Abstract
Brain-derived neurotrophic factor (BDNF) plays a role in cognition, as well as neural survival and plasticity. There are several common polymorphisms in the BDNF gene, one of which (rs6265) is an extensively studied non-synonymous coding polymorphism (Val66Met) which has been linked to cognitive performance in healthy controls and some clinical populations. We hypothesized that the Met allele of rs6265 would be associated with poorer cognitive performance in individuals with mild-to-moderate traumatic brain injury, and that other polymorphisms in the BDNF gene would also affect cognition. Genotype at 9 single-nucleotide polymorphisms (SNPs) in the BDNF gene, and measures of speed of information processing, learning, and memory were assessed in 75 patients with mTBI and 38 healthy subjects. Consistent with previous reports, the Met allele of rs6265 was associated with cognition (slower processing speed) in the entire group. Two other SNPs were associated with processing speed in the mTBI group, but both are in linkage disequilibrium with rs6265, and neither remained significant after adjustment for rs6265 status. Within the mTBI group, but not the controls, 4 SNPs, but not rs6265, were associated with memory measures. These associations were not affected by adjustment for rs6265 status. Polymorphisms in BDNF influence cognitive performance shortly after mTBI. The results raise the possibility that a functional polymorphism other than rs6265 may contribute to memory function after mTBI.
Key words: adult brain injury, genetic factors, head trauma, neuropsychology, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a significant public health problem. It is estimated that ∼ 1.5 million Americans suffer a TBI each year (Rutland-Brown et al., 2006), and over 3 million Americans are living with some TBI-related disability (Zaloshnja et al., 2008). Cognitive deficits, particularly in the domains of memory, attention, executive function, and speed of information processing, are frequently described by survivors and their family/caregivers as particularly troublesome (DeGuise et al., 2008).
Cognitive outcome varies according to the severity of the initial injury. However, individuals who suffer seemingly similar degrees of injury under similar circumstances may have very different outcomes. Although much of this variance probably relates to differences in the biomechanical forces causing the injury, our group and others have raised the question of whether differences in genes that modulate the neural response to trauma may also play a role (Jordan, 2007; McAllister, 2005,2009; McAllister et al., 2008).
One likely candidate is the gene for brain-derived neurotrophic factor (BDNF). BDNF is one of several trophic factors that influence the response of the central nervous system (CNS) to trauma and other disorders (Lipsky and Marini, 2007). BDNF mRNA is elevated in the hippocampus within 1 h of trauma and remains elevated for several days (Hicks et al., 1997,1998). BDNF plays an important role in neural survival, repair, and plasticity. Specifically, it appears to facilitate both early and late long-term potentiation, processes critical to the formation and maintenance of episodic and working memory (Egan et al., 2003; Lu and Gottschalk, 2000; Poo, 2001). BDNF is initially manufactured as a precursor protein (pro-BDNF), and then cleaved to BDNF, which is stored in and released from secretory vesicles, at least in part in response to neural activity.
There are many common single-nucleotide polymorphisms (SNPs) in the BDNF gene, one of which, rs6265, is a non-synonymous coding polymorphism in the pro-BDNF sequence, which results in a change in the amino acid at codon 66 from valine to methionine (Egan et al., 2003). This switch alters the processing and release of the pro-BDNF polypeptide in an as yet unclear fashion (Lipsky and Marini, 2007), and has been associated with altered neurocognitive functioning in a variety of circumstances. For example, in a study of 136 healthy controls, 106 individuals with schizophrenia spectrum disorders, and 138 unaffected siblings, Egan and associates (2003) found that the Met allele was associated with lower scores on the Wechsler Memory Scale-R (Wechsler, 1987), abnormal hippocampal activation on functional magnetic resonance imaging (fMRI), lower hippocampal n-acetyl acetate (NAA) levels on magnetic resonance spectroscopy, and abnormal storage and secretion of BDNF in an in vivo cell culture assay.
Although virtually all of the genetic association studies of BDNF polymorphisms done to date have focused on rs6265, there are hints in the literature that this polymorphism may not capture all of the biologically important genetic variation at this locus. For example, in their study of the global diversity of BDNF haplotypes, Petryshen and colleagues (2010) describe three common haplotypes that account for the vast majority of BDNF genotypes in individuals of European extraction. The Met allele at rs6265 is a marker for one of those haplotypes (Petryshen haplotype 7), and based on their data it appears that there has probably been a strong selective pressure favoring that haplotype in recent human evolutionary history. In addition, however, those authors found evidence for at least one other selection event, independent of genotype at rs6265.
