloid beta-protein of Alzheimer's disease. Am. J. Pathol. 1990;136:255–260. [PMC free article] [PubMed] [Google Scholar]
- Angot E. Steiner J.A. Hansen C. Li J.Y. Brundin P. Are synucleinopathies prion-like disorders? Lancet Neurol. 2010;9:1128–1138. doi: 10.1016/S1474-4422(10)70213-1. [DOI] [PubMed] [Google Scholar]
- Areza-Fegyveres R. Rosemberg S. Castro R.M. Porto C.S. Bahia V.S. Caramelli P. Nitrini R. Dementia pugilistica with clinical features of Alzheimer's disease. Arq. Neuropsiquiatr. 2007;65:830–833. doi: 10.1590/s0004-282x2007000500019. [DOI] [PubMed] [Google Scholar]
- Armstrong R.A. Ellis W. Hamilton R.L. Mackenzie I.R. Hedreen J. Gearing M. Montine T. Vonsattel J.P. Head E. Lieberman A.P. Cairns N.J. Neuropathological heterogeneity in frontotemporal lobar degeneration with TDP-43 proteinopathy: a quantitative study of 94 cases using principal components analysis. J. Neural. Transm. 2010;117:227–239. doi: 10.1007/s00702-009-0350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S.E. Han L.Y. Clark C.M. Grossman M. Trojanowski J.Q. Quantitative neurohistological features of frontotemporal degeneration. Neurobiol. Aging. 2000;21:913–919. doi: 10.1016/s0197-4580(00)00173-1. [DOI] [PubMed] [Google Scholar]
- Augustinack J.C. Schneider A. Mandelkow E.M. Hyman B.T. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- Belfor N. Amici S. Boxer A.L. Kramer J.H. Gorno-Tempini M.L. Rosen H.J. Miller B.L. Clinical and neuropsychological features of corticobasal degeneration. Mech. Ageing Dev. 2006;127:203–207. doi: 10.1016/j.mad.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Brandmeir N.J. Geser F. Kwong L.K. Zimmerman E. Qian J. Lee V.M. Trojanowski J.Q. Severe subcortical TDP-43 pathology in sporadic frontotemporal lobar degeneration with motor neuron disease. Acta Neuropathol. 2008;115:123–131. doi: 10.1007/s00401-007-0315-5. [DOI] [PubMed] [Google Scholar]
- Brundin P. Li J.Y. Holton J.L. Lindvall O. Revesz T. Research in motion: the enigma of Parkinson's disease pathology spread. Nat. Rev. Neurosci. 2008;9:741–745. doi: 10.1038/nrn2477. [DOI] [PubMed] [Google Scholar]
- Cagnin A. Rossor M. Sampson E.L. Mackinnon T. Banati R.B. In vivo detection of microglial activation in frontotemporal dementia. Ann. Neurol. 2004;56:894–897. doi: 10.1002/ana.20332. [DOI] [PubMed] [Google Scholar]
- Cairns N.J. Bigio E.H. Mackenzie I.R. Neumann M. Lee V.M. Hatanpaa K.J. White C.L., 3rd Schneider J.A. Grinberg L.T. Halliday G. Duyckaerts C. Lowe J.S. Holm I.E. Tolnay M. Okamoto K. Yokoo H. Murayama S. Woulfe J. Munoz D.G. Dickson D.W. Ince P.G. Trojanowski J.Q. Mann D.M. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007a;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns N.J. Neumann M. Bigio E.H. Holm I.E. Troost D. Hatanpaa K.J. Foong C. White C.L., 3rd Schneider J.A. Kretzschmar H.A. Carter D. Taylor-Reinwald L. Paulsmeyer K. Strider J. Gitcho M. Goate A.M. Morris J.C. Mishra M. Kwong L.K. Stieber A. Xu Y. Forman M.S. Trojanowski J.Q. Lee V.M. Mackenzie I.R. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am. J. Pathol. 2007b;171:227–240. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F. Bolmont T. Crowther R.A. Abramowski D. Frank S. Probst A. Fraser G. Stalder A.K. Beibel M. Staufenbiel M. Jucker M. Goedert M. Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsellis J.A. Boxing and the brain. BMJ. 1989;298:105–109. doi: 10.1136/bmj.298.6666.