Abstract
Aim
To investigate the clinical significance of Grave's ophthalmopathy-specific quality of life (GO-QOL) in Korean patients.
Methods
A cross-sectional study was conducted at the Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea, on 98 consecutive Grave's ophthalmopathy (GO) patients. The GO-QOL survey provided by Terwee and colleagues and suggested by the European group on Graves' orbitopathy (EUGOGO) was translated into Korean language and distributed to study participants. Clinical severity was judged by scores of the modified NOSPECS classification, and inflammatory activity was measured by a seven-point scale of clinical activity score (CAS).
Results
The mean GO-QOL scores were 73.7 (standard deviation (SD), 26) for visual functioning, 61.9 (SD 26) for appearance, and 67.8 for total quality of life (QOL; SD 22). The worse QOL scores for each part were significantly associated with the higher modified NOSPECS score and CAS after adjusting for confounders such as age and sex (P<0.05, respectively). In particular, decreased QOL scores for visual function were significantly correlated with a higher grade of extraocular muscle involvement (P<0.05). Lower QOL scores for appearance were associated with more severe soft-tissue involvement and proptosis (P<0.05, respectively).
Conclusions
GO-QOL suggested by EUGOGO showed correlation with objective clinical parameters. GO-QOL can be a simple and effective tool in the evaluation of the clinical and psychological illness of GO patients.
Keywords: Graves' ophthalmopathy, quality of life, questionnaire
Introduction
Grave's ophthalmopathy (GO) is an autoimmune-associated inflammatory disorder of the orbit causing disfiguring proptosis, pain, redness, and swelling of the eyelids, as well as grittiness of the eyes, diplopia, and sometimes even blindness.1, 2 GO may be severely disabling because of its effect on vision and appearance. About 25–50% of patients with Grave's thyroid disease suffer from GO.3 The management of GO has been well studied previously, but the impaired quality of life (QOL) caused by GO has been the focus of recent attention.4
QOL of patients with GO was investigated using a health questionnaire (Medical Outcome Study Short-Form General Health Survey and Sickness Impact Profile) by Gerding et al.4 The study showed little correlation between measures of activity or severity of disease and the scores on the QOL questionnaire. The authors reported that patients with mild or moderately severe GO had similar or worse QOL impairment than patients suffering from heart failure, pulmonary emphysema, or diabetes mellitus. General questionnaires are, however, not sensitive to some of the unique problems experienced by patients with GO.4
Bradley et al5 investigated the QOL of GO patients with 25 items of the National Eye Institute Visual Function Questionnaire (NEI VFQ-25). NEI VFQ-25 includes many items that are relevant to GO patients; however, it lacks items on other issues that are important to evaluate the QOL of GO patients, such as altered appearance and ocular discomfort.5
Terwee et al6 developed a Dutch language GO QOL instrument and proved it to be a reliable instrument in Dutch GO patients. They used the QOL questionnaire to evaluate the change in QOL score associated with clinical improvement after different treatment modalities.7 Park et al8 used the modified and translated GO-specific QOL survey in Australians. This was the first time that the questionnaire was applied to an English-speaking population, and the results showed significant correlation between impaired QOL score and disease severity.
In this study, we translated the GO-specific QOL survey into Korean and used the questionnaire in Korean GO patients. The purpose of this study was to assess GO-specific QOL in Korean patients and to determine the correlation of GO-specific QOL scores with disease severity and activity.
Materials and methods
Study design and recruitment of participants
After approval from the Institutional Review Board at Yonsei University College of Medicine, informed consent was obtained from all participants. This cross-sectional study included 98 patients with GO who were followed at the Department of Ophthalmology, Yonsei University College of Medicine between January and December 2010. The inclusion criteria were the presence of typical eye symptoms and signs in a patient with autoimmune Graves' disease. The patients who have ophthalmic diseases such as cataract, age-related macular degeneration, glaucoma, corneal opacity and so on that could affect the QOL were excluded. Clinical data collected include complete ophthalmic investigations, autoimmune Graves' disease progress and medication history, ophthalmological data of visual acuity, intraocular pressure, diplopia tests such as binocular single vision test or Hess screen, and proptosis measurement by Hertel exophthalmometer. All clinical observations and objective measurements were consistently performed by one ophthalmologist (JS Yoon).