The role of the Val66Met polymorphism and other polymorphisms in the BDNF gene in response to and recovery from human TBI has not been thoroughly characterized. Interestingly, Krueger and associates (2011) reported that the Met allele was associated with better measures of executive function in Viet Nam era combat veterans with combat-related focal frontal lobe lesions. However, there are no reports of the effect of BDNF genotype on cognitive function shortly after mild non-penetrating injuries.
Given the abundance of BDNF in the hippocampus, its role in both normal memory function and response to trauma, the vulnerability of the hippocampus to TBI (Farkas and Povlishock, 2007), and the frequency of memory, attention, and speed of information processing problems seen after TBI, we hypothesized that polymorphisms in this gene would influence performance on tests of memory, learning, and speed of information processing, 1 month after mild-to-moderate TBI.
Methods
Overview
This report is part of an ongoing longitudinal investigation of genetic predictors of cognitive outcome after TBI. All participants are given a battery of cognitive tests assessing general intellectual function; learning, verbal, visual, and working memory; executive and attentional functioning; and processing speed approximately 1 month and again 1 year after injury. From the cognitive outcome measures available, specific tests are chosen prior to data analysis as primary outcome variables on the basis of hypothesized effects of a given candidate allele on specific cognitive domains. This article reports on the role of BDNF SNPs in modulating memory, learning, and processing speed 1 month after injury. In addition, results of reassessment approximately 1 year after injury are reported for a smaller cohort of the TBI participants (n=44).
Participants
A cohort of 75 consecutive patients with mTBI was recruited from a Level 1 trauma center emergency department. mTBI was defined as initial Glasgow Coma Scale (GCS) scores of 9–15 when available, and/or duration of loss of consciousness not exceeding 24 h. The American Congress of Rehabilitation Medicine criteria for mild TBI were used as the minimum standard for having sustained a brain injury (American Congress of Rehabilitation Medicine, 1993). TBI participants were studied approximately 1 month (mean 38 days, SD 16) after injury. Healthy control subjects (n=38) were recruited through advertisements. Exclusion criteria included history of other neurological disorders, substantial systemic medical illness, or current Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) axis I psychiatric diagnosis disorder based on the Structured Clinical Interview for DSM-IV (First et al., 1997) with the exception of substance abuse. The study protocol and informed consent were approved by the Dartmouth College Committee for the Protection of Human Subjects. Written informed consent was obtained from all participating subjects. Over 95% of both the TBI and control groups were Caucasians of European descent.
Cognitive measures
Prior to analyzing the data the California Verbal Learning Test (CVLT; Delis et al., 1987,2000) and the Gordon Continuous Performance Test (CPT; Gordon, 1986) were selected as primary outcome measures based on the hypothesized role of BDNF in learning, memory, attention, and processing speed. Specifically, the Simple Reaction Time Reaction Time (SRTRT) of the CPT was chosen as a measure of processing speed, and the Short Delay (CVLT-SD) and Long Delay (CVLT-LD) tests of the CVLT were chosen as measures of learning and memory performance. The Wide-Range Achievement Test, Third edition, (WRAT-3) Reading subtest (Wilkinson, 1993), and the Wechsler Adult Intelligence Scale-III (WAIS-III) Block Design subtest (Wechsler, 1997) were used to estimate the baseline level of general intellectual function.
Genotyping
Qiagen's blood mini-kit (Qiagen, Alameda, CA) was used to isolate DNA from peripheral blood samples. Genotyping was done using a 3300-SNP targeted panel developed in a partnership between the Dartmouth Neurogenetics Group (DNG) and Affymetrix (Affymetrix Inc., Santa Clara, CA). Candidate genes and alleles were selected for inclusion on the chip after detailed manual curation by the DNG based on a review of the available human and animal literature, making use of multiple databases of genetic associations with neurological, psychiatric, neurodevelopmental, and neurodegenerative disorders of the CNS and relevant phenotypic markers. Selection criteria for specific alleles included (1) the gene had known or hypothesized involvement in critical pathways involved in cognition, neurotrauma, neurodegeneration, excitotoxic and injury cascades, neural plasticity and repair, inflammation, immune responsivity, stress response/reactivity, or depression/anxiety; (2) minor allele frequency of at least 5%; and (3) known functional effects or location in gene regions that might impact gene expression (e.g., promoter regions). The resulting array was implemented by Affymetrix using molecular inversion probe technology and consisted of 3300 SNPs (after quality control filters) from approximately 1000 candidate genes. Ancestral informative markers were also included to examine population substructure. We have successfully obtained genotype data on an initial sample of 1000 individuals with several neuropsychiatric disorders including TBI. Reliability and quality control were excellent. For this report we analyzed the 9 BDNF SNPs selected for inclusion in the targeted neuroscience panel.