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsellis J.A.N. Bruton C.J. Freeman-Browne D. The aftermath of boxing. Psychol. Med. 1973;3:270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- Cummings J.L. Mega M. Gray K. Rosenberg-Thompson S. Carusi D.A. Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- de Calignon A. Fox L.M. Pitstick R. Carlson G.A. Bacskai B.J. Spires-Jones T.L. Hyman B.T. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky S.T. Abrahamson E.E. Ciallella J.R. Paljug W.R. Wisniewski S.R. Clark R.S. Ikonomovic M.D. Association of increased cortical soluble abeta42 levels with diffuse plaques after severe brain injury in humans. Arch. Neurol. 2007;64:541–544. doi: 10.1001/archneur.64.4.541. [DOI] [PubMed] [Google Scholar]
- DeKosky S.T. Ikonomovic M.D. Gandy S. Traumatic brain injury—football, warfare, and long-term effects. N. Engl. J. Med. 2010;363:1293–1296. doi: 10.1056/NEJMp1007051. [DOI] [PubMed] [Google Scholar]
- Fonseca M.I. Kawas C.H. Troncoso J.C. Tenner A.J. Neuronal localization of C1q in preclinical Alzheimer's disease. Neurobiol. Dis. 2004;15:40–46. doi: 10.1016/j.nbd.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Forman M.S. Trojanowski J.Q. Lee V.M. TDP-43: a novel neurodegenerative proteinopathy. Curr. Opin. Neurobiol. 2007;17:548–555. doi: 10.1016/j.conb.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstl H. Haass C. Hemmer B. Meyer B. Halle M. Boxing-acute complications and late sequelae: from concussion to dementia. Dtsch. Arztebl. Int. 2010;107:835–839. doi: 10.3238/arztebl.2010.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B. Diamond M.I. Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 2010;11:155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B. Jacks R.L. Diamond M.I. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin T.C. Chen F. Zambrano A. Abraha A. Lagalwar S. Guillozet A.L. Lu M. Fu Y. Garcia-Sierra F. LaPointe N. Miller R. Berry R.W. Binder L.I. Cryns V.L. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavett B.E. Stern R.A. McKee A.C. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin. Sports Med. 2011;30:179–188. doi: 10.1016/j.csm.2010.09.007. xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes J.F. Vowles G.H. Nicoll J.A. Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999;98:171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- Gitcho M. Baloh R. Chakraverty S. Mayo K. Norton J. Levitch D. Hatanpaa K. White C. Bigio E. Caselli R. Baker M. Al-Lozi M. Morris J. Pestronk A. Rademakers R. Goate A. Cairns N. TDP-43 A315T mutation in familial motor neuron disease. Ann. Neurol. 2008;63:535–538. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. Clavaguera F. Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010;33:317–325. doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Griffin W.S. Sheng J.G. Gentleman S.M. Graham D.I. Mrak R.E. Roberts G.W. Microglial interleukin-1 alpha expression in human head injury: correlations with neuronal and neuritic beta-amyloid precursor protein expression. Neurosci. Lett. 1994;176:133–136. doi: 10.1016/0304-3940(94)90066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E. Starr A. Kim R.C. Parachikova A. Lopez G.E. Dick M. Cribbs D.H. Relapsing polychondritis with features of dementia with Lewy bodies. Acta Neuropathol. 2006;112:217–225. doi: 10.1007/s00401-006-0098-0. [DOI] [PubMed] [Google Scholar]
