Abstract
Zebrafish (Danio rerio) have an innate tendency to join shoals. Based on this, we refined visual choice tests to focus on social interaction and novelty preference. Our design follows mouse three-chamber sociability protocols, except testing is conducted under 940 Lux fluorescent lighting. Initially, we compared performance among zebrafish strains: inbred (AB) or wild-crossbred (WIK) from Zebrafish International Resource Center, to golden and short-fin from Petco stores. AB fish exhibited a preference for shoaling; they dwelled longest near transparent boxes containing zebrafish, while short-fin favored blue boxes without fish. AB and golden exhibited a strong preference for social novelty, not evident in short-fin or WIK fish. Serotonin and cannabinoids shape mammalian social behavior, and equivalents of both receptor types are expressed in the zebrafish brain. We examined the effects of the cannabinoid receptor agonist WIN55,212-2 (1 mg/L), or 5-HT1A agonist buspirone (10 mg/L) on Petco short-fin social choice. Fish were bath-exposed to test compounds for 10 min, under these conditions [3H]CP55,940 (4 nM) bound to brain with a concentration of 1.9-6.4 fmol/mg 5-30 min afterward. Social approach was measured 20 min following acclimation to the testing arena. WIN55,212-2 and buspirone increased short-fin dwelling near boxed zebrafish. In zebrafish whole brain homogenates, buspirone displaced [3H]8-OH-DPAT (dissociation constant, KD = 16r±1.2 nM) with an inhibition constant (Ki) of 1.8±1.0 nM lower than that of WAY100,635 (Ki ~1000 nM). These fish social choice tests may enhance social behavior research, and are useful for studying the effects of genetic manipulations, pharmaceuticals or environmental toxins.
Keywords: Danio rerio; social behavior; cannabinoid; 5-HT1A receptor; visual discrimination; anxiolytics; [3H]8-OH-DPAT binding; buspirone; WIN55,212-2
Introduction
Zebrafish (Danio rerio) are visually drawn to conspecifics, and adults instinctively aggregate into shoals (Krause et al., 2000; Savernio & Gerlai, 2008; Miller & Gerlai, 2011). We utilized this innate tendency to develop fish visual choice tests of preference for social interaction and novelty, based on the mouse three-chamber sociability tests utilized in autism research (Silverman et al., 2010). Initially, we compared performance among different zebrafish lines, paralleling screening of mouse strains for social behavior (e.g. Moy et al., 2004). Several established wild-type zebrafish lines (hereafter referred to as strains) are available from the Zebrafish International Resource Center (http://zebrafish.org). Of these, AB is the oldest inbred laboratory strain, while WIK is recently derived from wild-caught zebrafish (Spence et al., 2008). In contrast, short-fin “zebra Danios” from pet stores are outbred (Gerlai et al., 2009). Several pigment pattern mutations occur in short-fin zebrafish, including a ‘golden’ form, in which melanin pigmentation is reduced due to a recessive cation exchanger (slc24a5) gene polymorphism affecting melanosomes (Lamason et al., 2005). Anxiolytic-sensitive responses to novel environments differ among AB, WIK and outbred zebrafish with pigment mutations (Sackerman et al., 2010; Egan et al., 2009). Given the phenotypic and genotypic differences among these lines, we hypothesized that they would have different behavioral preferences for social interactions. We also examined whether zebrafish exhibit preference for social novelty in this context.
In contrast to mice, zebrafish are diurnal and are therefore more responsive to color cues. Favoring short wavelengths, they use color discrimination to perform associative learning tasks (Colwill et al., 2005; Risner et al., 2006). Zebrafish retinas have four cone types, sensitive to UV, violet, blue-green or yellow, and their range of spectral responses indicates a potential capacity for fine short-wavelength color discrimination (Connaughton & Nelson, 2010). To enhance color contrast for visual discrimination, zebrafish shoalmate seeking behavior was tested on a white background with lighting of greater intensity (940 Lux) than that in their home aquaria (178 Lux).
In rodents, social interaction behavior is enhanced by anxiolytics, including those targeting serotonin 5-HT1A receptors such as buspirone (File & Seth, 2003; Gould et al., 2011). Cannabinoid agonists such as WIN 55,212-2 have anxiolytic properties in mice, and the endogenous cannabinoid anandamide was found to enhance their social interaction (Haller et al., 2007, Umathe et al., 2009). Hence, we sought to determine if 5-HT1A and cannabinoid agonists could also enhance zebrafish social behavior. We hypothesized that bath exposure to buspirone or WIN55,212-2 would bind to sites in the zebrafish brain and promote shoalmate seeking. Equivalents of both cannabinoid and 5-HT1A receptors occur in zebrafish (Rodriguez-Martinez et al., 2007; Airhart et al., 2007). The pharmacological properties of cannabinoid receptors have been characterized in the zebrafish brain (Rodriguez-Martinez et al., 2007). However, the pharmacological properties of zebrafish central serotonin 5-HT1A-receptor-like binding sites were not well characterized. Hence, we measured the saturation binding of [3H] 8-hydroxy-N,N-dipropylaminotetralin (8-OH-DPAT) in zebrafish whole-brain membranes, and determined the relative affinity of busprione and WAY 100,635 for those sites.
Materials and Methods
Animals and housing
Adult zebrafish (> 6 months old) of the short-fin and golden long-fin variety were obtained from Petco (San Diego, CA, USA) local franchises (San Antonio, TX, USA). AB and WIK zebrafish adults (> 6 months) were obtained from the Zebrafish International Resource Center (ZIRC Eugene, OR, USA, http://zebrafish.org). The zebrafish were housed in groups of 6-8 within the 3L tanks of a benchtop aquatic habitat (Aquatic Eco-Systems, Apopka, FL, USA) filled with 25-27°C de-ionized water supplemented with 200 mg/L “Instant Ocean” salts (Aquarium Systems, Mentor, OH, USA). Light/dark cycles were 14:10 h (lights on at 0700 h and off at 2100 h). Fish acclimated to the housing tanks for two weeks or longer prior to behavioral testing, and were fed “Top Fin” tropical flakes (Pacific Coast Distributing, Phoenix, AZ, USA). Fish were of mixed gender, with equal proportions. All procedures were approved by the University of Texas Health Science Center Institutional Animal Care and Use Committee (IACUC), and were in accordance with National Institutes of Health (NIH) guidelines (http://oacu.od.nih.gov/ARAC). The zebrafish tested weighed 270 ± 23 mg on average.