Genetic analyses
Initially, Bayesian clustering as implemented in the program Structure (Pritchard et al., 2000) was used to look for significant genetic subpopulations within our sample, using all 3300 SNPs. Minor allele frequencies were estimated and used along with estimated genotype frequencies to test for departures from Hardy-Weinberg equilibrium (HWE). The extent of linkage disequilibrium (r2) between each pairwise combination of SNPs in the control subjects was estimated and visualized using Haploview (Barrett et al., 2005) for the 9 SNPs of interest in BDNF.
Statistical analyses
Demographics
Between-group differences in demographic characteristics were examined by t-tests or chi-square tests for continuous or categorical variables, respectively. Group differences in cognitive performance related to allele status and haplotype status were examined by linear regression and analysis of variance (ANOVA) with Tukey's Honestly Significant Difference corrections for multiple comparisons (Saville, 1990; Tukey, 1949).
Polymorphism associations
Each of the 9 allele variables was summarized as either a factor variable with three levels (0, 1, or 2), or as a genotype variable representing dominant, recessive, or additive models. Group differences for cognitive measures were analyzed by analysis of covariance (ANCOVA) with diagnosis, the allele variable, and the interaction between allele and diagnosis included in the model, covarying for age and education, as these factors have known effects on cognitive measures. Significance tests were examined for the main effects of diagnosis and allele status, and for the interaction between allele and diagnosis. p Values were adjusted for multiple comparisons using the false discovery rate (FDR) method of Benjamini and Hochberg (1995), using an FDR of 0.10. Two-tailed p values were used for significance thresholds.
Haplotype associations
Haplotype trend regressions (HTR) were performed in the TBI sample using PowerMarker (Liu and Muse 2005) with sliding windows of 3, 4, 5, and 6 SNPs. Haplotypes and their frequencies were estimated with the expectation-maximization (EM) algorithm (Excoffier and Slatkin, 1995; Long et al., 1995). The association of each estimated haplotype with the cognitive variables of interest was examined through HTR using the gap package in R (http://cran.r-project.org/web/packages/gap/; Zaykin et al., 2002). Linear regression was also used to confirm the results from the HTR.
Results
Demographics
Demographic characteristics of the TBI and healthy control groups are summarized in Table 1. There were no between-group differences in baseline intellectual function based on WRAT-3 reading score or WAIS-III Block Design scaled score. The two groups differed with respect to education. Although the differences are small in absolute terms and not clinically meaningful, subsequent analyses were adjusted for both age and education. The TBI group had a mean (SD) GCS score of 14.1 (1.7), post-traumatic amnesia of 34 (92) min, and loss of consciousness of 8.4 (11.7) min, suggesting a fairly mild injury profile. Nine (12%) of the cohort were judged to have complicated mild TBI on the basis of abnormal acute computed tomography (CT) scans, but none of these participants underwent neurosurgical procedures. Thirteen (17%) of the TBI participants were judged to have moderate TBI on the basis of GCS score, length of unconsciousness, and length of post-traumatic amnesia, when these measures were available and judged reliable by careful review of medical records. The mean injury-to-testing interval was 38 (16) days. None of the participants (controls or TBI patients) had substance dependence disorders. Only two of the TBI group met DSM-IV criteria for current substance abuse. The groups did not differ with respect to distribution of ancestral markers, suggesting that they came from a homogenous population. Ninety-six percent were Caucasians of European descent.
Table 1.
Demographic Characteristics of the Study Groups
| Characteristic | TBI mean (SD) n=75 | Control mean (SD) n=38 | p Value |
|---|---|---|---|
| Age, years | 33.1 (13.1) | 31.9 (10.1) | 0.61 |
| Male gender | 46 (61%) | 19 (50%) | 0.25 |
| READ | 105 (10) | 108 (7) | 0.08 |
| BD | 12.6 (3.0) | 11.7 (2.7) | 0.10 |
| Education (years) | 14.2 (2.6) | 15.3 (2.4) | 0.04 |
| Mother's education | 13.7 (2.5) | 14.1 (3.0) | 0.46 |
| Father's education | 13.9 (3.7) | 14.4 (3.1) | 0.44 |
BD, Block Design Scaled Score; READ, Wide-Range Achievement Test, Third edition (WRAT-3) Reading Standard Score; TBI, traumatic brain injury; SD, standard deviation.
Genetic analysis
Bayesian clustering as implemented in the program Structure [16] showed no differences in population structure between the TBI participants and healthy controls. Based on both the chi-square and exact test analyses, all SNPs were in Hardy-Weinberg equilibrium (p>0.34), and all SNPs had a minor allele frequency greater than 0.16.
