Abstract
Aims
To consider the cost implication of adopting epimacular brachytherapy (EMB) for the treatment of neovascular (wet) age-related macular degeneration (wAMD), compared with ranibizumab or bevacizumab monotherapy.
Methods
This analysis compared the cumulative 3-year costs of anti-VEGF (vascular endothelial growth factor) monotherapy to EMB combined with anti-VEGF therapy. Two patient groups were considered: newly diagnosed (treatment-naïve) patients; and patients already receiving chronic anti-VEGF therapy.
Results
In the treatment-naïve patients, the highest cumulative treatment costs were associated with ranibizumab monotherapy (£25 658), followed by bevacizumab monotherapy (£16 177), EMB with ranibizumab (£14 002), then EMB with bevacizumab (£10 289). In previously treated patients, the highest treatment costs were ranibizumab monotherapy (£18 355), followed by EMB with ranibizumab (£17 428), bevacizumab monotherapy (£16 177), then EMB with bevacizumab (£12 129).
Conclusion
EMB combined with anti-VEGF treatment has the potential to yield considerable cost savings, compared with anti-VEGF monotherapy. If the ongoing large studies of EMB confirm the published feasibility data, then adjuvant EMB may represent a cost-effective alternative to anti-VEGF monotherapy.
Keywords: epimacular brachytherapy, neovascular age-related macular degeneration, ranibizumab, bevacizumab, cost, health economics
Introduction
Neovascular (wet) age-related macular degeneration (wAMD) is the most common cause of blind registration in most developed nations.1 In the United Kingdom, patients are usually treated with intravitreal injections of ranibizumab (Lucentis, Novartis, Frimley, UK), a recombinant monoclonal antibody directed against vascular endothelial growth factor (VEGF). The initial Phase III trials of ranibizumab demonstrated impressive visual outcomes using a monthly dosing regimen,2, 3 but due to high drug costs, the UK's National Institute for Health and Clinical Excellence (NICE) recommended ‘as required' (p.r.n.) dosing.4 Most patients receive a loading dose of three consecutive monthly injections, followed by monthly review, and retreatment if there is evidence of disease activity. Despite this conservative treatment policy, the UK's National Health Service (NHS) spent £138 million on ranibizumab in 2010 alone.5
With an aging population, the cost of managing wAMD is set to increase. A recently published modelling exercise commissioned by the Royal National Institute of Blind People estimated that 414 561 people in the United Kingdom are affected by wAMD, which might increase to 515 509 by 2020.6 The authors predicted that 143 519 people would lose sight from wAMD, even if the United Kingdom could achieve 90% treatment coverage with ranibizumab. This figure will increase to 187 523 by 2020, because of an increased elderly population, which outweighs the beneficial effects of ranibizumab therapy.6
One means of reducing drug costs is to adopt bevacizumab instead of ranibizumab. Bevacizumab (Avastin, Roche, South San Francisco, CA, USA) is the parent molecule of ranibizumab, and is widely used off-licence to treat wAMD in the private sector and in other countries. The recently published CATT study compared ranibizumab with bevacizumab.7 At 1 year, bevacizumab and ranibizumab had equivalent effects on visual acuity, when administered according to the same dosing schedule. Bevacizumab is substantially cheaper than ranibizumab—in the CATT trial p.r.n. dosing resulted in a drug cost of US $23 400 for ranibizumab compared with US $595 for bevacizumab. Despite the lack of marketing authorisation for wAMD, or guidance from NICE, many healthcare commissioners are exerting pressure on UK clinicians to adopt bevacizumab as the treatment for wAMD.
Although both ranibizumab and bevacizumab produce visual outcomes far better than the natural history, both entail monthly hospital review and most patients require regular intravitreal injections. Even if drug costs are reduced, this remains an expensive and burdensome treatment regimen.
