Abstract
Enzymatic cleavage of Amyloid-β Protein Precursor (AβPP) produces amyloid-β (Aβ) peptides which form the insoluble cortical plaques characteristic of Alzheimer’s Disease (AD). AβPP is post-transcriptionally processed into three major isoforms with differential cellular and tissue expression patterns. Changes in AβPP isoform expression may be indicative of disease pathogenesis in AD, but accurately measuring AβPP gene isoforms has been difficult to standardize, reproduce, and interpret. In light of this, we developed a set of isoform specific absolute quantification real time PCR standards that allow for quantification of transcript copy numbers for total AβPP and all three major isoforms (AβPP695, AβPP751, and AβPP770) in addition to glyceraldehyde-3-dehydrogenase (GAPDH) and examined expression patterns in superior frontal gyrus (SFG) and cerebellar (CBL) samples from patients with (n=12) and without AD (n=10). Both total AβPP and AβPP695 transcripts were significantly decreased in superior frontal gyrus (SFG) of patients with AD compared to control (p= 0.037 and p=0.034, respectively). AβPP751 and AβPP770 transcripts numbers were not significantly different between AD and control (p>0.15). There was trend for decreased percentage AβPP695 (p=0.051) and increased percentage AβPP770 (p=0.013) expression in SFG of patients with AD. GAPDH transcripts levels were also decreased significantly in the SFG of patients with AD compared to control (p=0.005). Decreasing Total AβPP and AβPP695 copy number was associated with increased plaque burden and decreased cognitive function. In this study we describe a simple procedure for measuring AβPP isoform transcripts by real-time PCR and confirm previous studies showing altered AβPP isoform expression patterns in AD.
Keywords: amyloid-β protein precursor (AβPP), amyloid-β (Aβ), Alzheimers Disease (AD), dementia, kunitz, polymerase chain reaction, alternative splicing
Introduction
A hallmark pathological finding in Alzheimer’s Disease (AD) is the presence of central nervous system plaques containing insoluble amyloid-β (Aβ) resulting from proteolytic processing of Amyloid-β Protein Precursor (AβPP) [1]. AβPP is a ubiquitously expressed type 1 transmembrane protein that is alternatively spliced into several isoforms in a tissue specific manner. Each isoform can undergo processing along two major pathways to produce several peptide products in addition to Aβ, with multiple suggested functions [2, 3]. The three major isoforms of AβPP are derived from post-transcriptional alternative splicing that removes exon(s) 7 and/or 8; AβPP770 contains both, AβPP751 does not contain exon 8, and AβPP695 lacks exons 7 and 8. Exon 7 codes for a Kunitz-type protease inhibitor (KPI) domain known as Protease Nexin-2 (PN2) that can regulate thrombosis through binding to Factors Xa and XIa [4, 5]. AβPP695 has been described as the neuronally predominant isoform; AβPP751 is abundant in CNS tissue, mostly in astrocytes and glial cells, but is also found in high levels in peripheral tissues; AβPP770 is widely expressed in peripheral tissues but minimally in CNS tissue [3].
Over the past twenty years, several groups have documented variation in AβPP isoform expression in multiple regions of the brain affected by AD. In general, studies have found either no change in total AβPP transcript levels or modest reductions in AD-affected areas of the brain with simultaneous increases in KPI containing AβPP isoforms [6–8]. Alteration of AβPP isoform levels has also been observed in cortical tissues of subjects with other forms of dementia [9, 10]. There is some inconsistency between studies, however. Some of the variability between studies may be due to differences in anatomic location and sample quality. In addition, progress in the area has been hampered to some degree by the technical difficulty of in situ hybridization or nuclease protection assays and to a larger degree by the limitation that relative quantification PCR (RQ-PCR) cannot be used to compare isoform levels within studies or expression levels across independent studies.
In light of this, we developed a set of PCR primers to produce standard curves for measuring total AβPP, each of the three major AβPP isoforms, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by absolute quantification PCR (AQ-PCR). We then used commercially available real-time PCR probe sets in conjunction with our isoform-specific standards to measure AβPP isoform transcript levels in the frontal cortex of subjects with AD and normal controls. This method is easily executed and allows for direct comparison of gene copy number across isoforms, which one cannot do using relative quantification PCR (RQ-PCR). We found that total AβPP and AβPP695 levels are reduced in the frontal cortex in AD and the expression of AβPP770 as a percentage of total AβPP was elevated. Decreasing total AβPP and AβPP695 copy number was associated with increasing plaque burden and decreased cognitive function, suggesting the decrease was a result of neuronal death or dysfunction. Further, this study illustrates the utility of absolute quantification PCR as a research tool for understanding the biology of the AβPP system.