Outcome variable
Grave's Ophthalmopathy specific Quality of Life survey (GO-QOL survey) provided by Terwee et al7 from the Netherlands was translated into Korean language. It has 16 questions divided into two sections; visual functioning and appearance. The GO-QOL survey was converted into QOL scores in which 0 corresponds to worst and 100 to no disturbance in visual function and appearance. The same method was used as previously described by Terwee et al.7 We gave the translated GO QOL survey and informed consent to almost all patients who visited our oculoplastic clinic. Patients self-administered the questionnaire during outpatient clinic consultations.
Independent variable
Clinical activity was evaluated by one ophthalmologist (JS Yoon) and was described using a Clinical Activity Score (CAS) based on seven signs of inflammation of the orbit.9 The grading scale was from 0 to 7. GO patients were divided into three overall groups according to CAS grades: 0–1, 2–3, and 4–7 for statistical analysis. Patients with CAS 4 or more were considered as having clinically active inflammation. The severity of GO was described by the modified NOSPECS score obtained by adding NOSPECS grades for each of soft-tissue involvement, proptosis, extraocular muscle (EOM) involvement, lid retraction, site difference, corneal defect, and optic nerve compression. We quoted the modified NOSPECS classification that was used in the publication of Echstein et al10 as a basic concept, and added the grade 1 of EOM involvement if there was a limitation of motion at extreme gaze to make the grade of EOM involvement in numerical order.
The range of modified NOSPECS scores was from 0 (normal) to 17 (most severe). GO was divided into three grades based on the modified NOSPECS score: 0–3, 4–7, and 7–17, which correspond to mild, moderate, and severe disease categories, respectively. To evaluate internal consistency and reliability, the Cronbach's alphas of two sections were calculated.
Statistical analysis
Descriptive statistics of the study population were reported. Associations between QOL score and the modified NOSPECS score or CAS were analysed by regression analysis using Pearson's correlation and multiple regression analysis. Adjusted means of QOL score of visual functioning and appearance were calculated to assess the relationship of the modified NOSPECS score and CAS. To determine which component (soft-tissue involvement, proptosis, EOM involvement, lid retraction, site difference, corneal defect, or optic nerve compression) of the modified NOSPECS score was the strongest predictor of QOL score, adjusted means of QOL score were calculated and adjusted for the other variables mentioned above. Statistical calculation was done using the software program SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). In all analyses, we determined two-sided P-values and considered a P-value less than 0.05 to be significant.
Results
GO-QOL questionnaires were distributed to all 98 patients. The surveys of 12 patients contained one unanswered question, while three patients had two or more unanswered questions.
The clinical characteristics and demographic details of the patient population are shown in Table 1. The 98 patients included 79 women and 19 men with a mean age of 40.7 years. Eighty patients took anti-thyroid medications. Seven patients received radioiodine treatment and five patients had thyroidectomy. The mean CAS was 2.1, and the mean modified NOSPECS score was 4.4. The number of active GO patients, assuming active GO to be in participants with CAS at least 4, was 19 (19.4%).
Table 1. Characteristics of patients with Graves' ophthalmopathy at the time of the survey.
| GO patients | N=98 |
|---|---|
| Age (years) | 40.7 (15–72) |
| Gender (male/female) | 19/79 |
| Smokers | 17 (17.7%) |
| DM | 5 (5.1%) |
| HTN | 9 (9.2%) |
| Other autoimmune disease | 5 (5.1%) |
| Family history of thyroid disease | 18 (18.4%) |
| Treatment of thyroid dysfunction | |
| Anti-thyroid medication | 80/98 (81.6%) |
| Radioiodine therapy | 7/98 (7.1%) |
| Thyroidectomy | 5/98 (5.1%) |
| Clinical feature of GO patients | |
| Clinical activity score (CAS) | 2.1±1.6 (0–7)a |
| The modified NOSPECS score | 4.4±2.7 (0–17)a |
| Active GO (CAS≥4) | 19 (19.4%) |
| Optic nerve involvement | 7 (7.1%) |
Abbreviations: DM, diabetes mellitus; HTN, hypertension.
Mean±SD (range).
The frequencies of responses to each of the questions are shown in Table 2. In all, 71.4% of the patients felt that GO interfered with their visual function in some way and 95.9% patients felt their altered appearance interfered with psychosocial functioning. The most frequently limited activities were reading (59.2%), doing something they wanted to do (53.1%), watching TV (49.0%), and doing hobbies or pastimes (42.9%). The majority of participants perceived altered appearance (91.8%) and impaired self-confidence (70.4%).
