Abstract
Alcohol use disorders are characterized by compulsive drug-seeking and drug-taking, loss of control in limiting intake, and withdrawal syndrome in the absence of drug. The central amygdala (CeA) and neighboring regions (extended amygdala) mediate alcohol-related behaviors and chronic alcohol-induced plasticity. Acute alcohol suppresses excitatory (glutamatergic) transmission whereas chronic alcohol enhances glutamatergic transmission in CeA. Acute alcohol facilitates inhibitory (GABAergic) transmission in CeA, and chronic alcohol increases GABAergic transmission. Electrophysiology techniques are used to explore the effects of neuropeptides/neuromodulators (CRF, NPY, nociceptin, dynorphin, endocannabinoids, galanin) on inhibitory transmission in CeA. In general, pro-anxiety peptides increase, and anti-anxiety peptides decrease CeA GABAergic transmission. These neuropeptides facilitate or block the action of acute alcohol in CeA, and chronic alcohol produces plasticity in neuropeptide systems, possibly reflecting recruitment of negative reinforcement mechanisms during the transition to alcohol dependence. A disinhibition model of CeA output is discussed in the context of alcohol dependence- and anxiety-related behaviors.
Keywords: Central Amygdala, GABA, Glutamate, CRF, NPY, Nociceptin, Dynorphin, Galanin, Cannabinoids, Neuropeptides, Alcohol Dependence
1. Introduction
Alcoholism (i.e., substance dependence on alcohol) is a complex and dynamic disease process. The ICD-10 & DSM-IV describe alcohol dependence as a chronically relapsing disorder characterized by compulsive drug-seeking and drug-taking, loss of control in limiting intake (in terms of amount of drug per bout and number of drug-taking bouts), and the emergence of a withdrawal syndrome in the absence of the drug that includes, but is not limited to dysphoria, sleep disturbances, disruption of autonomic processes, as well as increases in anxiety and irritability (American Psychiatric Association, 1994; World Health Organization, 2007). These stages of alcohol abuse and dependence have been conceptualized in terms of cycling between alcohol binge intake and intoxication, physical and motivational withdrawal from alcohol in its absence, and preoccupation with and anticipation of alcohol consumption (Koob, 2003). These cycles are hypothesized to become progressively more exaggerated with time, whereby tolerance produces higher levels of alcohol intake, which produce gradually worsening withdrawal syndromes in the absence of the drug, thereby increasing the probability of subsequent alcohol-seeking and alcohol-drinking behavior (Koob, 2003). Many years of research have shaped the current view that excessive alcohol consumption is largely mediated by an organism’s past experience (e.g., pattern and frequency) with alcohol, and is driven by the emotional disturbances, rather than the physical disturbances, associated with alcohol withdrawal/abstinence (Heilig et al., 2010).
Animal models of alcoholism aim to mimic particular components, rather than the entirety of the addiction phenotype in humans. For example, genetic animal models have been used to model the heritable aspects of spontaneous and persistent high and low alcohol preference in the offspring of animals that consume either high or low quantities of alcohol (Grahame et al., 1999; Murphy et al., 2002). Alternatively, models of chronic high-dose alcohol exposure have been utilized to produce an array of neural, emotional and motivational disturbances that reflect symptoms of alcohol dependence. Each method (e.g., alcohol vapor, alcohol liquid-diet) of inducing alcohol dependence in rats has its own set of advantages and disadvantages (for reviews, see Lieber & DeCarli, 1973; Rogers et al., 1979). Most of the studies described below (behavior and slice electrophysiology) utilize chronic intermittent alcohol vapor inhalation for producing alcohol dependence in rats. The advantages of this procedure over others are that the dose, duration and pattern of alcohol exposure are easily controlled across rats (providing a singular independent variable) and the procedure reliably produces physical and motivational aspects of alcohol dependence. The major disadvantages of the alcohol vapor procedure are that alcohol exposure is not voluntary and that the route of administration is not the same as in humans, which can lead to a different set of deleterious effects on peripheral physiological systems.