Materials and Methods
Preparation of APP isoform specific standards for AQ-PCR
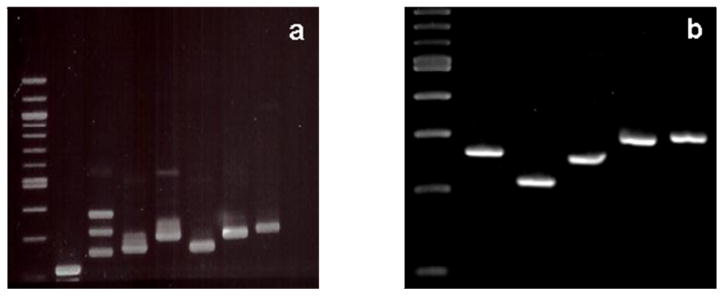
To create absolute quantification standard curves, PCR primer pairs were designed to amplify either total AβPP transcripts (AβPPcm), isoform specific transcripts for AβPP770, AβPP751, or AβPP695, and GAPDH from cDNA libraries. Series of primers for each standard were prepared to produce cDNAs with sequences encompassing the target region measured by isoform specific TaqMan assays (Applied Biosystems Inc). Testing of empiric combinations yielded pairs of specific primers (Table 1 and Figure 1) which produced cDNAs of known size that could be resolved by gel electrophoresis, physically isolated, and purified. This step was necessary as each set of primers could amplify more than one product (Figure 2a). However, after isolation and purification, only one cDNA was observed for each of the standards; the cDNAs amplified as standards for total AβPP, AβPP770, AβPP751, AβPP695, and GAPDH are 265, 215, 258, 300, and 305 bp long, respectively (Figure 2b). Purified samples were sequenced at the University of Vermont DNA Analysis Core to ensure the proper sequence had been produced (See Supplementary Figure 1). Concentration of the cDNA standards was determined by spectrometry (NanoDrop) and a standard curve with known standard copy number for each transcript was made by serial dilution.
Table 1.
Absolute Quantification PCR Standards Primer Sequences
| Reference Sequence | Forward Primer Sequence* | Reverse Primer Sequence* | |
|---|---|---|---|
| AβPP695 | NM_201414.2 | aggaggaagaagtggctgaggtgga | tctctcggtgcttggcctcaa |
| AβPP751 | NM_201413.2 | gccgagcaatgatctcccgctggta | tctctcggtgcttggcctcaa |
| AβPP770 | NM_000484.3 | gtgtgctctgaacaagccgagac | ggatctcgggcaagaggttc |
| Total AβPP / AβPP-cm | NM_000484.3 | catggccagagtggaagccatgctc | gagagactgattcatgcgctc |
| GAPDH | NM_002046.3 | tctgctcctcctgttcgacag | tggtcgttgagggcaatgccag |
All primers given in the 5′ – 3′ direction
Figure 1.
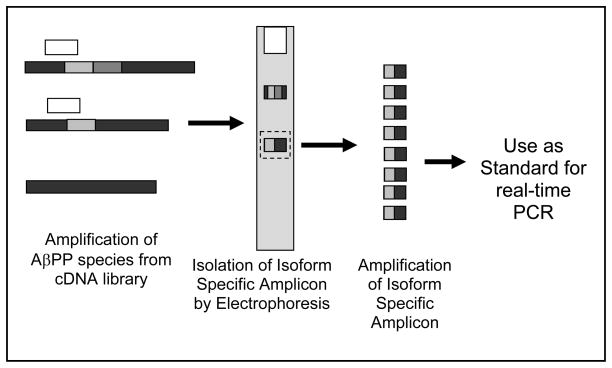
Schematic for preparation of AQ-PCR standards for AβPP isoforms. AβPP species are amplified from cDNA libraries using various primer combinations and resolved on an agarose gel. Using predicted sizes for each amplicon, the isoform specific amplicon is isolated and amplified. After sequencing the amplicon to ensure its fidelity, it can be used as an isoform specific standard. The example here is for AβPP751. Primers (white boxes) amplify multiple AβPP isoforms (Exon 7 - light grey, Exon 8 - dark grey, thus AβPP770, AβPP751, AβPP695 from top to bottom). The amplicon for AβPP751 is excised from the gel and re-amplified using the same primer set. This yields a large pool of the AβPP751-specific amplicon which can then be diluted and used as a real-time PCR standard.
Figure 2.
Preparation of Isoform Specific AβPP Standards. a) Agarose gel electrophoresis of isoform specific primer pairs showing multiple amplicons; from left: 100bp ladder, AβPP770, AβPP751, AβPP695 primer pair 1, AβPP695 primer pair 2, total AβPP primer pair 1, total AβPP primer pair 2, and GAPDH. b) Agarose gel electrophoresis of isolated and PCR amplified isoform specific standards; from left: 100 bp ladder, total AβPP, AβPP770, AβPP751, AβPP695, and GAPDH. No additional amplicons were noted in any lane.