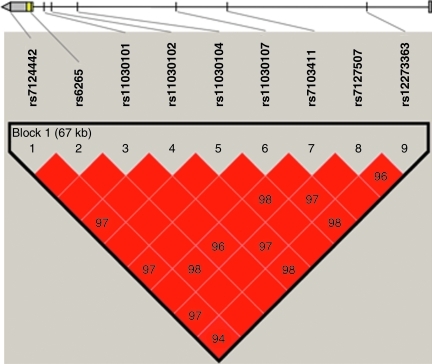
Figure 1 shows the relative location on the BDNF gene of the SNPs studied. The default setting in Haploview highlighted a single 67-kb region of high linkage disequilibrium.
FIG. 1.
Linkage disequilibrium between each pairwise combination of the 9 genotyped single-nucleotide polymorphisms (SNPs) in brain-derived neurotrophic factor (BDNF). The heavy black outline indicates that all the SNPs belong to a single haploblock. The numbers in the squares are measures of linkage disequilibrium, D’. Red squares indicate high linkage disequilibrium. Grey lines indicate the relative position in BDNF. Grey boxes in the gene diagram represent exons, and the line represents an intron. Vertical lines represent the locations of SNPs.
Effects of genotype on cognition
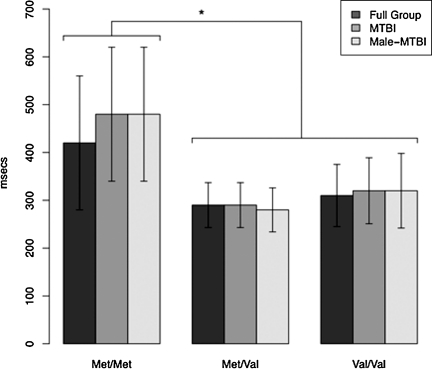
Effects of genotype on cognition are summarized in Tables 2 and 3. Using an FDR of 0.1, 3 of the 9 BDNF SNPs, including rs6265, showed a main effect on reaction time (SRTRT), a measure of information processing speed, on the CPT Simple Reaction Time trial 1 month after injury. Figure 2 shows that consistent with previous reports, the Met/Met condition was associated with the slowest reaction times. Two SNPs (rs7124442 and rs7127507) were associated with the Short Delay trial of the CVLT, a measure of learning and short-term recall.
Table 2.
Summary of Association of Allele Type with Cognitive Outcome Measures by Gender within Group
| |
|
|
|
|
TBI |
Controls |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
All subjects |
Males |
Females |
Males |
Females |
||||||||||
| SNP | Location | LD | SD | SRTRT | LD | SD | SRTRT | LD | SD | SRTRT | LD | SD | SRTRT | LD | SD | SRTRT |
| rs7124442 | 3′-UTR | 0.4 | 0.042 | 0.06 | 0.068 | 0.13 | 0.21 | 0.45 | 0.25 | 0.095 | 0.31 | 0.10 | 0.11 | 0.19 | 0.27 | 0.058 |
| rs6265 | Coding | 0.85 | 0.71 | 0.001 | 0.27 | 0.59 | 0.00082 | 0.68 | 0.57 | 0.14 | 0.32 | 0.057 | 0.24 | 0.70 | 0.83 | 0.16 |
| rs11030101 | Intron | 0.45 | 0.14 | 0.82 | 0.18 | 0.12 | 0.70 | 0.50 | 0.21 | 0.63 | 0.17 | 0.027 | 0.28 | 0.13 | 0.69 | 0.54 |
| rs11030102 | Intron | 0.15 | 0.081 | 0.28 | 0.019 | 0.021 | 0.41 | 0.63 | 0.46 | 0.21 | 0.53 | 0.93 | 0.31 | 0.22 | 0.87 | 0.058 |
| rs11030104 | Intron | 0.84 | 0.73 | 0.001 | 0.16 | 0.37 | 0.00082 | 0.96 | 0.71 | 0.097 | 0.32 | 0.057 | 0.24 | 0.95 | 0.48 | 0.0087 |
| rs11030107 | Intron | 0.11 | 0.064 | 0.29 | 0.022 | 0.024 | 0.40 | 0.63 | 0.46 | 0.21 | 0.53 | 0.93 | 0.31 | 0.22 | 0.87 | 0.058 |
| rs7103411 | Intron | 0.52 | 0.5 | 0.001 | 0.27 | 0.52 | 0.0015 | 0.96 | 0.71 | 0.097 | 0.32 | 0.057 | 0.24 | 0.95 | 0.48 | 0.0087 |
| rs7127507 | Intron | 0.23 | 0.017 | 0.10 | 0.02 | 0.037 | 0.30 | 0.45 | 0.25 | 0.095 | 0.20 | 0.10 | 0.041 | 0.19 | 0.27 | 0.058 |
| rs12273363 | 5′ near gene | 0.13 | 0.066 | 0.38 | 0.019 | 0.021 | 0.53 | 0.82 | 0.59 | 0.21 | 0.59 | 0.93 | 0.085 | 0.039 | 0.30 | 0.19 |
Bold indicates significant at false discovery rate (FDR) of 0.10, adjusted for age and education.