Behavioral testing arena and conditions
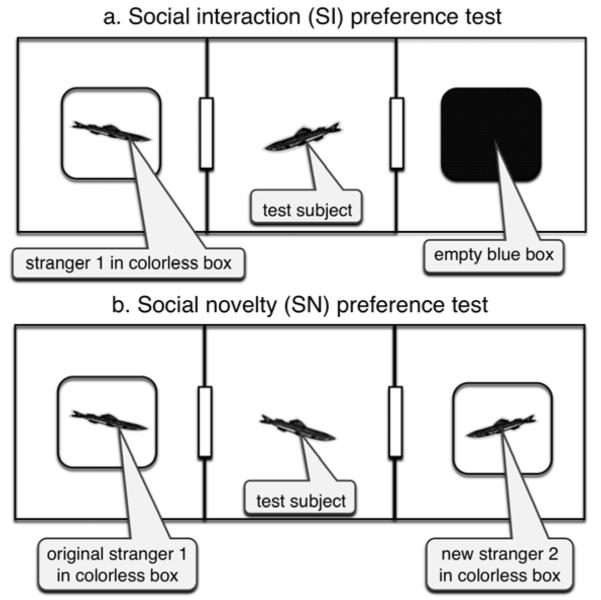
Social choice testing was conducted between 1000 and 1400 h under fluorescent lighting with an intensity of 940 Lux, as measured by light meter (Extech Instruments, Waltham, MA, USA). Testing took place in a 30 cm × 10 cm rectangular arena within a transparent acrylic fish offset cross maze (Ezra Scientific, San Antonio, TX, USA, http://www.ezrascientific.com). The 71 cm long × 51 cm wide × 10 cm deep maze can be subdivided into 10 cm2 units throughout by removable transparent acrylic drop-in doors. The maze arm was lined on the outsides and underneath by 3 mm thick white foam-board (Crafts Etc., Oklahoma City, OK, USA). The interior 3-unit rectangular testing arena (30 cm × 10 cm) was divided into 3 chambers (10 cm × 10 cm) by plastic canvas mesh inserts (3 mm holes #7, Darice Inc., Strongsville, OH, USA) with center and bottom aligned 3.2 cm2 openings that served as entryways when drop-in doors were removed (Figure 1). The maze was filled with habitat water to a depth of 5.2 cm, and was 6 cm deep when stimulus boxes were added. The maze water was refreshed between testing trials for each fish. Each zebrafish was only used once as a behavioral test subject. The offset cross-maze (Figure a) and side views of the testing arena (Figures b and c) are shown in the Wiley Online supplement to this manuscript.
Figure 1. Top view of the aquatic three-chamber arena set up for (a) social interaction (SI) and (b) social novelty (SN) tests.
In both tests, subject fish were free to explore the arena for 10 min while their behavior was video-recorded. In the SI test, subjects could approach and dwell near an empty blue box or a stranger zebrafish in a transparent box. In the SN preference test the blue box was replaced by a colorless one with a new stranger, so subjects could visit and linger near “old” stranger 1 or “new” stranger 2. Subjects and strangers were able to see each other, but due to relative depth of the water and box enclosures, odor cues were absent. Chambers were partially separated by mesh walls with small interconnecting doorways (represented by vertical rectangles in the diagrams) during testing. Three-dimensional side view figures of the test arena appear in a supplementary appendix to this manuscript on the Wiley Online Library accessible via link through Genes, Brain and Behavior.
Visual social interaction and novelty test protocols for lone zebrafish
Acclimation
Individual zebrafish subjects were transferred by net into the center chamber of the testing arena, in which the drop-in doors were closed. Subjects were confined to the center for 10 min to recover from the shock of transfer and acclimate to the arena and lighting. Then the doors to the side chambers were simultaneously removed so the fish could explore all three chambers for another 10 min. During this time, fish were observed for obvious physiological or drug-induced mobility impairments. Ideally, fish should visit both ends of the test arena during acclimation, as all fish in this study did. If not, acclimation time could be extended by a few minutes, or investigators may consider their possible exclusion. After the acclimation session, the fish were again confined to the center chamber by first closing the door to the unoccupied chamber. Most subject fish noticed this, and approached the closed door, so the second door was closed behind them. For less responsive fish, dipping the second door into the water behind the fish (repeatedly if necessary) induced swimming to the center. This process took 30 sec in most cases, but always less than 2 min. Importantly, subjects were not forced into the center by chasing them with nets or doors prior to testing. Chasing zebrafish with an object could establish a confounding side-avoidance bias (Levin & Chen, 2004; Lau et al., 2011).
Pilot Stimulus Preference Studies
To select dichotomous choice stimuli, the preferences of 40 Petco short-fin male and female zebrafish were measured. Colorless, orange or blue transparent acrylic boxes (5.7 cm × 5.7 cm × 10.5 cm tall, AMAC, Sausalito, CA, from the Container Store, Dallas, TX, USA) were filled to 6 cm deep with aquarium water. Male vs. female (both 260 mg), or large female (>300 mg) vs. small lt (<120 mg) short-fin zebrafish from a local pet store (Alamo Aquatics and Exotics, San Antonio, TX) were used as alternate stimuli in the colorless boxes. Preference for empty colorless, orange or blue boxes was also tested. The blue boxes resembled the shade and hue of the back and cover of fish home tanks. After acclimation, different stimuli boxes were placed at opposite ends of the arena in a randomized pattern, and the arena doors were opened for testing. The first arena end entered, and the latency to enter was recorded for each subject fish. Based on the results (see table 1), large female fish and blue boxes were used as stimuli in all subsequent tests.