Tissue Collection
Post-mortem brain samples were obtained from Banner Sun Health Research Institute (Sun City, AZ) where they had been collected under an ongoing institutional review board approved research protocol[11]. De-identified samples from human subjects clinically and neuropathologically classified as having Alzheimer’s Disease (AD; n = 12) or no dementia (ND; n = 10) (See Table 2) were tested in accordance with institutional review board policies at the University of Vermont. Subjects without dementia (8 males / 2 females) averaged 76.2 ± 16.3 (mean ± SD) years of age at expiry, and their last Mini-Mental Status Exam (MMSE) scores averaged 28.6 ± 1.5 (n=8; 2 subjects did not have a final MMSE score). AD subjects (5 males / 7 females) were 76.2 ± 9.2 years of age with last MMSE scores averaging 9 ± 6.5 (n = 8; 4 subjects had no final MMSE score). Post-mortem intervals for tissue collection averaged 3 hrs with no differences between ND and AD subjects (p=0.5). AD subjects had frequent plaques and tangles and were all classified as Braak Stage 5 or 6. ND subjects had few to no plaques, low tangle density, and were classified as Braak Stages 1 – 3. The ApoE4 genotype was present in 9 AD subjects, but only 2 ND subjects.
Table 2.
Subject Clinical Characteristics
| No Dementia | Alzheimer’s Disease | ||
|---|---|---|---|
| n | 10 | 12 | |
| Sex (M / F) | 8 / 2 | 5 / 7 | p=0.099** |
| Age (yrs ± SD) | 76.2 ± 16.3 | 76.2 ± 9.2 | p=0.69 |
| ApoE4 (Y / N) | 2 / 8 | 9 / 3 | p=0.015** |
| Last MMSE (± SD) | 28.6 ± 1.5* | 9 ± 6.5* | p≪0.001 |
| Post-Mortem Interval (hrs ± SD) | 3.0 ± 1.3 | 2.7 ± 0.6 | p=0.50 |
| All Plaque Score | 0.8 ± 1.8 | 13.3 ± 1.4 | p≪0.001 |
| Tangle Total | 2.2 ± 1.9 | 13.6 ± 2.9 | p≪0.001 |
| Braak Stage (± SD) | 1.6 ± 0.9 | 5.6 ± 0.5 | p≪0.001 |
n = 8
p-values from Fisher’s Exact test; all other p-values from unpaired Student’s t-test.
Extraction of RNA and preparation of cDNA from brain tissue
Frozen superior frontal gyrus (SFG) and cerebellar (CBL) samples were homogenized in QIAzol reagent and RNA was extracted using RNeasy Lipid tissue mini kit (Qiagen). During the extraction, RNA was treated with DNase I using RNase-free DNase sets (Qiagen) according to the manufacturer’s instructions. A cDNA library was made using Advantage RT-for-PCR kits (Clontech) from 1 μg of RNA with oligo-dT primers according to manufacturer’s instructions.
Real-time PCR
Absolute quantification real-time PCR was carried out in a standard fashion using human TaqMan gene expression assays (assay IDs; AβPP-common / total AβPP: Hs_00169098_m1; AβPP770: Hs_01552289_m1; AβPP751: Hs_01562342_m1; AβPP695: Hs_01562345_m1; GAPDH: 4326317E) on an Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems Inc, Foster City, CA). Relative quantification real-time PCR (RQ-PCR) was conducted using the same protocol for 18s rRNA (assay ID: 4319413E). All samples were brought to room temperature before use. The PCR conditions were as follows: one cycle at 50 °C for 2 min, one cycle at 95 °C for 10 min, and 40 cycles at 95 °C for 15 s and 60 °C for 1 min. All clinical samples were run in duplicate while serially diluted AQ-PCR standards were run in triplicate. Transcript copy numbers were interpolated using the ABI 7300 Sequence Detection Software. RQ-PCR data was analyzed using the 2−Ct method [12]. Unamplified and TaqMan amplified standards and samples were electrophoresed on a 4% agarose gel and visualized using ethidium bromide (Sigma) under UV excitation (See Supplementary Figure 2) to determine the fidelity of the TaqMan reactions. Relative amounts of products in the gels were determined from digital analysis of fluorescence intensity using ImageJ (National Institutes of Health).
Statistical analyses
Clinical data were analyzed using Fisher’s Exact test or unpaired, two-tailed Student’s t-test. All gene expression data were analyzed using unpaired, heteroscedastic, two-tailed Student’s t-tests. Data were natural logarithm transformed where noted. Simple linear regression was used to determine relationships between gene expression and clinical data. A p-value less than 0.05 was considered significant. Data are presented as mean ± SEM, except where noted.
