Abstract
Purpose
The purpose of this study is to compare the retrobulbar hemodynamic parameters in the ophthalmic artery (OA), central retinal artery (CRA), and posterior cilliary arteries (PCA), in open-angle glaucoma (OAG) and angle-closure glaucoma (ACG) patients.
Patients and methods
A total of 52 eyes from 52 patients with OAG and 25 eyes from 25 ACG patients who met the inclusion/exclusion criteria were included in this cross-sectional study. Peak-systolic velocity, end-diastolic velocity, and Pourcelot resistivity index (RI) were assessed in the OA, CRA, and PCA. Intraocular pressure (IOP) was measured both with the Goldmann applanation tonometer (GAT) and with the Dynamic Contour tonometer (DCT) three times, respectively. Ocular pulse amplitude was measured using DCT.
Results
The RI was significantly higher in both the ophthalmic and short PCA in the OAG patients as compared with that in those ACG patients, P=0.003 and 0.048, respectively. There was no correlation between the IOP measured with GAT and the retrobulbar hemodynamic parameters in either OAG or ACG.
Conclusions
There was an increased resistance to blood flow in the OA of OAG as compared with ACG patients. Additionally, the degree of circulatory disturbance was not related to either the IOP or the visual-field damage.
Keywords: open-angle glaucoma, angle-closure glaucoma, color Doppler imaging, ocular blood flow
Introduction
Although elevated intraocular pressure (IOP) is one of the major risk factors not only for the onset of glaucoma,1, 2 but also for the progression of the glaucomatous damage,3, 4 the term glaucoma covers a wide range of multifactorial optic neuropathies.
The evidence for vascular risk factors in glaucoma has continued to build over the past several years as evidence from dozens of prospective studies across the world that ocular blood flow (OBF) changes are involved both in the pathogenesis of glaucoma5, 6, 7, 8, 9, 10 and in progression of glaucomatous damage.11, 12, 13
However, the question of whether OBF disturbances are an independent primary factor remains.
The most widely utilized imaging technology for investigating OBF in humans is colour Doppler imaging (CDI).14 CDI combines B-scan grey scale imaging of tissue structure, colour representation of blow flow based on Doppler shift, and pulsed Doppler measurement of blood-flow velocities. This method is used in ophthalmology to measure blood-flow velocities in the retrobulbar vessels.7, 14, 15, 16
Angle-closure glaucoma (ACG) is relatively rare in European countries, but is very common in other parts of the world. Population-based prevalence studies among Asians and Eskimos showed that ACG is the most common type of glaucoma.17
Few studies have investigated the ocular circulatory changes in ACG patients.
Cheng et al18 reported that patients with chronic ACG may have decreased retrobulbar blood-flow velocities and increased vascular resistance in the central retinal artery (CRA) and temporal posterior cilliary arteries (PCA).
The purpose of this study is to compare the retrobulbar hemodynamic parameters in the ophthalmic artery (OA), CRA, and PCA, in primary open-angle glaucoma (POAG) and primary ACG (PACG) patients.
Materials and methods
A total of 52 OAG and 25 ACG patients were included in this prospective and cross-sectional study.
All participants signed an informed, written consent form approved by the ethics committee of the Clinical Centre of Serbia in Belgrade, which was in agreement with the provisions of the Declaration of Helsinki for biomedical research.
Institutional review board approval was obtained and the study adhered to the tenets of the Declaration of Helsinki. Only one eye that fulfilled all the inclusion criteria and none of the exclusion criteria was designed as the study eye in each patient; in patients in whom both eyes fulfilled all inclusion criteria and none of the exclusion criteria, one randomly chosen eye was included for the purpose of statistical analysis.
All participants were required to meet the following inclusion criteria: age equal or higher than 25 years, clinical diagnosis of OAG or ACG in early stage, IOP higher than 25 mm Hg, willingness to comply with the investigators and protocol indications.
Patients were excluded by the following criteria: previous treatment with ocular filtering surgery, history of previous refractive surgery, diabetes, history of progressive retinal or optic nerve disease of any cause, and pregnancy or lactation.