- Heilbronner R.L. Bush S.S. Ravdin L.D. Barth J.T. Iverson G.L. Ruff R.M. Lovell M.R. Barr W.B. Echemendia R.J. Broshek D.K. Neuropsychological consequences of boxing and recommendations to improve safety: a National Academy of Neuropsychology education paper. Arch. Clin. Neuropsychol. 2009;24:11–19. doi: 10.1093/arclin/acp005. [DOI] [PubMed] [Google Scholar]
- Hof P.R. Bouras C. Buee L. Delacourte A. Perl D.P. Morrison J.H. Differential distribution of neurofibrillary tangles in the cerebral cortex of dementia pugilistica and Alzheimer's disease cases. Acta Neuropathol. 1992;85:23–30. doi: 10.1007/BF00304630. [DOI] [PubMed] [Google Scholar]
- Hyman B.T. Caspase activation without apoptosis: insight into Abeta initiation of neurodegeneration. Nat. Neurosci. 2011;14:5–6. doi: 10.1038/nn0111-5. [DOI] [PubMed] [Google Scholar]
- Ikeda K. Akiyama H. Arai T. Nishimura T. Glial tau pathology in neurodegenerative diseases: their nature and comparison with neuronal tangles. Neurobiol. Aging. 1998;19:S85–S91. doi: 10.1016/s0197-4580(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Jellinger K.A. Head injury and dementia. Curr. Opin. Neurol. 2004;17:719–723. doi: 10.1097/00019052-200412000-00012. [DOI] [PubMed] [Google Scholar]
- Jiang H. Burdick D. Glabe C.G. Cotman C.W. Tenner A.J. β-Amyloid activates complement by binding to a specific region of the collagen-like domain of C1qA chain. J. Immunol. 1994;152:5050–5059. [PubMed] [Google Scholar]
- Jicha G.A. Bowser R. Kazam I.G. Davies P. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J. Neurosci. Res. 1997;48:128–132. doi: 10.1002/(sici)1097-4547(19970415)48:2<128::aid-jnr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Jordan B.D. Chronic traumatic brain injury associated with boxing. Semin. Neurol. 2000;20:179–185. doi: 10.1055/s-2000-9826. [DOI] [PubMed] [Google Scholar]
- Jordan B.D. Kanik A.B. Horwich M.S. Sweeney D. Relkin N.R. Petito C.K. Gandy S. Apolipoprotein E epsilon 4 and fatal cerebral amyloid angiopathy associated with dementia pugilistica. Ann. Neurol. 1995;38:698–699. doi: 10.1002/ana.410380429. [DOI] [PubMed] [Google Scholar]
- Jordan B.D. Relkin N.R. Ravdin L.D. Jacobs A.R. Bennett A. Gandy S. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278:136–140. [PubMed] [Google Scholar]
- King A. Sweeney F. Bodi I. Troakes C. Maekawa S. Al-Sarraj S. Abnormal TDP-43 expression is identified in the neocortex in cases of dementia pugilistica, but is mainly confined to the limbic system when identified in high and moderate stages of Alzheimer's disease. Neuropathology. 2010;30:408–419. doi: 10.1111/j.1440-1789.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- Kordower J.H. Chu Y. Hauser R.A. Freeman T.B. Olanow C.W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat. Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Kordower J.H. Dodiya H.B. Kordower A.M. Terpstra B. Paumier K. Madhavan L. Sortwell C. Steece-Collier K. Collier T.J. Transfer of host-derived alpha synuclein to grafted dopaminergic neurons in rat. Neurobiol. Dis. 2011;43:552–557. doi: 10.1016/j.nbd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh S. Van Broeckhoven C. Frontotemporal lobar degeneration: current concepts in the light of recent advances. Brain Pathol. 2007;17:104–114. doi: 10.1111/j.1750-3639.2007.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong L.K. Neumann M. Sampathu D.M. Lee V.M. Trojanowski J.Q. TDP-43 proteinopathy: the neuropathology underlying major forms of sporadic and familial frontotemporal lobar degeneration and motor neuron disease. Acta Neuropathol. 2007;114:63–70. doi: 10.1007/s00401-007-0226-5. [DOI] [PubMed] [Google Scholar]
