Abstract
Objective
To determine whether short-term antioxidant supplementation affects insulin sensitivity, endothelial adhesion molecule levels, and oxidative stress in overweight young adults.
Methods and Procedures
A randomized, double-blind, controlled study tested the effects of antioxidants (AOX) on measures of insulin sensitivity (homeostasis model assessment, HOMA and QUICKI), endothelial adhesion molecules (sICAM-1, sVCAM-1, sE-selectin), adiponectin and oxidative stress (lipid hydroperoxides, PEROX) in overweight and normal weight individuals (N=48, 18-30 years). Participants received either AOX (vitamin E 800IU, vitamin C 500mg, β-carotene 10mg) or placebo (PLC) for 8 weeks.
Results
HOMA values were initially higher in the overweight subjects and were lowered with AOX by week 8 (15% reduction, p=0.02). Adiponectin increased in both AOX groups. sICAM-1 and sE-selectin decreased in overweight AOX treated groups by 6% and 13%, respectively (p<0.05). Plasma PEROX were reduced by 0.31 and 0.70 nmol/ml in the normal weight and overweight AOX treated groups, respectively, by week 8 (p<0.05).
Discussion
AOX supplementation moderately lowers HOMA and endothelial adhesion molecule levels in overweight young adults. A potential mechanism to explain this finding is the reduction in oxidative stress by AOX. Long term studies are needed to determine whether AOX are effective in suppressing diabetes or vascular activation over time.
Keywords: obesity, antioxidant, inflammation, adiponectin
Introduction
Insulin resistance and endothelial dysfunction are two reciprocally related processes that precede metabolic and cardiovascular disease.(1) While insulin has important metabolic actions, it also regulates vascular reactivity, activation and endothelial cellular adhesion molecule (CAM) expression.(2) Both insulin resistance and endothelial dysfunction are features of obesity.(3) Oxidative stress is a common mechanism underlying insulin resistance and endothelial dysfunction, and is elevated in obesity.(4) Adiponectin is an adipocyte-derived, anti-atherogenic insulin regulating protein(5) that might in part be regulated by oxidative stress related endothelial dysfunction.(6) Thus, correcting oxidative stress may be a good strategy to combat endothelial dysfunction, insulin resistance and low adiponectin in obese individuals.
Obesity is associated with a low antioxidant defense compared with normal weight.(4) Tissue antioxidant levels are lower in obese than normal weight adults.(7, 8) Also, total antioxidant status is lower in obesity, and this antioxidant imbalance favors systemic oxidative stress.(4) Dietary antioxidants may attenuate oxidative stress. However, a controversy exists in the literature whether antioxidant supplementation actually protects against the onset of cardiovascular disease or diabetes. Small studies have shown that supplementation with individual nutrients such as vitamins C, E or A lowers against oxidative stress, CAM expression, insulin resistance and improves glucose disposal.(9-16) Other studies, however, show enhanced oxidative stress and increased insulin resistance with vitamin E alone.(17) Most of the major antioxidant trials in large cardiovascular diseased cohorts have yielded an overall lack of protection against disease in humans.(18-22) The discrepancy among these studies may in part be explained by the facts that the major dietary antioxidants work together within intracellular antioxidant defense systems(23) and may be most effective when administered together instead of one antioxidant alone.(24) In addition, the trial populations were generally older (≥40 years)(18) and/or have advanced stages of disease,(19, 20, 25) or had major confounding variables that negated any potential positive supplementation effect.(21) Finally, the timing, type and dosage of antioxidant supplementation may not have been optimal for the specific cardiovascular populations studied.(22) One possibility is that antioxidants may not have been used early enough to offer protection before disease fulminates. Therefore, several questions remain unanswered regarding the efficacy of antioxidants on early disease processes in the vulnerable overweight adult.
Available experimental evidence in diabetic animals and humans showed that short-term supplementation with vitamins C and/or E decreased insulin levels(15, 26) and reduced CAM concentrations and oxidative stress.(27),(28) To our knowledge, it is unknown whether the combination vitamins E, C and β-carotene can attenuate pre-disease processes of insulin insensitivity, elevated endothelial adhesion molecule levels and oxidative stress levels in a younger, overweight population who is at risk for disease. To address this issue, this study examined the effects of short-term combined antioxidant supplementation on insulin sensitivity, CAM levels and oxidative stress in overweight young adults compared to normal weight controls.
Methods and Procedures
A randomized, double-blinded, controlled study design was used. Forty-eight apparently healthy young men and women (18-30 years) volunteered for this study. Participants were recruited by flyers, newspaper, institutional internet sites and clinic advertisements from the Central Virginia region. All participants had to meet the following criteria prior to enrollment in the study: no participation in regular physical activity (vigorous exercise 2 times or more per week), no chronic health problems or current smoking, no history of cardiovascular, metabolic or respiratory disease, no consumption of antioxidant supplements within the past 6 months, no current usage of any form of hormonal forms of contraceptives. All participants read and signed a written informed consent statement consistent with university policy on protection of human subjects. The protocol of the study was approved by the Institutional Review Board for Studies Involving Human Subjects at the University of Virginia (UVA).
Participants and Study Groups
After the investigators had determined BMI values, participants were stratified into normal weight or overweight groups [normal weight, (BMI<25 kg/m2) and overweight and obese (BMI≥25 kg/m2)]. Within each group, participants were randomized by the UVA investigational pharmacist to receive either antioxidant (AOX) treatment or placebo. Hence, there were a total of four participant groupings: normal weight, antioxidant-treated (N-AOX), normal weight, placebo (N-PL), overweight antioxidant-treated (O-AOX), and overweight placebo (O-PL). Adiposity status is represented by normal weight (N) and overweight (O). A total of 135 individuals responded to the advertisements and were screened for eligibility. Those who were not enrolled either did not meet all inclusion criteria, were too old, or who were beginning a new weight loss program at the same time. A total of 65 participants enrolled into the study; 11 were withdrawn due to overcommitted schedules, failure to comply with the study visits, failure to respond to investigator contact and child care issues.