For glaucoma diagnosis, visual-field (VF) examinations were performed using the 24-2 Swedish Interactive Thresholding Algorithm Standard strategy (SITA standard) on the Humphrey VF analyser (Carl Zeiss Meditec, Dublin, CA, USA). Glaucomatous VF defects were defined as follows: a minimum of one location in the paracentral or nasal step regions corresponding to sectors 1 or 2, or to the inferior 3 location in sector 3 of the Glaucoma Hemifield Test,19 exhibiting repeatable abnormality at the P<0.5% level by pattern deviation probability analysis, or by two or more locations in a cluster exhibiting repeatable abnormality at P<2% by pattern deviation probability analysis, excluding any location in the cluster located in the opposite horizontal hemifield.
Early glaucoma was defined using the criteria developed by Hodapp et al.20
Subjects were diagnosed as having ACG if they had history of elevated IOP, occludable angles and VF defects in one or both eyes, and were free from symptoms/signs of acute attack. An occludable angle was defined as an angle in which the pigmented trabecular meshwork was not visible throughout three-quarters or more of the angle circumference without indentation and manipulation.
At baseline, all subjects underwent a standard ophthalmic examination including visual acuity (Snellen chart), slit-lamp biomicroscopy, gonioscopy, IOP measurement with Goldmann applanation (GAT) and Dynamic Contour tonometer (DCT), central corneal thickness (CCT) measurement with ultrasound pachymeter, fundus examination using a lens, and the CCT three times, consecutively with the Ultrasound Pachymetry (Palm Scan AP 2000, ophthalmic ultrasound, Micro Medical Devices, Inc., Calabasas, CA, USA).
IOP was determined three times, each consecutively, using DCT (Swiss Microtechnology AG, Port, Switzerland) and GAT (Goldmann tonometer; Haag Streit AG, Koeniz, Switzerland). Ocular pulse amplitude (OPA) appeared during the DCT measurement.
All CDI examinations (model Antares; Siemens, Munich, Germany) were performed at all study visits by the same experienced observer, who was masked to the diagnosis.
A 7.5–10 MHz vector-array transducer was applied to the closed eyelid using a coupling gel; any pressure on the eye itself was avoided. Measurements were taken using the conventional technique,21 which is identical to the technique we have used in many previous studies.13, 22, 23, 24, 25 In brief, OA flow measurements were performed approximately 10–15 mm posterior to the globe, where ultrasound signals are stronger. Short PCA (SPCA) images were taken temporally and nasally to the optic nerve just behind the posterior pole of the eye. The angle between transducer and vessel orientation was corrected. CRA measurements were taken at the optic nerve head level.
Peak-systolic velocity (PSV) and end-diastolic velocity (EDV) were measured in the OA, CRA, and medial and lateral SPCAs. Although the medial and lateral PCAs were individually assessed, the mean value of both was used for the statistical analysis. PSV and EDV were used to calculate the Pourcelot resistivity index (RI), using the following equation: RI=PSV−EDV/PSV.26 Evaluations of blood pressure and radial pulse were obtained in a supine position after 10 min of rest. Systolic blood pressure and diastolic blood pressure were measured in the upper right arm using a mercury sphygmomanometer and the heart rate was measured by palpation of the radial pulse. These parameters were obtained every 10 min, during Doppler examination.
Statistical analysis
Before the study, it was determined that a sample of at least 22 patients was required to detect a difference of 0.05 units in the mean RI in the OA, SPCA, and CRA, at a significance level of 0.05 with a power of 0.90, and assuming an SD of 0.05 units. The adjusted power of the study was 0.73 (0.9 × 0.9 × 0.9).
Descriptive statistics (mean (SD)) and 95% confidence intervals were used to report demographic and ocular baseline characteristics. Data were tested for normal distribution using a Kolmogorov–Smirnov test. As data were normally distributed, a two-tailed, independent samples Student's t-test was used to evaluate the IOP and the hemodynamic parameters by intergroup comparisons.
Because of the large number of tests, simultaneous inference using the Bonferroni correction was used to correct the P-value (α/9). Statistical significance was accepted for P<0.0055.
To analyse the correlation between the retrobulbar hemodynamics and in IOP assessed with GAT, Pearson's correlation coefficients were calculated for every parameter.