This paper considers the cost implications of adopting an alternative treatment modality, epimacular brachytherapy (EMB). EMB combines a standard vitreoretinal operation, pars plana vitrectomy, with intraocular delivery of radiation. The radiation is delivered using a CE-marked device containing a Strontium-90 source (VIDION, NeoVista, Newark, CA, USA). The device is held directly over the AMD lesion, delivering a single dose of 24 Gy in ∼3–4 min. Reports suggest that EMB reduces the number of anti-VEGF injections required to control wAMD, while maintaining or sometimes improving vision.8, 9, 10
This report aims to model the cumulative 3-year cost to the NHS of anti-VEGF monotherapy with either ranibizumab or bevacizumab, and then compare this to treatment using adjunctive EMB.
Materials and methods
Two patient groups were considered—newly diagnosed treatment-naïve patients, and those already on ranibizumab treatment. In both the groups, costs were estimated using treatment with ranibizumab or bevacizumab, with and without EMB.
Treatment frequency assumptions
The analysis was predicated on the assumptions shown in Table 1. Based on the UK subset of the MERITAGE study (clinicaltrials.gov: NCT00809419), it was assumed that previously treated patients had received 8 anti-VEGF injections at the start of the cost comparison.10
Table 1. Treatment frequency assumptions.
| AMD therapy |
Year 1 |
Years 2–3 |
||||
|---|---|---|---|---|---|---|
| Baseline | Low | High | Baseline | Low | High | |
| Anti-VEGF Injections | ||||||
| Number of injections per patient per year (ranibizumab)a | 7 | 6 | 8 | 7 | 6 | 8 |
| Number of injections per patient per year (bevacizumab)a | 8 | 7 | 9 | 8 | 7 | 9 |
| Number of follow-up visits per patient per yearb | 12 | 10 | 12 | 12 | 10 | 12 |
| EMB | ||||||
| Number of EMB treatments per patient per year | 1 | 1 | 1 | 0 | 0 | 0 |
| Number of anti-VEGF injections per patient per year (treatment naive)c | 2 | 1 | 3 | 1 | 0 | 2 |
| Number of anti-VEGF injections per patient per year (previously treated)d | 3.2 | 2 | 4 | 2 | 1 | 3 |
Abbreviations: EMB, epimacular brachytherapy; VEGF, vascular endothelial growth factor.
Based on the CATT study.7
Low value obtained from 2007 Royal College of Ophthalmologists Guide to Commissioning AMD services;14 high value obtained from NICE (National Institute for Health and Clinical Excellence) costing template and equivalent to baseline value.11
Based on data modified from Avila et al9 (see text).
Based on data from the MERITAGE study.10
Anti-VEGF monotherapy
Participants in the CATT trial received 6.9 p.r.n. ranibizumab injections per year compared with 7.7 for bevacizumab. Therefore, the present study assumed 7 p.r.n. ranibizumab injections and 8 p.r.n. bevacizumab injections, per year.
Epimacular brachytherapy
The EMB procedure combines a standard pars plana vitrectomy, and the use of a surgical device to deliver beta radiation to the macula. The procedure is performed only once.
In a study of treatment-naïve disease, participants received, on average, three anti-VEGF injections in the 3 years following EMB.9 This included two per-protocol injections around the time of EMB and one subsequent p.r.n. injection. Therefore, we estimated two injections in year 1 following EMB, and one further injection in each of years 2 and 3.
The MERITAGE study reported that the previously treated patients averaged 3.2 p.r.n. ranibizumab injections in the 12 months following EMB, with fewer injections over time.10 It was therefore assumed that patients receive 3.2 injections in year 1, and two further injections in each of years 2 and 3.
Cost assumptions
Based on the above assumptions, the costs associated with each treatment are shown in Table 2. These costs were estimated in June 2011. Inflation was not applied over the 3-year projection.