- Lampert P.W. Hardman J.M. Morphological changes in brains of boxers. JAMA. 1984;251:2676–2679. [PubMed] [Google Scholar]
- Langer F. Eisele Y.S. Fritschi S.K. Staufenbiel M. Walker L.C. Jucker M. Soluble Aβ seeds are potent inducers of cerebral β-amyloid deposition. J. Neurosci. 2011;31:14488–14495. doi: 10.1523/JNEUROSCI.3088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.Y. Englund E. Holton J.L. Soulet D. Hagell P. Lees A.J. Lashley T. Quinn N.P. Rehncrona S. Bjorklund A. Widner H. Revesz T. Lindvall O. Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat. Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Lucas S.M. Rothwell N.J. Gibson R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006;147(Suppl. 1):S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K.T. Wang Y.W. Yang J.T. Yang Y.L. Chen H.I. Effect of interleukin-1 on traumatic brain injury-induced damage to hippocampal neurons. J. Neurotrauma. 2005;22:885–895. doi: 10.1089/neu.2005.22.885. [DOI] [PubMed] [Google Scholar]
- Martland H.S. Punch drunk. JAMA. 1928;91:1103–1107. [Google Scholar]
- Matser E. J. Kessels A.G. Lezak M.D. Jordan B.D. Troost J. Neuropsychological impairment in amateur soccer players. JAMA. 1999;282:971–973. doi: 10.1001/jama.282.10.971. [DOI] [PubMed] [Google Scholar]
- Matser J.T. Kessels A.G. Lezak M.D. Troost J. A dose-response relation of headers and concussions with cognitive impairment in professional soccer players. J. Clin. Exp. Neuropsychol. 2001;23:770–774. doi: 10.1076/jcen.23.6.770.1029. [DOI] [PubMed] [Google Scholar]
- McGeer P.L. Rogers J. Anti-inflammatory agents as a therapeutic approach to Alzheimer's disease. Neurology. 1992;42:447. doi: 10.1212/wnl.42.2.447. [DOI] [PubMed] [Google Scholar]
- McKee A.C. Cantu R.C. Nowinski C.J. Hedley-Whyte E.T. Gavett B.E. Budson A.E. Santini V.E. Lee H.S. Kubilus C.A. Stern R.A. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie J.E. Roberts G.W. Royston M.C. Comparative investigation of neurofibrillary damage in the temporal lobe in Alzheimer's disease, Down's syndrome and dementia pugilistica. Neurodegeneration. 1996;5:259–264. doi: 10.1006/neur.1996.0034. [DOI] [PubMed] [Google Scholar]
- McKhann G.M. Albert M.S. Grossman M. Miller B. Dickson D. Trojanowski J.Q. Clinical and pathological diagnosis of frontotemporal dementia: Report of the work group on frontotemporal dementia and Pick's disease. Arch. Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Mendez M.F. The neuropsychiatric aspects of boxing, particularly from a neurological standpoint. Br. Med. J. 1995;25:249–262. [Google Scholar]
- Meyer-Luehmann M. Coomaraswamy J. Bolmont T. Kaeser S. Schaefer C. Kilger E. Neuenschwander A. Abramowski D. Frey P. Jaton A.L. Vigouret J.M. Paganetti P. Walsh D.M. Mathews P.M. Ghiso J. Staufenbiel M. Walker L.C. Jucker M. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- Morales R. Duran-Aniotz C. Castilla J. Estrada L.D. Soto C. De novo induction of amyloid-beta deposition in vivo. Mol. Psychiatry. 2011 doi: 10.1038/mp.2011.120. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Moseley I.F. The neuroimaging evidence for chronic brain damage due to boxing. Neuroradiology. 2000;42:1–8. doi: 10.1007/s002340050001. [DOI] [PubMed] [Google Scholar]