LD, California Verbal Learning Test Long Delay Free Recall; SD, California Verbal Learning Test Short Delay Free Recall; SRTRT, Gordon Continuous Performance Test Simple Reaction Time Test Reaction Time; SNP, single-nucleotide polymorphism; TBI, traumatic brain injury.
Table 3.
Summary of Association of Allele with Cognitive Outcome Measures in mTBI and Control Groups Adjusted for Age, Education, and rs6265 Allele Status
| |
|
TBI |
Controls |
||||
|---|---|---|---|---|---|---|---|
| SNP | Location | LD | SD | SRTRT | LD | SD | SRTRT |
| rs7124442 | 3′-UTR | 0.1 | 0.099 | 0.4 | 0.36 | 0.73 | 0.93 |
| rs6265 | Coding | NA | NA | NA | NA | NA | NA |
| rs11030101 | Intron | 0.24 | 0.12 | 0.78 | 0.67 | 0.91 | 0.35 |
| rs11030102 | Intron | 0.029 | 0.029 | 0.71 | 0.32 | 0.94 | 0.74 |
| rs11030104 | Intron | 0.66 | 0.95 | 9E-05 | 0.23 | 0.13 | 0.042 |
| rs11030107 | Intron | 0.017 | 0.012 | 0.71 | 0.32 | 0.94 | 0.74 |
| rs7103411 | Intron | 0.64 | 0.91 | 0.85 | 0.23 | 0.13 | 0.042 |
| rs7127507 | Intron | 0.047 | 0.027 | 0.5 | 0.45 | 0.76 | 0.71 |
| rs12273363 | 5′ near gene | 0.025 | 0.011 | 0.67 | 0.25 | 0.67 | 0.55 |
Bold indicates significant at false discovery rate (FDR) of 0.10, adjusted for age and education.
LD, California Verbal Learning Test Long Delay Free Recall; SD, California Verbal Learning Test Short Delay Free Recall; SRTRT, Gordon Continuous Performance Test Simple Reaction Time Test Reaction Time; SNP, single-nucleotide polymorphism; TBI, traumatic brain injury.
FIG. 2.
Effect of rs6265 allele status on processing speed (Simple Reaction Time Reaction Time, SRTRT). Effect of rs6265 genotype on a measure of processing speed in milliseconds (Gordon Continuous Performance Test, CPT) in all participants, the male mild-to-moderate traumatic brain injury (mTBI) group only, and the mTBI group. Higher values suggest slower processing speed. Statistically significant differences were found between the Met/Met homozygotes and the groups with a Val allele (full group: p=0.0013; mTBI: p=0.0003; mTBI males: p=0.0008).
Within the mTBI group 4 SNPs were associated with the Long Delay trial of the CVLT, a measure of learning and memory recall performance, 4 SNPs with the Short Delay trial of the CVLT, and 3 SNPs were associated with the Simple Reaction Time RT measure. Interestingly, Tables 2 and 3 suggest that these results were being largely driven by associations within the male mTBI group. Within the control group, none of the SNPs were associated with either the reaction time or learning/memory measures.
Given the previously reported effects of rs6265 on cognition in some populations (Egan et al., 2003), and the high linkage disequilibrium of the SNPs studied, the analyses were repeated adjusting for rs6265 allele status. Table 3 shows that the previously noted associations between information processing speed and rs11030104 and rs7127507 were largely accounted for by rs6265 allele status. However, even after adjusting for rs6265 status, three SNPs were associated with both the Long Delay and Short Delay trial conditions of the CVLT (rs11030102, rs11030107, and rs12273363), and a fourth SNP (rs712507) was also associated with the Short Delay trial.
Forty-four participants with mTBI were available for reassessment approximately 1 year after injury. In this smaller sample, modest associations were found after FDR correction in male mTBI participants between rs11030101 and the CVLT LD condition (p<0.02), and rs7103411 and SRTRT (p<0.01). After accounting for rs6265 status and after FDR correction, a significant association remained in male mTBI participants between rs11030102 and the Long Delay condition of the CVLT (p<0.02).