Table 2. Cost assumptions.
| AMD therapy |
Year 1 |
Years 2–3 |
||||
|---|---|---|---|---|---|---|
| Baseline | Low | High | Baseline | Low | High | |
| Anti-VEGF Injections | ||||||
| Attendance cost per injectiona | £500 | £400 | £600 | £500 | £400 | £600 |
| Attendance cost per follow-up visita | £65 | £52 | £78 | £65 | £52 | £78 |
| Cost of ranibizumab per injectionb | £913 | £913 | £913 | £913 | £913 | £913 |
| Cost of bevacizumab per injectionc | £75 | £55 | £107 | £75 | £55 | £107 |
| Allowance for possible future cataract procedurea,d | £13 | £10 | £16 | £12 | £9 | £14 |
| EMB | ||||||
| Cost of vitrectomy per patienta | £1124 | £900 | £1349 | £0 | £0 | £0 |
| Cost of radiotherapy physics support per patienta | £200 | £160 | £240 | £0 | £0 | £0 |
| Cost of RDM per patiente | £125 | £100 | £150 | £0 | £0 | £0 |
| Cost of DDM per patientf | £4200 | £4200 | £4200 | £0 | £0 | £0 |
| Allowance for possible future cataract procedurea,d | £309 | £247 | £371 | £26 | £21 | £31 |
Abbreviations: DDM, disposable delivery module; EMB, epimacular brachytherapy; RDM, reusable delivery module; VEGF, vascular endothelial growth factor.
Low/high values are ±20% compared with the baseline value.
Cited as book price for Lucentis plus VAT (value-added tax; 20%).
Low/high values are based on the lower and higher values (plus VAT) quoted for providing bevacizumab in a pre-filled 1.25 mg syringe, from three UK compounding pharmacies.
The allowance for cataract surgery is based on the cost of surgery and the percentage of phakic patients predicted to develop cataract over the 3-year projection.2, 3 The details of the assumptions are provided in the text.
Baseline value assumes that a single RDM unit (rental cost of £250 per month) is used for 24 patients over 1 year. Low/high values are ±20% compared with the baseline value.
Cost of DDM is £3500 (plus VAT) and is set by the manufacturer, and is assumed not to vary.
Anti-VEGF therapy
The administering of ranibizumab is outside the scope of the ‘Payment by Results' (PbR) national tariff system and therefore is subject to local negotiation. In its costing template accompanying the Technology Appraisal Guidance for ranibizumab in wAMD,11 NICE proposed a fee of 75% of the cost of a day-case vitreoretinal (VR) procedure and 25% of the cost of an outpatient VR procedure. Based on the 2011–2012 PbR Road Test Tariff Information for VR procedures (code BZ23Z),12 the cost of administering ranibizumab was estimated at £500 per injection. This concurs with our knowledge of current commissioning in 20 UK hospitals participating in the MERLOT study of EMB (clinicaltrials.gov: NCT01006538). Outpatient visits were estimated to cost £65 each.12 The price of ranibizumab is £913.44, inclusive of value added tax (VAT). It is important to note that the NICE Technology Appraisal Guidance requires that the manufacturer is responsible for the drug cost of ranibizumab in a given patient, beyond their 14th injection.4 The NHS reimbursement scheme applies only if a patient receives their first three ranibizumab injections at between 28 and 35 days apart. Conservatively, it was assumed that 100% of patients qualify.
Owing to a lack of published data on the cost of bevacizumab for the treatment of wAMD, we conducted an informal market survey of pharmacies currently dispensing pre-filled syringes of 1.25 mg bevacizumab and estimated the cost at £75 per dose (VAT inclusive). This includes the cost of compounding and dispensing.
Based on the MARINA study it was assumed that 52% of the eyes were pseudophakic, and 48% were phakic, and that 3.5% of phakic eyes require cataract surgery per year.3 The PbR Road Test Tariff Information lists phacoemulsification cataract surgery (HRG code BZ02Z) at £748.12 Cataract surgery was therefore costed at £13 per patient in year 1, and £12 in both years 2 and 3.
Epimacular brachytherapy
The EMB vitrectomy is of low complexity, involving no macular manipulation, and was costed at £1124 (2011/12 PbR Admitted Patient Care and Outpatient Procedure tariff for HRG code BZ22Z, vitreous retinal procedures—Category 2).12
There is no published data on the cost of the physics support required for EMB. We undertook an informal survey of the costs applied in MERLOT sites, and thereby estimated £200 per patient.