Statistical analysis was performed using Med-Calc11.5.1.0 (MedCalc Software, Mariakerke, Belgium).
Results
Subject demographic characteristics are shown in Table 1. There was no statistically significant difference between the two groups in age, sex, IOP, refractive status, CCT, and VF damage.
Table 1. Baseline demographic characteristics.
| OAG (52) | ACG (25) | P-value | |
|---|---|---|---|
| Sexa | |||
| Male | 22 (423%) | 13 (52.0%) | 0.578 |
| Female | 30 (57.7%) | 12 (48.0%) | 0.578 |
| Age (years) | |||
| Mean (SD) | 65.9 (12.4) | 66.5 (10.5) | 0.836 |
| 95% CI | 62.4–69.3 | 62.2–70.8 | |
| IOP (mm Hg) | |||
| Mean (SD) | 33.0 (8.3) | 34.7 (10.2) | 0.199 |
| 95% CI | 30.7–35.3 | 30.5–38.9 | |
| CCT (μ) | |||
| Mean (SD) | 553.8 (38.6) | 557.9 (55.6) | 0.702 |
| 95% CI | 543.0–564.5 | 534.9–580.9 | |
| MD (dB) | |||
| Mean (SD) | −2.96 (3.2) | −2.31 (2.43) | 0.371 |
| 95% CI | −3.96 to −2.07 | −3.31 to −1.30 | |
| PSD (dB) | |||
| Mean (SD) | 2.59 (2.05) | 2.57 (1.69) | 0.972 |
| 95% CI | 2.02–3.16 | 1.88–3.27 | |
Abbreviations: POAG, open-angle glaucoma; PACG, angle-closure glaucoma; 95% CI, 95% confidence interval; IOP, intraocular pressure; MD, mean defect; PSD, pattern SD.
P-values were calculated comparing the parameters at baseline between the two study groups (one-way ANOVA test); P-values were considered statistically significant if lower than 0.05.
χ2-test. P-value was considered statistically significant if lower than 0.05.
There was no significant difference in OPA between OAG and ACG, 4.1 (1.3) and 3.9 (1.0), respectively, P=0.202.
The retrobulbar hemodynamic parameters are summarized in Table 2.
Table 2. Retrobulbar hemodynamic parameters in OAG and ACG patients.
|
OAG |
ACG |
P-value | |||
|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | ||
| PSV OA (cm/s) | 56.4 (25.2) | 49.4–63.5 | 62.3 (33.2) | 48.6–76.0 | 0.389 |
| EDV OA (cm/s) | 17.1 (12.6) | 13.6–20.6 | 21.9 (11.2) | 17.3–26.6 | 0.101 |
| RI OA | 0.72 (0.02) | 0.68–0.75 | 0.65 (0.02) | 0.61–0.69 | 0.003 |
| PSV CRA (cm/s) | 39.8 (23.4) | 33.4–46.4 | 39.4 (9.3) | 35.5–43.2 | 0.917 |
| EDV CRA (cm/s) | 12.8 (8.0) | 10.5–15.0 | 13.0 (5.4) | 10.8–15.3 | 0.881 |
| RI CRA | 0.67 (0.12) | 0.64–0.71 | 0.67 (0.08) | 0.64–0.71 | 0.979 |
| PSV PCA (cm/s) | 30.8 (14.5) | 26.8–34.9 | 28.1 (12.3) | 22.9–33.1 | 0.412 |
| EDV PCA (cm/s) | 9.2 (4.2) | 8.1–10.4 | 10.6 (4.2) | 8.9–12.3 | 0.185 |
| RI PCA | 0.66 (0.14) | 0.62–0.70 | 0.59 (0.09) | 0.56–0.64 | 0.048 |
Abbreviations: OAG, open-angle glaucoma; ACG, angle-closure glaucoma; 95% CI, 95% confidence interval; PSV, peak systolic velocity; EDV, end-diastolic velocity; RI, resistivity index; OA, ophthalmic artery; CRA, central retinal artery; PCA, posterior cilliary artery.