Haplotype analysis
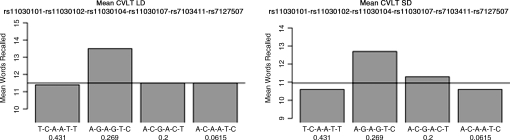
To investigate whether haplotypes in this region are more explanatory of outcome measures than individual SNPs, we performed an HTR using the program PowerMarker (Liu and Muse, 2005). Potential haplotypes between 3 and 6 SNPs in length were defined and tested for associations with the Short and Long Delay recall trials of the CVLT. The most significant association found was for a 6-SNP block spanning rs11030101, rs11030102, rs11030104, rs11030107, rs7103411, and rs7127507, and the Short Delay (p=0.02) and Long Delay (p=0.01) conditions in male but not female participants (Fig. 3). Males with the haplotype A-G-A-G-T-C (frequency 0.27) scored significantly better on both the Short Delay and Long Delay conditions relative to participants with other haplotypes. This effect was absent in females alone and controls alone. There was a trend for both conditions in the male mTBI group (LD p=0.07, SD p=0.05).
FIG. 3.
Haplotype analysis. Mean values of California Verbal Learning Test Long Delay (CVLT LD) and CVLT Short Delay (SD) by haplotype in male subjects. The SNPs included in the haploblock are listed at the top of the graph and haplotypes are listed along the x axis. Numbers below each haplotype represent the frequency of each haplotype among males. The horizontal line indicates the mean SD value or LD value for all males.
Discussion
These results are consistent with our hypothesis that polymorphisms in the BDNF gene influence cognitive function 1 month after mild-to-moderate TBI. Consistent with previous literature, the known functional polymorphism (rs6265) was associated with cognition in this cohort, a measure of speed of information processing. Perhaps the most important result of this study is that other SNPs and a haplotype not containing rs6265 were associated with learning and memory function. Adjusting for allele status at rs6265 did not remove the associations between allele status at rs11030102, rs11030107, and rs12273363, and the memory and learning measures, nor was rs6265 in the 6-SNP window associated with memory and learning measures. To our knowledge this is the first demonstration that the Met/Val polymorphism does not capture all of the functionally important genetic variation at this locus.
The explanation of this effect is not known. As previously noted, the hippocampus is particularly vulnerable to the effects of TBI, perhaps in part related to the disproportionate expression of NMDA receptors and presumed resultant sensitivity to TBI-induced release of excitatory amino acids. BDNF is robustly expressed in both the hipppocampus and frontal cortices; thus it is uniquely positioned to modulate both the acute response to neurotrauma, as well as repair processes. Given the multiple roles BDNF plays as a trophic factor during development, as a neurotransmitter mediating synaptic plasticity in the adult, and as a neuronal survival factor following injury, it is perhaps not surprising that the association of BDNF polymorphisms with changes in cognitive function after TBI is a complicated one. BDNF polymorphisms might, for example, be associated with differences in brain structure affecting the susceptibility of the brain to traumatic injury, with variation in neuronal survival at the time of injury, or with alterations in synaptic plasticity allowing more or less recovery of function after injury. Polymorphisms at different sites in the gene might be expected to affect these functions of BDNF differently, and to have differential effects on different aspects of cognition.
Although we did not find a statistically significant sex×diagnosis×allele interaction in our data, the observation that the effects of BDNF on cognition appear particularly robust in males is of interest in that TBI occurs with a 2:1 male:female ratio. The explanation of this effect is not clear and needs further confirmation. However, it is interesting to note recent work suggesting a neuroprotective effect of estrogen and progesterone in various models of neurotrauma including TBI (Schumacher et al., 2007). Furthermore, in some trauma models females appear to benefit from normally-occurring higher levels of endogenous progesterone (Robertson et al., 2006; Roof et al., 1993; Schumacher et al., 2007), and BDNF expression has been shown to be upregulated by progesterone (Gonzalez et al., 2004). Thus it is possible that the observed BDNF effects in our cohort are more apparent in males due to the lower endogenous levels of progesterone.
There are several limitations of this study that should be noted in interpreting the results. This is a cohort of individuals with predominantly mild TBI. The results may not be generalizable to more severely injured populations. Some of the cell sizes with particular genotypes are relatively small. Although the analytic approach we used accounts for cell size in determining association significance, these results clearly need replication in a larger sample. Cognitive outcome after TBI is a complex interaction of pre-injury capacities, the profile and type of brain injury, effects of injury on other areas, post-injury treatment, and various psychosocial factors. Thus the contribution of a single polymorphism to outcome may be quite modest. In more severely injured individuals, or in longer injury-to-outcome intervals, these other contributing factors may overwhelm the contribution of a single polymorphism. This is why we chose to look initially in a cohort of individuals with mild injuries. In this study we used healthy (non-injured) individuals as our control group, reflecting our interest in studying the effects of our candidate alleles on normal cognition as well as outcome after TBI. This approach limits our ability to attribute the observed genetic effects in our TBI group to the TBI alone, as opposed to additional contributions from non-specific effects of injury in general. Future studies would benefit from inclusion of an “other injury” control group. Our cohort is from a largely northern European Caucasian population, reflecting the demographics of our catchment area; further work is needed to determine the generalizability to other racial and ethnic populations, as there are significant differences at this locus in different ethnic groups. For example, the Met allele-containing haplotype found in Caucasians is not present in other populations; the Met allele is present on a different haplotype in Asian populations and is essentially absent in African populations (Petryshen et al., 2010). Finally, although mildly injured as a group, we cannot exclude the possibility that there are subtle but significant differences in injury severity between individuals. It is often difficult to accurately ascertain injury severity indicators such as duration of loss of consciousness (e.g., many of the events are unwitnessed accidents), or duration of post-traumatic amnesia (e.g., self-reports cannot be considered reliable in this population, and medical records are often not sufficiently detailed for accurate determination).
