Abstract
Hypoxia frequently occurs in patients with traumatic brain injury (TBI) and is associated with increased morbidity and mortality. This study examined the effects of immediate or delayed post-traumatic hypoxia (fraction of inspired oxygen [FiO2] 11%) on acute neuronal degeneration and long-term neuronal survival in hippocampal fields after moderate fluid percussion injury in rats. In Experiment 1, hypoxia was induced for 15 or 30 min alone or immediately following TBI. In Experiments 2 and 3, 30 min of hypoxia was induced immediately after TBI or delayed until 60 min after TBI. In Experiment 1, acute neurodegeneration was evaluated in the hippocampal fields 24 h after insults using Fluoro-Jade staining and stereological quantification. During hypoxia alone, or in combination with TBI, mean arterial blood pressure was significantly reduced by approximately 30%, followed by a rapid return to normal values upon return to pre-injury FiO2. Hypoxia alone failed to cause hippocampal neuronal degeneration when measured at 24 h after insult. TBI alone resulted in neuronal degeneration in each ipsilateral hippocampal field, predominantly in CA2–CA3 and the dentate gyrus. Compared to TBI alone, TBI plus immediate hypoxia for either 15 or 30 min significantly increased neuronal loss in most ipsilateral hippocampal fields and in the contralateral hilus and dentate gyrus. In Experiment 2, TBI plus hypoxia delayed 30 min significantly increased degeneration only in ipsilateral CA2–CA3. In Experiment 3, 30 min of immediate hypoxia significantly reduced the numbers of surviving neurons in the CA3 at 14 days after TBI. The greatly increased vulnerability in all hippocampal fields by immediate 30 min post-traumatic hypoxia provides a relevant model of TBI complicated with hypoxia/hypotension. These data underscore the significance of the secondary insult, the necessity to better characterize the range of injuries experienced by the TBI patient, and the importance of strictly avoiding hypoxia in the early management of TBI patients.
Key words: hippocampal fields, hippocampus, hypoxia, neuronal degeneration, neuronal survival, traumatic brain injury
Introduction
Traumatic brain injury (TBI) occurs in approximately 1.7 million people each year in the United States and remains a leading cause of death and disability (Faul et al., 2010). One quarter to a third of severe TBI patients arrive in the emergency department with significant hypoxia and hypotension (Manley et al., 2001). Chestnut and colleagues, using the Traumatic Coma Data Bank, showed that hypoxia (partial arterial oxygen pressure [PaO2]≤60 mm Hg) or hypotension (systolic blood pressure [SBP]<90 mm Hg) were independently associated with increased morbidity and mortality following severe TBI (Glasgow Coma Scale [GCS] score ≤ 8; Chesnut et al., 1993). Additionally, the association between hypoxemia and poor clinical outcome from TBI is well documented (Chi et al., 2006; Davis et al., 2004,2009; Jiang et al., 2002). Several adverse effects of TBI plus secondary hypoxia have been described, including increased neuronal damage (Bauman et al., 2000; Clark et al., 1997; Nawashiro et al., 1995), exacerbated axonal pathology and neuroinflammatory response (Goodman et al., 2010; Hellewell et al., 2010), aggravated brain edema (Van Putten et al., 2005), and exacerbated sensorimotor and cognitive deficits (Bauman et al., 2000; Clark et al., 1997). However, whether hippocampal neuronal loss correlates closely with the duration and timing of post-traumatic hypoxia has not been clearly addressed (Chesnut et al., 1993; Manley et al., 2001).
In experimental TBI, hippocampal neurons are thought to be extremely vulnerable to post-traumatic insults, including hypoxia and ischemia, which are considered important pathophysiological mechanisms leading to neuronal death and behavioral deficits (Aoyama et al., 2008; Bauman et al., 2000; Clark et al., 1997; Mikrogianakis et al., 2007; Nawashiro et al., 1995). The hippocampus plays an essential role in memory, with specific hippocampal fields supporting unique behavioral functions (Amaral and Witter, 1995; Gilbert et al., 2001; Kesner et al., 2004; Okada and Okaichi, 2009). Quantitative analysis of the distribution of degenerating neurons in the hippocampus following experimental TBI over the course of 1 week demonstrated significant cell death in the CA3 and dentate gyrus following mild or moderate injury, with very little cell death in the CA1 (Anderson et al., 2005; Lowenstein et al., 1992). While previous animal studies have characterized patterns of cell death in the hippocampus following TBI and the effect of 30 min of hypoxia on hippocampal cell death, the present study examined the effects of both the duration (15 or 30 min), and timing (immediate or after a 1-h delay) of post-traumatic hypoxia on acute neuronal degeneration and long-term neuronal survival in hippocampal fields following moderate lateral fluid percussion TBI in the rat.
Methods
Subjects
One hundred male Sprague-Dawley rats weighing 310–360 g were used in this study. Animals were housed in individual cages in a temperature- (22°C) and humidity-controlled (50% relative) animal facility with a 12-h light/dark cycle. Animals had free access to food and water during the duration of the experiments. The animals remained in the animal facility for at least 7 days prior to surgery. The Institutional Animal Care and Use Committee at the University of California at Davis approved all animal procedures in these experiments.
Experimental design
Experiment 1 was designed to evaluate the effect of 15 and 30 min of hypoxia (FiO2=11%) alone or immediately following TBI on acute neuronal degeneration in the hippocampus and physiological parameters, including PaO2, arterial oxygen saturation (SaO2), hematocrit, and mean arterial blood pressure (MABP). Animals were subjected to surgical procedures and then randomly assigned to five groups: 15 min or 30 min hypoxia alone (n=6 in each group), TBI with normoxia (FiO2=33%; n=6), and TBI coupled with 15 min (n=7) or 30 min (n=8) of hypoxia induced immediately (within 5 sec) post-TBI.
Experiment 2 was designed to evaluate the effects of immediate hypoxia or hypoxia delayed by 30 min after TBI on acute neuronal loss across hippocampal fields: CA1, CA2–CA3, CA3c, the hilus, and the granular layer of the dentate gyrus. Three groups of animals were subjected to fluid percussion injury, followed by 30 min of immediate hypoxia (FiO2=11%; n=6) or 30 min of hypoxia (FiO2=11%) initiated at 60 min post-injury (n=7), or maintained at normoxia (FiO2=33%) after TBI (n=8). All animals were euthanized 24 h after TBI, and brain tissue was processed for analysis of acute neuronal degeneration.
Experiment 3 was designed to evaluate cell death at 2 weeks following moderate TBI with hypoxia (FiO2=11%). After receiving moderate TBI or sham injury, animals were separated into five groups: sham (n=8), normoxia (n=6), 15 min (n=6) or 30 min (n=7) of immediate hypoxia, and 30 min of delayed hypoxia (60–90 min post-TBI; n=7). Serial coronal sections were stained with cresyl violet and surviving neurons in the hippocampal CA3 and CA1 were quantified using stereological procedures.
Surgical procedure
Rats were anesthetized with 4% isoflurane in a 2:1 nitrous oxide/oxygen mixture, intubated, and mechanically ventilated with a rodent volume ventilator (model 683; Harvard Apparatus, Holliston, MA). A surgical level of anesthesia was maintained with 2% isoflurane. The rats were mounted in a stereotaxic frame, a scalp incision was made along the midline, and a 4.8-mm-diameter craniectomy was performed on the right parietal bone (centered at −4.5 mm from bregma and 3.0 mm right lateral). A rigid plastic injury tube (a modified Luer-Loc needle hub, 2.6-mm inside diameter) was secured with cyanoacrylate adhesive over the exposed intact dura. Two skull screws (2.1 mm diameter, 6.0 mm length) were placed into burr holes to secure the injury tube. One screw was located 1 mm rostral to the bregma and 1 mm right lateral; the second screw was located 1 mm caudal to lambda on the midline. The assembly was secured to the skull with cranioplastic cement (Plastics One, Roanoke, VA). Rectal temperature was continuously monitored and maintained within the normal range during surgical preparation by a feedback temperature controller pad (model TC-1000; CWE, Ardmore, PA). Temporalis muscle temperature was measured by insertion of a needle temperature probe (unit TH-5, probe MT-29/2; Physitemp, Clifton, NJ) between the skull and temporalis muscle. The right femoral artery was cannulated with PE 50 tubing for monitoring blood pressure and obtaining samples for blood gas analysis.
Traumatic brain injury
Experimental TBI was produced using a fluid percussion device (VCU Biomedical Engineering, Richmond, VA; Dixon et al., 1987) with the lateral orientation (McIntosh et al., 1989). The device consists of an acrylic glass cylindrical reservoir filled with isotonic saline. One end of the reservoir has an acrylic glass piston mounted on O-rings, and the opposite end has a transducer housing with a 2.6-mm inside diameter male Luer-Loc opening. Injury is induced by the descent of a pendulum striking the piston, which injects a small volume of saline epidurally into the closed cranial cavity, producing a brief displacement and deformation of neural tissue. The resulting pressure pulse was measured in atmospheres (atm) by an extracranial transducer (model SPTmV0100PG5W02; SenSym ICT, Milpitas, CA), and recorded on a digital storage oscilloscope (model TDS 1002; Tektronix Inc., Beaverton, OR).