Antioxidant Intervention and Testing Schedule
Groups assigned to the AOX treatment were administered vitamin E (800 IU/d), vitamin C (500 mg/d) and β-carotene (10 mg/d). The supplements or placebo were administered by the UVA investigational pharmacist in opaque bottles, and were taken once a day. Each supplement was provided in an individual capsule forms; the vitamin C and β-carotene were provided in yellow and green polysaccharide capsules mixed with inert microcrystalline cellulose. The vitamin E was supplied in an opaque gel capsule, containing a mixture of the eight tocopherols and tocotrienols found naturally in food. The content of each antioxidant was independently verified using an external quality control laboratory contracted by the institution. The placebos were provided in identical opaque bottles with color matched (yellow, green and opaque), odorless capsules similar to those of the AOX. Placebos consisted of microcrystalline cellulose (90%) contained within a natural polysaccharide capsule (capsule derived from water, gelatin and titanium dioxide). Vitamin E softgel capsule placebos contained cornstarch. The dosages of antioxidants were chosen based on previous work indicating a 27% reduction in lipid peroxidation with this range of combined supplements.(29) Treatment was administered for 8 weeks, a time by which plasma concentrations of vitamin E stabilize with supplementation.(30)
All participants visited the testing area three times (two pre-intervention, one post-intervention). During visit one, participants were acclimated to the UVA Exercise Physiology Laboratory at the General Clinical Research Center (GCRC), body composition and vital signs were measured, and 3-day dietary record forms were provided to participants. During visit two, aerobic fitness levels (VO2peak) were measured using a load-incremented cycle ergometer protocol. Visit 3 was a repeat visit of 1 and 2 combined that occurred after the supplementation period. Visits 1 and 2 were conducted within one week.
Anthropometric Measures
Height and weight were measured using a standard medical grade scale. For classification of obesity, waist and hip girths were measured using a soft, cloth measuring tape at anatomical landmarks described by ACSM.(31) Body mass index (BMI) values were determined by the following: BMI = weight(kg)/height(m)2. Body volume was estimated using air displacement plethysmography in a BodPod® device (Bod-Pod, Life Measurement Instruments, Concord, CA) corrected for thoracic gas volume; body density was calculated and used to predict body fat using the Siri equation.(32)
Dietary Analysis
Three-day dietary record forms were provided to each participant with standard instructions on how to complete the record. Participants were instructed to estimate servings of foods using household measurements (volume) as described in national dietary guidance documents as previously described.(33) Each participant received individual training sessions with the same investigator with regard to measuring technique and volume estimation. Picture books of portion sizes were also provided after the dietary estimation training session.(33) Diet records were assessed by the same investigator using Nutritionist Pro® Software (v 2.1.13, First DataBank, SanBruno CA) and were analyzed for macronutrient, antioxidant and caloric intake. To ensure the stability of the habitual diet of the subjects, all subjects completed a second three-day dietary record eight weeks later.(33) To further improve dietary stability, we performed two steps: 1) during screening and the initial days of the study, we indicated to each participant the importance of maintaining normal dietary patterns for the two months of the supplementation period, and 2) during the bimonthly periodic check in visits during the study, we discussed with each participant whether any unusual dietary deviations occurred, or whether there had been any systematic changes in food intake (volume or type of food) since the previous visit.
Peak Oxygen Consumption (VO2peak)/Lactate Threshold (LT) test
A peak oxygen consumption test was conducted to measure the aerobic fitness level of each participant. After a 12 hour overnight fast, participants arrived at the GCRC. A venous catheter was inserted into a forearm vein. Participants completed a peak oxygen consumption (VO2 peak)/lactate threshold (LT) test on an electronically braked cycle ergometer (Ergo Metrics 800S; Sensor Medics, Yorba Linda, CA). The initial power output (PO) was set at 20 W, and the PO was increased 15 W every 3 minutes until volitional fatigue. Blood samples were taken at rest and during the last 15 seconds of each exercise stage for the measurement of blood lactate concentration (HLa)(model 2700, YSI Instruments, Yellow Springs, OH). Heart rates (HR), blood pressures and rating of perceived exertion (RPE) were collected at every exercise stage.(31) Metabolic data were collected during both the VO2peak test using standard open-circuit spirometric techniques (Vmax 229; Sensor Medics, Yorba Linda, CA). HR was determined electrocardiographically (Marquette Max-1 electrocardiograph, Marquette, WI).
Blood Sampling
Fasting blood samples were collected from a catheter from an antecubital vein into heparinized vacutainer tubes during visits 1 and 3. Blood samples were analyzed for glucose, insulin, PEROX, glycated hemoglobin (HbA1c) levels, and inflammatory cytokines and adiponectin. All samples were batched within each subject and run in the same assay. A portion of the blood was immediately centrifuged at 1,500 X g for 5 minutes to separate plasma from red blood cell pellets. Plasma samples were immediately frozen and stored at −70°C until analysis. Insulin sensitivity biomarkers, vascular endothelial adhesion molecules and adiponectin were kindly performed by the General Clinical Research Center at UVa (UVa GCRC). Cholesterol, glucose and HbA1c samples were collected in the UVa GRCR and sent to the UVA Health System Clinical and Toxicology Laboratories in the same medical building for processing. PEROX assays were performed by a member of the investigational team (HKV).
Insulin Sensitivity Estimates
The homeostasis model assessment (HOMA) calculation was calculated from fasting glucose (G0) and insulin (I0) concentrations using the following formula: (G0 X I0)/ 22.5.(34, 35) The quantitative insulin sensitivity check index (QUICKI) was determined by the following calculation: 1/(log (fasting glucose) + log (fasting insulin)).(36)
Lipid Peroxidation Measurements
PEROX were quantified using the colorimetric ferrous oxidation/xylenol orange spectrophotometric technique previously described, where cumene hydroperoxide was used as the standard for this assay. Hydroperoxide formation was reflected by the accumulation of a purple chromatophore within the sample that was read at 580nm.(37) All samples were performed in triplicate in a single batch analysis. The coefficient of variation for this assay was 4%.