Although not the primary focus of this study, it is also of interest that some of the associations between genotype and cognitive measures persisted 1 year after injury in a smaller cohort. The pattern of results overlapped with the 1-month data, but was not identical. This could be related to the smaller sample size available for analysis 1 year after injury, the complexities of genotype interacting with intervening life events after injury, a time-dependent role of BDNF in cognitive outcome (e.g., primary effects on repair early after injury, or predominant effects on memory function later after injury), the fact that these SNPs are in linkage disequilibrium, or other undetermined factors. Nevertheless, these results suggests that SNPs in the BDNF gene continue to play a role in recovery from TBI; however, the story is complex and may be influenced by time. Additional studies with larger sample sizes may help clarify this further. Our results do suggest that injury-to-assessment interval is important to address in future study designs. The observation that multiple polymorphisms in the BDNF gene are independently associated with measures of memory and processing speed in mBTI patients is an important one, suggesting mechanistic hypotheses which could be exploited in the development of novel therapies for this important group of patients.
Acknowledgments
This work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (R01HD048176), and the National Institute of Neurological Disorders and Stroke (R01NS055020). The authors would like to thank Reid Kehoe, Ph.D., and Ashlee Robbins, B.A., who assisted with some of the analyses in this work.
Author Disclosure Statement
No competing financial interests exist.
References
- American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993;8:86–87. [Google Scholar]
- Barrett J. Fry B. Maller J. Daly M. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Benjamini Y. Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Roy. Stat. Soc., Ser. B. 1995;57:289–300. [Google Scholar]
- DeGuise E. LeBlanc J. Feyz M., et al. Long-term outcome after severe traumatic brain injury: the McGill interdisciplinary prospective study. J. Head Trauma Rehabil. 2008;23:294–303. doi: 10.1097/01.HTR.0000336842.53338.f4. [DOI] [PubMed] [Google Scholar]
- Delis D.C. Kramer J.H. Kaplan E. Ober B.A. California Verbal Learning Test: Adult Version. The Psychological Corporation; New York: 1987. [Google Scholar]
- Delis D.C. Kramer J.H. Kaplan E. Ober B.A. California Verbal Learning Test—Second Edition: Adult Version Manual. The Psychological Corporation; San Antonio: 2000. [Google Scholar]
- Egan M.F. Kojima M. Callicott J.H. Goldberg T.E. Kolachana B.S. Bertolino A. Zaitsev E. Gold B. Goldman D. Dean M. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Excoffier L. Slatkin M. Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol. Biol. Evol. 1995;12:921–927. doi: 10.1093/oxfordjournals.molbev.a040269. [DOI] [PubMed] [Google Scholar]
- Farkas O. Povlishock J.T. Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage. Prog. Brain Res. 2007;161:43–59. doi: 10.1016/S0079-6123(06)61004-2. [DOI] [PubMed] [Google Scholar]
- First M.B. Spitzer R.L. Gibbon M. Williams J.B.W. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Press; Washington D.C.: 1997. [Google Scholar]
- Gonzalez S.L. Labombarda F. Gonzalez Deniselle M.C. Guennoun R. Schumacher M. De Nicola A.F. Progesterone up-regulates neuronal brain-derived neurotrophic factor expression in the injured spinal cord. Neuroscience. 2004;125:605–614. doi: 10.1016/j.neuroscience.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Gordon M. The Gordon Diagnostic System. Gordon Systems, Inc.; New York: 1986. [Google Scholar]
- Hicks R.R. Numan S. Dhillon H.S. Prasad M.R. Seroogy K.B. Alterations in BDNF and NT-3 mRNAs in rat hippocampus after experimental brain trauma. Brain Res. Molec. Brain Res. 1997;48:401–406. doi: 10.1016/s0169-328x(97)00158-7. [DOI] [PubMed] [Google Scholar]
- Hicks R.R. Zhang L. Dhillon H.S. Prasad M.R. Seroogy K.B. Expression of trkB mRNA is altered in rat hippocampus after experimental brain trauma. Brain Res. Molec. Brain Res. 1998;59:264–268. doi: 10.1016/s0169-328x(98)00158-2. [DOI] [PubMed] [Google Scholar]
- Jordan B.D. Genetic influences on outcome following traumatic brain injury. Neurochemical Res. 2007;32:905–915. doi: 10.1007/s11064-006-9251-3. [DOI] [PubMed] [Google Scholar]