Results
Characterization of AβPP isoform standards
Absolute quantification standards for each transcript amplified with high fidelity and reproducibility; mean r2 for GAPDH standard curves was 0.9931 ± 0.0051 (mean ± SD; n=5), mean r2 for total AβPP (AβPPcm) standards was 0.9970 ± 0.0015 (n=6) (See Supplemental Figure 3), mean r2 for AβPP695 standards was 0.9972 ± 0.0023 (n=4), mean r2 for AβPP751 standards was 0.9967 ± 0.0026 (n=4), and mean r2 for AβPP770 standards was 0.9909 ± 0.0064 (n=4). Primer efficiencies were calculated and any run with an efficiency <1.8 or >2.2 was excluded from further analysis [12]. Gel electrophoresis of TaqMan amplified AQ-PCR standards showed that only one amplicon was produced for each standard (See Supplementary Figure 2a, lanes 7 – 11).
Decreased GAPDH expression in cortex of subjects with Alzheimer’s Disease
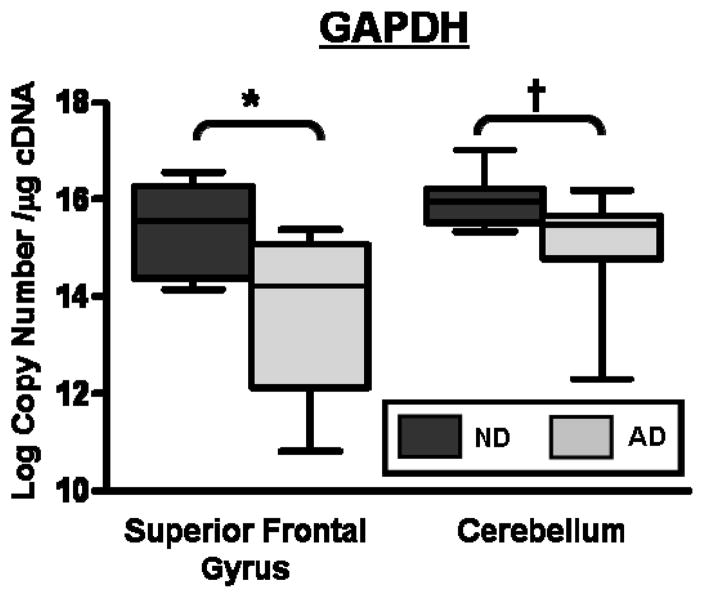
Using these AQ-PCR standards, we found average ln GAPDH expression was decreased by almost 70% in the SFG of subjects with AD compared to ND subjects (p=0.0053). A decrease in GAPDH transcripts between CBL samples from AD or ND subjects was also noted (p=0.017; Figure 3 and Table 3). Gel electrophoresis of TaqMan amplified samples showed that only one amplicon was produced (See Supplementary Figure 2a, lanes 17 – 20). These data suggest a fundamental metabolic change in the AD-affected brain. To further characterize this observation we examined expression of 18s rRNA using RQ-PCR. Mean relative quantities, calculated using 2−Ct, were not significantly different between AD and ND SFG samples (1.1E-06 ± 2.4E-07 vs. 1.5E-06 ± 3.7E-07, p = 0.29) or between CBL samples (1.2E-06 ± 3.4E-07 vs. 8.0E-07 ± 1.4E-07, p = 0.31). Based on these data we concluded that our samples were intact, but that normalization of cerebral gene expression to GAPDH is not appropriate in the setting of AD.
Figure 3.
GAPDH transcript numbers are decreased in superior frontal gyrus in subjects with Alzheimer’s Disease (AD; light grey) by ~70% compared to control subjects without dementia (ND; dark grey)(*p=0.0053). A slight, but significant reduction in GAPDH was found in cerebellar samples from subjects with AD compared to control (†p=0.0168).
Table 3.