The VIDION EMB system consists of reusable and disposable components. The reusable delivery module (RDM) contains the radioactive material and lasts for 200 patients or 3 years. This is rented from the manufacturer at a cost of £250 per month. Based on a conservative estimate that a single RDM unit is used for 24 patients over 1 year, it was estimated that the per patient rental cost was £125. The disposable delivery modules (DDMs) cost £4200 each (£3500 +VAT), and treat one patient.
The cost of administering anti-VEGF treatment, and outpatient visits, were assumed to be the same as the anti-VEGF group.
Cataract is a well-known effect of vitrectomy. The MERITAGE study reported that 86% of phakic eyes required cataract surgery within 1 year of EMB.10 Based on a £748 tariff for cataract surgery,12 assuming 48% of eyes were phakic eyes3 and 86% of these required cataract surgery each year,10 the per patient allowance was £309 in year 1, and £26 per annum in years 2 and 3.
Results
Treatment-naïve patients
The costs of the four treatment options in treatment-naïve patients are provided in Table 3, with a summary of the total annual costs for years 1–3 provided in Table 4.
Table 3. Baseline annual cost components.
| Anti-VEGF therapy | Year 1 | Year 2 | Year 3 |
|---|---|---|---|
| Attendance cost per injection | £500 | £500 | £500 |
| Number of injections per patient (ranibizumab) | 7 | 7 | 7 |
| Number of injections per patient (bevacizumab) | 8 | 8 | 8 |
| (a) Total cost of administering injections per patient (ranibizumab) | £3500 | £3500 | £3500 |
| (a) Total cost of administering injections per patient (bevacizumab) | £4000 | £4000 | £4000 |
| Attendance cost per follow-up visit | £65 | £65 | £65 |
| Number of follow-up visits per patient | 12 | 12 | 12 |
| (b) Total cost of follow-up visits per patient | £780 | £780 | £780 |
| Drug cost of ranibizumab per injection | £913 | £913 | £913 |
| Treatment-naïve patients | |||
| (c)(i) Total drug cost of ranibizumab per patienta | £6391 | £6391 | £0 |
| Previously treated patients | |||
| (c)(i) Total drug cost of ranibizumab per patienta | £5478 | £0 | £0 |
| Drug cost of bevacizumab per injection | £75 | £75 | £75 |
| (c)(ii) Total drug cost of bevacizumab per patient | £600 | £600 | £600 |
| EMB | Year 1 | Year 2 | Year 3 |
|---|---|---|---|
| Cost of vitrectomy per patient | £1124 | £0 | £0 |
| Cost of radiotherapy physics support per patient | £200 | £0 | £0 |
| Cost of RDM per patient | £125 | £0 | £0 |
| Cost of DDM per patient | £4200 | £0 | £0 |
| (a) Total cost of EMB treatment per patient | £5649 | £0 | £0 |
| Treatment-naïve patients | |||
| Number of injections per patient | 2 | 1 | 1 |
| Previously treated patients | |||
| Number of injections per patient | 3.2 | 2 | 2 |
| Treatment-naïve patients | |||
| (b) Total cost of administering injections per patientb | £1000 | £500 | £500 |
| Previously treated patients | |||
| (b) Total cost of administering injections per patientb | £1600 | £1000 | £1000 |
| Treatment-naïve patients | |||
| (c)(i) Total drug cost of ranibizumab per patientc | £1826 | £913 | £913 |
| Previously treated patients | |||
| (c)(i) Total drug cost of ranibizumab per patientc | £2922 | £1826 | £1826 |
| Treatment-naïve patients | |||
| (c)(ii) Total drug cost of bevacizumab per patientc | £150 | £75 | £75 |
| Previously treated patients | |||
| (c)(ii) Total drug cost of bevacizumab per patientc | £240 | £150 | £150 |
Abbreviations: DDM, disposable delivery module; EMB, epimacular brachytherapy; RDM, reusable delivery module; VEGF, vascular endothelial growth factor.