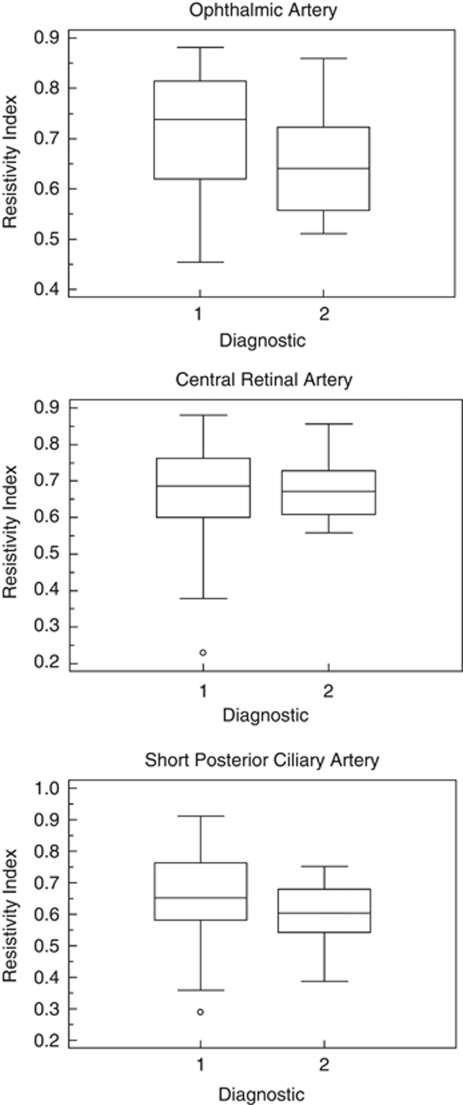
The RI was significantly higher in the OAG patients as compared with that in that ACG, in both ophthalmic and SPCAs patients, P=0.003 and 0.048, respectively, Table 2 and Figure 1.
Figure 1.
Distribution plot of the RI in OA, CRA, and SPCA in OAG and ACG glaucoma patients. Diagnostic 1=OAG, diagnostic 2=ACG. RI is in arbitrary units.
However, when Bonferroni correction was applied retrobulbar hemodynamic parameters only, the RI in the OA was significantly different Table 2.
Pearson correlation coefficients between retrobulbar hemodynamic parameters in the retrobulbar vessels and IOP, assessed with GAT, are shown in Table 3.
Table 3. Pearson's correlation coefficient between IOP, assessed with GAT, and OPA, and the retrobulbar hemodynamic parameters in the retrobulbar vessels in OAG and ACG glaucoma patients.
|
OAG (52) |
ACG (25) |
|||
|---|---|---|---|---|
| r (95% CI) | P-value | r (95% CI) | P-value | |
| PSV OA | 0.05 (−0.22 to 0.31) | 0.747 | 0.12 (−0.29 to 0.49) | 0.118 |
| EDV OA | 0.12 (−0.15 to 0.38) | 0.393 | −0.08 (−0.46 to 0.32) | 0.698 |
| RI OA | −0.07 (−0.34 to 0.20) | 0.594 | 0.20 (−0.20 to 0.56) | 0.318 |
| PSV CRA | 0.00 (−0.27 to 0.27) | 0.985 | 0.24 (−0.17 to 0.58) | 0.246 |
| EDV CRA | 0.12 (−0.16 to 0.38) | 0.408 | 0.05 (−0.36 to 0.43) | 0.829 |
| RI CRA | −0.04 (−0.31 to 0.24) | 0.773 | 0.22 (−0.19 to 0.57) | 0.287 |
| PSV SPCA | 0.02 (−0.26 to 0.29) | 0.889 | −0.02 (−0.41 to 0.38) | 0.917 |
| EDV SPCA | 0.29 (−0.01 to 0.53) | 0.053 | 0.07 (−0.34 to 0.45) | 0.743 |
| RI SPCA | −0.21 (−0.46 to 0.06) | 0.131 | −0.07 (−0.46 to 0.33) | 0.714 |
| OPA | 0.15 (−0.13 to 0.40) | 0.298 | 0.39 (−0.01 to 0.68) | 0.052 |
Abbreviations: 95% CI, 95% confidence interval; PSV, peak systolic velocity; EDV, end-diastolic velocity; RI, resistivity index; OA, ophthalmic artery; CRA, central retinal artery; PCA, posterior cilliary artery; OPA, ocular pulse amplitude; GAT, Goldmann applanation tonometer.