Vascular Endothelial Adhesion Molecules and Adiponectin
Soluble intercellular adhesion molecule-1 (sICAM), vascular adhesion molecule (sVCAM-1) and endothelial-leukocyte adhesion molecule-1 (sE-selectin) were assessed by the GCRC Core Laboratory using enzyme linked immunosorbent assays. R&D Quantikine® ELISA kits (Minneapolis, MN: Cat #s BBE 2B,BBE 1B, BB3) were used to measure adhesion molecules. Monoclonal antibodies specific for sVCAM-1, sIUCAM-1 and sE-selectin are precoated onto microplates; controls, standards and samples are pipetted into each microplate and the biomarkers are sandwiched between the plate bound antibody and the enzyme-linked monoclonal specific antibody for each biomarker. A substrate solution specific for each assay is added and generates color in the well in proportion to the amount of biomarker bound to the plate. All samples were performed in duplicate.
Cholesterol, Glucose and HbA1c
To document levels of lipid substrates in the blood available for oxidation, plasma cholesterol subfractions (total cholesterol, high density lipoproteins (HDL-C), triglycerides) were analyzed using standard automated spectrophotometric laboratory procedures (Olympus AU640, Olympus calibrator Cat #DR0040 and Genzyme HDL-C calibrator Cat#80-4529-00). Low density lipoproteins (LDL-C) were estimated from the following equation: LDL-C = total cholesterol – HDL-C – (triglycerides/5). Blood glucose was assessed using an Olympus AU640 procedure, in which glucose was phosphorylated by hexokinase in the presence of ATP and magnesium. The resultant glucose-6-phosphonate (G-6-P) dehydrogenase oxidized G-6-P to 6-phosphogluconate and reduced nicotinamide adenine dinucleotide (NAD+) to NADH. The change in absorbance at 340/380 was proportional to the amount of glucose in the sample (Olympus Glucose Reagent, Calibrator Cat# DR0040). HbA1c was analyzed using automated high performance liquid chromatography (Tosoh G7 Automated HPLC Analyzer, using TSKgel G& HSi elution columns). All samples were performed in duplicate.
Statistics
All data are expressed in mean ± standard error (SE). Data were analyzed using Statistical Package for the Social Sciences (v. 12.0, Chicago, IL). Percent changes (percent change from baseline) were calculated for several blood measures. Descriptive variables were analyzed using a two way analysis of variance (ANOVA). If differences did not exist between groups at baseline, repeated measures ANOVAs were performed for the change scores (Δ values from pre-post CLPO exercise at baseline and 8 weeks) for the inflammatory cytokines, blood lipids and PEROX. The between group factors were adiposity status (non-obese, overweight) and treatment (antioxidants, placebo) and the within group factor was time (pre and post-exercise, baseline and 8 weeks). When baseline differences existed for blood measures, two way analyses of covariance (ANCOVAs) were performed using the baseline value as the covariate. The between group factors were adiposity status (non-obese, overweight) and treatment (antioxidants, placebo). The level of significance was set at 0.05 for all statistical tests.
Results
Subject Characteristics at Baseline
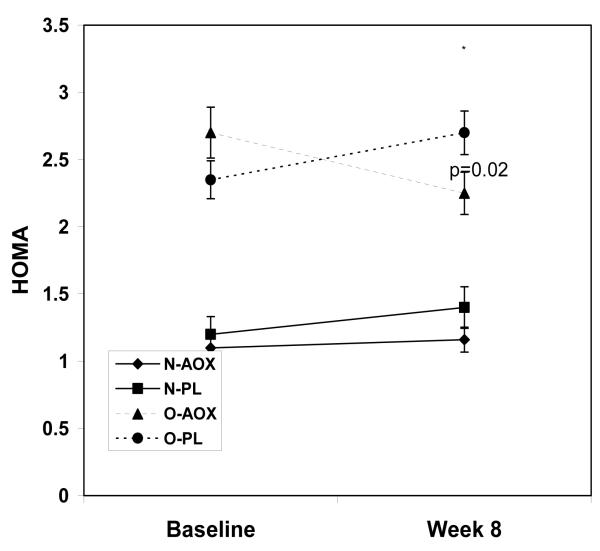
As expected, several indices of body composition were significantly different between the normal weight and overweight group (all p<0.05; Table 1). Aerobic fitness was lower in the overweight group (p<0.0001). Fasting measures of blood glucose were not different between groups at baseline, but HOMA and QUICKI scores were different in the overweight group than in the normal weight group (p<0.024). LDL-C concentrations were higher in the overweight group than the normal weight group (p<0.05). sICAM-1 and sE-selectin tended to be higher in the overweight group than the normal weight group (p=0.077).
Table 1.
Subject characteristics at baseline. Values are means ± SE.