As described above, alcohol addiction is defined by three recurring and cyclical phases. The binge/intoxication phase has been modeled in rodents using alcohol self-administration, conditioned place preference and brain stimulation reward (i.e. intracranial self-stimulation) techniques. The withdrawal/negative affect phase has been modeled using withdrawal-induced alcohol self-administration and conditioned place aversion techniques, as well as an array of assays intended to measure anxiety-like behavior in rodents. Finally, the preoccupation/anticipation phase has been modeled using reinstatement procedures wherein animals exhibit increases in drug-seeking behavior triggered by a small priming alcohol dose, exposure to stressor, or presentation of a drug-related cue (Koob, 2009). The current review will focus on neurotransmission in the central nucleus of the amygdala (CeA) as it is relevant to the withdrawal/negative affect phase of the alcohol addiction cycle. A major conclusion of this review is that neuropeptide systems in the CeA interact with each other and with alcohol in a complex way to modulate synaptic transmission, largely by modulating pre-synaptic release, and that the transition to alcohol dependence is marked by neuroadaptations that functionally up-regulate many of these neuropeptide systems in the CeA.
2. Negative Emotional State Circuitry
Chronic consumption of large quantities of drugs, including alcohol, promotes a transition from casual drug use to drug dependence that is defined neurally by downregulation of dopamine signaling in the mesocorticolimbic reward system, hyperactivity of glutamate signaling, and dysregulation of brain stress systems (Koob & Volkow, 2010). Chronic alcohol effects on brain stress systems can refer to either alcohol-induced changes in neuroendocrine function (i.e. hypothalamic-pituitary-adrenal [HPA] axis; Clarke et al., 2008; Kiefer & Wiedemann, 2004) or the recruitment of extra-hypothalamic brain stress systems. The current review will focus on the circuitry of extra-hypothalamic stress systems involved in excessive alcohol-drinking phenotypes, with the ultimate goal of guiding future research on the neurobiological mechanisms of alcohol dependence. This review will detail the effects of acute and chronic alcohol on synaptic transmission and plasticity in the CeA and neighboring regions that exhibit high connectivity with the CeA, and the role of these regions in mediating alcohol-related behaviors. Throughout this review, acute alcohol exposure refers to in vitro application of ethanol to the slice preparation, whereas chronic alcohol exposure refers to long-duration (at least several weeks) in vivo alcohol exposure. Furthermore, the chronic alcohol exposure protocols utilized here reliably produce somatic and motivational signs of alcohol dependence (Gilpin et al., 2009), therefore, the terms chronic alcohol exposure and dependence will be used interchangeably in this review. Finally, we will review the existing literature on peptidergic modulation of synaptic transmission in the central (and extended) amygdala, particularly because these peptides share a common cellular target, and they interact with each other and alcohol.
Many of the long-term emotional disturbances associated with alcohol abuse and dependence have been attributed to neurotransmission within a conceptual macrostructure in the basal forebrain called the “extended amygdala” (Koob, 2008). In the context of drug addiction, the major constituents of the extended amygdala are the CeA, bed nucleus of the stria terminalis (BNST), and the nucleus accumbens (NAc) shell (Heimer & Alheid, 1991). These regions exhibit similar morphology, a high degree of interconnectivity, and overlapping afferents from limbic cortices, hippocampus, and basolateral amygdala (BLA). Data from the BNST and BLA will be used throughout the review to support findings in the CeA, but full review of alcohol effects on synaptic transmission in those regions is beyond the scope of this review and can be found elsewhere (e.g., McCool et al., 2010). The outputs of the extended amygdala project largely to effector regions, including lateral hypothalamus and various brainstem regions that produce changes in behavior, most prominently those related to fear and anxiety responses (Davis, 1998).