Table 1. Initial preference of short-fin zebrafish in dichotomous choice tests.
| Stimulus variables |
Latency (sec) |
Zebrafish subject choices*: | |
|---|---|---|---|
| Option 1 | Option 2 | ||
| Fish gender (colorless box) |
55 ± 10 |
Female (62.5%) 3 males 2 females |
Male (37.5%) 1 male 2 females |
| Fish size (colorless box) |
53 ±13 |
Large (75%) 3 males 3 females |
Small (25%) 1 male 1 female |
| Box color (blue vs. orange) |
71 ± 12 |
Blue (71%) 2 males 3 females |
Orange (29%) 2 males 0 females |
| Box color (blue vs. colorless) |
76 + 9 |
Blue (80%) 4 males 4 females |
Colorless (20%) 1 male 1 female |
Overall preferences, based on initial approach, are for large fish and females, and blue boxes, as opposed to small fish or males and orange or colorless boxes. Male zebrafish preferred females; females had no gender preference. Females and males preferred blue over colorless, while males approached blue and orange boxes equally.
Social interaction (SI) and social novelty (SN) preference tests
Shoalmate-seeking behavior of each subject fish was observed and video-recorded by digital camera (Photosmart R742, Hewlett-Packard, Palo Alto, CA, USA) in two consecutive 10 min tests, social interaction followed by social novelty. In social interaction (SI) tests, following subject acclimation and confinement to the center, a colorless water-filled transparent acrylic box containing a stranger fish, and a blue water-filled transparent acrylic box without a fish, were each placed at opposite ends of the testing arena. Stranger zebrafish were female short-fin adults (> 9 months old, 311 ± 7 mg, N = 8) obtained from a different branch store. Strangers were housed in a separate tank on a higher shelf, hidden from the test subjects. Stranger fish were used repeatedly, and box placements were randomized between trials. Once the boxes were situated on either end, the central chamber doors were removed so the subject was free to explore the boxes in the arena for 10 min. Figure 1a is a top view illustration of the SI test setup.
After the SI test, the subject was confined to the central chamber. The door to the chamber unoccupied by the fish was closed first, and the second door was gently and repeatedly dipped until submerged by 2 cm into the water at least 2 cm behind the subject fish until it swam to the arena center (≥ 30 sec). For social novelty (SN) testing the blue box was replaced by a colorless box containing a “new” stranger zebrafish, while the “old” stranger box was repositioned at the opposite end (Figure 1b). Again, the central chamber doors were removed so the subject could explore the arena for 10 min while their behavior was video-recorded.
Effects of acute drug exposure on zebrafish SI and SN preference
Naïve Petco short-fin zebrafish were exposed to drugs dissolved in 250 mL of habitat water for 10 min prior to social choice behavior testing. Afterward, they were transferred by net to a holding beaker containing home-tank water for 5 min, and then introduced into the testing arena for acclimation, SI and SN testing (see Figure 1). The following drug treatments were used: 1 mg/L WIN55,212-2 dissolved in 0.1% dimethyl sulfoxide (DMSO), or 10 mg/L buspirone dissolved in tank water (Sigma, St. Louis, MO, USA). Habitat water with and without 0.1% DMSO was used in control treatments.
Video and Statistical Analysis
The video-recorded behavior was observed for each subject’s chamber entries and the total amount of time spent in each chamber by at least two independent observers who were unaware of the treatment groupings. The resultant data were averaged together for each fish to produce a single data set for statistical analysis. Inter-group means and variance for the zebrafish line comparisons and the drug treatment groups were compared by repeated measures (mixed model) MANOVA for time spent in chamber with the empty blue box vs. the stranger fish in a box in SI tests and for time spent with old vs. new strangers in SN tests. Significant results were analyzed further by test phase with mixed model ANOVA followed by Fisher’s LSD post-hoc using Statistica (Statsoft, Tulsa, OK, USA). This analysis is also used for mouse three-chamber sociability tests, as reviewed in Silverman et al. (2010).
Radioligand Uptake in Bath Exposures
Zebrafish were bath-exposed to 4 nM of tritiated cannabinoid agonist [3H]CP55,940 (Perkin-Elmer, Waltham, MA) for 10 min, then transferred to ligand-free water for either 5 min or 30 min prior to sacrifice. Fish brains, viscera and muscle samples were weighed, homogenized in scintillation fluid (Ecolume, Fisher Scientific, USA) and agitated on a shaker (setting 4) for 12 h. [3H] CP55,940 content was measured on a liquid scintillation counter (Beckman, Brea, CA).
Saturation and displacement binding to 5-HT1A-like sites in zebrafish brain
[3H]8-Hydroxy-N,N-dipropylaminotetralin (8-OH-DPAT) binding was performed in adult zebrafish brain homogenates using methods adapted from Chamberlain et al. (1993). Whole brains from 12-16 adult zebrafish of mixed gender were homogenized in 50 mM Tris-HCl wash buffer, pH 7.4 at 4°C, and centrifuged for 10 min at 30,600 × g at 4°C. Resultant pellets were re-suspended in buffer, pre-incubated for 20 min at 37°C to remove endogenous serotonin, and centrifuged again. Final membrane pellets were suspended in 12 ml of 50 mM Tris-HCl, 2.5 mM MgCl2 assay buffer, pH 7.4 at 37°C. Protein content was measured spectrophotometrically using Bradford reagent (Sigma, St. Louis, MO). Binding assays were carried out in triplicate in assay buffer for 1 hour at 37°C. [3H] 8-OH-DPAT (Perkin-Elmer, Boston, MA) concentrations ranged from 0.1 to 12 nM for saturation, and was 1 nM for displacement assays. Non-specific binding was defined by adding 20 μM serotonin to subsets of tubes in the saturation assays. Buspirone and WAY100,635 (Sigma) at concentrations from 0.5 pM - 5 μM were used to displace [3H] 8-OH-DPAT in competition assays. Incubations were terminated with 4 mL of wash buffer, pH 7.4 at 4°C. Labeled homogenates were captured by vacuum filtration onto 0.5% polyethyleneimine-soaked glass fiber filters with a tissue harvester (Brandel, Gaithersburg, MD), and were washed twice more with 4 mL buffer each time. Tritium-bound to tissue trapped on filters was counted using a liquid scintillation counter (Packard, Downers Grove, IL) with 60% efficiency. Data were analyzed by non-linear regression using DeltaGraph (Red Rock, Salt Lake City, UT).