AβPP and GAPDH Transcript Copy Numbers
| Superior Frontal Gyrus | Cerebellum | |||||
|---|---|---|---|---|---|---|
| ND | AD | ND | AD | |||
| GAPDH | 6,755 ± 1,635 (15.4 ± 0.3) | 1,780 ± 494 (13.6 ± 0.5) | p=0.0053 | 9,875 ± 2,035 (16.0 ± 0.2) | 4,703 ± 850 (15.0 ± 0.3) | p=0.0168 |
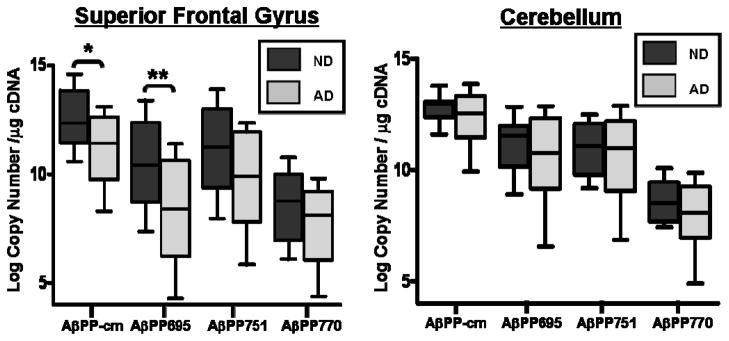
| Total AβPP | 614 ± 231 (12.6 ± 0.4) | 152 ± 46 (11.1 ± 0.5) | p=0.037 | 406 ± 82 (12.8 ± .02) | 388 ± 100 (12.4 ± 0.3) | p=0.34 |
| AβPP695 | 151 ± 69 (10.5 ± 0.7) | 21 ± 9 (8.3 ± 0.7) | p=0.034 | 119 ± 38 (11.2 ± 0.4) | 117 ± 40 (10.5 ± 0.6) | p=0.36 |
| AβPP751 | 267 ± 114 (11.1 ± 0.7) | 67 ± 24 (9.7 ± 0.6) | p=0.14 | 99 ± 32 (11.0 ± 0.4) | 110 ± 36 (10.5 ± 0.5) | p=0.54 |
| AβPP770 | 13 ± 5 (8.5 ± 0.5) | 5 ± 2 (7.6 ± 0.5) | p=0.25 | 8 ± 2 (8.6 ± 0.3) | 6 ± 2 (8.0 ± 0.4) | p=0.21 |
Copy Number ×103 per μg cDNA ± SEM (Natural log transformed copy numbers per μg cDNA ± SEM) p-values from two-tailed Student’s t-test of natural log transformed data with adjustment for unequal variance where necessary.
Decreased AβPP transcripts in the frontal cortex of subjects with Alzheimer’s Disease
A decrease in ln-transformed total AβPP transcript number was found in the SFG of subjects with AD compared to ND (p=0.037), mirrored by decreased expression of the AβPP695 isoform (p=0.034). Transcript levels of AβPP751 and AβPP770 in the SFG of subjects with AD did not differ from ND subjects (p=0.15 and p=0.25 respectively) (Figure 4a; Table 3). CBL samples served as controls as this area of the brain does not typically exhibit substantial amyloid plaque burden in AD [13, 14]. No differences were found in total AβPP copy number from subjects with AD compared to ND in the cerebellar samples. Likewise, no differences were found in copy numbers of AβPP695, AβPP751, or AβPP770 between AD and ND CBL samples (p>0.2 for all; Figure 4b; Table 3). Post-TaqMan amplification gel electrophoresis showed a single amplicon for reactions using AβPPcm and AβPP770 assays. AβPP751 reactions consistently produced a second amplicon at very low levels and AβPP695 reactions produced a second amplicon at low but variable levels (See Supplementary Figure 2b). These secondary amplicons constituted ~5% of the UV fluorescence signal and did not affect overall interpretation of the data (See Discussion).
Figure 4.
AβPP expression is altered in the frontal cortex in subjects with Alzheimer’s Disease (AD) compared to subjects without dementia (ND). There were significant decreases in total AβPP (AβPP-cm) and AβPP695 in the superior frontal gyrus from subjects with AD (*p=0.037 and **p=0.034, respectively). AβPP751 and AβPP770 tended to be decreased in AD, but were not statistically different compared to ND (p=0.14 and p=0.25, respectively). No differences were observed in cerebellar samples from either group (p = 0.21 – 0.54).
Altered AβPP isoform ratios in frontal cortex of subjects with Alzheimer’s Disease
For each tissue sample, the copies of the three measured isoforms accounted for 45.1 ± 4.0% of total AβPP transcript numbers. The AβPP695 isoform transcript was expressed at 15.7 ± 2.9% of total AβPP in the SFG of ND subjects and at 8.3 ± 1.8% in the SFG of AD subjects, a near significant decrease (p=0.051; see Table 4). The AβPP751 isoform comprised 30.0 ± 5.6% of total AβPP in SFG of ND subjects and 30.9 ± 5.7% in SFG of AD subjects (p=0.92). The AβPP770 isoform was elevated in the SFG of AD subjects (3.3 ± 0.4% of AβPPcm) compared to the SFG of ND subjects (1.9 ± 0.3% of AβPPcm; p=0.013). Collectively, the KPI-containing isoforms (AβPP751 and 770) accounted for 32.0 ± 5.8% of total AβPP in the SFG of ND subjects and 34.2 ± 6.0% in SFG from AD subjects(p=0.8). Expression ratios were not different between CBL samples from AD or ND subjects.
Table 4.