Our study found a lack of correlation between the mean defect and the blood-flow parameters in both OAG and ACG Table 4.
Table 4. Pearson's correlation coefficient between VF damage, assessed with the MD, and OPA and the retrobulbar hemodynamic parameters in the retrobulbar vessels in OAG and ACG patients.
|
OAG (52) |
ACG (25) |
|||
|---|---|---|---|---|
| r (95% CI) | P-value | r (95% CI) | P-value | |
| PSV OA | 0.02 (−0.26 to 0.29) | 0.896 | 0.05 (−0.35 to 0.43) | 0.823 |
| EDV OA | −0.07 (−0.34 to 0.21) | 0.621 | 0.00 (−0.39 to 0.39) | 0.991 |
| RI OA | −0.13 (−0.39 to 0.15) | 0.348 | −0.02 (−0.41 to 0.38) | 0.915 |
| PSV CRA | 0.12 (−0.16 to 0.38) | 0.399 | −0.05 (−0.44 to 0.35) | 0.798 |
| EDV CRA | 0.07 (−0.21 to 0.34) | 0.627 | −0.29 (−0.12 to 0.61) | 0.165 |
| RI CRA | −0.05 (−0.32 to 0.22) | 0.693 | −0.21 (−0.53 to 0.05) | 0.086 |
| PSV SPCA | 0.03 (−0.25 to 0.30) | 0.839 | 0.18 (−0.24 to 0.53) | 0.401 |
| EDV SPCA | 0.16 (−0.11 to 0.42) | 0.242 | 0.3 (−0.37 to 0.42) | 0.883 |
| RI SPCA | −0.07 (−0.34 to 0.20) | 0.589 | −0.30 (−0.62 to 0.10) | 0.141 |
| OPA | −0.04 (−0.32 to 0.23) | 0.729 | −0.01 (−0.40 to 0.39) | 0.968 |
Abbreviations: CI, confidence interval; PSV, peak systolic velocity; EDV, end-diastolic velocity; RI, resistivity index; OA, ophthalmic artery; CRA, central retinal artery; PCA, posterior cilliary artery; OPA, ocular pulse amplitude; MD, mean defect.
Discussion
The present study found that the RI in the OA was significantly higher in the OAG as compared with the ACG patients. Additionally, our study also suggests a lack of correlation between either the retrobulbar hemodynamic parameters and the IOP, or the retrobulbar hemodynamic parameters and the VF damage in either OAG or ACG.
There are relatively few studies that have evaluated the hemodynamic parameters of the ACG patients.
Cheng et al18 reported that patients with well-controlled CACG may have decreased retrobulbar blood-flow velocities and increased vascular resistance in the CRA and temporal PCA, as compared with healthy subjects.
It is very difficult to compare our results with those published by Cheng et al,18 because our study did not include a healthy control group and Cheng et al's18 study did not evaluate the OA.
Additionally, the inclusion criteria were different. Cheng et al18 included patients with an IOP within normal limits (equal or lower than 19 mm Hg) without medical treatment, and our study included newly diagnosed patients with either OAG or ACG without medical treatment, but with elevated IOP.
Furthermore, the retrobulbar hemodynamic parameters found in our investigation seem to differ significantly to those reported by Cheng et al.18
On the other hand, Bonferroni correction was not used in the Cheng et al18 study.
Nong and Ninghua27 investigated the hemodynamic changes of the ophthalmic and CRA in PACG and the effects of IOP on the retrobulbar hemodynamics. The results of this study suggested that compared with the normal subjects, the hypertension group of ACG showed significant reduction in the EDV and increases in pulsatility index and RI of CRA, whereas normal tension group of ACG also showed remarkable increase in RI of CRA.27 However, these authors concluded that the elevated IOP was a major cause of the vascular resistance increased in PACG, which will make the end-diastolic blood flow of CRA decreased.