The rats were disconnected from the ventilator, the injury tube was connected to the fluid percussion cylinder, and a moderate fluid percussion pulse (2.13–2.31 atm) was delivered within 10 sec. Immediately after TBI the rats were returned to ventilation with 1% isoflurane in either normoxic or hypoxic carrier gas conditions. The plastic injury tube and skull screws were removed immediately after TBI, and the scalp incision was closed with 4-0 braided silk sutures.
Ventilation and controlled hypoxia
While true normoxia in room air has an FiO2 ∼21%, we elected to maintain “experimental normoxia” with a ventilatory mixture of nitrous oxide/oxygen (2:1) to produce an FiO2=33%, which is typically used in rat TBI studies (Bales et al., 2010; Bao et al., 2011; Hall and Lifshitz, 2010). Henceforth in this manuscript “normoxia” refers to our “experimental normoxia,” with an FiO2=33%. Hypoxia was maintained with a carrier gas mixture of 1:1 nitrous oxide/air (hypoxia FiO2=11%). In Experiment 1, animals receiving hypoxia alone were ventilated with 2% isoflurane to maintain surgical anesthesia. Animals receiving 15 min of hypoxia received an additional 15 min of normoxia before ventilation was discontinued. Animals receiving 30 min of hypoxia were removed from ventilation immediately following the termination of hypoxia. All animals receiving a TBI were ventilated with 1% isoflurane following TBI in order to maintain surgical anesthesia. Animals in the TBI normoxia group were ventilated for 30 min. Animals in the TBI+15 min immediate hypoxia group were subjected to 15 min of hypoxia, followed by an additional 10 min of normoxia. The TBI+30 min immediate hypoxia group was subjected to 30 min of hypoxia, followed by 15 min of normoxia. In the TBI+delayed hypoxia group, the rats were extubated after injury as soon as spontaneous breathing was observed (∼2 min), and then re-intubated at 60 min post-TBI and immediately ventilated with 1% isoflurane. The animals were made hypoxic for 30 min, and then ventilation was terminated. At the conclusion of ventilation, isoflurane was discontinued and the animals were monitored for the return of spontaneous breathing. The animals were extubated as soon as spontaneous breathing was observed and were returned to a heated cage for recovery.
Physiological monitoring
In Experiment 1, the left femoral artery was cannulated with PE 50 tubing under 2% isoflurane anesthesia in order to measure blood pressure and sample arterial blood gases. Blood pressure was continuously monitored and recorded every 5 min. Blood gas (PaO2, PaCO2, and O2 saturation) and hematocrit were assessed at 5 min pre-hypoxia, after 15 min of hypoxia, and 5 min post-hypoxia. In the TBI normoxia group, the assessments were carried out correspondingly at 5 min pre-TBI, at 15 min post-TBI, and 35 min post-TBI.
Electroencephalogram recording and analysis
Continuous electroencephalography (EEG) was recorded (amplifier 7P5; Grass Instruments, Quincy, MA) in two groups of rats: TBI with 30 min of hypoxia (FiO2=11%) immediately after TBI (n=6), and TBI+30 min hypoxia (FiO2=11%) delayed until 60 min after TBI (n=6). The EEG was recorded between the two skull screws used to secure the fluid percussion injury tube. EEG data were filtered between 1 and 3 kHz, digitized at 100 Hz, and stored on a computer (PolyView 16 version 1.1 software; Grass Instruments). Isoflurane anesthesia was reduced to 1.25% during baseline recording to eliminate an EEG burst-suppression phenomenon. Following TBI, isoflurane was maintained at 1.0% during EEG recording. EEG was recorded for 10 min prior to TBI and served as a pre-TBI baseline.
Spectral power was measured using standard fast Fourier transform (FFT) analysis by PolyView software. The raw EEG data were recorded over the entire experiment and divided into 10-min epochs and the FFT for each epoch was calculated. From these FFT functions, spectral EEG power was defined as the total power between 1 and 3 kHz. The FFT epochs were then averaged for animals in each group at baseline (prior to TBI) and 90–100 min after TBI. Changes in EEG power after TBI were normalized to the percentage of baseline power for each group.
Tissue collection and sectioning
The rats were euthanized at either 24 h or 14 days after TBI by deep sodium pentobarbital anesthesia (100 mg/kg IP), followed by transcardial perfusion with 100 mL of 0.1 M sodium phosphate buffer (PB; pH=7.4), and then 350 mL of 4% paraformaldehyde (pH 7.4). The brains were removed and post-fixed for 1 h in 4% paraformaldehyde at 4°C. The brains were cryoprotected in 10% sucrose solution for 24 h, and then transferred to a 30% sucrose solution for 48 h. The brains were frozen on powdered dry ice, and 45-μm coronal sections were cut using a sliding microtome. Every serial section starting at −2.12 mm bregma and ending at −4.80 mm bregma was saved in 0.1 M PB with sodium azide in 24-well cell culture plates and stored at 4°C. Systematic random sampling techniques were used for selecting tissue sections for staining and stereological analysis.
Quantification of acute neuronal degeneration
Acute neuronal degeneration was assessed at 24 h after TBI using the histofluorescent stain Fluoro-Jade B. Tissue sections were selected by taking every fifth section starting at −3.1 mm bregma and ending at −4.7 mm bregma for a total of 7 sample sections per brain. Tissue sections were mounted on gelatin-coated slides in a 1:1 ratio of 0.1 M PB and distilled H2O (dH2O) and air-dried overnight. The slide-mounted tissue sections were subsequently immersed in 100% alcohol (3 min), 70% alcohol (1 min), dH2O (1 min), and 0.006% potassium permanganate (15 min). The sections were rinsed in dH2O (1 min), incubated in 0.001% Fluoro-Jade B (Histo-Chem Inc., Pine Bluff, AK) staining solution in 0.1% acetic acid for 30 min, rinsed again in dH2O (3 min), and air-dried. Finally, the sections were immersed in xylene and cover-slipped with DePeX mounting medium (Electron Microscopy Sciences, Fort Washington, PA).
Neuronal degeneration was assessed in five hippocampal fields: CA1, CA2–CA3, CA3c, the hilus, and the granule layer in the dentate gyrus (DG). The medial boundary point of CA1 was recognized at the corner of the stratum pyramidale adjacent to the midline, and the lateral boundary point of CA1 was the widening of the stratum pyramidale at the border of the CA2, which also served as the superior boundary of CA2–CA3. A line connecting the lateral tips of the superior and inferior blades of the granule layer of the DG was set as the inferior boundary of CA2–CA3. The pyramidal neurons between the superior and inferior blades of dentate granular cells were counted as neurons in CA3c. Degenerating polymorphic neurons in the hilar field and granular neurons in the DG were also assessed.
Fluoro-Jade-positive cell counts were made by investigators uninformed of the injury and hypoxia conditions. Sections stained with Fluoro-Jade B were examined under a mercury arc lamp with an FITC fluorescence filter cube (Schmued and Hopkins, 2000; Schmued et al., 1997) on an epifluorescence microscope (Nikon E600; Nikon, Tokyo, Japan) with a motorized stage (MS-2000; Applied Scientific Instruments, Eugene, OR) and analyzed by computer software (Stereologer version 1.3; Systems Planning & Analysis, Inc., Alexandria, VA). Criterion for counting degenerating neurons (Fluoro-Jade B positive) included green fluorescing, morphologically distinct cell bodies. Neuronal identification and cell counting were performed with a 20× objective. The total number of Fluoro-Jade B-positive degenerating neurons was quantified using optical fractionator stereological methods (West et al., 1991). In this study, the spacing of the optical dissectors was modified to produce an area sampling fraction (ASF) of 1.0 in order to more accurately estimate the target cells, which were not uniformly distributed within the regions of interest (ROI). The guard height was set at 0.40 μM, producing a thickness sampling fraction (TSF) of 0.71. Target cells in every fifth section were counted, producing a section sampling fraction (SSF) of 0.20.
Quantification of long-term neuronal survival
Long-term neuronal survival was assessed on day 14 post-injury using cresyl violet-stained tissue from animals perfused on post-injury day 14. Tissue sections were selected by taking every fifth section starting at −2.6 mm bregma and ending at −4.2 mm bregma, for a total of 7 sample sections per brain. Tissue sections were mounted on gelatin-coated slides and dried overnight before staining. The sections were dehydrated at room temperature by a series of ethanol immersions: 70% (2 min×1), 95% (2 min×2), and 100% (2 min×2), followed by immersion in xylene (16 min). The sections were then rehydrated in a series of ethanol immersions: 100% (2 min×2), 95% (2 min×2), and 75% (2 min×1), then rinsed with dH2O (30 sec×2). The sections were next stained with cresyl violet acetate (0.1%) for 6 min, followed by rinsing in dH2O (15 sec×2), differentiated by immersion in 95% ethanol with 0.15% acetic acid (8 min), and dehydrated in a series of ethanol immersions: 95% (30 sec×2) and 100% (30 sec×2), and cleared by immersion in xylene (5 min×2). The sections were cover-slipped with Permount (Fisher Scientific, Hampton, NH). Neuronal survival was assessed in the CA1 and CA3 hippocampal fields.