AβPP Isoform Expression Ratios
| Superior Frontal Gyrus | Cerebellum | |||||
|---|---|---|---|---|---|---|
| ND | AD | ND | AD | |||
| % AβPP695 | 15.7% ± 2.9 | 8.3% ± 1.8 | p=0.051 | 24.9% ± 4.1 | 20.9% ± 4.1 | p=0.41 |
| % AβPP751 | 30.0% ± 5.6 | 30.9% ± 5.7 | p=0.92 | 21.4% ± 5.4 | 21.1% ± 4.0 | p=0.86 |
| % AβPP770 | 1.9% ± 0.3 | 3.3% ± 0.4 | p=0.013 | 1.8% ± 0.3 | 1.3% ± 0.2 | p=0.12 |
| % AβPP-KPI | 32.0% ± 5.8 | 34.2% ± 6.0 | p=0.80 | 23.2% ± 5.3 | 22.5% ± 4.1 | p=0.80 |
| AβPP695 / AβPP-KPI | 0.50 ± 0.04 | 0.22 ± 0.02 | p≪0.001 | 1.21 ± 0.14 | 0.94 ± 0.09 | p=0.12 |
% Total AβPP ± SEM
AβPP-KPI = AβPP751 + AβPP770
p-values in from two-tailed Student’s t-test with adjustment for unequal variance where necessary.
Decreasing AβPP transcripts correlate with neuroanatomic pathology and mental status
Linear regression analysis showed that decreased ln-transformed total AβPP and AβPP695 transcript numbers in SFG were correlated with higher amyloid plaque counts (r = 0.45, p = 0.035 and r = 0.45, p=0.038 respectively). Decreasing total AβPP and AβPP695 was also associated with lower MMSE scores (r = 0.53, p = 0.034 and r = 0.54, p = 0.03 respectively). Likewise, decreasing GAPDH copy numbers were significantly correlated to increasing tangle density (r = 0.46, p = 0.032) and plaque counts (r = 0.54, p = 0.01) and with decreased MMSE score (r = 0.60, p = 0.013). These associations suggest the decreasing AβPP expression may be a result of neuronal cell loss or dysfunction.
Discussion
Using a refined method for quantitative analysis of gene expression levels of total AβPP, the three major AβPP isoforms, and GAPDH by AQ-PCR, we found significantly decreased transcripts for neuronal AβPP (AβPP695) and total AβPP in the frontal cortex of subjects with AD compared to subjects without dementia. Further examination of isoform expression showed increased percent AβPP770 in the superior frontal gyrus of subjects with AD. We also found significantly decreased levels of GAPDH in the frontal cortex of subjects with AD.
An important technical consideration for this type of study is the quality of tissue and extracted RNA sample. CNS tissue samples degrade rapidly, so short PMI times are necessary to ensure sample quality; PMI times were very low for all subjects in this study. A limitation to this technique is the production of minor PCR products in samples by TaqMan primers. While no secondary products are produced in our standard sets, the amplification of a secondary product by the AβPP751 and AβPP695 TaqMan assays reduces the overall accuracy of the method. Because these products accounted for only ~5% of the total product, we did not adjust final transcript numbers based on the secondary product, but future improvements in TaqMan primers and post-amplification correction of transcript numbers will further refine our ability to accurately quantify AβPP isoforms using AQ-PCR. Another limitation to consider is the sample size of this study, which is relatively small. Therefore these results need to be confirmed in larger, independent datasets.
Understanding the biology of AβPP regulation and the expression of specific isoforms is critical to unraveling the pathology of AD. Preece et al found altered levels of AβPP isoforms in the SFG of individuals with AD compared to ND by relative PCR, but this study relied on primers which amplified multiple isoforms at one time and utilized a non-standard ANCOVA-based multiple reference gene normalization making clear interpretation of the data difficult[8]. Using a solution hybridization-RNase protection assay, Johnston et al found decreased total AβPP expression and increased proportions of KPI-containing AβPP isoforms in the mid-temporal gyrus of subjects with AD[6]. It should be noted that this technique used total nuclear extracts and gave subtly different results based on normalization to total RNA or DNA. Similarly, Liang et al showed increased total AβPP expression in laser-capture microdissected neurons from the mid-temporal gyrus and posterior cingulate of subjects with AD using a gene expression microarray, but confirmatory RQ-PCR found significant decreases in total AβPP expression normalized to β-glucuronidase[15, 16]. Using AQ-PCR, Matsui et al found significantly increased levels of KPI-containing AβPP in the mid-temporal gyrus of subjects with AD with a trend toward increased total AβPP expression[7]. We found a small increase in KPI-containing AβPP770 in the cortex of subjects with AD, but no change in AβPP751 levels. The change of AβPP isoform expression in our study was driven by the reduced AβPP695 level, which they did not measure. There are two important differences between studies: the technique presented here resolves cross-amplification of AβPP species in the standards by isolating the isoform-specific amplicons following gel electrophoresis, and, while both studies noted a significant decrease in GAPDH expression, we chose not to use it to normalize our copy number data. Recent evidence suggests that GAPDH is functionally altered and decreased in AD through oxidative mechanisms[17]. Our data support previous studies showing decreased expression of total AβPP in the frontal cortex of patients with AD. We extend these data by showing that these changes are due to decreased AβPP695. This decrease in both AβPP695 and GAPDH suggests a loss of neurons and synaptic density. Future studies comparing AβPP isoform expression with neuropathological and anatomic changes may yield new insights into normal ageing and dementia.