- Neary D. Snowden J.S. Gustafson L. Passant U. Stuss D. Black S. Freedman M. Kertesz A. Robert P.H. Albert M. Boone K. Miller B.L. Cummings J. Benson D.F. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Neumann M. Sampathu D.M. Kwong L.K. Truax A.C. Micsenyi M.C. Chou T.T. Bruce J. Schuck T. Grossman M. Clark C.M. McCluskey L.F. Miller B.L. Masliah E. Mackenzie I.R. Feldman H. Feiden W. Kretzschmar H.A. Trojanowski J.Q. Lee V.M. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Newman J. Rissman R.A. Sarsoza F. Kim R.C. Dick M. Bennett D.A. Cotman C.W. Rohn T.T. Head E. Caspase-cleaved tau accumulation in neurodegenerative diseases associated with tau and alpha-synuclein pathology. Acta Neuropathol. (Berl.) 2005;110:135–144. doi: 10.1007/s00401-005-1027-3. [DOI] [PubMed] [Google Scholar]
- Nguyen M.D. Julien J.P. Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat. Rev. Neurosci. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Nowak L.A. Smith G.G. Reyes P.F. Dementia in a retired world boxing champion: case report and literature review. Clin. Neuropathol. 2009;28:275–280. [PubMed] [Google Scholar]
- Otvos L., Jr. Feiner L. Lang E. Szendrei G.I. Goedert M. Lee V.M. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J. Neurosci. Res. 1994;39:669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Petrushina I. Ghochikyan A. Mktrichyan M. Mamikonyan G. Movsesyan N. Davtyan H. Patel A. Head E. Cribbs D.H. Agadjanyan M.G. Alzheimer's disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric Abeta species in amyloid precursor protein transgenic mice. J. Neurosci. 2007;27:12721–12731. doi: 10.1523/JNEUROSCI.3201-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman R.A. Poon W.W. Blurton-Jones M. Oddo S. Torp R. Vitek M.P. LaFerla F.M. Rohn T.T. Cotman C.W. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J. Clin. Invest. 2004;114:121–130. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A.H. Brain Damage in Boxers. Pitman Publishing; London: 1969. [Google Scholar]
- Roberts G.W. Immunocytochemistry of neurofibrillary tangles in dementia pugilistica and Alzheimer's disease: evidence for common genesis. Lancet. 1988;2:1456–1458. doi: 10.1016/s0140-6736(88)90934-8. [DOI] [PubMed] [Google Scholar]
- Rohn T.T. Head E. Caspase activation in Alzheimer's disease: early to rise and late to bed. Rev. Neurosci. 2008;19:383–393. doi: 10.1515/revneuro.2008.19.6.383. [DOI] [PubMed] [Google Scholar]
- Schmidt M.L. Zhukareva V. Newell K.L. Lee V.M. Trojanowski J.Q. Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer's disease. Acta Neuropathol. 2001;101:518–524. doi: 10.1007/s004010000330. [DOI] [PubMed] [Google Scholar]
- Schofield E. Kersaitis C. Shepherd C.E. Kril J.J. Halliday G.M. Severity of gliosis in Pick's disease and frontotemporal lobar degeneration: tau-positive glia differentiate these disorders. Brain. 2003;126:827–840. doi: 10.1093/brain/awg085. [DOI] [PubMed] [Google Scholar]
- Scully R.E. Mark E.J. McNeely W.F. Ebeling S.H. Weekly clinicopathological exercises: Case 12-1999. N. Engl. J. Med. 1999;340:1269–1277. doi: 10.1056/NEJM199706263362608. [DOI] [PubMed] [Google Scholar]