The cresyl violet cell counts were made by investigators uninformed of the injury and hypoxia conditions. The sections were examined with a microscope (Nikon E600) with a motorized stage (MAC5000 System; Ludl Electronic Products, Ltd., Hawthorne, NY) using computer software (Stereo Investigator™ 8.0; Microbrightfield, Inc., Williston, VT). The criterion for counting neurons required morphologically distinct cell bodies. Neuronal cell counting was performed with a 100× oil objective (Plan Apo, NA 1.40; Nikon). The total number of neurons was quantified using the optical fractionator stereological method (West et al., 1991). The spacing of the optical dissectors produced an average ASF of 0.032. The guard height was set at 0.40 μM, producing a TSF of 0.71. Target cells in every fifth section were counted, producing a SSF of 0.20.
Statistical analysis
Data analysis was performed using SPSS software (Version 17; SPSS, Chicago, IL), which adheres to a general linear model. Alpha level for Type I error was set at 0.05 for rejecting null hypotheses. Data for body weights, fluid percussion injury magnitudes, and temperature measurements were expressed as mean±standard deviation (SD). All other data were expressed as mean±standard error of the mean (SEM). Body weight, TBI magnitude, temperatures, PaO2, PaCO2, pH, and hematocrit were analyzed with one-way analyses of variance (ANOVAs) at each measurement time point, followed by a Dunnett post-hoc analysis comparing each mean with the TBI group. MABP was analyzed by ANOVA for each group, followed by a Dunnett post-hoc analysis comparing each mean with baseline. MABP was also analyzed with repeated-measures ANOVA for the TBI, 15 min hypoxia, and TBI+15 min hypoxia groups, and again for the TBI, 30 min hypoxia, and TBI+30 min hypoxia groups, followed by Tukey's Honestly Significant Difference post-hoc analysis. Degenerating neuronal counts from Fluoro-Jade B staining and surviving neuronal cell counts from cresyl violet staining were analyzed using separate one-way ANOVAs for each field, followed by Dunnett post-hoc analysis comparing each mean with the TBI normoxia group. EEG power was analyzed with repeated-measures ANOVA.
Results
There were no significant differences between groups in body weight, injury magnitude, temporalis muscle temperature, and rectal temperature in Experiments 1, 2, or 3 (Table 1).
Table 1.
Groups and Parameters in Experiments (Values Are Mean±Standard Deviation)
| |
|
|
Temporalis temperature (°C) |
Rectal temperature (°C) |
||||
|---|---|---|---|---|---|---|---|---|
| Group (n) | Weight (g) | TBI (atm) | Pre-TBI | 1 min post-TBI | Pre-TBI | 1 min post-TBI | 15 min hypoxia | 30 min hypoxia |
| Experiment 1: Physiological parameters and acute neuronal degeneration | ||||||||
| 15 min hypoxia (6) | 332±5 | 0 | 36.4±0.2 | 36.3±0.3 | 37.0±0.3 | 36.9±0.5 | 37.1±0.4 | 37.2±0.4 |
| 30 min hypoxia (6) | 342±16 | 0 | 36.4±0.2 | 36.0±0.4 | 37.1±0.1 | 36.9±0.3 | 36.9±0.1 | 37.0±0.3 |
| TBI normoxia (6) | 335±11 | 2.29±0.02 | 36.4±0.2 | 36.0±0.4 | 37.1±0.3 | 37.2±0.3 | 37.1±0.2 | 37.1±0.2 |
| TBI+15 min hypoxia (7) | 352±10 | 2.25±0.07 | 36.4±0.3 | 36.1±0.1 | 37.1±0.2 | 36.7±0.3 | 36.9±0.5 | 36.9±0.5 |
| TBI+30 min hypoxia (8) | 340±21 | 2.31±0.03 | 36.7±0.3 | 36.3±0.3 | 37.2±0.3 | 37.1±0.4 | 37.3±0.2 | 37.4±0.2 |
| Experiment 2: Acute neuronal degeneration | ||||||||
| TBI normoxia (8) | 327±13 | 2.15±0.01 | 35.6±0.3 | 35.5±0.3 | 37.1±0.3 | 37.0±0.3 | 37.0±0.3 | 37.1±0.2 |
| TBI+30 min hypoxia (6) | 340±12 | 2.14±0.02 | 35.7±0.3 | 35.7±0.2 | 36.9±0.3 | 37.0±0.2 | 37.0±0.2 | 36.9±0.3 |
| aTBI+delayed 30 min hypoxia (7) | 337±14 | 2.13±0.02 | 35.9±0.4 | 35.7±0.4 | 37.3±0.3 | 37.1±0.4 | 37.0±0.1 | 37.0±0.3 |
| Experiment 3: Long-term neuronal survival | ||||||||
| Sham (8) | 329±15 | 0 | 36.0±0.1 | 35.9±0.1 | 37.1±0.2 | 37.1±0.3 | - | - |
| TBI normoxia (6) | 298±6 | 2.15±0.01 | 35.8±0.3 | 35.9±0.2 | 36.9±0.3 | 37.1±0.5 | - | - |
| TBI+15 min hypoxia (6) | 311±7 | 2.14±0.01 | 35.6±0.3 | 35.7±0.3 | 37.2±0.3 | 37.1±0.4 | - | - |
| TBI+30 min hypoxia (7) | 296±12 | 2.14±0.01 | 35.7±0.3 | 35.7±0.3 | 37.2±0.5 | 37.3±0.3 | - | - |
| aTBI+delayed 30 min hypoxia (7) | 309±13 | 2.14±0.01 | 35.7±0.3 | 35.9±0.4 | 37.1±0.4 | 37.2±0.5 | - | - |
Hypoxia started at 60 min post-TBI, ended at 90 min post-TBI.
TBI, traumatic brain injury; atm, atmosphere.
Experiment 1
Physiological parameters
Fifteen minutes of hypoxia significantly altered PaO2 between groups [F(4,31)=162.9, p<0.001]. After 15 min of hypoxia (with or without TBI) PaO2 ranged between 29±1 and 33±1 mm Hg (p<0.01 compared to normoxia), and SaO2 decreased from 100% to 55–60%. Mean PaCO2, pH, and hematocrit measurements were within normal ranges throughout the experiment and were not significantly different between groups. The SaO2 remained at 100% during normoxia in all groups (Table 2).
Table 2.
Arterial PaO2 and Saturation Values
| |
Pao2 mm Hg mean±SEM (Sao2) |
||
|---|---|---|---|
| Group | Pre-hypoxia | 15 min hypoxia | 5 min post-hypoxia |
| 15 min hypoxia | 160±12 (100%) | 30±3 (57%) | 155±13 (100%) |
| 30 min hypoxia | 152±10 (100%) | 29±3 (55%) | 161±9 (100%) |
| TBI normoxia | 155±9 (100%) | 149±10 (100%) | 147±12 (100%) |
| TBI+15 min hypoxia | 135±10 (100%) | 29±1 (55%) | 162±14 (100%) |
| TBI+30 min hypoxia | 156±9 (100%) | 33±1 (60%) | 169±17 (100%) |
Pao2, partial arterial oxygen pressure; Sao2, arterial oxygen saturation; SEM, standard error of the mean.
In the TBI normoxia group, MABP was reduced [F(8,45)=3.5, p<0.01] from a baseline of 101.2±1.8 mm Hg to 91.6±2.5 mm Hg at 5 min post-injury (p<0.05), and remained significantly below baseline for the duration of the experiment (89.8±1.5 mm Hg at 40 min post-injury; Fig. 1).
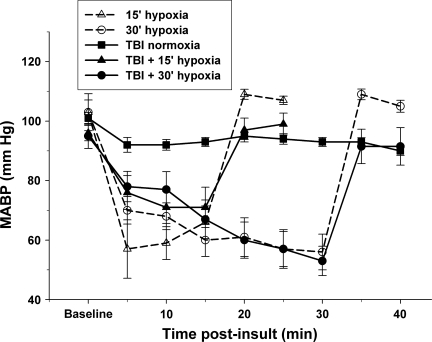
FIG. 1.
Hypoxia reduces mean arterial blood pressure (MABP). Hypoxia (fraction of inspired oxygen [FiO2]=11%), whether alone or combined with traumatic brain injury (TBI), produced a significant 30–40 mm Hg decrease in MABP. Blood pressure rapidly recovered to baseline within 5 min of return to ventilation with FiO2=33%. Values are mean±standard error of the mean.