While a great deal is known about neural AβPP and Aβ, AβPP is also widely expressed in peripheral tissues from skin, intestinal epithelia, and skeletal muscle to leukocytes, platelets, pancreas, and adipose tissue. The function and regulation of peripheral AβPP are not well understood [18–25]. Decreased total AβPP expression and a decreased ratio of AβPP695/751 to AβPP770 has been documented in the platelets of subjects with AD by immunoblot, suggesting a role for AβPP as a peripheral biomarker for AD progression[26, 27]. Increased AβPP levels and aberrant processing was also observed in lymphoblastoid cells from subjects with familial AD in parallel with increased pro-inflammatory cytokine expression[28]. These data are similar to our observation that AβPP is increased in adipocytes of obese individuals and is correlated with a pro-inflammatory cytokine expression profile [24, 29, 30]. Collectively, these data suggest peripheral changes in AβPP expression may be related to the progression of AD.
Absolute quantification of AβPP isoforms using the method presented is technically easy and can be implemented in a standardized fashion allowing AβPP isoform expression data to be compared between experiments and across studies conducted by independent research groups. This robust system could therefore have utility in developing biomarkers of AD from CSF, blood or peripheral tissues.
Supplementary Material
Acknowledgments
WGT is supported by a fellowship award from the National Institutes of Health (F30 DK084605) and by the University of Vermont College of Medicine Department of Medicine. The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901, and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Frankfort SV, Tulner LR, van Campen JP, Verbeek MM, Jansen RW, Beijnen JH. Amyloid beta protein and tau in cerebrospinal fluid and plasma as biomarkers for dementia: a review of recent literature. Curr Clin Pharmacol. 2008;3:123–131. doi: 10.2174/157488408784293723. [DOI] [PubMed] [Google Scholar]
- 3.Ling Y, Morgan K, Kalsheker N. Amyloid precursor protein (APP) and the biology of proteolytic processing: relevance to Alzheimer’s disease. Int J Biochem Cell Biol. 2003;35:1505–1535. doi: 10.1016/s1357-2725(03)00133-x. [DOI] [PubMed] [Google Scholar]
- 4.Xu F, Davis J, Miao J, Previti ML, Romanov G, Ziegler K, Van Nostrand WE. Protease nexin-2/amyloid beta-protein precursor limits cerebral thrombosis. Proc Natl Acad Sci U S A. 2005;102:18135–18140. doi: 10.1073/pnas.0507798102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu F, Previti ML, Nieman MT, Davis J, Schmaier AH, Van Nostrand WE. AbetaPP/APLP2 family of Kunitz serine proteinase inhibitors regulate cerebral thrombosis. J Neurosci. 2009;29:5666–5670. doi: 10.1523/JNEUROSCI.0095-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston JA, Norgren S, Ravid R, Wasco W, Winblad B, Lannfelt L, Cowburn RF. Quantification of APP and APLP2 mRNA in APOE genotyped Alzheimer’s disease brains. Brain Res Mol Brain Res. 1996;43:85–95. doi: 10.1016/s0169-328x(96)00161-1. [DOI] [PubMed] [Google Scholar]
- 7.Matsui T, Ingelsson M, Fukumoto H, Ramasamy K, Kowa H, Frosch MP, Irizarry MC, Hyman BT. Expression of APP pathway mRNAs and proteins in Alzheimer’s disease. Brain Res. 2007;1161:116–123. doi: 10.1016/j.brainres.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 8.Preece P, Virley DJ, Costandi M, Coombes R, Moss SJ, Mudge AW, Jazin E, Cairns NJ. Amyloid precursor protein mRNA levels in Alzheimer’s disease brain. Brain Res Mol Brain Res. 2004;122:1–9. doi: 10.1016/j.molbrainres.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Barrachina M, Dalfo E, Puig B, Vidal N, Freixes M, Castano E, Ferrer I. Amyloid-beta deposition in the cerebral cortex in Dementia with Lewy bodies is accompanied by a relative increase in AbetaPP mRNA isoforms containing the Kunitz protease inhibitor. Neurochem Int. 2005;46:253–260. doi: 10.1016/j.neuint.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Panegyres PK, Zafiris-Toufexis K, Kakulas BA. Amyloid precursor protein gene isoforms in Alzheimer’s disease and other neurodegenerative disorders. J Neurol Sci. 2000;173:81–92. doi: 10.1016/s0022-510x(99)00311-1. [DOI] [PubMed] [Google Scholar]