| Normal Weight | Overweight | |||
|---|---|---|---|---|
| AOX (n=12) | PL (n=13) | AOX (n=12) | PL (n=11) | |
| Sex (M/F) | 7/5 | 3/10 | 3/9 | 4/7 |
| Age | 22 ± 1 | 22 ± 1 | 23 ± 1 | 27 ± 1 |
| Height (cm) | 174 ± 3 | 170 ± 2 | 171 ± 3 | 171 ± 2 |
| Weight (kg) | 69 ± 3 | 62 ± 2 | 94 ± 6 * | 100 ± 7 * |
| Fat mass (kg) | 14 ± 1 | 14 ± 1 | 46 ± 5 * | 48 ± 6 * |
| Fat free mass (kg) | 55 ± 23 | 48 ± 2 | 57 ± 4 | 55 ± 4 |
| BMI (kg/m2) | 23 ± 1 | 21 ± 1 | 32 ± 2 | 34 ± 2 |
| Waist circumference (cm) | 76 ± 2 | 70 ± 2 | 96 ± 4 * | 100 ± 4 * |
| WHR | 0.76 ± 0.1 | 0.74 ± 0.1 | 0.83 ± 0.1 * | 0.85 ± 0.1 * |
| VO2peak (ml/kg*min) | 33 ± 2 | 31 ± 2 | 21 ± 2 * | 19 ± 1 * |
| Glucose (mmol/L) | 4.4 ± 0.1 | 4.4 ± 0.1 | 4.9 ± 0.3 | 5.0 ± 0.3 |
| HbA1c (%) | 5.0 ± 0.1 | 5.0 ± 0.1 | 4.9 ± 0.1 | 5.0 ± 0.1 |
| Insulin (μIU/ml) | 5.6 ± 0.7 | 5.9 ± 0.4 | 11.7 ± 1.8 * | 11.6 ± 4.0 * |
| HOMA | 1.1 ± 0.1 | 1.2 ± 0.1 | 2.6 ± 0.5 * | 2.6 ± 0.8 * |
| QUICKI | 0.39 ± 0.1 | 0.38 ± 0.1 | 0.35 ± 0.1 * | 0.33 ± 0.2 * |
| Total cholesterol (mmol/L) | 4.3 ± 0.1 | 3.8 ± 0.2 | 4.0 ± 0.3 | 4.3 ± 0.3 |
| HDL-C (mmol/L) | 1.5 ± 0.2 | 1.4 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.4 |
| LDL-C (mmol/L) | 2.4 ± 0.4 | 2.0 ± 0.6 | 2.3 ± 0.7 * | 2.7 ± 0.8 * |
| Triglycerides (mmol/L) | 0.8 ± 0.4 | 0.9 ± 0.5 | 1.3 ± 0.8 | 1.0 ± 0.6 |
different from normal weight group at p<0.05; BMI = body mass index; WHR = waist to hip ratio; SBP; VO2peak = peak rate of oxygen consumption; PL = placebo; AOX = antioxidant treated
Dietary Intake
Correlation values (r) between specific macro and micronutrient intake between the first and second dietary records ranged from 0.7-0.93, indicating a stable intrasubject dietary pattern. The only significant differences with regard to dietary intakes were found to be with saturated fat (Table 2) and copper. The O-PL had higher saturated fat intake than the N-PL group (p<0.05). Copper intakes were (in mg) 1.1 ± 0.1, 0.8 ± 0.1, 0.7 ± 0.1, and 1.0 ± 0.1 in the N-AOX, N-PL, O-AOX and O-PL groups respectively (O-AOX were different; p<0.05). Manganese intakes were (in mg) 2.9 ± 0.6, 2.4 ± 0.5, 1.5 ± 0.3 and 1.8 ± 0.4 in the N-AOX, N-PL, O-AOX and O-PL groups respectively. We presented the raw values for the calculated micronutrient intakes here for the reader, however we acknowledge that the level of precision reported for these values is likely much lower given the subject reporting of dietary intake.
Table 2.
Average dietary intakes of major macronutrient normal weight and overweight groups by AOX treatment. Values are means ± SE.
| Normal Weight | Overweight | |||
|---|---|---|---|---|
| AOX (n=12) | PL (n=13) | AOX (n=12) | PL (n=11) | |
| Energy intake (kcal) | 2357 ± 171 | 1940 ± 210 | 2104 ± 213 | 2422 ± 223 |
| Protein (g/day) | 83 ± 11 | 72 ± 7 | 77 ± 9 | 98 ± 13 |
| Carbohydrate (g/day) | 286 ±17 | 262 ± 26 | 288 ± 28 | 282 ± 32 |
| Fat (g/day) | 96 ± 3 | 65 ± 12 | 74 ± 10 | 101 ± 13 |
| Saturated fat (g/day) | 31 ± 5 | 20 ± 3 | 24 ± 4 | 36 ± 5 * |
| Vitamin C (mg) | 124 ± 34 | 113 ± 29 | 103 ± 28 | 98 ± 26 |
| Vitamin E (mg) | 11 ± 2 | 5 ± 1 | 7 ± 2 | 6 ± 1 |
| α-tocopherol (mg) | 4 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 1 |
| β-carotene (mg) | 2847 ±1193 | 743 ± 312 | 807 ± 201 | 1267 ± 387 |
| Zinc (mg) | 10 ± 1 | 7.0 ± 1 | 9 ± 1 | 11 ± 2 |
| Selenium (μg) | 86 ± 15 | 57 ± 9 | 67 ± 13 | 95 ± 18 |
different from N-PL at p<0.05
Post- AOX supplementation
There were no changes in body weight, fat or fat free mass, waist to hip ratio (WHR) after the treatment period in either the placebo or AOX group. No significant changes occurred in exercise capacity (VO2peak) or cholesterol profiles by week 8 of the intervention in any experimental group, indicating that these potential confounders remained stable from pre- to post-intervention.
Insulin Sensitivity Related Variables
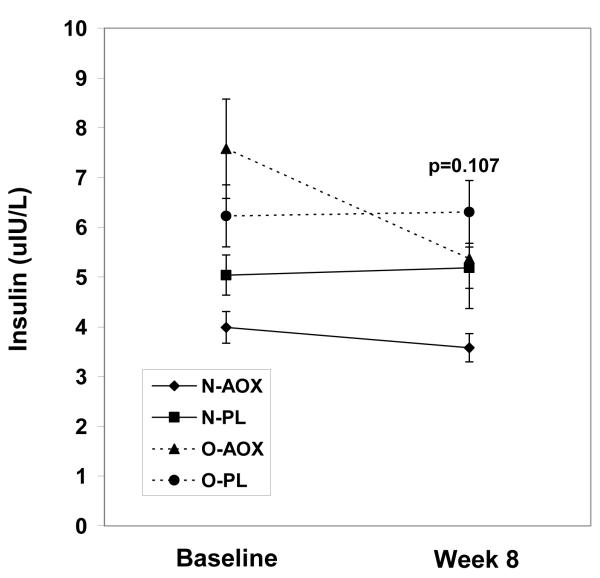
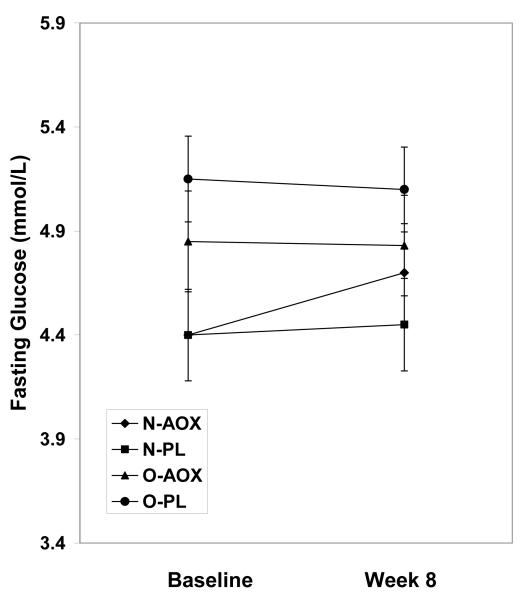
While the fasting insulin levels increased in the placebo group by week 8, insulin levels decreased in the O-AOX group, but this decrease did not achieve statistical significance (p=0.107; Figure 1a). Relative to participants treated with placebo, HOMA scores in the AOX group were significantly lower in the overweight group at week 8 (p=0.02; Figure 1c). HOMA scores in the normal weight group were not changed by AOX at 8 weeks. By week 8, the QUICKI scores were improved in the AOX overweight and normal weight groups, by 2.5% and 1%, respectively. QUICKI scores were reduced in the PL treated overweight and normal weight groups by 3.0% and 2.5%, respectively by week 8. These QUICKI changes however, were not significant (p=0.407). Fasting glucose levels were not different between groups or over time (p=0.973; Figure 1b).