Fifteen minutes of hypoxia in the absence of TBI produced systemic hypotension [F(5,18)=19.8, p<0.01], reducing MABP from a baseline of 98.4±4.8 to 66.2±5.5 mm Hg (p<0.01; Fig. 1). Thirty minutes of hypoxia reduced MABP [F(8,42)=17.4, p<0.01] from a baseline of 103.2±4.1 to 56.3±6.0 mm Hg (p<0.01). Five minutes after resumption of normoxia, the MABP increased to 109.3±1.7 and 108.9.0±1.8 mm Hg, respectively, for the 15- and 30-min hypoxia alone groups. TBI plus 15 or 30 min of hypoxia also produced systemic hypotension [F(5,65)=7.8, p<0.01] and [F(8,53)=9.0, p<0.01], respectively (Fig. 1), reducing MABP to 70.5±2.5 (p<0.01) and 53.3±4.9 (p<0.01) mm Hg at 15 and 30 min, respectively after TBI (baseline=96.2±2.6 and 95.4±4.2 mm Hg, respectively). Five minutes after resumption of normoxia, the MABP increased to 97.2±4.0 and 98.2.0±5.8 mm Hg, respectively, for the TBI+15 min hypoxia and TBI+30 min hypoxia groups.
Between-group analyses of MABP revealed a significant difference between the TBI, 15-min hypoxia, and TBI+15-min hypoxia groups [F(2,19)=5.69, p<0.05], and between the TBI, 30-min hypoxia, and TBI+30-min hypoxia groups [F(2,16)=13.30, p<0.01]. All groups receiving hypoxia experienced a significant reduction in MABP compared to TBI+normoxia. There were no significant differences between the 15-min hypoxia and TBI+15-min hypoxia groups, or between the 30-min hypoxia and TBI+30-min hypoxia groups.
Acute neuronal degeneration in hippocampal fields
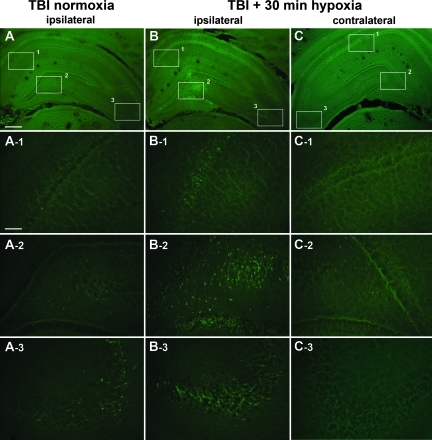
In the 15- and 30-min hypoxia-only groups, no Fluoro-Jade B-positive cells were observed in the hippocampal fields 24 h post-hypoxic insult. Visual inspection of the TBI normoxia group revealed a typical pattern of neuronal degeneration (Fluoro-Jade B-positive staining), evident primarily in the ipsilateral CA2–CA3 and granular layer of the DG, with some scattered degenerating neurons in the ipsilateral CA1, CA3c, and hilus (Fig. 2A, A-1, A-2, and A-3). Visual inspection of the TBI+30 min immediate hypoxia group revealed a greater number of degenerating neurons in ipsilateral hippocampal fields (Fig. 2B, B-1, B-2, and B-3), and recruitment of scattered degenerating neurons in the contralateral granular layer and hilar fields (Fig. 2C-2); no neuronal degeneration was observed in the contralateral CA1, CA2–CA3, and CA3c (Fig. 2C, C-1, and C-3).
FIG. 2.
Fluoro-Jade B histofluorescence of degenerating neurons 24 h after traumatic brain injury (TBI) in the hippocampus. The micrographs are representative coronal sections (–3.6 mm bregma) of the dorsal hippocampus stained with Fluoro-Jade, which labels degenerating neurons with bright green fluorescence. The numbered rectangular frames in panels A, B, and C delineate the higher-magnification micrographs shown below, and respectively labeled as A-1 (CA1), A-2 (hilus/dentate gyrus [DG]/CA3c), A-3 (CA2–CA3), and so forth. In the TBI normoxia group, degenerating neurons visualized by Fluoro-Jade staining were found primarily in the ipsilateral CA2–CA3 (A-3), some in hilus/DG/CA3c (A-2), and a few scattered degenerating neurons in CA1 (A-1). In the TBI+30-min hypoxia group, a large increase in the number of degenerating neurons was observed in the ipsilateral CA-1 (B-1), hilus/DG/CA3c (B-2), and CA2–CA3 (B-3). In the contralateral hippocampus of the TBI+30-min hypoxia group, degenerating neurons were only found in the hilus/DG (C-3; scale bars in A, B, and C=100 μm; in A-1, A-2, A-3, B-1, B-2, B-3, C-1, C-2, and C-3=20 μm). Color images available online at www.liebertonline.com/neu
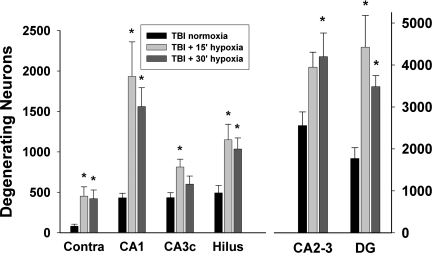
Based on our visual inspection, neuronal degeneration was then quantified in five ipsilateral hippocampal fields. The largest number of degenerating neurons, regardless of injury type, was found in the CA2–CA3 and DG granule cells. No degenerating neurons were detected with Fluoro-Jade B in the contralateral CA1, CA2–CA3, or CA3c. Scattered degenerating neurons were detected in the contralateral hilus and DG granule cells, and were combined and labeled as “Contra” in Figure 3. In the ipsilateral ROIs, the mean CE was 0.051 (range 0.040–0.064).
FIG. 3.
Quantification of degenerating hippocampal neurons 24 h after traumatic brain injury (TBI) with immediate hypoxia. Degenerating neurons were quantified in hippocampal fields of the ipsilateral hippocampus using stereological techniques. In the contralateral hippocampus degenerating neurons were only observed in the hilar and dentate gyrus (DG) fields; their quantification was combined and labeled “Contra.” Compared to the TBI normoxia group, both durations of hypoxia (15 or 30 min) immediately following TBI significantly increased the number of degenerating neurons in most of the ipsilateral hippocampal fields and in the combined contra group. Values are mean±standard error of the mean (*p<0.05 compared to TBI normoxia).
An analysis by group determined that the numbers of degenerating neurons were significantly different between the TBI normoxia and TBI+15 or 30 min hypoxia groups in the ipsilateral CA1 [F(2,18)=6.48, p<0.01], CA2–CA3 [F(2,18)=3.49, p<0.05], CA3c [F(2,18)=3.99, p<0.05], hilus [F(2,18)=4.921, p<0.05], DG granule cells [F(2,18)=6.96, p<0.01], and in the contralateral hippocampus [F(2,18)=4.06, p<0.05]. In the TBI+15 min hypoxia group, Dunnett post-hoc tests revealed significant increases (p<0.05) of degenerating neurons in the TBI+15 min post-traumatic hypoxia group in the ipsilateral CA1, C3c, hilus, granular layer, and contralateral hippocampus, compared to the TBI normoxia group (Fig. 3). In the TBI+30 min hypoxia group, Dunnett post-hoc tests revealed significant increases (p<0.05) in degenerating neurons in the ipsilateral CA1, CA2–CA3, hilus, DG granule cells, and the contralateral hippocampus, compared to the TBI normoxia group (Fig. 3)
Experiment 2
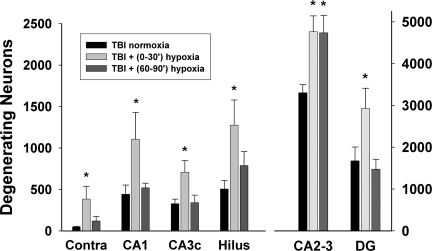
In Experiment 2, the numbers of degenerating neurons were significantly different between groups in all fields [CA1: F(2,18)=3.92, p<0.05; CA2–CA3: F(2,18)=6.85, p<0.01; CA3c: F(2,18)=4.90, p<0.05; hilus: F(2,18)=4.06, p<0.05; granular layer: F(2,18)=4.59, p<0.05; combination of contralateral hilus and granular layer: F(2,18)=4.36, p<0.05]. Comparing all TBI+hypoxia groups to the TBI normoxia group, a post-hoc Dunnett test indicated a significant increase of degenerating neurons in the TBI+immediate 30 min hypoxia group in all ipsilateral hippocampal fields, as well as in the combined contralateral hilus and granular layer (each p<0.05). In the TBI+delayed 30 min hypoxia group, only the number of degenerating neurons in the ipsilateral CA2–CA3 was significantly increased compared to the TBI normoxia group (p<0.05; Fig. 4). The mean CE was 0.070 (range 0.034–0.084).
FIG. 4.
Quantification of degenerating hippocampal neurons 24 h after traumatic brain injury (TBI) with delayed hypoxia. Degenerating neurons were quantified in hippocampal fields of the ipsilateral hippocampus using stereological techniques. In the contralateral hippocampus degenerating neurons were limited to the hilar and dentate gyrus (DG) fields, and their quantification was combined and labeled as “Contra.” Thirty minutes of hypoxia initiated at 60 min after TBI [labeled “TBI+(60–90’) hypoxia”] only increased the number of degenerating neurons in the ipsilateral CA2–CA3. The TBI+immediate 30 min hypoxia condition [labeled “TBI+(0–30’) hypoxia”] significantly increased the number of degenerating neurons in all hippocampal fields, essentially replicating the data seen in Figure 3. Values are mean±standard error of the mean (*p<0.05 compared to TBI normoxia).