- 11.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 13.Causevic M, Farooq U, Lovestone S, Killick R. beta-Amyloid precursor protein and tau protein levels are differently regulated in human cerebellum compared to brain regions vulnerable to Alzheimer’s type neurodegeneration. Neurosci Lett. doi: 10.1016/j.neulet.2010.08.088. [DOI] [PubMed] [Google Scholar]
- 14.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 15.Liang WS, Dunckley T, Beach TG, Grover A, Mastroeni D, Ramsey K, Caselli RJ, Kukull WA, McKeel D, Morris JC, Hulette CM, Schmechel D, Reiman EM, Rogers J, Stephan DA. Neuronal gene expression in non-demented individuals with intermediate Alzheimer’s Disease neuropathology. Neurobiol Aging. 31:549–566. doi: 10.1016/j.neurobiolaging.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang WS, Dunckley T, Beach TG, Grover A, Mastroeni D, Ramsey K, Caselli RJ, Kukull WA, McKeel D, Morris JC, Hulette CM, Schmechel D, Reiman EM, Rogers J, Stephan DA. Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: a reference data set. Physiol Genomics. 2008;33:240–256. doi: 10.1152/physiolgenomics.00242.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butterfield DA, Hardas SS, Lange ML. Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer’s disease: many pathways to neurodegeneration. J Alzheimers Dis. 20:369–393. doi: 10.3233/JAD-2010-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bush AI, Martins RN, Rumble B, Moir R, Fuller S, Milward E, Currie J, Ames D, Weidemann A, Fischer P, et al. The amyloid precursor protein of Alzheimer’s disease is released by human platelets. J Biol Chem. 1990;265:15977–15983. [PubMed] [Google Scholar]
- 19.Galloway S, Jian L, Johnsen R, Chew S, Mamo JC. beta-amyloid or its precursor protein is found in epithelial cells of the small intestine and is stimulated by high-fat feeding. J Nutr Biochem. 2007;18:279–284. doi: 10.1016/j.jnutbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Hansel DE, Rahman A, Wehner S, Herzog V, Yeo CJ, Maitra A. Increased expression and processing of the Alzheimer amyloid precursor protein in pancreatic cancer may influence cellular proliferation. Cancer Res. 2003;63:7032–7037. [PubMed] [Google Scholar]
- 21.Herzog V, Kirfel G, Siemes C, Schmitz A. Biological roles of APP in the epidermis. Eur J Cell Biol. 2004;83:613–624. doi: 10.1078/0171-9335-00401. [DOI] [PubMed] [Google Scholar]
- 22.Joachim CL, Mori H, Selkoe DJ. Amyloid beta-protein deposition in tissues other than brain in Alzheimer’s disease. Nature. 1989;341:226–230. doi: 10.1038/341226a0. [DOI] [PubMed] [Google Scholar]
- 23.Kuo YM, Kokjohn TA, Watson MD, Woods AS, Cotter RJ, Sue LI, Kalback WM, Emmerling MR, Beach TG, Roher AE. Elevated abeta42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AbetaPP metabolism. Am J Pathol. 2000;156:797–805. doi: 10.1016/s0002-9440(10)64947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YH, Tharp WG, Maple RL, Nair S, Permana PA, Pratley RE. Amyloid precursor protein expression is upregulated in adipocytes in obesity. Obesity (Silver Spring) 2008;16:1493–1500. doi: 10.1038/oby.2008.267. [DOI] [PubMed] [Google Scholar]
- 25.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 26.Colciaghi F, Marcello E, Borroni B, Zimmermann M, Caltagirone C, Cattabeni F, Padovani A, Di Luca M. Platelet APP, ADAM 10 and BACE alterations in the early stages of Alzheimer disease. Neurology. 2004;62:498–501. doi: 10.1212/01.wnl.0000106953.49802.9c. [DOI] [PubMed] [Google Scholar]
- 27.Di Luca M, Pastorino L, Bianchetti A, Perez J, Vignolo LA, Lenzi GL, Trabucchi M, Cattabeni F, Padovani A. Differential level of platelet amyloid beta precursor protein isoforms: an early marker for Alzheimer disease. Arch Neurol. 1998;55:1195–1200. doi: 10.1001/archneur.55.9.1195. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto A, Fujiwara Y. Aberrant proteolysis of the beta-amyloid precursor protein in familial Alzheimer’s disease lymphoblastoid cells. Eur J Biochem. 1993;217:21–27. doi: 10.1111/j.1432-1033.1993.tb18213.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee YH, Martin JM, Maple RL, Tharp WG, Pratley RE. Plasma Amyloid-beta Peptide Levels Correlate with Adipocyte Amyloid Precursor Protein Gene Expression in Obese Individuals. Neuroendocrinology. 2009 doi: 10.1159/000235555. [DOI] [PubMed] [Google Scholar]
- 30.Lee YH, Nair S, Rousseau E, Allison DB, Page GP, Tataranni PA, Bogardus C, Permana PA. Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia. 2005;48:1776–1783. doi: 10.1007/s00125-005-1867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.