Results
Pilot Preference Studies
Short-fin zebrafish initially entered arena chambers containing large or female fish, and blue boxes more often in pilot preference tests. Four female and 3 male fish did not enter any chamber within 5 min. First box entrances by 33 zebrafish, as well as mean ± S.E.M. latency to enter are summarized in table 1.
Global line differences in social interaction vs. preference for social novelty
Between the social interaction and social novelty tests, repeated measures MANOVA revealed significant strain differences in behavioral patterns, and an overall shift in time spent by subjects from the arena side containing the stranger 1 fish in the first phase, to the side containing the new stranger 2 in the second phase (SN) of testing, with no significant interactions (STRAIN λ 6,76 = 0.72, R = 2.3, P < 0.047; CHAMBER λ 2,38 = 0.74, R = 6.6, P < 0.003; INTERACTION λ 6,76 = 0.90, R = 0.70, P = 0.65). One social novelty test video was lost due to camera malfunction, hence AB and Petco standard zebrafish sample size was reduced to 11 for the analysis of the SN test.
Line differences in social interaction (SI)
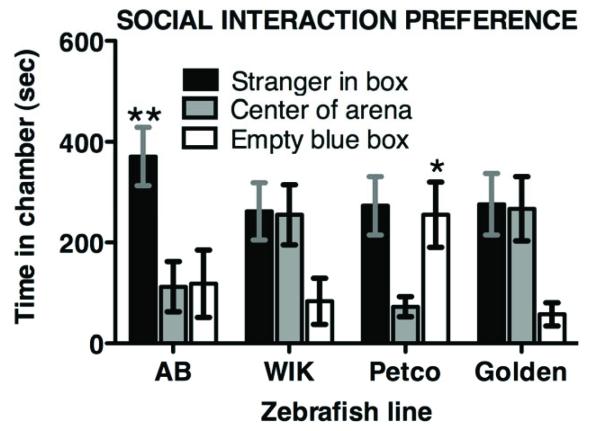
For the SI test, mixed model ANOVA indicated differences in time spent in the chamber with the stranger fish vs. the empty blue box among zebrafish lines, without a significant interaction effect (STRAIN F 3,41 = 3.9, P < 0.016; CHAMBER F 1,41 = 9.41, P < 0.004; INTERACTION F 3,41 = 0.96, P = 0.42). Specifically, as shown in Figure 2, AB fish spent proportionately more time in the chamber with the stranger fish than the other zebrafish lines (Fisher’s LSD P < 0.05). Also, Petco short-fin zebrafish spent proportionately more time in the chamber with the empty blue box than either golden or WIK fish (P < 0.01). Time in arena center is typically not analyzed in rodent social interaction tests (Silverman et al. 2010), however both WIK and golden spent more time in the center than AB or Petco short-fin (ONE-WAY F 3,41 = 3.88, P < 0.015), and this appears to have contributed to the observed line differences. Chamber entries did not differ significantly among lines (F 3,41 = 1.4, P = 0.27), they ranged from 18 ± 6 for AB, to 41 ± 10 entries for golden zebrafish.
Figure 2. Behavioral differences among zebrafish lines in the SI preference test.
AB line zebrafish spent a significantly greater proportion of time in the chamber with the stranger fish (**p < 0.05), while Petco short-fin zebrafish spent more time with the empty blue box (*p < 0.01). N = 12 fish, except N = 9 for golden. The differences observed among lines were not due to differences in the number of chamber entries the fish made, which averaged 29 ± 7 for all lines.
Social novelty preference trends among strains
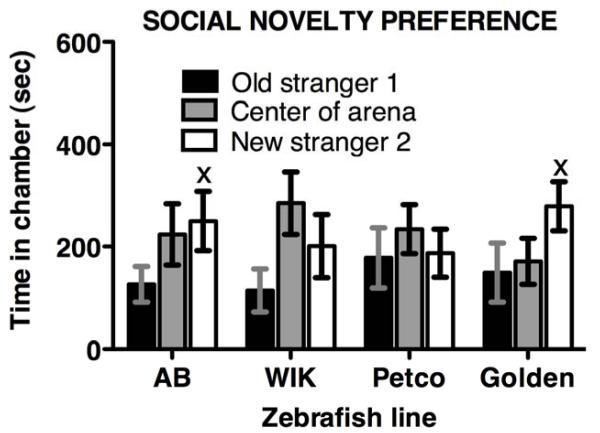
For the test of preference for social novelty, mixed model ANOVA indicated no significant performance differences among zebrafish lines, but a strong trend toward more time spent with the new (stranger 2) vs. old (stranger 1) fish was observed, without a significant interaction (STRAIN F 3,39 = 0.68, P = 0.57; CHAMBER F 1,39 = 3.86, P ≤ 0.056; INTERACTION F 3,39 = 0.39, P = 0.76). As shown in Figure 3, AB and golden behavior contributed the most to the trend toward new stranger preference (Fisher’s LSD P = 0.056), while Petco short-fin spent an equivalent amount of time with stranger 1 and stranger 2. The average number of chamber entries during the social novelty test did not differ among lines (F 3,39 = 0.83, P = 0.48), and ranged from 42 ± 10 in WIK to 65 ± 13 entries in golden zebrafish.