In contrast to the study of Nong and Ninghua,27 our study did not find either blood-flow disturbances in the retrobulbar vessels of the ACG patients or any correlation between the IOP and the retrobulbar hemodynamic parameters.
As our study did not find any significant difference in IOP between the groups, the differences in retrobulbar hemodynamics should be related to other factors.
In this study, there was no significant difference in either the IOP or the VF damage. Thus, it is likely that the hemodynamic changes seen in the OAG group were primary, but it is not possible to ascertain from our results.
Our study failed to find differences between OAG and ACG patients in other retrobulbar hemodynamic parameters. It might be explained by technical problems. Measurements of SPCA show greater variability than those of the OA, and although all CDI measurements were made by the same experienced user, the SD of the measurements in the SPCA was much higher.
Our study did not find any correlation the between VF damage, assessed with the mean defect, and OPA or the retrobulbar hemodynamic parameters in either the OAG or the ACG patients. Nevertheless, these findings might be related to the VF inclusion criterion.
Our study has some limitations. The first results from our use of a singular ocular-imaging technology to study OBF, without providing data on the retina or other vascular beds. We know that blood-flow velocity is not equivalent to blood flow. However, studies have shown that Doppler ultrasonography can be used to correlate changes in blood flow and provide a meaningful trend analysis in the central nervous system.28 We believe that flow velocities and RI measured from CDI may give us some clues to the status of ocular circulation. The second limitation of our study is that it is a single-center study, with a limited number of patients. Nevertheless, the sample size was calculated before the study. It should be also taken in consideration the fact that we included OAG patients with high pressures and early VF damage. Appropriate caution is therefore recommended when extending the results to other populations.
In summary, the results of our study suggest that the RI in the OA was significantly higher in the OAG as compared with the ACG patients. Additionally, the degree of circulatory disturbance was not related to either the IOP or the VF damage.
The authors declare no conflict of interest.
References
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- Advanced Glaucoma Intervention Study Investigators The Advanced Glaucoma Intervention Study (AGIS) (7). The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- Carter CJ, Brooks DE, Doyle DL, Drance SM. Investigation into a vascular aetiology for low tension glaucoma. Ophthalmology. 1990;97:49–55. doi: 10.1016/s0161-6420(90)32627-1. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Progress in the understanding of the vascular aetiology of glaucoma. Curr Opin Ophthalmol. 1994;5:26–35. [Google Scholar]
- Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Metzner-Serra L, et al. The impact of ocular blood fl ow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- Harris A, Kagemann L, Ehrlich R, Rospigliosi C, Moore D, Siesky B. Measuring and interpreting ocular blood flow and metabolism in glaucoma. Can J Ophthalmol. 2008;43 (3:328–336. doi: 10.3129/i08-051. [DOI] [PubMed] [Google Scholar]
- Werne A, Harris A, Moore D, BenZion I, Siesky B. The circadian variations in systemic blood pressure, ocular perfusion pressure, and ocular blood flow: risk factors for glaucoma. Surv Ophthalmol. 2008;53 (6:559–567. doi: 10.1016/j.survophthal.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Harris A.(eds).Ocular Blood Flow in Glaucoma: The 6th Consensus Report of the World Glaucoma Association. Section II: Clinical Measurement of Ocular Blood Flow Kugler Publications: Amsterdam, The Netherlands; 2009. p59 [Google Scholar]