NHS (National Health Service) policy requires that the drug cost of treatment beyond 14 injections of ranibizumab per patient is met by the manufacturer, thus resulting in zero drug cost in year 3 for both treatment-naïve and previously treated patients. The drug cost is zero in year 2 for previously treated patients as they enter the reimbursement scheme earlier, having started anti-VEGF therapy before the cost comparison.
Calculated as the number of injections per patient multiplied by the attendance cost per anti-VEGF injection.
Calculated as the number of injections per patient multiplied by the cost of ranibizumab/bevacizumab per injection.
Table 4. Summary of total annual costs.
| Year 1 | Year 2 | Year 3 | Total | |
|---|---|---|---|---|
| Treatment-naïve patients | ||||
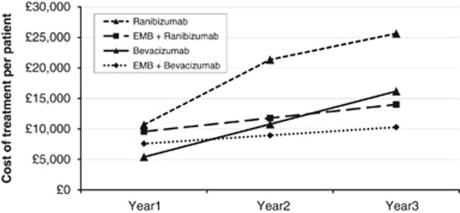
| Ranibizumab | £10 684 | £10 683 | £4292 | £25 659 |
| EMB + ranibizumab | £9564 | £2219 | £2219 | £14 002 |
| Bevacizumab | £5393 | £5392 | £5392 | £16 177 |
| EMB + bevacizumab | £7579 | £1355 | £1355 | £10 289 |
| Previously treated patients | ||||
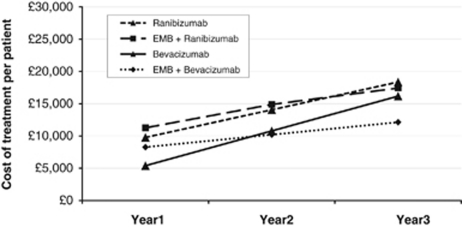
| Ranibizumab | £9771 | £4292 | £4292 | £18 355 |
| EMB + ranibizumab | £11 260 | £3632 | £2536 | £17 428 |
| Bevacizumab | £5393 | £5392 | £5392 | £16 177 |
| EMB + bevacizumab | £8269 | £1930 | £1930 | £12 129 |
Abbreviation: EMB, epimacular brachytherapy.
Treatment with EMB plus ranibizumab was consistently cheaper than ranibizumab monotherapy throughout years 1–3 (Figure 1). By the end of year 3, it was 45% cheaper. The cost of EMB plus bevacizumab was initially higher than bevacizumab monotherapy, but had equalised by the end of year 1 (Figure 1). By the end of year 3, it was 36% lower.
Figure 1.
Cumulative treatment costs over a 3-year projection, in treatment naïve patients.
The highest cumulative treatment costs were associated with ranibizumab monotherapy, followed by bevacizumab monotherapy, EMB plus ranibizumab, and then EMB plus bevacizumab.
Previously treated patient
The treatment costs for previously treated patients are provided in Table 3, with a summary of the total annual costs for years 1–3 provided in Table 4. The cost of ranibizumab monotherapy was initially less than EMB plus ranibizumab, but by the middle of year 2 this position reversed (Figure 2). At the end of 3 years, EMB plus ranibizumab cost 5% less than ranibizumab monotherapy. If the assumptions were unaltered this difference would increase over time.
Figure 2.
Cumulative treatment costs over 3 years, in patients who had previously been treated with ranibizumab.
The cost of bevacizumab monotherapy was initially less than EMB plus bevacizumab, but within the second year there was a strong divergence in favour of EMB (Figure 2). At the end of 3 years, EMB cost 25% less than bevacizumab monotherapy.
The highest cumulative treatment costs were associated with ranibizumab monotherapy, followed by EMB plus ranibizumab, bevacizumab monotherapy, and lastly EMB plus bevacizumab.