Figure 3. Performance of different zebrafish lines in the SN preference test.
There were no significant differences among lines in the time spent in chambers with new vs. old strangers. However, there was a strong trend among AB and golden zebrafish toward spending more time in the chamber with the new stranger (1) vs. old stranger (2) (Xp = 0.06). Mean chamber entries were similar among lines and averaged 48 ± 11.
Acute effects of anxiolytics on zebrafish social preferences
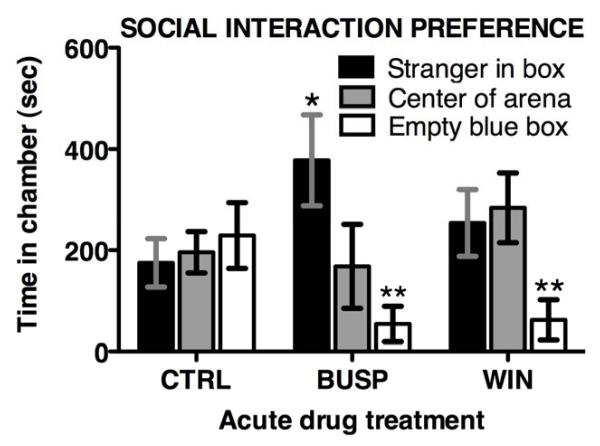
In the social interaction test, there was a significant difference in the proportional time in chambers among exposure groups (CHAMBERF 1,18 = 5.4, P < 0.035). Petco short-fin zebrafish exposed to either 10 mg/L of the 5-HT1A agonist buspirone (N = 5) or 1 mg/L of the synthetic cannabinoid receptor agonist WIN 55,212-2 (N = 7) spent less time in the chambers with the blue boxes, and buspirone-treated fish dwelled longer by stimulus fish than controls (N = 9) (F 2,18 = 3.35, P ≤ 0.058; Fisher’s LSD P < 0.05), as shown in Figure 4. Chamber entries did not differ among drug treatment groups, and ranged from 21 ± 9 in buspirone-treated fish to 33 ± 8 in control fish. The vehicle treatment group was pooled from fish treated with tank-water, either containing (N = 4) or without (N = 5) 0.1% DMSO. There was no difference in mean chamber entries or time spent in each chamber among these control groups (F1,7 > 1.3, P ≥ 0.28). In the social novelty test, there were no significant drug effects, chamber side preferences or interactions (DRUGTMT F 2,18 = 0.64, P = 0.53; CHAMBER F 1,18 = 0.09, P = 0.77; INTERACTION F 2,18 = 0.3, P = 0.74). These data appear as a graph (figure d) in the Wiley Online supplement.
Figure 4. Acute effects of anxiolytic treatments on Petco short-fin zebrafish performance in the SI Test.
Buspirone (BUSP) increased time spent in the chamber with a stranger fish (*p < 0.05), and both buspirone and WIN 55,212 (WIN) reduced time spent near the blue box (*p< 0.05). N=5-9. Entries for all treatment groups were similar and averaged 29 ± 9.
Radioligand uptake from bath exposures
Zebrafish (4, mean weight 260 ± 10 mg) bath-exposed to 4 nM of the cannabinoid agonist [3H]CP55,940 for 10 min had a mean ± S.E.M. content of 6.4 ± 1.5 fmol/mg in brain, 1.9 ± 0.2 fmol/mg in viscera, and 2.3 ± 0.6 fmol/mg in lateral muscles when sacrificed 5 min after exposure. Another set of 4 zebrafish, mean weight of 380 ± 40 mg, had a [3H]CP55,940 content of 1.6 ± 0.3 fmol/mg in brain, 2.5 ± 0.3 fmol/mg in viscera, and 0.6 ± 0.1 fmol/mg in muscles when sacrificed 30 min after exposure. Assuming a similar, linear relationship, approximately 1 pmol/mg of WIN55,212-2 may have been in the zebrafish brain at the time of testing.
Binding to 5-HT1A-like sites in zebrafish brain
Saturation binding of [3H]8-OH-DPAT to its binding sites in adult zebrafish whole-brain homogenates fit a single-site binding curve with dissociation constant (KD) of 16 ± 5.2 nM and maximal binding (Bmax) = 973 ± 451 fmol/mg protein by nonlinear regression (Wiley Online supplement figure e), and a KD = 16 ± 1.2 nM and Bmax = 890 ± 225 fmol/mg protein by Scatchard analysis. Displacement of [3H]8-OH-DPAT by buspirone and WAY 100,635 revealed inhibition constants (Ki) of 1.8 ± 1 nM and 1034 ± 253 nM, respectively (supplement figure f).
Discussion
Rationale for refinement of zebrafish visual social behavior testing
Learned social preference for shoalmates with different pigment patterns was initially characterized for nacre mutant (albino), heterozygote and wild-type AB zebrafish in a dichotomous choice paradigm (Engeszer et al., 2004). It has also been used to examine neurohormonal effects on social choice in zebrafish from a pet store (Braida et al., 2011). Zebrafish shoaling preferences become fixed in juveniles, and they depend upon the predominant shoalmate phenotype present during this time (Engeszer et al., 2007). Olfactory cues that zebrafish utilize for individual recognition (Gerlach et al., 2008) should not have influenced the subjects in these, or the present study, because stimulus fish were in watertight containers.
In a targeted study of zebrafish visual preference in which images of zebrafish were graphically manipulated, the unaltered image was generally preferred, and yellow-hued fish were also favored (Saverino & Gerlai, 2008). Given that zebrafish have rich short-wavelength color vision capacity, and use colors to perform discriminative learning tasks, it is not surprising that they swim toward and linger near blue striped “wild-type” zebrafish as a product of their strong instinct to shoal, combined with visual imprinting and acuity (Risner et al., 2006; Miller & Gerlai, 2011).