- Galassi F, Sodi A, Ucci F, Renieri G, Pieri B, Baccini M. Ocular hemodynamics and glaucoma prognosis: a color Doppler imaging study. Arch Ophthalmol. 2003;121:1711–1715. doi: 10.1001/archopht.121.12.1711. [DOI] [PubMed] [Google Scholar]
- Satilmis M, Orgul S, Doubler B, Flammer J. Rate of progression of glaucoma correlates with retrobulbar circulation and intraocular pressure. Am J Ophthalmol. 2003;135:664–669. doi: 10.1016/s0002-9394(02)02156-6. [DOI] [PubMed] [Google Scholar]
- Martinez A, Sanchez M. Predictive value of color Doppler imaging in a prospective study of visual field progression in primary open-angle glaucoma. Acta Ophthalmol Scand. 2005;83:716–723. doi: 10.1111/j.1600-0420.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Harris A.(eds).Ocular Blood Flow in Glaucoma: The 6th consensus report of the World Glaucoma Association. Section II: Clinical Measurement of Ocular Blood Flow Kugler Publications: Amsterdam, The Netherlands; 200921–22. [Google Scholar]
- Lieb WE. lor Doppler ultrasonography of the eye and orbit. Curr Opin Ophthalmol. 1993;4 (3:68–75. doi: 10.1097/00055735-199306000-00012. [DOI] [PubMed] [Google Scholar]
- Butt Z, O'Brien C, McKillop G, Aspinall P, Allan P. Color Doppler imaging in untreated high- and normal-pressure open-angle glaucoma. Invest Ophthalmol Vis Sci. 1997;38 (3:690–696. [PubMed] [Google Scholar]
- Congdon N, Wang F, Tielsch JM. Issues in the epidemiology and population-based screening of primary angle-closure glaucoma. Surv Ophthalmol. 1992;36:411–423. doi: 10.1016/s0039-6257(05)80022-0. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Liu CJ, Chiou HJ, Chou JC, Hsu WM, Liu JH. Color Doppler imaging study of retrobulbar hemodynamics in chronic angle-closure glaucoma. Ophthalmology. 2001;108 (8:1445–1451. doi: 10.1016/s0161-6420(01)00603-0. [DOI] [PubMed] [Google Scholar]
- Asman P, Heijl A. Glaucoma hemifield test. Automated visual field evaluation. Arch Ophthalmol. 1992;110:812–819. doi: 10.1001/archopht.1992.01080180084033. [DOI] [PubMed] [Google Scholar]
- Hodapp E, Parrish R, Anderson D. Clinical Decisions in Glaucoma. Mosby-Year Book, Inc.: St Louis, Missouri; 1993. [Google Scholar]
- Lieb WE, Cohen SM, Merton DA, Shields JA, Mitchell DG, Goldberg BB. Colour Doppler imaging of the eye and orbit: technique and normal vascular anatomy. Arch Ophthalmol. 1991;109:527–531. doi: 10.1001/archopht.1991.01080040095036. [DOI] [PubMed] [Google Scholar]
- Martinez A, González F, Capeans C, Pérez R, Sánchez-Salorio M. Dorzolamide effect on ocular blood flow. Invest Ophthalmol Vis Sci. 1999;40 (6:1270–1275. [PubMed] [Google Scholar]
- Martinez A, Sanchez M. A comparison of the effects of 0.005% latanoprost and fixed combination dorzolamide/timolol on retrobulbar haemodynamics in previously untreated glaucoma patients. Curr Med Res Opin. 2006;22:67–73. doi: 10.1185/030079906X80215. [DOI] [PubMed] [Google Scholar]
- Martinez A, Sanchez M. Effects of dorzolamide 2% added to timolol maleate 0.5% on intraocular pressure, retrobulbar blood flow, and progression of visual field damage in patients with primary open angle glaucoma: a single-centre, 4-year, open-label study. Clin Ther. 2008;30:1120–1134. doi: 10.1016/j.clinthera.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Martinez A, Sanchez M. Ocular haemodynamics in pseudoexfoliative and primary open-angle glaucoma. Eye. 2008;22 (4:515–520. doi: 10.1038/sj.eye.6702676. [DOI] [PubMed] [Google Scholar]
- Pourcelot L. Indications de l'ultrasonographie Doppler dans l'etude des vaisseaux peripheriques. Rev Prat. 1975;25:4671–4680. [PubMed] [Google Scholar]
- Nong T, Ninghua F. Color Doppler imaging in the study of retrobulbar hemodynamic changes of primary angle-closure glaucoma. Yan Ke Xue Bao. 1997;13 (3:113–115. [PubMed] [Google Scholar]
- Hansen NB, Stonestreet BS, Rosenkrantz TS, Oh W. Validity of Doppler measurements of anterior cerebral artery blood flow velocity: correlation with brain blood flow in piglets. Pediatrics. 1983;72:526–531. [PubMed] [Google Scholar]