Experiment 3
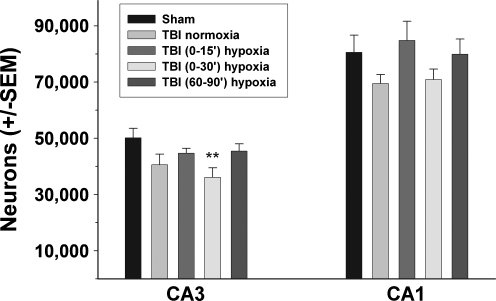
In Experiment 3, the numbers of long-term surviving neurons were significantly different between groups in the CA3 field [F(4,29)=3.048, p<0.05]. Post-hoc Dunnett's test revealed that the TBI group with 30 min of immediate hypoxia had a significant reduction in surviving pyramidal neurons compared to the sham TBI group (p<0.01; Fig. 5). The number of surviving neurons in the CA1 field were not significantly different between groups [F(4,29)=1.436, p=0.25]. The mean CE was 0.067 (range 0.050–0.090).
FIG. 5.
Quantification of surviving hippocampal neurons 14 days after traumatic brain injury (TBI) with immediate or delayed hypoxia. Surviving neurons were quantified in the CA1 and CA3 hippocampal fields using stereological techniques. Thirty minutes of hypoxia initiated immediately after TBI [labeled “TBI+(0–30’) hypoxia”] significantly decreased the numbers of surviving neurons in the CA3 compared to the TBI normoxia group. Values are mean±standard error of the mean (SEM; **p<0.01 compared to sham TBI).
EEG analysis
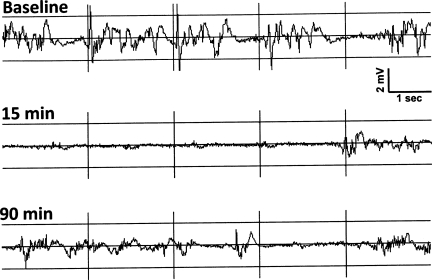
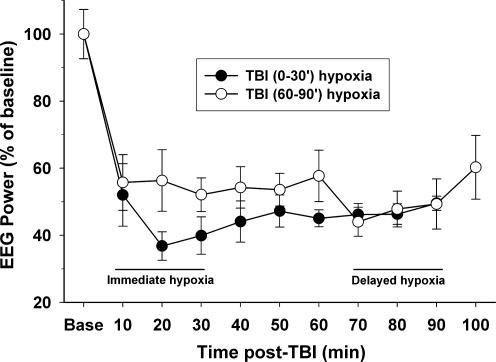
Raw EEG traces of TBI with 30 min of immediate hypoxia showed a pronounced reduction in amplitude after TBI with some recovery of amplitude by 90 min after TBI (Fig. 6). EEG power across all time points was significantly reduced after TBI (main effect of time [F(9,73)=15.87, p<0.001]). In the TBI with delayed hypoxia group, the EEG power deceased to ∼55% of baseline for the 60 min of recording after TBI, and was further reduced to 45–50% during the delayed hypoxia phase, and then recovered to ∼55% during the 10 min of return to normoxia (Fig .7). In the TBI with immediate hypoxia group, the EEG power deceased to below 40% of baseline at 20 and 30 min after hypoxia. Upon return to normoxia, the immediate hypoxia group remained between 45–50% of baseline. During the first 30 min after TBI, there was a trend for the immediate hypoxia group to have greater suppression of EEG power compared to TBI alone (delayed hypoxia group) [F(1,10)=2.94, p=0.117]. There were no statistical differences between groups [F(1,8)=0.117, p=0.742].
FIG. 6.
Cortical electroencephalogram (EEG) trace of traumatic brain injury (TBI) with 30 min of immediate hypoxia. The EEG was recorded from two skull screws located 1 mm rostral and 1 mm right lateral to the bregma and a second located 1 mm caudal to the lambda on the midline. The traces are from a single representative rat subjected to TBI with immediate hypoxia (FiO2=11%) for 30 min. The upper trace is a 10-sec sample of a baseline EEG prior to TBI. The middle trace is 15 min into the hypoxia phase after TBI and shows a robust reduction in EEG amplitude with a burst of EEG suggestive of burst suppression. The lower trace is 90 min after TBI (60 min after return to normoxia) and shows some recovery of EEG amplitude, but it is still reduced compared to baseline.
FIG. 7.
Cortical electroencephalogram (EEG) power analysis following traumatic brain injury (TBI) with hypoxia. Spectral power between 1 and 3 kHz was measured using standard fast Fourier transform (FFT) analysis. The raw EEG data were recorded over the entire experiment and divided into 10-min epochs. The FFT epochs were averaged for animals in each group at baseline (Base; prior to TBI), and for 90 or 100 min after TBI. Changes in EEG power after TBI were normalized to the percentage of baseline power for each group. There was a significant (p<0.001) reduction in power from baseline for both groups over time. During the first 30 min after TBI, there was a trend for the immediate hypoxia group to have greater suppression of EEG power compared to TBI alone (delayed hypoxia group).
Discussion
In this study, 15 or 30 min of hypoxia (FiO2=11%) alone or immediately following moderate lateral fluid percussion TBI in the rat caused a significant 20–30 mm Hg decrease in MABP, a decreased arterial PaO2 to ∼30 mm Hg, and a low oxygen saturation of 55–60%. Both durations of hypoxia immediately after TBI significantly increased the number of degenerating neurons detected with Fluoro-Jade B staining in all hippocampal fields examined in the ipsilateral hippocampus, and additional degenerating neurons were detected in the contralateral hilus and dentate granular cell fields where there was no evidence of degenerating neurons following TBI or hypoxia alone. When the initiation of a 30-min hypoxia period was delayed for 60 min post-TBI, even though there were significant effects of hypoxia on all of the physiological measurements, the increased neuronal vulnerability was limited to the CA2–CA3 fields. At 2 weeks post-TBI, only the TBI with 30 min of immediate hypoxia resulted in a significant decrease in surviving neurons in the CA3 field. An important aspect of these findings is that hypoxia occurring immediately after TBI is especially detrimental to neuronal survival, and that even delayed hypoxia can exacerbate damage to particularly vulnerable brain regions. Analysis of the EEG after TBI revealed a large decrease in EEG power with the immediate hypoxia group trending toward greater depression of EEG power compared to the delayed hypoxia group. The increased vulnerability to even 15 min of immediate hypoxia (FiO2=11%) after TBI has implications for the importance of maintaining proper ventilation and oxygenation in emergency response situations, while the delayed hypoxia further substantiates the importance of maintaining proper oxygenation in the acute hospital care and intensive care units for TBI patients.
Systemic hypoxia changed blood pressure
The level of hypoxia used in this study produced a rapid and significant reduction in systemic blood pressure in the hypoxia-alone conditions and in combination with TBI. The hypoxia/hypotension alone condition did not produce detectable neuronal degeneration as evaluated with Fluoro-Jade B staining 24 h later. These results are consistent with previous studies reporting that short periods of systemic hypoxia in the rat induce significant hypotension in the absence of pathological brain lesions (Matsushita et al., 2000), and produce no cell death or indicators of apoptosis in mice (Mikrogianakis et al., 2007). Furthermore, multiple short (15 min) exposures to hypoxia in the immature rat produced minimal functional alterations or morphological changes in the hippocampus either acutely or chronically (Owens et al., 1997). The ability of the mature uninjured brain to withstand short periods of hypoxia/hypotension (15 or 30 min in the present study) is likely due to cerebral pressure autoregulation that maintains adequate cerebral blood flow (CBF) during alterations in systemic blood pressure and oxygenation (Czosnyka et al., 2009). In contrast, the traumatically injured brain is more susceptible to episodes of hypoxia/hypotension since cerebral pressure autoregulation is compromised by TBI in a number of species (DeWitt and Prough, 2009), including rats (Engelborghs et al., 2000; Prat et al., 1997), cats (DeWitt et al., 1992; Lewelt et al., 1980), and humans (Bouma and Muizelaar, 1990; Bouma et al., 1992; Czosnyka et al., 2001; Kirkness et al., 2001).
However, a recent study of fluid percussion with controlled hemorrhagic hypotension in rats reported that cerebral pressure autoregulation became impaired only when systemic blood pressure was below 60 mm Hg (Bedell et al., 2004). In the present study, MABP fell below 60 mm Hg only during the last 5 min of the 30-min hypoxia condition, suggesting that cerebral pressure autoregulation may have only been compromised during the later part of the more severe hypoxia/hypotension condition. However, the presence of low blood oxygen saturation (55–60%) during hypoxia/hypotension in the present study may have exacerbated the alterations in cerebrovascular autoregulation responses seen after TBI, and subsequently contributed to increased neuronal cell death in the hippocampus.