However, there may be an element of novelty-induced anxiety in rodent social behavior tests (File & Seth, 2003), and also in our zebrafish social preference tests. Anxiolytics can enhance mouse social behavior that is otherwise impaired (e.g. Gould et al., 2011; Pobbe et al., 2011). In zebrafish, oxytocin, isotocin, arginine-vasopressin and vasotocin influenced both social behavior and fear response with inverted U-shape dose-response relationships (Braida et al., 2011). White backgrounds activate fear centers in the zebrafish brain, and are initially avoided by 90% of adults (Lau et al., 2011). White avoidance by zebrafish is more pronounced under high intensity lighting (Stephenson et al., 2011). In this context, we gave zebrafish the choice of approaching another zebrafish, or a blue box resembling elements of their home tank. Zebrafish behavior is consistent with preference for the color blue in absence of other fish (Table 1 and Colwill et al., 2005).
The 20 min acclimation in our protocol allows subject fish to recover from the initial stress of being netted and transferred to the novel test arena before testing. Zebrafish introduced into a novel tank have initially low swimming rates that increase over the first three minutes of observation (Levin et al., 2007). Also, following 1 min of air exposure and return to water, zebrafish whole body cortisol levels recovered to within 30% of baseline values after 20 min (Fuzzen et al., 2010). Acclimation also allows full recovery of visual acuity in the well-lit (940 Lux) testing arenas after transfer from mesoptic to scotopic lighting conditions (178 Lux) in home tanks. Some mutations or drug treatments (such as cannabinoid ligands) may alter cone responses to light onset or offset (Struik et al., 2006; Yazulla, 2008). Zebrafish retinas exhibit CB1 and TRPV1-like immunoreactivity, so targeting these receptors could alter initial ability to discriminate visual targets if light intensity changes (Adams et al., 1978; Yazulla, 2008). Hence, acclimation before testing can eliminate some potentially confounding factors.
Finally, in our paradigm zebrafish must enter end chambers through narrow doorways to approach strangers or the blue box, preserving this element of mouse three-chambered sociability tests (Silverman et al., 2010 and Wiley Online supplement, figures b & c). In contrast, conventional fish dichotomous choice tests measure dwelling at either end of an open tank, devoid of any retreat-inhibiting stenosis (e.g. Engeszer et al., 2004; Grossman et al., 2010; Lau et al., 2011). These modifications could make social behavior testing in zebrafish and other teleosts more sensitive and robust.
Social and anxiety-related behavioral differences among zebrafish lines
Among zebrafish lines, we observed that AB spent more time in the arena chamber with a stranger fish than others, while Petco short-fin zebrafish spent more time in the chamber with the empty blue box (see Figure 2). AB and golden zebrafish exhibited a trend toward preference for social novelty that was absent in WIK and Petco fish (see Figure 3). In other studies, short-fin zebrafish from pet shops had strong social preferences (Grossman et al., 2010; Braida et al., 2011). However, the specific choice among another fish or a larger blue object was not presented in those studies. We could speculate that Petco short-fin zebrafish dwelled near blue boxes longer due to an anxiety prone-phenotype, consistent with our earlier findings of more bottom dwelling and scototaxis by them in novel environments (Sackerman et al., 2010). Yet in this same study, AB and Petco short-fin behaved in an “anxious” manner, while WIK behavior was more consistent with a “low anxiety” state. Thus, performance in anxiety tests does not necessarily indicate how zebrafish will behave in social preference tests. Preference of Petco short-fin zebrafish for blue boxes could be influenced by factors such as their environment during juvenile imprinting (Engeszer et al., 2007), and warrants further investigation.
Behavioral effects of anxiolytic CB1 or 5-HT1A agonists
Buspirone or WIN 55,212 treated Petco short-fin zebrafish spend more time in the chamber with the stranger fish vs. the blue box, consistent with findings of their sociability promotion in mice (Gould et al., 2011; Umathe et al., 2009). These agonists of CB1 and 5-HT1A G-protein coupled receptors have been shown to reduce anxiety in zebrafish and rodent models by other measures (Patel & Hillard, 2006; Bencan et al., 2009; Lalonde & Strazielle, 2010; Lau et al., 2011). Our 10 mg/L buspirone dose was based on a prior study in which only doses above 6 mg/L were anxiolytic in zebrafish (Bencan et al., 2009). Two buspirone-treated fish were dropped from our analysis because they appeared to be sedated; each made ≤ 2 box entries and spent > 580 sec touching the arena floor. Examination of a wider range of buspirone doses, and teasing apart its pharmacological effects at 5-HT1A like-receptors from the dopamine and adrenergic receptors that it may be targeting would clarify its properties in zebrafish.
Serotonin 5-HT1A transcripts can be measured in zebrafish, and are downregulated in the spinal cord following juvenile fluoxetine exposures (Airhart et al., 2007). However, in vitro 5-HT1A-like binding properties had not been characterized in the zebrafish brain prior to our study. In the teleost arctic charr (Salvelinus alpinus), high affinity [3H] serotonin binding to brain membrane homogenates was inhibited by 8-OH-DPAT at two sites (Ki1 = 1.7 and Ki2 = 82 nM), and by buspirone at one site (Ki = 32 nM) (Winberg & Nilsson, 1996). These affinities are comparable to what we found in zebrafish. Paradoxically, the 5-HT1A antagonist WAY 100,635 had lower affinity in zebrafish brain homogenates (Ki ~ 1000 nM) relative to its affinity (Ki ~ 1 nM) in mice. Yet lower affinity of WAY100,635 in teleost fish is consistent with findings in electric fish, wherein concentrations of 2.5 mM of WAY100,635 increased electric organ discharge, while 8-OH-DPAT robustly diminished it at 0.025 mM (Allee et al., 2008). 50% of [3H] 8-OH-DPAT binding was non-specific, and only partially displaced by buspirone and WAY 100,635 in our assays. [3H] 8-OH-DPAT may be binding to other non-5-HT1A sites in the zebrafish brain, including transporters of serotonin and other receptor types, consistent with what occurs in the rat brain (Assié & Koek, 2000).