Table 2. Frequencies of responses from questions on visual functioning and appearance.
| Visual functioning Limitation in carrying out the following activity | Severely limited (%) | Little limited (%) | Not limited (%) | Missing response (%) |
|---|---|---|---|---|
| Q1. Riding a bicyclea | 5.1 | 5.1 | 52.0 | 5.1 |
| Q2. Drivingb | 6.1 | 24.5 | 31.6 | 5.1 |
| Q3. Moving around the house | 0 | 16.3 | 81.6 | 2.0 |
| Q4. Walking outdoors | 6.1 | 23.5 | 68.4 | 2.0 |
| Q5. Reading | 16.3 | 42.9 | 39.8 | 1.0 |
| Q6. Watching TV | 9.2 | 38.8 | 51.0 | 1.0 |
| Q7. Hobbies or pastimes | 9.2 | 30.6 | 57.1 | 3.1 |
| Q8. Hindered from doing something they wanted to do | 19.4 | 33.7 | 43.9 | 3.1 |
|
Appearance |
Very much (%) |
A little (%) |
No (%) |
Missing response (%) |
| Q9. Changed appearance | 56.1 | 35.7 | 8.2 | 0.0 |
| Q10. Stared in the streets | 15.3 | 42.9 | 41.8 | 0.0 |
| Q11. People react unpleasantly | 2.0 | 25.5 | 72.5 | 0.0 |
| Q12. Influence on self confidence | 28.6 | 41.8 | 29.6 | 0.0 |
| Q13. Socially isolated | 4.0 | 22.4 | 73.6 | 0.0 |
| Q14. Influence on making friends | 9.2 | 26.5 | 64.3 | 0.0 |
| Q15. Appear less often on photos than before | 38.8 | 31.6 | 29.6 | 0.0 |
| Q16. Mask changes in your appearance | 23.5 | 31.6 | 44.9 | 0.0 |
Cannot ride a bicycle: 32.7%.
No drivers' licence: 32.7%.
Cronbach's alpha: 0.90 for visual functioning/0.88 for appearance.
Cronbach's alphas were 0.90 for visual functioning and 0.88 for appearance. The consistency and reliability of the questionnaire were both well preserved, compared with Cronbach's alphas of the Dutch GO-QOL survey made by Terwee et al.6
The mean QOL score of visual function-associated questionnaire was 73.7±26 (mean±SD). The mean QOL score of appearance-related questionnaire was 61.9±26. The mean QOL score of total questionnaire was 67.8±22.
The modified NOSPECS score and CAS on QOL score
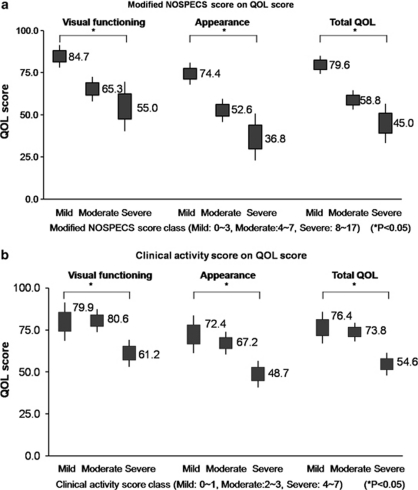
Regression analysis showed that the QOL scores of visual functioning were significantly negatively correlated with age (Pearson coefficient=−0.254, P=0.012), the modified NOSPECS score (Pearson coefficient=−0.410, P<0.001), and CAS (Pearson coefficient=−0.266, P=0.008) (Figure 1). The QOL scores of appearance and total QOL scores were also negatively correlated with the modified NOSPECS score (Pearson coefficient=−0.347, P=0.001) and CAS (Pearson coefficient=0.382, P<0.01) in Pearson Correlation analyses (Table 3).
Figure 1.
The effect of (a) modified NOSPECS scores and (b) clinical activity score on QOL scores of visual functioning and appearance in multivariate linear regression analysis adjusted for age and gender. Vertical line ranges from maximum to minimum value. Bold square presents one standard error range. The number represents the mean of adjusted QOL scores for each section. CAS, clinical activity score; QOL, quality of life.