Immediate post-traumatic hypoxia exacerbated hippocampal neuronal degeneration
Immediate post-traumatic hypoxia for 15 min or 30 min significantly increased the number of degenerating neurons in all dorsal hippocampal fields examined compared to TBI alone. Furthermore, both durations of immediate post-traumatic hypoxia recruited degenerating neurons in the contralateral hilus and dentate granular cell fields. Degenerating neurons were detected with Fluoro-Jade B, an anionic fluorochrome capable of selectively staining neurons including cell bodies and their processes in brain tissue undergoing degeneration resulting from a variety of neurotoxic insults (Anderson et al., 2005; Schmued and Hopkins, 2000; Schmued et al., 1997). The 24-h end-point was chosen as a time point of maximal neuronal degeneration based on previous TBI studies that examined Fluoro-Jade staining ranging from 30 min to 7 days post-injury (Hallam et al., 2004; Zhao et al., 2003). It is interesting that 15 or 30 min of hypoxia immediately after TBI produced very similar degrees of acute neuronal degeneration, indicating that a period as short as 15 min of hypoxia is sufficient to exacerbate pathological changes associated with TBI. However, examination of the ipsilateral hippocampal fields at 14 days after TBI revealed that the number of surviving neurons were significantly decreased only in the TBI with 30 min of immediate hypoxia condition, and only in the CA3 field. This evidence indicates that 30 min of immediate hypoxia after TBI is a more damaging insult than either shorter periods of immediate hypoxia (15 min) or 30 min of delayed hypoxia.
It is clear from the data that in both the TBI with 15 or 30 min of hypoxia groups there were significant increases in Fluoro-Jade B-positive neurons at 24 h that did not correspond with significant decreases in long-term cell survival at 14 days after injury. One should also bear in mind that the significant differences seen between groups in the acute neuronal degeneration evaluations encompassed several hundred dying neurons, while the differences between groups in cumulative cell survival, whether statistically significant or not, was at least one order of magnitude greater. For example, an increase of 1500 Fluoro-Jade-positive neurons after TBI with 30 min of immediate hypoxia compared to TBI alone was found to be significant. However, a decrease of 10,000 surviving cells between these same groups at 14 days after injury was not statistically significant. Thus, the longer-term neuronal survival comparison is likely a more conservative evaluation than a comparison of acute neuronal cell death.
Although not measured in this study, the fluid percussion model is associated with acute elevations in intracranial pressure (ICP) that are further elevated with combined hypoxia via hypoventilation (Anderson and Atkinson, 2003). The combination of reduced MABP from the hypoxia and increased ICP from the TBI may have been sufficient to reduce cerebral perfusion pressure (CPP) to a level approaching ischemia in the vulnerable hippocampal fields where increased neuronal degeneration was detected. Even short periods of reduced blood flow can have an impact on the survival of neurons in the hippocampus after TBI. For example, a previous study demonstrated that combining a short duration of forebrain ischemia with a mild fluid percussion TBI produced extensive hippocampal neuronal death, even though neither insult alone was sufficient to produce hippocampal cell death (Jenkins et al., 1989). Ito and colleagues demonstrated that weight-drop TBI in rats produced a rapid rise in ICP that reached a maximum value (28±3 mm Hg) at 30 min (Ito et al., 1996). When hypoxia and hypotension were associated with weight-drop TBI, a major increase in ICP and decreases in MABP, CPP, and local CBF were observed (Ito et al., 1996; Ract et al., 2001). Combined effects of hypoxia and hypotension have also been confirmed clinically in a recent meta-analysis by the International Mission on Prognosis and Analysis of Clinical Trials in TBI (IMPACT) group. In a review of over 5600 patients from 7 different clinical trials, a single episode of hypoxia or hypotension early after injury dramatically increased the likelihood of unfavorable outcome at 6 months (McHugh et al., 2007). The effects of both hypoxia and hypotension were found to be sub-additive; the combined effect of both insults was significantly higher than either insult alone, but less than the sum of the two effects. Therefore, a combination of reduced MABP, increased ICP, and reduced CPP, coupled with impairment of cerebral autoregulation and reduced blood oxygen saturation, could contribute to the detrimental effects of the imposed secondary hypoxia following TBI in this study.
The EEG analysis in the present study indicates that imposed hypoxia did not result in a flat-line EEG indicative of cerebral ischemia. However, EEG power was significantly reduced in both the immediate and delayed hypoxia TBI groups, indicative of disruption of normal brain function. There was a trend for TBI with immediate hypoxia to produce a greater suppression of EEG power. Although the EEG patterns were not indicative of classic cerebral ischemia, the trend for greater EEG suppression with immediate hypoxia, along with the dampened EEG recovery after return to normoxia with this group, suggests a more severe insult compared to the delayed hypoxia group. These findings are consistent with the greater degree of acute and cumulative cell death seen in the immediate hypoxia group.
Regional patterns of neuronal degeneration in the hippocampus have been documented following fluid percussion injury in the rat with differing degrees of detail, revealing that the CA2–CA3 fields, hilus, and the DG granule cells are the most vulnerable populations of neurons (Anderson et al., 2005; Hallam et al., 2004; Sato et al., 2001; Zhong et al., 2005). Several studies have reported that post-traumatic hypoxia increased the number of degenerating neurons in the hippocampus (Bramlett et al., 1999b; Clark et al., 1997; Nawashiro et al., 1995), but they did not compare changes in cell death at acute and chronic time points. In the present study we performed detailed stereological quantification of acute (24 h) degenerating neurons in each of the dorsal hippocampal fields. The results demonstrated that TBI combined with immediate hypoxia/hypotension causes exacerbation of acute neuronal degeneration across all hippocampal fields, but a 1-h delay of hypoxia following TBI produced increased vulnerability only in the CA2–CA3 area. These findings have important implications, since specific hippocampal fields contribute differentially to behavioral components of memory (Amaral and Witter, 1995). Studies have illustrated that specific hippocampal fields subsume different cognitive functions. For example, the DG in conjunction with the CA3 participates in spatial pattern separation, the CA3 supports short-term memory, while the CA1 field supports processes associated with temporal pattern association and intermediate-term memory (Gilbert et al., 2001; Kesner et al., 2004; Okada and Okaichi, 2009). The results of our investigation indicate that TBI without hypoxia resulted in marked neuronal degeneration, primarily in the CA2–CA3 and DG fields, and minimal neuronal degeneration in other fields at 24 h after injury. The recruitment of significant numbers of degenerating neurons in all hippocampal fields when hypoxia is combined with TBI provides anatomical evidence for the worse cognitive performance reported by others when TBI is followed hypoxia (Bramlett et al., 1999a). Furthermore, these data suggest that future behavioral studies that are related to region-specific cell death are warranted.
One critical mechanism of post-traumatic hypoxia contributing to exacerbated hippocampal damage is excitatory amino acid-related neurotoxicity. It is well recognized that excessive excitatory amino acid elevation is strongly associated with excitotoxicity and neuronal degeneration. Glutamate, the principal excitatory neurotransmitter in the central nervous system, when released in excess causes excitotoxic damage to neurons through activation of the AMPA and NMDA glutamate receptors (Faden et al., 1989; Globus et al., 1995; Katayama et al., 1990; Meldrum, 2000). Studies show that extracellular glutamate is elevated immediately after TBI (Faden et al., 1989; Globus et al., 1995; Katayama et al., 1990; Zhong et al., 2006). Real-time monitoring found that both the concentration and duration of excessive glutamate were strongly increased with post-traumatic hypoxia (FiO2=10%; Matsushita et al., 2000), whereas periods of hypoxia alone fail to cause significant elevation of glutamate in the extracellular space (Katoh et al., 1997). Thus immediate hypoxia following TBI is likely to exacerbate glutamate excitotoxicity.
Delayed post-traumatic hypoxia selectively increased neuronal degeneration in CA2–CA3
The present study also evaluated the effect of delayed post-traumatic hypoxia on neuronal degeneration in hippocampal fields. The majority of previous post-traumatic hypoxia studies employed immediate secondary hypoxia and reported deleterious effects on cell survival, cognitive function, and long-term outcome (Bauman et al., 2000; Bramlett et al., 1999a; Clark et al., 1997; Matsushita et al., 2000; Mikrogianakis et al., 2007; Nawashiro et al., 1995). Delaying the initiation of the 30-min hypoxia insult for 1 h after TBI failed to enhance the neuronal degeneration in all but one of the hippocampal fields studied. Only in the CA2–CA3 fields did the delayed hypoxia result in an increase in neuronal degeneration compared to TBI alone. The reduction in vulnerability in most of the hippocampal fields later after experimental TBI may be due to the subsiding of acute events such as indiscriminate depolarization and accompanying neurotransmitter release (e.g., glutamate and catecholamines). However, the time course of neurotransmitter release may be extended in clinical situations. For example, clinical studies of TBI using microdialysis have documented very high concentrations of extracellular glutamate persisting for days after injury (Bullock et al., 1998). Nevertheless, the persisting elevation in cell death in the CA2–CA3 indicates that this field is particularly vulnerable to secondary insults.