Cannabinoid CB1 receptors were characterized in zebrafish, and CP55,940 binds to this population, while WIN 55,212-2 bind to CB1, and equivalents of CB2 receptors too (Rodriguez-Martinez et al., 2007). We utilized 1 mg/L of WIN 55,212-2, because synthetic cannabinoid ligands generally have greater affinities for their receptors and behavioral potencies than buspirone has for 5-HT1A receptors (Patel & Hillard, 2006; Haller at al., 2007; Rodriguez-Martinez et al., 2007). WIN 55,212-2 reduced time spent near the blue boxes in our study, but did not significantly increase dwelling near other zebrafish (Figure 4). We also demonstrated that cannabinoid ligands reach sites in the zebrafish brain through 10 min bath exposures, through the use of [3H] CP55,940 in this study, and [3H] citalopram in our prior study (Sackerman et al., 2010). This finding is important, since bath exposures of zebrafish and other teleosts are commonly utilized in pharmacology and toxicology studies to administer test compounds.
Conclusion: Potential utility of zebrafish social preference tests
Our fish social choice protocols may meet an emerging need for behavioral screens to examine the neural correlates of developmental psychiatric disorders, such as autism and schizophrenia, wherein social behavior impairments are prominent (Tropepe & Sive, 2003; Mathur & Guo, 2010; Kabashi et al., 2011). Such behavioral tests can compliment their use as a versatile genetic tool to explore candidate genes, neurodevelopmental factors, and the acute or chronic effects of pharmaceuticals and toxicants on cognitive and emotional behaviors (Ton et al., 2006; Airhart et al., 2007; Egen et al., 2009; Guo et al., 2009; Grossman et al., 2010; Wright & Washborne 2011).
Supplementary Material
Acknowledgements
This research was supported by a sub-award from T42CCT610417 from the National Institute for Occupational and Environmental Health (NIOSH)/Centers for Disease Control and Prevention to the Southwest Center for Occupational and Environmental Health, a NIOSH Education and Research Center, and an NIH/NIMH R03MH086708 award. We thank Xavier Escobedo, Charles Freeman, Monica Gamez, Peter Guerra, Victor Ordonez, and Jennifer K. Rodriguez for their technical assistance in collecting data from digital video-recordings, Dr. Lynette Daws for helpful suggestions and use of her laboratory facilities, and Irene Chapa, Director of Recruitment and Science Outreach, for the collegiate volunteer program at the University of Texas Health Science Center at San Antonio.
Footnotes
The authors have no conflicts of interest to disclose.
References
- Adams AJ, Brown B, Haegerstrom-Portnoy G, Flom MC, Jones RT. Marijuana, alcohol, and combined drug effects on the time course of glare recovery. Psychopharmacology. 1978;56:81–6. doi: 10.1007/BF00571413. [DOI] [PubMed] [Google Scholar]
- Airhart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (Prozac) Neurotoxicol Teratol. 2007;29:652–64. doi: 10.1016/j.ntt.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Allee SJ, Markham MR, Salazar VL, Stoddard PK. Opposing actions of 5HT1A and 5HT2-like serotonin receptors on modulations of the electric signal waveform in the electric fish Brachyhypopomus pinnicaudatus. Horm Behav. 2008;53:481–8. doi: 10.1016/j.yhbeh.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assié MB, Koek W. [(3)H]-8-OH-DPAT binding in the rat brain raphe area: involvement of 5-HT(1A) and non-5-HT(1A) receptors. Br J Pharmacol. 2000;130:1348–52. doi: 10.1038/sj.bjp.0703426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Donzelli A, Martucci R, Capurro V, Busnelli M, Chini B, Sala M. Neurohypophyseal hormones manipulation modulate social and anxiety-related behavior in zebrafish. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2482-2. In press PMID: 21956239. [DOI] [PubMed] [Google Scholar]
- Chamberlain J, Offord SJ, Wolfe BB, Tyau LS, Wang HL, Frazer A. Potency of 5-hydroxytryptamine1a agonists to inhibit adenylyl cyclase activity is a function of affinity for the “low-affinity” state of [3H] 8-hydroxy-N,N-dipropylaminotetralin ([3H] 8-OH-DPAT) binding. J Pharmacol Exp Ther. 1993;266:618–25. [PubMed] [Google Scholar]
- Colwill RM, Raymond MP, Ferreira L, Escudero H. Visual discrimination learning in zebrafish (Danio rerio) Behav Processes. 2005;70:19–31. doi: 10.1016/j.beproc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Nelson R. Spectral responses in zebrafish horizontal cells include a tetraphasic response and a novel UV-dominated triphasic response. J Neurophysiol. 2010;104:2407–22. doi: 10.1152/jn.00644.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeszer RE, Ryan MJ, Parichy DM. Learned social preference in zebrafish. Curr Biol. 2004;14:881–4. doi: 10.1016/j.cub.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Engeszer RE, Barbiano LA, Ryan MJ, Parichy DM. Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Anim Behav. 2007;74:1269–1275. doi: 10.1016/j.anbehav.2007.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Fuzzen ML, Van Der Kraak G, Bernier NJ. Stirring up new ideas about the regulation of the hypothalamic-pituitary-interrenal axis in zebrafish (Danio rerio) Zebrafish. 2010;7:349–58. doi: 10.1089/zeb.2010.0662. [DOI] [PubMed] [Google Scholar]
- Gerlach G, Hodgins-Davis A, Avolio C, Schunter C. Kin recognition in zebrafish: a 24-hour window for olfactory imprinting. Proc Biol Sci. 2008;275:2165–70. doi: 10.1098/rspb.2008.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Chatterjee D, Pereira T, Sawashima T, Krishnannair R. Acute and chronic alcohol dose: population differences in behavior and neurochemistry of zebrafish. Genes Brain Behav. 2009;8:586–99. doi: 10.1111/j.1601-183X.2009.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T±tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman L, Utterback E, Stewart A, Gaikwad S, Chung KM, Suciu C, Wong K, Elegante M, Elkhayat S, Tan J, Gilder T, Wu N, Dileo J, Cachat J, Kalueff AV. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav Brain Res. 2010;214:277–84. doi: 10.1016/j.bbr.2010.05.039. [DOI] [PubMed] [Google Scholar]