Conclusion
This analysis shows that combined EMB and anti-VEGF treatment has the potential to yield considerable cost savings, compared with anti-VEGF monotherapy. For example, compared with ranibizumab monotherapy, EMB reduced treatment costs by nearly £12 000 in the first 3 years of treatment—a 45% cost saving. Total treatment costs were reduced further, if EMB was combined with bevacizumab. In treatment-naïve patients, cost savings are yielded earlier (within year 1) when compared with previously treated patients. However, even in the latter group, cost savings are realised within a 2–3 year window.
If these figures are extrapolated to regional or national populations then there are substantial costs savings. For example, based on assumptions used by NICE,11 and a typical UK regional commissioning base of 200 000 people,13 there will be 78 newly diagnosed patients with wAMD each year. If 20% of these patients are treated with EMB and the remaining 80% are treated with anti-VEGF monotherapy, the potential savings would be approximately £180 000 over 3 years with ranibizumab, and £92 000 with bevacizumab, in treatment-naïve patients. If 50% of patients were treated with EMB, the savings would be approximately £455 000 with ranibizumab, and £230 000 with bevacizumab, over 3 years. If these assumptions are applied to the total estimated population of new cases each year in the United Kingdom, for 20% uptake of EMB, the savings would be approximately £61 million with ranibizumab and £31 million with bevacizumab, over 3 years. For 50% uptake the savings would be approximately £151 million with ranibizumab and £76 million with bevacizumab.
This paper has deliberately made some conservative assumptions to estimate cost, and it is possible that cost savings may be higher than those presented for several reasons. First, the model assumes monthly hospital review, yet studies in treatment-naïve patients show that a majority of patients are injection free out to year 3.9 If the results of these preliminary studies are validated in larger studies, then it appears that monthly hospital review would not be required for most patients, reducing not only the cost, but also the burden of care. Second, treatment-naive patients on anti-VEGF monotherapy were assumed to receive as many injections as those entering the CATT study.7 The CATT study commenced participants on a p.r.n. dosing regimen from the outset, whereas UK patients have three mandated injections at the start of treatment. This ranibizumab loading phase is likely to increase the number of injections in the first year. These three loading injections are not required with EMB. Third, the assumptions used for post EMB anti-VEGF retreatment were based on the MERITAGE study,10 yet the MERITAGE study enrolled patients with particularly active disease, and fewer injections may be required if EMB was used in a more typical patient population.
Anti-VEGF monotherapy is a treatment paradigm that is increasingly difficult to sustain in an NHS where resources are limited. NICE has estimated that 26 000 new cases of wAMD will be eligible for anti-VEGF treatment each year in the United Kingdom alone.11 As a result, wAMD clinic numbers are growing by as much as 30% a year, and costs will continue to escalate, even if the NHS adopts bevacizumab as the standard of care.
Interestingly, the cost saving to the NHS of changing from ranibizumab monotherapy to bevacizumab monotherapy was less than might have been expected in previously treated patients: over 3 years ranibizumab treatment cost £18 355 vs £16 177 with bevacizumab. As can be seen in Figure 2, the costs of the two treatments converge and if the assumptions remain unchanged, ranibizumab would become the less expensive option in future years.15 This was primarily due to the UK's ranibizumab reimbursement scheme, but it was also partly the result of assuming more bevacizumab injections per year than ranibizumab. The CATT study found that patients treated with bevacizumab required 7.7 p.r.n. injections per year, significantly more than those treated with ranibizumab, who received an average of 6.9 per year (P=0.003).7 Therefore, in previously treated patients, it is not certain that a switch to bevacizumab would produce substantial cost savings. By contrast, in treatment-naïve patients a switch to bevacizumab produces a 37% cost saving over the 3-year projection.