In summary, this study quantitatively evaluated neuronal death in the rat hippocampal fields following TBI combined with immediate or delayed hypoxia. Hypoxia was associated with a significant reduction in MABP that recovered quickly after cessation of the hypoxia condition. Fifteen or 30 min of hypoxia in the absence of TBI did not cause neuronal degeneration. However, both durations of hypoxia initiated immediately after TBI caused significant exacerbation of neuronal degeneration compared to TBI alone. When 30 min of hypoxia alone was delayed for 1 h after TBI, increased neuronal degeneration was only observed in the CA2–CA3 fields. This model of fluid percussion TBI coupled with controlled secondary hypoxia may be useful in evaluating therapeutic interventions targeted at excitotoxicity and hippocampal vulnerability. The difference between hippocampal field vulnerability to the timing of secondary hypoxia indicates that the time course of pathological mechanisms may differ after TBI alone and TBI with hypoxia, and that different therapeutic strategies may be needed. Two of the limitations of the lateral fluid percussion model have been severity of injury and the lack of co-morbidities that are frequently experienced by patients suffering moderate and severe TBI. This model addresses both of those concerns, as TBI+hypoxia injured animals have a significant increase in cell death throughout the hippocampus, and this appears to be a reliable model for TBI in conjunction with a clinically relevant co-morbidity. Finally, given the prevalence and importance of hypotension and hypoxia in clinical TBI, these data strongly support the importance of monitoring and promptly managing secondary hypoxia.
Acknowledgments
This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke grant NS29995, and the Center for Biophotonics Science and Technology, a designated NSF Science and Technology Center, managed by the University of California at Davis, under cooperative agreement number PHY 0120999. Expert technical assistance was provided by Rahil Ghiasvand and Darrin Lee.
Author Disclosure Statement
No competing financial interests exist.
References
- Amaral D.G. Witter M.P. The hippocampal formation. In: The Rat Nervous System. In: Paxinos G., editor. Academic Press; San Diego: 1995. pp. 443–493. [Google Scholar]
- Anderson K.J. Miller K.M. Fugaccia I. Scheff S.W. Regional distribution of fluoro-jade B staining in the hippocampus following traumatic brain injury. Exp. Neurol. 2005;193:125–130. doi: 10.1016/j.expneurol.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Anderson R.E. Atkinson J.L. Intracranial pressure response to severe head injury induced apnea and catecholamine surge. J. Trauma. 2003;54:550–554. doi: 10.1097/01.TA.0000047049.64695.69. [DOI] [PubMed] [Google Scholar]
- Aoyama N. Lee S.M. Moro N. Hovda D.A. Sutton R.L. Duration of ATP reduction affects extent of CA1 cell death in rat models of fluid percussion injury combined with secondary ischemia. Brain Res. 2008;1230:310–319. doi: 10.1016/j.brainres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales J.W. Ma X. Yan H.Q. Jenkins L.W. Dixon C.E. Expression of protein phosphatase 2B (calcineurin) subunit A isoforms in rat hippocampus after traumatic brain injury. J. Neurotrauma. 2010;27:109–120. doi: 10.1089/neu.2009.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y.H. Bramlett H.M. Atkins C.M. Truettner J.S. Lotocki G. Alonso O.F. Dietrich W.D. Post-traumatic seizures exacerbate histopathological damage after fluid-percussion brain injury. J. Neurotrauma. 2011;28:35–42. doi: 10.1089/neu.2010.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman R.A. Widholm J.J. Petras J.M. McBride K. Long J.B. Secondary hypoxemia exacerbates the reduction of visual discrimination accuracy and neuronal cell density in the dorsal lateral geniculate nucleus resulting from fluid percussion injury. J. Neurotrauma. 2000;17:679–693. doi: 10.1089/089771500415427. [DOI] [PubMed] [Google Scholar]
- Bedell E.A. DeWitt D.S. Uchida T. Prough D.S. Cerebral pressure autoregulation is intact and is not influenced by hypothermia after traumatic brain injury in rats. J. Neurotrauma. 2004;21:1212–1222. doi: 10.1089/neu.2004.21.1212. [DOI] [PubMed] [Google Scholar]
- Bouma G.J. Muizelaar J.P. Relationship between cardiac output and cerebral blood flow in patients with intact and with impaired autoregulation. J. Neurosurg. 1990;73:368–374. doi: 10.3171/jns.1990.73.3.0368. [DOI] [PubMed] [Google Scholar]
- Bouma G.J. Muizelaar J.P. Stringer W.A. Choi S.C. Fatouros P. Young H.F. Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J. Neurosurg. 1992;77:360–368. doi: 10.3171/jns.1992.77.3.0360. [DOI] [PubMed] [Google Scholar]
- Bramlett H.M. Dietrich W.D. Green E.J. Secondary hypoxia following moderate fluid percussion brain injury in rats exacerbates sensorimotor and cognitive deficits. J. Neurotrauma. 1999a;16:1035–1047. doi: 10.1089/neu.1999.16.1035. [DOI] [PubMed] [Google Scholar]
- Bramlett H.M. Green E.J. Dietrich W.D. Exacerbation of cortical and hippocampal CA1 damage due to posttraumatic hypoxia following moderate fluid-percussion brain injury in rats. J. Neurosurg. 1999b;91:653–659. doi: 10.3171/jns.1999.91.4.0653. [DOI] [PubMed] [Google Scholar]
- Bullock R. Zauner A. Woodward J.J. Myseros J. Choi S.C. Ward J.D. Marmarou A. Young H.F. Factors affecting excitatory amino acid release following severe human head injury. J. Neurosurg. 1998;89:507–518. doi: 10.3171/jns.1998.89.4.0507. [DOI] [PubMed] [Google Scholar]
- Chesnut R.M. Marshall L.F. Klauber M.R. Blunt B.A. Baldwin N. Eisenberg H.M. Jane J.A. Marmarou A. Foulkes M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Chi J.H. Knudson M.M. Vassar M.J. McCarthy M.C. Shapiro M.B. Mallet S. Holcroft J.J. Moncrief H. Noble J. Wisner D. Kaups K.L. Bennick L.D. Manley G.T. Prehospital hypoxia affects outcome in patients with traumatic brain injury: a prospective multicenter study. J. Trauma. 2006;61:1134–1141. doi: 10.1097/01.ta.0000196644.64653.d8. [DOI] [PubMed] [Google Scholar]
- Clark R.S. Kochanek P.M. Dixon C.E. Chen M. Marion D.W. Heineman S. DeKosky S.T. Graham S.H. Early neuropathologic effects of mild or moderate hypoxemia after controlled cortical impact injury in rats. J. Neurotrauma. 1997;14:179–189. doi: 10.1089/neu.1997.14.179. [DOI] [PubMed] [Google Scholar]
- Czosnyka M. Brady K. Reinhard M. Smielewski P. Steiner L.A. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit. Care. 2009;10:373–386. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- Czosnyka M. Smielewski P. Piechnik S. Steiner L.A. Pickard J.D. Cerebral autoregulation following head injury. J. Neurosurg. 2001;95:756–763. doi: 10.3171/jns.2001.95.5.0756. [DOI] [PubMed] [Google Scholar]
- Davis D.P. Dunford J.V. Poste J.C. Ochs M. Holbrook T. Fortlage D. Size M.J. Kennedy F. Hoyt D.B. The impact of hypoxia and hyperventilation on outcome after paramedic rapid sequence intubation of severely head-injured patients. J. Trauma. 2004;57:1–8. doi: 10.1097/01.ta.0000135503.71684.c8. discussion 8–10. [DOI] [PubMed] [Google Scholar]
- Davis D.P. Meade W. Sise M.J. Kennedy F. Simon F. Tominaga G. Steele J. Coimbra R. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J. Neurotrauma. 2009;26:2217–2223. doi: 10.1089/neu.2009.0940. [DOI] [PubMed] [Google Scholar]
- DeWitt D.S. Prough D.S. Blast-induced brain injury and posttraumatic hypotension and hypoxemia. J. Neurotrauma. 2009;26:877–887. doi: 10.1089/neu.2007.0439. [DOI] [PubMed] [Google Scholar]
- DeWitt D.S. Prough D.S. Taylor C.L. Whitley J.M. Deal D.D. Vines S.M. Regional cerebrovascular responses to progressive hypotension after traumatic brain injury in cats. Am. J. Physiol. 1992;263:H1276–H1284. doi: 10.1152/ajpheart.1992.263.4.H1276. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Lyeth B.G. Povlishock J.T. Findling R.L. Hamm R.J. Marmarou A. Young H.F. Hayes R.L. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Engelborghs K. Haseldonckx M. Van Reempts J. Van Rossem K. Wouters L. Borgers M. Verlooy J. Impaired autoregulation of cerebral blood flow in an experimental model of traumatic brain injury. J. Neurotrauma. 2000;17:667–677. doi: 10.1089/089771500415418. [DOI] [PubMed] [Google Scholar]