- Guo S. Using zebrafish to assess the impact of drugs on neural development and function. Expert Opin Drug Discov. 2009;4:715–726. doi: 10.1517/17460440902988464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Mátyás F, Soproni K, Varga B, Barsy B, Németh B, Mikics E, Freund TF, Hájos N. Correlated species differences in the effects of cannabinoid ligands on anxiety and on GABAergic and glutamatergic synaptic transmission. Eur J Neurosci. 2007;25:2445–56. doi: 10.1111/j.1460-9568.2007.05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashi E, Brustein E, Champagne N, Drapeau P. Zebrafish models for the functional genomics of neurogenetic disorders. Biochim Biophys Acta. 2011;1812:335–45. doi: 10.1016/j.bbadis.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Krause J, Butlin RK, Peuhkuri N, Pritchard VL. The social organization of fish shoals: a test of the predictive power of laboratory experiments for the field. Biol Rev Camb Philos Soc. 2000;75:477–501. doi: 10.1111/j.1469-185x.2000.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Lamason RL, Mohideen MA, Mest JR, Wong AC, Norton HL, Aros MC, Jurynec MJ, Mao X, Humphreville VR, Humbert JE, Sinha S, Moore JL, Jagadeeswaran P, Zhao W, Ning G, Makalowska I, McKeigue PM, O’donnell D, Kittles R, Parra EJ, Mangini NJ, Grunwald DJ, Shriver MD, Canfield VA, Cheng KC. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–6. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- Lau BY, Mathur P, Gould GG, Guo S. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc Natl Acad Sci. 2011;108:2581–6. doi: 10.1073/pnas.1018275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. Relations between open-field, elevated plus-maze, and emergence tests in C57BL/6J and BALB/c mice injected with GABA- and 5HT-anxiolytic agents. Fundam Clin Pharmacol. 2010;24:365–76. doi: 10.1111/j.1472-8206.2009.00772.x. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chen E. Nicotinic involvement in memory function in zebrafish. Neurotoxicol Teratol. 2004;26:731–5. doi: 10.1016/j.ntt.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90:54–8. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Mathur P, Guo S. Use of zebrafish as a model to understand mechanisms of addiction and complex neurobehavioral phenotypes. Neurobiol Dis. 2010;40:66–72. doi: 10.1016/j.nbd.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NY, Gerlai R. Shoaling in zebrafish: what we don’t know. Rev Neurosci. 2011;22:17–25. doi: 10.1515/RNS.2011.004. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–11. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Pobbe RL, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T± tf/J mouse strain. Behav Brain Res. 2011;216:446–51. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner ML, Lemerise E, Vukmanic EV, Moore A. Behavioral spectral sensitivity of the zebrafish (Danio rerio) Vision Res. 2006;46:2625–35. doi: 10.1016/j.visres.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martin I, de Velasco EM, Rodriguez RE. Characterization of cannabinoid-binding sites in zebrafish brain. Neurosci Lett. 2007;413:249–54. doi: 10.1016/j.neulet.2006.11.057. [DOI] [PubMed] [Google Scholar]
- Sackerman J, Donegan JJ, Cunningham CS, Nguyen NN, Lawless K, Long A, Benno RH, Gould GG. Zebrafish Behavior in Novel Environments: Effects of Acute Exposure to Anxiolytic Compounds and Choice of Danio rerio Line. Int J Comp Psychol. 2010;23:43–61. [PMC free article] [PubMed] [Google Scholar]
- Saverino C, Gerlai R. The social zebrafish: behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev Camb Philos Soc. 2008;83:13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- Stephenson JF, Whitlock KE, Partridge JC. Zebrafish preference for light or dark is dependent on ambient light levels and olfactory stimulation. Zebrafish. 2011;8:17–22. doi: 10.1089/zeb.2010.0671. [DOI] [PubMed] [Google Scholar]
- Struik ML, Yazulla S, Kamermans M. Cannabinoid agonist WIN 55212-2 speeds up the cone response to light offset in goldfish retina. Vis Neurosci. 2006;23:285–93. doi: 10.1017/S0952523806232127. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav. 2003;2:268–81. doi: 10.1034/j.1601-183x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Ton C, Lin Y, Willett C. Zebrafish as a model for developmental neurotoxicity testing. Birth Defects Res A Clin Mol Teratol. 2006;76:553–67. doi: 10.1002/bdra.20281. [DOI] [PubMed] [Google Scholar]
- Umathe SN, Manna SS, Utturwar KS, Jain NS. Endocannabinoids mediate anxiolytic-like effect of acetaminophen via CB1 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1191–9. doi: 10.1016/j.pnpbp.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Winberg S, Nilsson G. Multiple high-affinity binding sites for [3H] serotonin in the brain of a telost fish, the Arctic charr (Salvelinus alpinus) J Exp Biol. 1996;199:2429–35. doi: 10.1242/jeb.199.11.2429. [DOI] [PubMed] [Google Scholar]
- Wright GJ, Washbourne P. Neurexins, neuroligins and LRRTMs: synaptic adhesion getting fishy. J Neurochem. 2011;117:765–78. doi: 10.1111/j.1471-4159.2010.07141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazulla S. Endocannabinoids in the retina: from marijuana to neuroprotection. Prog Retin Eye Res. 2008;27:501–26. doi: 10.1016/j.preteyeres.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.