One weakness of this study is that it relied on clinical trial data, and that it is not certain that the treatment patterns observed within a clinical trial would be replicated in a non-trial setting. The NVI-111 study8 of EMB in treatment-naive patients and the MERITAGE study10 of previously treated patients enrolled only 34 and 53 patients, respectively. Larger trials are needed to validate these early results. Although the CATT study7 of anti-VEGF monotherapy was large, it was undertaken outside the United Kingdom, and to date only 12-month data are available. Other than cataract surgery, surgical complications that might occur following vitrectomy were not costed. Any surgical complications requiring treatment, such as retinal detachment, would tend to reduce the cost-effectiveness of EMB. Complications would also factor into the risk-benefit analysis. It is not possible to determine the incidence of surgical complications without larger studies. Cost modelling necessarily involves a number of assumptions—in general these assumptions were conservative in nature, but the resulting predictions can only be fully validated retrospectively.
In summary, cost modelling of EMB suggests that it has the potential to reduce treatment costs in a UK setting, in combination with either ranibizumab or bevacizumab. If the ongoing large studies of EMB confirm the published feasibility data, then adjuvant EMB may represent a cost-effective alternative to anti-VEGF monotherapy.
T Jackson has received research funding from NeoVista, Novartis, Oraya and Thrombogenics. He is on the advisory board of Bausch and Lomb, DORC and Oraya, and has been a consultant to Merck and NicOx. He has received conference support from DORC and NeoVista. S Prasad is a consultant for Bausch and Lomb, and Nidek. He has also received a conference support from Alcon, Allergan, and Novartis. L Kirkpatrick and Quorum Consulting received funding from NeoVista.
References
- Bunce C, Wormald R. Leading causes of certification for blindness and partial sight in England & Wales. BMC Public Health. 2006;6:58. doi: 10.1186/1471-2458-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- NICE Technology Appraisal Guidance 155: ranibizumab and pegaptanib for the treatment of age-related macular degeneration, August 2008.
- IMS data ( ( http://www.imshealth.com ).
- Minassian DC, Reidy A, Lightstone A, Desai P. Modelling the prevalence of age-related macular degeneration (2010–2020) in the UK: expected impact of anti-vascular endothelial growth factor (VEGF) therapy. Brit J Ophth. 2011;95 (10:1433–1436. doi: 10.1136/bjo.2010.195370. [DOI] [PubMed] [Google Scholar]
- The CATT Research Group Ranibizumab and bevacizumab for neovascular age-related macular degeneration. New Engl J Med. 2011;364 (20:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávila M, Farah ME, Santos A, Duprat JP, Woodward BW, Nau J. Twelve-month short-term safety and visual-acuity results from a multicentre prospective study of epiretinal strontium-90 brachytherapy with bevacizumab for the treatment of subfoveal choroidal neovascularisation secondary to age-related macular degeneration. Brit J Ophth. 2009;93:305–309. doi: 10.1136/bjo.2008.145912. [DOI] [PubMed] [Google Scholar]
- Ávila M, Farah ME, Santos A, Carla L, Fuji G, Rossi J, et al. Three year safety and visual acuity results of epiretinal strontium-90 brachytherapy with bevacizumab for the treatment of subfoveal choroidal neovascularization secondary to age-related macular degeneration. Retina. 2012;32:10–18. doi: 10.1097/IAE.0b013e31822528fc. [DOI] [PubMed] [Google Scholar]
- Dugel P, Petrarca R, Bennett M, Barak A, Weinberger D, Nau J, et al. Macular EpiRetinal brachytherapy in Treated AGE-related macular degeneration (MERITAGE): 12 month safety and efficacy results Ophthalmology(in press). [DOI] [PubMed]
- NICE Technology Appraisal Guidance 155: ranibizumab and pegaptanib for age-remacular degeneration. Costing template and report - implementing NICE guidance, August 2008.
- 2011-12 PbR Tariff Information ( ( http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/ PublicationsPolicyAndGuidance/DH_122717 ).
- MHP . The Path To GP Commissioning. MHP: London; 2011. [Google Scholar]
- The Royal College of Ophthalmologists Commissioning contemporary AMD services: a guide for commissioners and cliniciansJuly 2007.
- Jackson TL, Kirkpatrick L. Cost comparison of ranibizumab and bevacizumab. BMJ. 2011;23:343:d5058. doi: 10.1136/bmj.d5058. [DOI] [PubMed] [Google Scholar]