- Faden A.I. Demediuk P. Panter S.S. Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- Faul M. Xu L. Wald M.M. Coronado V.G. Centers for Disease Control and Prevention, Control NCfIPa. U.S. Department of Health and Human Services; Atlanta: 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. [Google Scholar]
- Gilbert P.E. Kesner R.P. Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Globus M.Y. Alonso O. Dietrich W.D. Busto R. Ginsberg M.D. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J. Neurochem. 1995;65:1704–1711. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- Goodman M.D. Makley A.T. Huber N.L. Clarke C.N. Friend L.A. Schuster R.M. Bailey S.R. Barnes S.L. Dorlac W.C. Johannigman J.A. Lentsch A.B. Pritts T.A. Hypobaric hypoxia exacerbates the neuroinflammatory response to traumatic brain injury. J. Surg. Res. 2010 doi: 10.1016/j.jss.2010.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K.D. Lifshitz J. Diffuse traumatic brain injury initially attenuates and later expands activation of the rat somatosensory whisker circuit concomitant with neuroplastic responses. Brain Res. 2010;1323:161–173. doi: 10.1016/j.brainres.2010.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T.M. Floyd C.L. Folkerts M.M. Lee L.L. Gong Q.Z. Lyeth B.G. Muizelaar J.P. Berman R.F. Comparison of behavioral deficits and acute neuronal degeneration in rat lateral fluid percussion and weight-drop brain injury models. J. Neurotrauma. 2004;21:521–539. doi: 10.1089/089771504774129865. [DOI] [PubMed] [Google Scholar]
- Hellewell S.C. Yan E. Bye N. Agyapomaa D. Morganti-Kossmann C. Post-traumatic hypoxia exacerbates brain tissue damage: Analysis of axonal injury and glial responses. J. Neurotrauma. 2010 doi: 10.1089/neu.2009.1245. [DOI] [PubMed] [Google Scholar]
- Ito J. Marmarou A. Barzo P. Fatouros P. Corwin F. Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J. Neurosurg. 1996;84:97–103. doi: 10.3171/jns.1996.84.1.0097. [DOI] [PubMed] [Google Scholar]
- Jenkins L.W. Moszynski K. Lyeth B.G. Lewelt W. DeWitt D.S. Allen A. Dixon C.E. Povlishock J.T. Majewski T.J. Clifton G.L. Young H.F. Becker D.P. Hayes R.L. Increased vulnerability of the mildly traumatized rat brain to cerebral ischemia: the use of controlled secondary ischemia as a research tool to identify common or different mechanisms contributing to mechanical and ischemic brain injury. Brain Res. 1989;477:211–224. doi: 10.1016/0006-8993(89)91409-1. [DOI] [PubMed] [Google Scholar]
- Jiang J.Y. Gao G.Y. Li W.P. Yu M.K. Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J. Neurotrauma. 2002;19:869–874. doi: 10.1089/08977150260190456. [DOI] [PubMed] [Google Scholar]
- Katayama Y. Becker D.P. Tamura T. Hovda D.A. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- Katoh H. Sima K. Nawashiro H. Wada K. Chigasaki H. The effect of MK-801 on extracellular neuroactive amino acids in hippocampus after closed head injury followed by hypoxia in rats. Brain Res. 1997;758:153–162. doi: 10.1016/s0006-8993(97)00213-8. [DOI] [PubMed] [Google Scholar]
- Kesner R.P. Lee I. Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev. Neurosci. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Kirkness C.J. Mitchell P.H. Burr R.L. Newell D.W. Cerebral autoregulation and outcome in acute brain injury. Biol. Res. Nurs. 2001;2:175–185. doi: 10.1177/109980040100200303. [DOI] [PubMed] [Google Scholar]
- Lewelt W. Jenkins L.W. Miller J.D. Autoregulation of cerebral blood flow after experimental fluid percussion injury of the brain. J. Neurosurg. 1980;53:500–511. doi: 10.3171/jns.1980.53.4.0500. [DOI] [PubMed] [Google Scholar]
- Lowenstein D.H. Thomas M.J. Smith D.H. McIntosh T.K. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J. Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley G. Knudson M.M. Morabito D. Damron S. Erickson V. Pitts L. Hypotension, hypoxia, and head injury: frequency, duration, and consequences. Arch. Surg. 2001;136:1118–1123. doi: 10.1001/archsurg.136.10.1118. [DOI] [PubMed] [Google Scholar]
- Matsushita Y. Shima K. Nawashiro H. Wada K. Real-time monitoring of glutamate following fluid percussion brain injury with hypoxia in the rat. J. Neurotrauma. 2000;17:143–153. doi: 10.1089/neu.2000.17.143. [DOI] [PubMed] [Google Scholar]
- McHugh G.S. Engel D.C. Butcher I. Steyerberg E.W. Lu J. Mushkudiani N. Hernandez A.V. Marmarou A. Maas A.I. Murray G.D. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J. Neurotrauma. 2007;24:287–293. doi: 10.1089/neu.2006.0031. [DOI] [PubMed] [Google Scholar]
- McIntosh T.K. Vink R. Noble L. Yamakami I. Fernyak S. Soares H. Faden A.L. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Meldrum B.S. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J. Nutr. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Mikrogianakis A. Shaye R.E. Griffin P. Kawesa S. Lockwood J. Gendron N.H. Gaboury I. Merali Z. Mackenzie A.E. Hutchison J.S. Hypoxia alters the expression of inhibitor of apoptosis proteins after brain trauma in the mouse. J. Neurotrauma. 2007;24:338–353. doi: 10.1089/neu.2006.003615. [DOI] [PubMed] [Google Scholar]
- Nawashiro H. Shima K. Chigasaki H. Selective vulnerability of hippocampal CA3 neurons to hypoxia after mild concussion in the rat. Neurol. Res. 1995;17:455–460. [PubMed] [Google Scholar]
- Okada K. Okaichi H. Functional differentiation and cooperation among the hippocampal subregions in rats to effect spatial memory processes. Behav. Brain Res. 2009;200:181–191. doi: 10.1016/j.bbr.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Owens J., Jr. Robbins C.A. Wenzel H.J. Schwartzkroin P.A. Acute and chronic effects of hypoxia on the developing hippocampus. Ann. Neurol. 1997;41:187–199. doi: 10.1002/ana.410410210. [DOI] [PubMed] [Google Scholar]
- Prat R. Markiv V. Dujovny M. Misra M. Evaluation of cerebral autoregulation following diffuse brain injury in rats. Neurol. Res. 1997;19:393–402. doi: 10.1080/01616412.1997.11740832. [DOI] [PubMed] [Google Scholar]
- Ract C. Vigue B. Bodjarian N. Mazoit J.X. Samii K. Tadie M. Comparison of dopamine and norepinephrine after traumatic brain injury and hypoxic-hypotensive insult. J. Neurotrauma. 2001;18:1247–1254. doi: 10.1089/089771501317095287. [DOI] [PubMed] [Google Scholar]
- Sato M. Chang E. Igarashi T. Noble L.J. Neuronal injury and loss after traumatic brain injury: time course and regional variability. Brain Res. 2001;917:45–54. doi: 10.1016/s0006-8993(01)02905-5. [DOI] [PubMed] [Google Scholar]
- Schmued L.C. Albertson C. Slikker W., Jr. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Schmued L.C. Hopkins K.J. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Van Putten H.P. Bouwhuis M.G. Muizelaar J.P. Lyeth B.G. Berman R.F. Diffusion-weighted imaging of edema following traumatic brain injury in rats: effects of secondary hypoxia. J. Neurotrauma. 2005;22:857–872. doi: 10.1089/neu.2005.22.857. [DOI] [PubMed] [Google Scholar]
- West M.J. Slomianka L. Gundersen H.J. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zhao X. Ahram A. Berman R.F. Muizelaar J.P. Lyeth B.G. Early loss of astrocytes after experimental traumatic brain injury. Glia. 2003;44:140–152. doi: 10.1002/glia.10283. [DOI] [PubMed] [Google Scholar]
- Zhong C. Zhao X. Sarva J. Kozikowski A. Neale J.H. Lyeth B.G. NAAG peptidase inhibitor reduces acute neuronal degeneration and astrocyte damage following lateral fluid percussion TBI in rats. J. Neurotrauma. 2005;22:266–276. doi: 10.1089/neu.2005.22.266. [DOI] [PubMed] [Google Scholar]
- Zhong C. Zhao X. Van K.C. Bzdega T. Smyth A. Zhou J. Kozikowski A.P. Jiang J. O'Connor W.T. Berman R.F. Neale J.H. Lyeth B.G. NAAG peptidase inhibitor increases dialysate NAAG and reduces glutamate, aspartate and GABA levels in the dorsal hippocampus following fluid percussion injury in the rat. J. Neurochem. 2006;97:1015–1025. doi: 10.1111/j.1471-4159.2006.03786.x. [DOI] [PubMed] [Google Scholar]