Table 3. Correlation analysis between QOL scores and clinical parameters of disease severity and activity.
| Age | Soft-tissue sign | Proptosis | EOM involvement | Lid retraction | Corneal defects | Site difference | Optic nerve compression | Modified NOSPECS Score | CAS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Visual function score | ||||||||||
| Pearson coefficient | −0.254 | −0.211 | −0.223 | −0.404 | −0.107 | −0.103 | −0.109 | −0.185 | −0.410 | −0.266 |
| P-value | 0.012a | 0.037a | 0.028a | 0.000b | 0.293 | 0.315 | 0.287 | 0.068 | 0.000b | 0.008a |
| Appearance score | ||||||||||
| Pearson coefficient | −0.019 | −0.304 | −0.261 | −0.168 | −0.000 | −0.124 | −0.152 | −0.147 | −0.347 | −0.390 |
| P-value | 0.849 | 0.002a | 0.010a | 0.098 | 0.998 | 0.224 | 0.136 | 0.148 | 0.001b | 0.000b |
| Total QOL score | ||||||||||
| Pearson coefficient | −0.160 | −0.300 | −0.282 | −0.334 | −0.058 | −0.142 | −0.161 | −0.196 | −0.450 | −0.382 |
| P-value | 0.116 | 0.003a | 0.005a | 0.001a | 0.570 | 0.163 | 0.114 | 0.053 | 0.000b | 0.000b |
Abbreviation: CAS, clinical activity score.
P<0.05.
P<0.001.
Multivariate linear regression analysis with adjustment for age and gender showed that patients belonging to the more severe disease category based on the modified NOSPECS score showed significantly less mean QOL scores of visual function (P<0.05) and appearance (P<0.05) than those in the mild disease category (Figure 1a). Similar trends were observed when the effect of CAS on QOL score was analysed. Patients belonging to the more active disease category based on CAS showed significantly less mean QOL scores of visual function (P<0.05) and appearance (P<0.05) than those with mild disease (Figure 1b).
Soft-tissue involvement, proptosis and EOM involvement in the modified NOSPECS score on QOL score
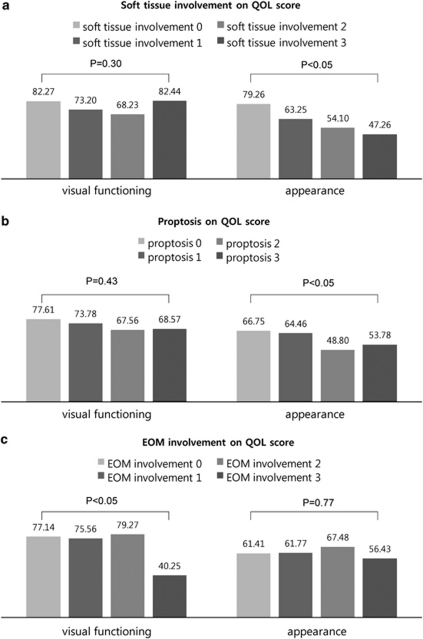
The associations between each component of the modified NOSPECS classification such as soft-tissue involvement, proptosis, or EOM involvement and the QOL scores are shown in Figure 2. Multivariate linear regression analysis with adjustment for age, gender, and each component of the modified NOSPECS score showed that worse soft-tissue involvement and proptosis was significantly associated with decreased QOL scores of appearance (P=0.011 and 0.034 respectively) (Figures 2a and b), whereas the degree of EOM involvement was correlated with decreased QOL scores of visual functioning (P=0.001, Figure 2c). Other components of the modified NOSPECS classification such as lid retraction, site difference, corneal defect, and optic nerve compression showed no significant association with each QOL score.
Figure 2.
The effect of (a) soft-tissue involvement, (b) proptosis, and (c) extraocular muscle involvement by modified NOSPECS score on QOL scores of visual functioning and appearance in multivariate linear regression analysis adjusted for age, gender, and each component of the modified NOSPECS score. EOM, extraocular muscle; QOL, quality of life.