Figure 1a.
Fasting insulin values for normal weight and overweight young adults treated with antioxidants (AOX, vitamins E, C and β-carotene) or placebo (PL) at baseline and 8 weeks. Values are means ± SE.
Figure 1c.
Fasting plasma glucose concentrations for normal weight and overweight young adults treated with antioxidants (AOX, vitamins E, C and β-carotene) or placebo (PL) at baseline and 8 weeks. Values are means ± SE.
Figure 1b.
HOMA values for normal weight and overweight young adults treated with antioxidants (AOX, vitamins E, C and β-carotene) or placebo (PL) at baseline and 8 weeks. Values are means ± SE.
Lipid peroxidation, adhesion molecules and adiponectin
Data regarding lipid peroxidation, adhesion molecules and adiponectin are shown in Table 3. Relative to participants receiving placebo, those treated with AOX (both N-AOX and O-AOX) showed significant reductions in PEROX (p=0.013). sE-selectin and sICAM-1 concentrations also decreased over time in participants treated with AOX, although these reductions were significant only in the overweight group (p=0.023, 0.14, respectively). Adiponectin increased in normal weight and overweight participants treated with AOX, while the placebo treated groups demonstrated decreases in adiponectin over time (p=0.05).
Table 3.
Fasting measurements of oxidative stress, vascular inflammation and adiponectin in normal weight and overweight groups by AOX treatment at baseline. Change values are the raw differences between baseline and week 8 values for each measure. Values are means ± SE.
| Normal Weight | Overweight | |||||||
|---|---|---|---|---|---|---|---|---|
| AOX (n=12) | PL (n=13) | AOX (n=12) | PL (n=11) | |||||
| Baseline | Week 8 | Baseline | Week 8 | Baseline | Week 8 | Baseline | Week 8 | |
| PEROX | 2.7 ± 0.2 | 2.3 ± 0.2 | 2.6 ± 0.2 | 2.8 ± 0.2 | 2.5 ± 0.2 | 2.2 ± 0.2 | 2.6 ± 0.2 | 2.6 ± 0.2 |
| change | −0.3 ± 0.2 * | 0.6 ± 0.4 | −0.7 ± 0.3 * | 0.1 ± 0.1 | ||||
| sE-selectin | 37 ± 5 | 39 ± 5 | 48 ± 4 | 47 ± 4 | 52 ± 8 | 45 ± 5 * | 49 ± 6 | 48 ± 6 |
| change | −1 ± 2 | −1 ± 1 | −9 ± 3 * | -2 ± 6 | ||||
| sICAM-1 | 220 ± 13 | 209 ± 13 | 213 ± 14 | 214 ± 15 | 231 ± 15 | 216 ± 14 * | 254 ± 18 | 254 ± 19 |
| change | −12 ± 8 | 0.5 ± 0.8 | −14 ± 5 * | −0.2 ± 6 | ||||
| sVCAM-1 | 546 ± 45 | 524 ± 46 | 551 ± 42 | 556 ± 43 | 570 ± 42 | 537 ± 42 | 676 ± 45 | 643 ± 48 |
| change | −25 ± 12 | 9 ± 32 | −33 ± 26 | −10 ± 24 | ||||
| Adiponectin | 9,730 ± 1,242 | 11,339 ± 1,286 | 9,903 ± 1,189 | 9,152 ± 1,231 | 9,103 ± 1,142 | 9,534 ± 1,182 | 9,157 ± 1,302 | 8,662 ± 1,348 |
| change | 1,748 ± 629 * | -751 ± 938 | 194 ± 446 * | -495 ± 583 | ||||
Lipid hydroperoxides (PEROX) expressed as nmol/ml; sE-selectin, sICAM-1, sVCAM-1 and adiponectin are expressed in ng/ml
different from PL groups at p<0.05
Discussion
The combination of vitamins C, E and β-carotene during an 8 week supplementation period moderately reduced HOMA values, and sICAM-1 and sE-selectin levels in overweight young adults. Oxidative stress was also reduced by week 8, and may be a potential mechanism underlying these favorable changes in cardiovascular disease and diabetes precursors. These preliminary findings suggest that obesity-induced early developmental stages of insulin resistance and endothelial dysfunction might be influenced by combined antioxidant administration.
Previous supplementation studies of individual antioxidants C and E in humans and animals have shown inconsistent effects on insulin sensitivity, with some showing improvements, (15, 38, 39) whereas others do not.(39, 40) It is not known whether isolated β-carotene affects insulin sensitivity in obese young adults. A limitation to these and other studies is that antioxidants do not work optimally in isolation, but rather serve as part of an antioxidant system where optimal protection against disease processes occurs with several antioxidants together, as found in natural foods. In the present study, the combination of vitamins E, C and β-lowered the HOMA index in overweight but not in normal weight young adults. This moderate change reflects an improvement in the responsiveness to insulin in the overweight participants. We propose that the antioxidants work in concert to act at several sites in the insulin metabolism pathways. For example, vitamin C supplementation increases antioxidant defenses in tissue (41) and potentiates insulin action and improves glucose uptake in humans.(42) Vitamin E protects against oxidative modification of insulin metabolism mediating proteins such as glucose transporters and insulin kinases.(41) Inhibition of free radical production and restoration of cell redox status with vitamins E, C and β-carotene might preserve insulin receptor structure and function, and improve insulin sensitivity in obesity.(43) The fact that the normal weight participants did not demonstrate significant improvements is not surprising, given that oxidative stress was not elevated in this group, and fasting insulin and glucose values were already within appropriate ranges and likely had little room for improvement with the AOX treatment.