- Spillane J.D. Five boxers. BMJ. 1962:1205–1210. doi: 10.1136/bmj.2.5314.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones T.L. de Calignon A. Matsui T. Zehr C. Pitstick R. Wu H.Y. Osetek J.D. Jones P.B. Bacskai B.J. Feany M.B. Carlson G.A. Ashe K.H. Lewis J. Hyman B.T. In vivo imaging reveals dissociation between caspase activation and acute neuronal death in tangle-bearing neurons. J. Neurosci. 2008;28:862–867. doi: 10.1523/JNEUROSCI.3072-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J.H. Cummings B.J. Cotman C.W. Early phosphorylation of tau in Alzheimer's disease occurs at Ser-202 and is preferentially located within neurites. Neuroreport. 1994;5:2358–2362. doi: 10.1097/00001756-199411000-00037. [DOI] [PubMed] [Google Scholar]
- Thal D.R. Capetillo-Zarate E. Schultz C. Rub U. Saido T.C. Yamaguchi H. Haass C. Griffin W.S. Del Tredici K. Braak H. Ghebremedhin E. Apolipoprotein E co-localizes with newly formed amyloid beta-protein (Abeta) deposits lacking immunoreactivity against N-terminal epitopes of Abeta in a genotype-dependent manner. Acta Neuropathol. 2005;110:459–471. doi: 10.1007/s00401-005-1053-1. [DOI] [PubMed] [Google Scholar]
- Tokuda T. Ikeda S. Yanagisawa N. Ihara Y. Glenner G.G. Re-examination of ex-boxers' brains using immunohistochemistry with antibodies to amyloid beta-protein and tau protein. Acta Neuropathol. 1991;82:280–285. doi: 10.1007/BF00308813. [DOI] [PubMed] [Google Scholar]
- Uboga N.V. Price J.L. Formation of diffuse and fibrillar tangles in aging and early Alzheimer's disease. Neurobiol. Aging. 2000;21:1–10. doi: 10.1016/s0197-4580(00)00091-9. [DOI] [PubMed] [Google Scholar]
- Uhl G.R. McKinney M. Hedreen J.C. White C.L., III Coyle J.T. Whitehouse P.J. Price D.L. Dementia puglisitica: Loss of basal forebrain cholinergic neurons and cortical cholinergic markers. Ann. Neurol. 1982;12:99. [Google Scholar]
- Uryu K. Laurer H. McIntosh T. Pratico D. Martinez D. Leight S. Lee V.M. Trojanowski J.Q. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 2002;22:446–454. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek J. Elward K. Gasque P. Activation of complement in the central nervous system: roles in neurodegeneration and neuroprotection. Ann NY Acad. Sci. 2003;992:56–71. doi: 10.1111/j.1749-6632.2003.tb03138.x. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley L.A. Luk K.C. Patel T.P. Tanik S.A. Riddle D.M. Stieber A. Meaney D.F. Trojanowski J.Q. Lee V.M. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster S.D. Bradt B. Rogers J. Cooper N. Aggregation state-dependent activation of the classical complement pathway by amyloid beta peptide. J. Neurochem. 1997;69:388–398. doi: 10.1046/j.1471-4159.1997.69010388.x. [DOI] [PubMed] [Google Scholar]
- Wilson A.C. Dugger B.N. Dickson D.W. Wang D.S. TDP-43 in aging and Alzheimer's disease—a review. Int. J. Clin. Exp. Pathol. 2011;4:147–155. [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y. Uryu K. Higuchi M. Longhi L. Hoover R. Fujimoto S. McIntosh T. Lee V.M. Trojanowski J.Q. Enhanced neurofibrillary tangle formation, cerebral atrophy, and cognitive deficits induced by repetitive mild brain injury in a transgenic tauopathy mouse model. J. Neurotrauma. 2005;22:1134–1141. doi: 10.1089/neu.2005.22.1134. [DOI] [PubMed] [Google Scholar]
- Zhang L. Heier L.A. Zimmerman R.D. Jordan B. Ulug A.M. Diffusion anisotropy changes in the brains of professional boxers. Am. J. Neuroradiol. 2006;27:2000–2004. [PMC free article] [PubMed] [Google Scholar]
- Zhang L. Ravdin L.D. Relkin N. Zimmerman R.D. Jordan B. Lathan W.E. Ulug A.M. Increased diffusion in the brain of professional boxers: a preclinical sign of traumatic brain injury? Am. J. Neuroradiol. 2003;24:52–57. [PMC free article] [PubMed] [Google Scholar]