- Krueger F. Pardini M. Huey E.D. Raymont V. Solomon J. Lipsky R.H. Hodgkinson C.A. Goldman D. Grafman J. The role of the Met66 brain-derived neurotrophic factor allele in the recovery of executive functioning after combat-related traumatic brain injury. J. Neurosci. 2011;31:598–606. doi: 10.1523/JNEUROSCI.1399-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky R. Marini A. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann NY Acad. Sci. 2007;1122:130–143. doi: 10.1196/annals.1403.009. [DOI] [PubMed] [Google Scholar]
- Liu K. Muse S.V. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21:2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- Long J.C. Williams R.C. Urbanek M. An E-M algorithm and testing strategy for multiple-locus haplotypes. Am. J. Hum. Genet. 1995;56:799–810. [PMC free article] [PubMed] [Google Scholar]
- Lu B. Gottschalk W. Modulation of hippocampal synaptic transmission and plasticity by neurotrophins. Prog. Brain Res. 2000;126:231–241. doi: 10.1016/S0079-6123(00)28020-5. [DOI] [PubMed] [Google Scholar]
- McAllister T. Flashman L.A. Rhodes C.H. Tyler A.L. Moore J.H. Saykin A.J. McDonald B.C. Tosteson T.D. Tsongalis G.J. Single nucleotide polymorphisms in ANKK1 and the dopamine D2 receptor genes effect acute cognitive outcome after traumatic brain injury: A replication and extension study. Brain Inj. 2008;22:705–714. doi: 10.1080/02699050802263019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T. Mild brain injury and postconcussive symptoms. In: Silver J., editor; Yudofsky S., editor; McAllister T., editor. Textbook of Traumatic Brain Injury. American Psychiatric Press; New York: 2005. pp. 279–308. [Google Scholar]
- McAllister T. Polymorphisms in genes modulating the dopamine system: Do they influence outcome after traumatic brain injury? J. Head Trauma Rehabil. 2009;24:65–68. doi: 10.1097/HTR.0b013e3181996e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryshen T.L. Sabeti P.C. Aldinger K.A. Fry B. Fan J.B. Schaffner S.F. Waggoner S.G. Tahl A.R. Sklar P. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Molec. Psychiatry. 2010;15:810–815. doi: 10.1038/mp.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M.-M. Neurotrophins as synaptic modulators. Nature Rev. Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Pritchard J. Stephens M. Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C.L. Soane L. Siegel Z.T. Fiskum G. The potential role of mitochondria in pediatric traumatic brain injury. Developmental Neurosci. 2006;28:432–446. doi: 10.1159/000094169. [DOI] [PubMed] [Google Scholar]
- Roof R.L. Duvdevani R. Stein D.G. Gender influences outcome of brain injury—progesterone plays a protective role. Brain Res. 1993;607:333–336. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- Rutland-Brown W. Langlois J.A. Thomas K.E. Xi Y.L. Incidence of traumatic brain injury in the United States, 2003. J. Head Trauma Rehabil. 2006;21:544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- Saville D.J. Multiple comparison procedures: The practical solution. Am. Statistician. 1990;44:174–180. [Google Scholar]
- Schumacher M. Guennoun R. Stein D. De Nicola A. Progesterone: Therapeuric opportunities for neuroprotection and myelin repair. Pharmacol. Therapeurics. 2007;116:77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Tukey J.W. Comparing individual means in the analysis of variance. Biometrics. 1949;5:99–114. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised (WMS-R) Psychological Corporation; New York: 1987. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Third Edition WMS-III Administration and Scoring Manual. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Wilkinson G.S. The Wide Range Achievement Test (WRAT3): Administration Manual. Wide Range, Inc.; Wilmington, DE: 1993. [Google Scholar]
- Zaloshnja E. Miller T. Langlois J. Selassie A. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehabil. 2008;23:394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]
- Zaykin D.V. Westfall P.H. Young S.S. Karnoub M.A. Wagner M.J. Ehm M.G. Testing association of statistically inferred haplotypes with discrete and continuous traits in samples of unrelated individuals. Hum. Hered. 2002;53:79–91. doi: 10.1159/000057986. [DOI] [PubMed] [Google Scholar]