The role of the extended amygdala in fear and anxiety behaviors has been well described (Davis et al., 2010). Many years of work have contributed to the notion that the CeA and BNST play prominent roles in phasic fear and sustained fear (i.e., anxiety). The BLA receives significant sensory input from thalamus and cortex, sends prominent glutamatergic projections to CeA and BNST, and is integral in both conditioning (Phelps & LeDoux, 2005) and extinction (Quirk & Mueller, 2008) processes. The CeA is composed mostly of gamma-aminobutyric acid (GABA)ergic projection neurons and interneurons (Sun & Cassell, 1993; Veinante & Freund-Mercier, 1998), and a major target of CeA projection neurons is the BNST (Krettek & Price, 1978; Sun et al., 1991; Sun & Cassell, 1993; Veinante & Freund-Mercier, 1998; Weller & Smith, 1982). Of major relevance for the data discussed below, connections between CeA and BNST often contain neuropeptide co-transmitters, for example, the CeA is a major source of corticotropin-releasing factor (CRF) in the BNST (Sakanaka et al., 1986).
Neuropeptides in the extended amygdala have been attributed a prominent role in the negative affective aspects of addiction to drugs, including alcohol (Koob, 2008). More specifically, these peptides have been conceptually divided into pro-stress peptides and anti-stress peptides that respectively promote and rescue negative affective disturbances during drug abstinence following heavy drug use. Pro-stress peptides include CRF, dynorphin, hypocretin/orexin, and vasopressin, whereas anti-stress peptides include neuropeptide Y (NPY) and nociceptin. It is becoming increasingly evident that these peptides interact in a complex way in the extended amygdala to modulate excitatory and inhibitory transmission, and that dysregulation of these peptide systems by alcohol alters the way in which they modulate synaptic neurotransmission (see Figure 1).
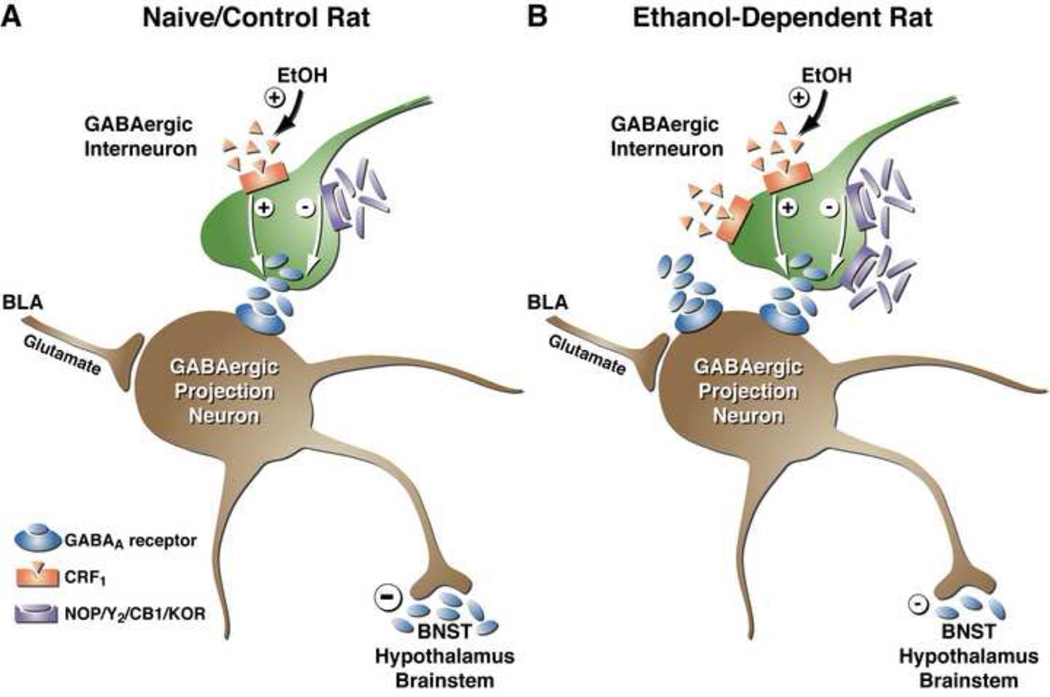
Figure 1.