The effects of combined AOX on early disease biomarkers such as endothelial adhesion molecules or adiponectin in overweight humans are not clear. We hypothesized that the AOX may work synergistically to combat the stimulus of obesity-induced oxidative damage to the endothelium, and may indirectly suppress high levels of sVACM-1, sICAM-1 and sE-selectin. Previous studies in healthy adults(11) have shown that vitamin E supplementation suppresses sVCAM-1, sICAM-1 and sE-selectin levels, whereas studies of uncomplicated type I diabetics treated with vitamin E did not show changes in sVCAM-1.(44) Other data have shown that vitamins E (300 g/d) and C (250 g/d) and n-acetylcysteine can rapidly affect change by attenuating sVCAM-1 levels after a high fat meal.(45) This finding may be especially relevant for obese individuals who might consume more dietary fat and fewer antioxidants (e.g., copper), as observed in some of the overweight participants in this study. Previous studies have shown reductions in lipid peroxidation (estimate of oxidative stress) and concurrent reductions in VCAM-1,(45) E-Selectin,(11) and ICAM-1(46) with supplementation of individual or combined antioxidants. Supplementation with antioxidants such as vitamins E, C or β-carotene are purported to either squelch free radicals such as superoxide, directly within the endothelium, to disrupt TNF-α intracellular signaling cascades, or to reduce oxidative modification of LDL-C, all of which would otherwise initiate expression of endothelial adhesion molecules.(47)
AOX significantly lowered sICAM-1 and sE-selectin in the healthy young overweight adults in this study, but not normal weight adults. While sVCAM-1 levels decreased in the AOX group regardless of adiposity status, this change was not statistically significant. Eight weeks of treatment may not be sufficient to produce statistical reductions in sVCAM-1 such as those reductions observed in supplementation studies that were 6 months.(48) Our normal weight participants demonstrated some improvement in these endothelial adhesion molecule levels, although these improvements were not significant. Given that these participants were healthy, there was likely a ceiling effect of the AOX treatment on adhesion molecule levels.
Data are sparse regarding the effects of AOX on circulating adiponectin levels. However, one recent study indicated that vitamin E alone (800-1200IU/d) for 6 months did not significantly change adiponectin levels in middle aged, overweight subjects.(5) One animal study that used a vitamin C rich extract reduced oxidative stress and increased adiponectin levels in experimentally induced diabetic rats.(49) Here, combined AOX moderately increased adiponectin and decreased oxidative stress; these data suggest that combined antioxidants may be required to appreciably increase adiponectin levels in the overweight human. The incorporation of multiple AOX into multiple physiologic mechanisms at the level of the endothelium likely suppresses oxidative stress pathways that inhibit adiponectin expression.(5)
Limitations
Limitations to this study deserve comment. This was a small exploratory study, and the study should be expanded to include a larger sample. The dietary intake data have accuracy limitations, and contain a wide variation of inter-individual differences in macro and micronutrient intake; the reported differences in copper and saturated fat might simply reflect individual dietary choices of calorically richer or less nutrient rich foods (e.g., fewer fresh foods) in the overweight groups during the time frame of the dietary record. Also, the N-AOX group contained a few vegetarians, a participant pool who normally consumed higher amounts of vitamin and mineral rich fresh foods. A longer dietary record period might have identified additional dietary differences between the normal weight and overweight groups, or washed out the differences. The supplementation period was short, and longer term and larger studies in young overweight or obese adults would reveal whether AOX can consistently and permanently reduce endothelial adhesion molecule levels. Ultimately, this intervention should be tested to determine whether the onset of cardiovascular disease and diabetes is influenced in the overweight adult population.
Conclusion
Short term AOX supplementation moderately lowers HOMA and endothelial adhesion molecule levels and increased adiponectin in overweight but not normal weight young adults despite no change in body composition or body weight. A potential mechanism to explain this finding is the reduction in oxidative stress by AOX.
Acknowledgements
This study was supported in part by Grant Numbers T32-AT00052 and K30-AT-00060 from the National Center for Complementary and Alternative Medicine (NCCAM), and from the UVA General Clinical Center Grant Number 5 M01 RR000847 and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, or the National Institutes of Health.