Discussion
GO influences patients' activities of daily lives such as reading, watching television, and pastimes, as well as dysfunction of social role and impaired self-image because of the altered appearance. Previous studies reported a low correlation between generic QOL survey and the severity of disease.4, 5, 11 Our group published on the QOL of GO patients measured with the Korean version of the 36-item Short-Form General Health Survey (SF-36) questionnaire.11 According to that study, the QOL of Korean patients with threatened vision or significant diplopia was significantly low. However, overall scores of QOL on the SF-36 were not highly correlated with the severity of disease, probably because the generic QOL questionnaires such as Medical Outcome Study, SF-36, and NEI VFQ were not generated specifically for GO. The GO-specific QOL survey provided by Terwee et al,7 characterized well the GO-specific conditions that impair the QOL. In this study, we found that GO-QOL scores of Korean patients were significantly correlated with disease severity and inflammatory activity measured by the modified NOSPECS score and CAS, respectively. In addition, as the Cronbach alphas of the Korean language survey used in this study were comparable with those of the Dutch GO-QOL survey (visual functioning, 0.86 in reference vs 0.90 in this study; and appearance, 0.82 in reference vs 0.88 in this study),6 it is assumed that the validity and internal consistency of the Korean language questionnaire were also well preserved.
The mean QOL scores measured in this study were much higher than those measured in the Dutch and Australian GO-QOL survey (visual functioning, 54.7 in Dutch survey, 59.0 in Australian survey and 73.7 in this study; appearance, 60.1 in Dutch survey, 54.5 in Australian survey and 61.9 in this study). The difference of QOL scores between these studies might be associated with the different characteristics of the enrolled patients. The mean CAS was 2.6 in the Dutch survey and 2.1 in this study, meaning that the Korean GO patients who were enrolled in this study had a milder form of GO than the patients enrolled in the Dutch survey. The GO severity of each survey group could not be compared because the three surveys each had used different methods for calculating the GO severity. Another possible cause of the different QOL score might be the ethnic difference. It is well known that the prevalence and clinical features of GO have racial difference.12, 13 Tsai et al14 reported that exophthalmos values of patients with Chinese Graves' disease in Taiwan tend to be lower than those of Caucasians and black-American people. The ethnic characteristics of GO, which appears to be milder in Asians than Caucasians, could attribute to the difference between the QOL scores.
The mean QOL score of visual functioning was correlated with age. Older participants gave low scores on visual functioning than younger participants (Pearson coefficient=−0.254, P=0.012). This tendency was also observed in the Australian GO-QOL survey. Park et al8 suggested that older participants are more concerned about limitations in vision-related tasks and less concerned about changes in appearance. However, there is also a possibility that actual visual difficulties resulting from the aging process could have a negative influence on the QOL scores of visual functioning. To confirm the association of the modified NOSPECS score and CAS with QOL scores, effects of age and gender were controlled for using multivariate linear regression analysis. As shown in Figure 1, CAS and the modified NOSPECS score still had statistical significant associations with QOL scores.
The mean QOL scores showed a significant correlation with CAS, which is well known and currently used as a clinical parameter of disease activity. When patients are in the active phase of GO, the inflammatory symptoms and signs such as swelling of preorbital area, injection of eye ball and retro orbital pain associated with eye movement can have negative influence on cosmesis and also induce decreased visual function, both of which make patients nervous and frustrated, deteriorating psychosocial well-being.
Interestingly, visual functional scores were strongly correlated with EOM involvement scores, whereas appearance scores were significantly correlated with soft-tissue involvement and proptosis scores. To our knowledge, this is the first report of the association between the specific parameters of severity and GO-QOL scores. As the patients exhibit a heterogeneous clinical course, GO is very difficult to manage. Unfortunately the tools to evaluate patients' status in the outpatient clinic have been limited thus far. The GO-QOL would be helpful for patient follow-up and useful in making decisions about management in clinically heterogeneous GO. Either visual functioning questionnaires or appearance questionnaires can be, respectively, applied to corresponding patients with muscle-predominant disease or cosmetically disfigured patients with soft-tissue involvement or proptosis in the outpatient clinic.
Our study has some limitations. First, this was a cross-sectional study, which makes it hard to clarify the causal relationship. Second, our results were derived from a relatively specific group of patients seen at a single academic institution, and the condition of patients in our clinic could be different from that of patients in the community setting. Third, the fact that GO severity and activity were observed by one clinician could be good for reliability; however, this could create systemic bias.