Acute and chronic effects of alcohol on GABAergic synaptic transmission in the medial aspect of the CeA. Schematic diagram of a medial CeA GABAergic synapse, including pre-synaptic GPCRs that modulate neurotransmitter release and post-synaptic ionotropic receptors located at synapses. GABAergic afferents in the medial CeA arise largely from the lateral aspect of the CeA and the intercalated GABA cells. Many of these neurons contain CRF and/or other neuropeptides as co-transmitters. Glutamatergic inputs to medial CeA arise largely from BLA. (A) In the medial CeA synapse of an alcohol-naïve rat, the predominant pre-synaptic effect of acute alcohol is potentiation of GABA release, most likely via effects on neuromodulators such as CRF. It is thought that activation of pre-synaptic CRF1Rs and downstream signaling pathways (e.g., AC and PKCε) increases the probability of vesicle fusion and GABA release. Acute alcohol enhances GABA release from CeA interneurons or afferent projections to the CeA (green neuron) either via release of CRF onto the pre-synaptic terminal or via direct activation of CRF1Rs. Thus, CRF and acute alcohol both augment the inhibition of CeA projection neurons (brown neuron), leading to excitation (i.e., disinhibition) of downstream (e.g., BNST, hypothalamus, PAG, LC) neurons. Activation of pre-synaptic nociceptin (NOP) receptors, NPY-Y2 receptors, CB1 receptors, and KORs reduces GABA release onto CeA inhibitory projection neurons, increasing inhibition of downstream targets. (B) Following chronic alcohol exposure (i.e., the development of alcohol dependence), the GABA release is increased at medial CeA synapses, leading to more inhibition of GABA projection neurons out of CeA and net disinhibition of downstream target regions (smaller negative symbol in right panel). This increased GABA release may be due to increased CRF levels in the CeA, since CRF1R antagonists reverse basal increases in GABA release of alcohol-dependent rats. There is also evidence for increased function/quantity of pre-synaptic NOP and Y2Rs in the medial CeA of alcohol-dependent rats.
3. Inhibitory (GABAergic) Transmission in Amygdala & Alcohol
For the slice electrophysiology data presented in this section, it is important to note that in the preparation used in our lab, inhibitory transmission is pharmacologically isolated and post-synaptic potentials are evoked by local electrical stimulation in the CeA (Figure 1). Because projection neurons out of the CeA are also inhibitory, evoked GABAergic transmission from CeA afferents and interneurons inhibits the activity of GABAergic neurons projecting out of CeA. Conversely, decreases in GABAergic transmission from CeA afferents and interneurons would increase the activity of GABAergic neurons projecting out of CeA, thereby facilitating the release of GABA onto downstream targets. As such, recorded increases in GABAergic transmission reflect a disinhibition of downstream target regions (e.g., hypothalamus, periaqueductal grey, locus coeruleus, nucleus of the solitary tract, pedunculopontine tegmental nucleus), whereas recorded decreases in GABAergic transmission reflect a net inhibition of downstream target regions. The overall hypothesis, then, is that increases and decreases in local GABAergic transmission in CeA produce decreases and increases in inhibitory output from the CeA to downstream effector regions, and increases and decreases in anxiety-like behavior, respectively.
3.1 GABAergic Transmission & Alcohol-Related Behaviors
GABA, the major inhibitory transmitter in the brain, acts on two classes of GABA receptors: GABAA (which includes GABAA-rho subclass -formerly GABAC) and GABAB. GABAA receptors are ligand-gated ion channels, whereas GABAB receptors are G protein-coupled receptors. There is considerable evidence that GABAergic transmission mediates some aspects of alcohol-drinking behavior, but there is some ambiguity in the literature with respect to the directions of these effects. Early studies showed that systemic administration of GABAAR agonists increased voluntary alcohol drinking whereas GABAAR antagonists and benzodiazepine inverse agonists decreased alcohol drinking (Boyle et al., 1993; Rassnick et al., 1993a). A subsequent experiment showed that infusion of both GABAAR agonists and antagonists into the NAc suppress alcohol drinking by non-dependent rats (Hodge et al., 1995). A more recent study showed that systemic administration of a GABABR agonist suppresses alcohol drinking by all rats, but that alcohol-dependent rats are more sensitive to this effect, suggesting an upregulation of GABABR function in those animals (Walker & Koob, 2008). These studies confirm the long-standing notion that chronic alcohol produces neuroadaptations in inhibitory neurotransmission, as supported by findings that [1] GABA mediates somatic disturbances associated with acute alcohol withdrawal (Cooper et al., 1979; Frye et al., 1983) and [2] animals exhibit changes in sensitivity to GABAergic compounds following chronic alcohol exposure (Martz et al., 1983; Rassnick et al., 1993a).