Abbreviations
- HOMA
homeostasis model assessment
- AOX
antioxidant
- sICAM
soluble intercellular adhesion molecule-1
- sVCAM-1
vascular adhesion molecule
- sE-selectin
endothelial-leukocyte adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kim J, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction. Circulation. 2006;113:1888–904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 2.Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, et al. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. Journal of Biological Chemistry. 2002;277(3):1794–9. doi: 10.1074/jbc.M103728200. [DOI] [PubMed] [Google Scholar]
- 3.Couillard C, Ruel G, Archer WR, Pomerleau S, Bergeron J, Couture P, et al. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. Journal of Clinical Endocrinology and Metabolism. 2005;90(12):6454–9. doi: 10.1210/jc.2004-2438. [DOI] [PubMed] [Google Scholar]
- 4.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. International Journal of Obesity. 2006;30:400–18. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 5.Sutherland WH, Manning PJ, Walker RJ, de Jong SA, Ryalls AR, Berry EA. Vitamin E supplementation and plasma 8-isoprostane and adiponectin in overweight subjects. Obesity (Silver Spring) 2007;15(2):386–191. doi: 10.1038/oby.2007.546. [DOI] [PubMed] [Google Scholar]
- 6.Hattori S, Hattori Y, Kasai K. Hypoadiponectinemia is caused by chronic blockade of nitric oxide synthesis in rats. Metabolism. 2005;54(4):482–7. doi: 10.1016/j.metabol.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Facchini FS, Humphreys MH, DoNascimento CA, Abbasi F, Reaven GM. Relation between insulin resistance and plasma concentrations of lipid hydroperoxides, carotenoids, and tocopherols. American Journal of Clinical Nutrition. 2000;72(3):776–9. doi: 10.1093/ajcn/72.3.776. [DOI] [PubMed] [Google Scholar]
- 8.Strauss RS, National Health and Nutrition Examination Survey Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III) Journal of Pediatrics. 1999;134(2):160–5. doi: 10.1016/s0022-3476(99)70409-9. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann A, Niess AM, Grunert-Fuchs M, Poch B, Speit G. Vitamin E prevents exercise-induced DNA damage. Mutation Research. 1995;346(4):195–202. doi: 10.1016/0165-7992(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 10.Goldfarb AH, Patrick SW, Bryer S, You T. Vitamin C supplementation affects oxidative-stress blood markers in response to a 30-minute run at 75% VO2max. International Journal of Sport Nutrition, Exercise and Metabolism. 2005;15(3):279–90. doi: 10.1123/ijsnem.15.3.279. [DOI] [PubMed] [Google Scholar]
- 11.van Dam B, van Hinsbergh VW, Stehouwer CD, Versteilen A, Dekker H, Buytenhek R, et al. Vitamin E inhibits lipid peroxidation-induced adhesion molecule expression in endothelial cells and decreases soluble cell adhesion molecules in healthy subjects. Cardiovascular Research. 2003;57(2):563–71. doi: 10.1016/s0008-6363(02)00699-5. [DOI] [PubMed] [Google Scholar]
- 12.Rayment SJ, Shaw J, Woollard KJ, Lunec J, Griffiths HR. Vitamin C supplementation in normal subjects reduces constitutive ICAM-1 expression. Biochemical & Biophysical Research Communications. 2003;308(2):339–45. doi: 10.1016/s0006-291x(03)01383-4. [DOI] [PubMed] [Google Scholar]
- 13.Metin G, Atukeren P, Gumustas MK, Belce A, Kayserilioglu A. The effect of vitamin E treatment on oxidative stress generated in trained rats. Tohoku Journal of Experimental Medicine. 2002;198(1):47–53. doi: 10.1620/tjem.198.47. [DOI] [PubMed] [Google Scholar]
- 14.Manning PJ, Sutherland WH, Walker RJ, Williams SM, De Jong SA, Ryalls AR, et al. Effect of high-dose vitamin E on insulin resistance and associated parameters in overweight subjects. Diabetes Care. 2004;27(9):2166–71. doi: 10.2337/diacare.27.9.2166. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Wahab YH, O’Harte FP, Mooney MH, Barnett CR, Flatt PR. Vitamin C supplementation decreases insulin glycation and improves glucose homeostasis in obese hyperglycemic (ob/ob) mice. Metabolism. 2002;51(4):514–7. doi: 10.1053/meta.2002.30528. [DOI] [PubMed] [Google Scholar]
- 16.Facchini F, Coulston AM, Reaven GM. Relation between dietary vitamin intake and resistance to insulin-mediated glucose disposal in healthy volunteers. American Journal of Clinical Nutrition. 1996;63(6):946–9. doi: 10.1093/ajcn/63.6.946. [DOI] [PubMed] [Google Scholar]
- 17.Skrha J, Sindelka G, Kvasnicka J, Hilgertova J. Insulin action and fibrinolysis influenced by vitamin E in obese Type 2 diabetes mellitus. Diabetes Research & Clinical Practice. 1999;44(1):27–33. doi: 10.1016/s0168-8227(99)00010-8. [DOI] [PubMed] [Google Scholar]
- 18.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. Journal of the National Cancer Institute. 1993;85(18):1483–92. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 19.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361(9374):2017–23. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P, The Heart Outcomes Prevention Evaluation Study Investigators Vitamin E supplementation and cardiovascular events in high-risk patients. New England Journal of Medicine. 2000;342(3):154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 21.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. New England Journal of Medicine. 1996;334(18):1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 22.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110(5):637–41. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- 23.Tauler P, Aguilo A, Gimeno I, Fuentespina E, Tur JA, Pons A. Response of blood cell antioxidant enzyme defences to antioxidant diet supplementation and to intense exercise. European Journal of Nutrition. 2005;22 doi: 10.1007/s00394-005-0582-7. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Abudu N, Miller JJ, Attaelmannan M, Levinson SS. Vitamins in human arteriosclerosis with emphasis on vitamin C and vitamin E. Clinica Chimica Acta. 2004;339:11–25. doi: 10.1016/j.cccn.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000;356(9237):1213–8. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 26.Boshtam M, Rafiei M, Golshadi ID, Ani M, Shirani Z, Rostamshirazi M. Long term effecrs of oral vitamin E supplement in type II diabetic patients. International Journal of Vitamin and Nutrition Research. 2005;75(5):341–6. doi: 10.1024/0300-9831.75.5.341. [DOI] [PubMed] [Google Scholar]