Nowadays, GO is understood not only as a vision-threatening disease but also a psychosocially debilitating disease. Such limitations to daily living and impaired self-confidence caused by GO could be improved after appropriate treatment. However, it is difficult to determine how we can evaluate these illnesses and when to start treatment. The GO-specific QOL survey is a simple and easily applicable item that can be used in the clinic, and could be a valuable clinical parameter that helps in the evaluation of GO activity and severity, allowing treatment planning and measurement of the efficacy of treatment.
The authors declare no conflict of interest.
References
- Burch HB, Wartofsky L. Graves' ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993;14 (6:747–793. doi: 10.1210/edrv-14-6-747. [DOI] [PubMed] [Google Scholar]
- Weetman AP. Thyroid-associated eye disease: pathophysiology. Lancet. 1991;338 (8758:25–28. doi: 10.1016/0140-6736(91)90013-f. [DOI] [PubMed] [Google Scholar]
- Bartalena L, Pinchera A, Marcocci C. Management of Graves' ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21 (2:168–199. doi: 10.1210/edrv.21.2.0393. [DOI] [PubMed] [Google Scholar]
- Gerding MN, Terwee CB, Dekker FW, Koornneef L, Prummel MF, Wiersinga WM. Quality of life in patients with Graves' ophthalmopathy is markedly decreased: measurement by the medical outcomes study instrument. Thyroid. 1997;7 (6:885–889. doi: 10.1089/thy.1997.7.885. [DOI] [PubMed] [Google Scholar]
- Bradley EA, Sloan JA, Novotny PJ, Garrity JA, Woog JJ, West SK. Evaluation of the National Eye Institute visual function questionnaire in Graves' ophthalmopathy. Ophthalmology. 2006;113 (8:1450–1454. doi: 10.1016/j.ophtha.2006.02.060. [DOI] [PubMed] [Google Scholar]
- Terwee CB, Gerding MN, Dekker FW, Prummel MF, Wiersinga WM. Development of a disease specific quality of life questionnaire for patients with Graves' ophthalmopathy: the GO-QOL. Br J Ophthalmol. 1998;82 (7:773–779. doi: 10.1136/bjo.82.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwee CB, Dekker FW, Mourits MP, Gerding MN, Baldeschi L, Kalmann R, et al. Interpretation and validity of changes in scores on the Graves' ophthalmopathy quality of life questionnaire (GO-QOL) after different treatments. Clin Endocrinol (Oxf) 2001;54 (3:391–398. doi: 10.1046/j.1365-2265.2001.01241.x. [DOI] [PubMed] [Google Scholar]
- Park JJ, Sullivan TJ, Mortimer RH, Wagenaar M, Perry-Keene DA. Assessing quality of life in Australian patients with Graves' ophthalmopathy. Br J Ophthalmol. 2004;88 (1:75–78. doi: 10.1136/bjo.88.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the European group on Graves' orbitopathy (EUGOGO) on management of Graves' orbitopathy. Thyroid. 2008;18 (3:333–346. doi: 10.1089/thy.2007.0315. [DOI] [PubMed] [Google Scholar]
- Eckstein AK, Plicht M, Lax H, Neuhauser M, Mann K, Lederbogen S, et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves' ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91 (9:3464–3470. doi: 10.1210/jc.2005-2813. [DOI] [PubMed] [Google Scholar]
- Lee H, Roh HS, Yoon JS, Lee SY. Assessment of quality of life and depression in Korean patients with Graves' ophthalmopathy. Korean J Ophthalmol. 2010;24 (2:65–72. doi: 10.3341/kjo.2010.24.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez M, Cooper J, Edmonds C. Graves' ophthalmopathy in relation to cigarette smoking and ethnic origin. Clin Endocrinol (Oxf) 1992;36 (3:291–294. doi: 10.1111/j.1365-2265.1992.tb01445.x. [DOI] [PubMed] [Google Scholar]
- K-Jafari A, Sadeghi-Tari A, Minaee-Noshahr N, Ameri A, Anvari F, Ali-Mahmoudi A, et al. Ocular movement disorders and extraocular muscle involvement in Iranian Graves' ophthalmopathy patients. Binocul Vis Strabismus Q. 2010;25 (4:217–230. [PubMed] [Google Scholar]
- Tsai CC, Kau HC, Kao SC, Hsu WM. Exophthalmos of patients with Graves' disease in Chinese of Taiwan. Eye (Lond) 2006;20 (5:569–573. doi: 10.1038/sj.eye.6701925. [DOI] [PubMed] [Google Scholar]