- 27.Tousoulis D, Antoniades C, Tentolouris C, Tsioufis C, Toutouza M, Toutouzas P, et al. Effects of combined administration of vitamins C and E on reactive hyperemia and inflammatory process in chronic smokers. Atherosclerosis. 2003;170(2):261–7. doi: 10.1016/s0021-9150(03)00250-8. [DOI] [PubMed] [Google Scholar]
- 28.Koo JR, Ni Z, Oviesi F, Vaziri ND. Antioxidant therapy potentiates antihypertensive action of insulin in diabetic rats. Clinical and Experimental Hypertension. 2002;24(5):333–44. doi: 10.1081/ceh-120004795. [DOI] [PubMed] [Google Scholar]
- 29.Schroder H, Navarro E, Mora J, Galiano D, Tramullas A. Effects of alpha-tocopherol, beta-carotene and ascorbic acid on oxidative, hormonal and enzymatic exercise stress markers in habitual training activity of professional basketball players. European Journal of Nutrition. 2001;40(4):178–84. doi: 10.1007/s003940170006. [DOI] [PubMed] [Google Scholar]
- 30.Fischer CP, Hiscock NJ, Penkowa M, Basu S, Vessby B, Kallner A, et al. Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. Journal of Physiology. 2004;558(Pt 2):633–45. doi: 10.1113/jphysiol.2004.066779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medicine ACoS . ACSM’s Guidelines for Exercise Testing and Prescription. 6 ed Lippincott Williams and Wilkins; Philedelphia: 2000. [Google Scholar]
- 32.Siri WE. Body composition from fluid spaces and density: analysis of methods. In: J B, A H, editors. Techniques for measuring body composition. National Academy of Sciences; Washington DC: 1961. pp. 223–44. [Google Scholar]
- 33.Freese R, Alfthan G, Jauhiainen M, Basu S, Erlund I, Salminen I, et al. High intakes of vegetables, berries, and apples combined with a high intake of linoleic or oleic acid only slightly affect markers of lipid peroxidation and lipoprotein metabolism in healthy subjects. American Journal of Clinical Nutrition. 2002;76(5):950–60. doi: 10.1093/ajcn/76.5.950. [DOI] [PubMed] [Google Scholar]
- 34.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 35.Straczkowski M, Stepien A, Kowalska I, Kinalska I. Comparison of simple indices of insulin sensitivity using the euglycemic hyperinsulinemic clamp technique. Medical Science Monitor. 2004;10(8):CR480–CR4. [PubMed] [Google Scholar]
- 36.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. Journal of Clinical Endocrinology & Metabolism. 2000;85(7):2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 37.Hermes-Lima M, Willmore WG, Storey KB. Quantification of lipid peroxidation in tissue extracts based on Fe(III)xylenol orange complex formation. Free Radicals in Biology and Medicine. 1995;19(3):271–80. doi: 10.1016/0891-5849(95)00020-x. [DOI] [PubMed] [Google Scholar]
- 38.Paolisso G, Balbi V, Volpe C, Varricchio G, Gambardella A, Saccomanno F, et al. Metabolic benefits deriving from chronic vitamin C supplementation in aged non-insulin dependent diabetics. Journal of the American College of Nutrition. 1995;14(4):387–92. doi: 10.1080/07315724.1995.10718526. [DOI] [PubMed] [Google Scholar]
- 39.Barbagallo M, Dominguez LJ, Tagliamonte MR, Resnick LM, Paolisso G. Effects of vitamin E and glutathione on glucose metabolism: role of magnesium. Hypertension. 1999;34(4 Pt 2):1002–6. doi: 10.1161/01.hyp.34.4.1002. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Karne RJ, Hall G, Campia U, Panza JA, Cannon RO, 3rd, et al. High-dose oral vitamin C partially replenishes vitamin C levels in patients with Type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. American Journal of Physiology - Heart & Circulatory Physiology. 2006 Jan;290(1):H137–45. doi: 10.1152/ajpheart.00768.2005. [DOI] [PubMed] [Google Scholar]
- 41.Vinayaga Moorthi R, Bobby Z, Selvaraj N, Sridhar MG. Vitamin E protects the insulin sensitivity and redox balance in rat L6 muscle cells exposed to oxidative stress. Clinica Chimica Acta. 2006 Feb 1; doi: 10.1016/j.cca.2005.12.004. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 42.Paolisso G, D’Amore A, Balbi V, Volpe C, Galzerano D, Giugliano D, et al. Plasma vitamin C affects glucose homeostasis in healthy subjects and in non-insulin-dependent diabetics. American Journal of Physiology. 1994;266(2 Pt 1):E261–8. doi: 10.1152/ajpendo.1994.266.2.E261. [DOI] [PubMed] [Google Scholar]
- 43.Maziere C, Morliere P, Santus R, Marcheux V, Louandre C, Conte MA, et al. Inhibition of insulin signaling by oxidized low density lipoprotein. Protective effect of the antioxidant Vitamin E. Atherosclerosis. 2004;175(1):23–30. doi: 10.1016/j.atherosclerosis.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Skyrme-Jones RA, Meredith IT. Soluble adhesion molecules, endothelial function and vitamin E in type 1 diabetes. Coronary Artery Disease. 2001;12(1):69–75. doi: 10.1097/00019501-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Neri S, Signorelli SS, Torrisi B, Pulvirenti D, Mauceri B, Abate G, et al. Effects of antioxidant supplementation on postprandial oxidative stress and endothelial dysfunction: a single-blind, 15-day clinical trial in patients with untreated type 2 diabetes, subjects with impaired glucose tolerance, and healthy controls. Clinical Therapy. 2005;27(11):1764–73. doi: 10.1016/j.clinthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Desideri G, Croce G, Tucci M, Passacquale G, Broccoletti S, Valeri L, et al. Effects of bezafibrate and simvastatin on endothelial activation and lipid peroxidation in hypercholesterolemia: evidence of different vascular protection by different lipid-lowering treatments. Journal of Clinical Endocrinology and Metabolism. 2004;89(4):1978. doi: 10.1210/jc.2003-030724. [DOI] [PubMed] [Google Scholar]
- 47.Frei B. On the role of vitamin C and other antioxidants in atherogenesis and vascular dysfunction. Proceedings of the Society for Experimental Biology and Medicine. 1999;222(3):196–204. doi: 10.1046/j.1525-1373.1999.d01-136.x. [DOI] [PubMed] [Google Scholar]
- 48.Tahir M, Foley B, Pate G, Crean P, Moore D, McCarroll N, et al. Impact of vitamin E and C supplementation on serum adhesion molecules in chronic degenerative aortic stenosis: a randomized controlled trial. American Heart Journal. 2005;150(2):302–6. doi: 10.1016/j.ahj.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Rao TP, Sakaguchi N, Juneja LR, Wada E, Yokozawa T. Amla (Emblica officinalis Gaertn.) extracts reduce oxidative stress in streptozotocin-induced diabetic rats. Journal of Medicinal Foods. 2005;8(3):362–8. doi: 10.1089/jmf.2005.8.362. [DOI] [PubMed] [Google Scholar]