Behavioral studies have highlighted a role for brain GABA circuitry in alcohol-drinking behavior, particularly in regions implicated in the negative reinforcing properties of the drug (i.e., extended amygdala). One such study examined the effects of GABAAR antagonism in the major constituent regions of the extended amygdala: CeA, BNST, and NAc shell (Hyytia and Koob, 1995). In each of these regions, antagonism of GABAARs suppressed alcohol drinking by non-dependent rats, but this suppressive effect on alcohol consumption was most potent and most selective for alcohol when infused into the CeA. Another study showed that antagonism of GABA receptors in the BNST reverses decreases in alcohol drinking produced by D2 receptor antagonism in the ventral tegmental area (VTA) of alcohol-preferring (P) rats (Eiler & June, 2007). Interestingly, infusion of a GABAA agonist directly into the amygdala suppresses alcohol drinking by alcohol-dependent rats without affecting intake by non-dependent controls (Roberts et al., 1996). There are considerable methodological differences between these studies, but what is clear is that GABAergic neurotransmission modulates alcohol drinking, and in the case of excessive alcohol consumption by alcohol-dependent rats, the amygdala is a strong candidate region for localization of these effects.
3.2 Acute Alcohol Effects on Inhibitory Transmission in CeA
In vitro brain slice preparations provide a number of highly sensitive experimental strategies that allow for investigation of the synaptic effects of ethanol (for reviews of these approaches, see Weiner, 2002; Siggins et al., 2005). Unlike many cell culture models, the interneuronal connections between neurons are largely preserved. While not all the afferent and efferent projections are maintained in brain slices, the synapses that are preserved have developed in their native environment and are representative of the anatomical relationships that are observed in vivo. The stability and experimental access afforded by the brain slice preparation facilitates single-cell and whole-cell recordings that are extremely difficult to carry out in intact animals.
Acute alcohol potentiation of GABAA receptor function has been extensively studied (for review, see Lovinger & Roberto, in press). Alcohol (1–100 mM) selectively potentiates the function of GABAA receptors that contain particular subunit compositions, but findings have been inconsistent across laboratories examining alcohol effects in heterologous systems (reviewed in (Lovinger and Homanics, 2007; Aguayo et al., 2002). In general, alcohol enhances synaptic inhibition by increasing GABA release from pre-synaptic terminals (reviewed in Siggins et al., 2005) and also by activating post-synaptic GABAA receptors. Alcohol increases both evoked and spontaneous GABAergic synaptic transmission in the CeA (Roberto et al., 2003; see Figure 2) and BLA (Zhu and Lovinger, 2006). More specifically, ethanol increases the amplitudes of evoked GABAA-mediated inhibitory post-synaptic potentials and currents (IPSP/Cs) (Fig. 2) and miniature IPSC (mIPSC) frequencies (Fig. 3). These effects are rapid and reversible, and have a significant pre-synaptic component. The post-synaptic contribution to alcohol effects in the CeA is evidenced by acute alcohol-induced increases in mIPSC amplitudes as well as increases in the response to exogenous GABA in the presence of tetrodotoxin (TTX; in approximately two-thirds of cells tested; Roberto et al., 2003)
Figure 2.
Histogram summarizing the effects of ethanol (44 mM), nociceptin (500 nM), NPY (500 nM), and various CRF1R antagonists on evoked GABA IPSP/Cs in medial CeA. Also depicted are the effects of ethanol in combination with nociceptin, NPY, and CRF1R antagonists on evoked GABA IPSP/Cs in CeA. Ethanol produces increases in evoked CeA IPSP/Cs that are reliably prevented and reversed by nociceptin, NPY and CRF1R antagonists.
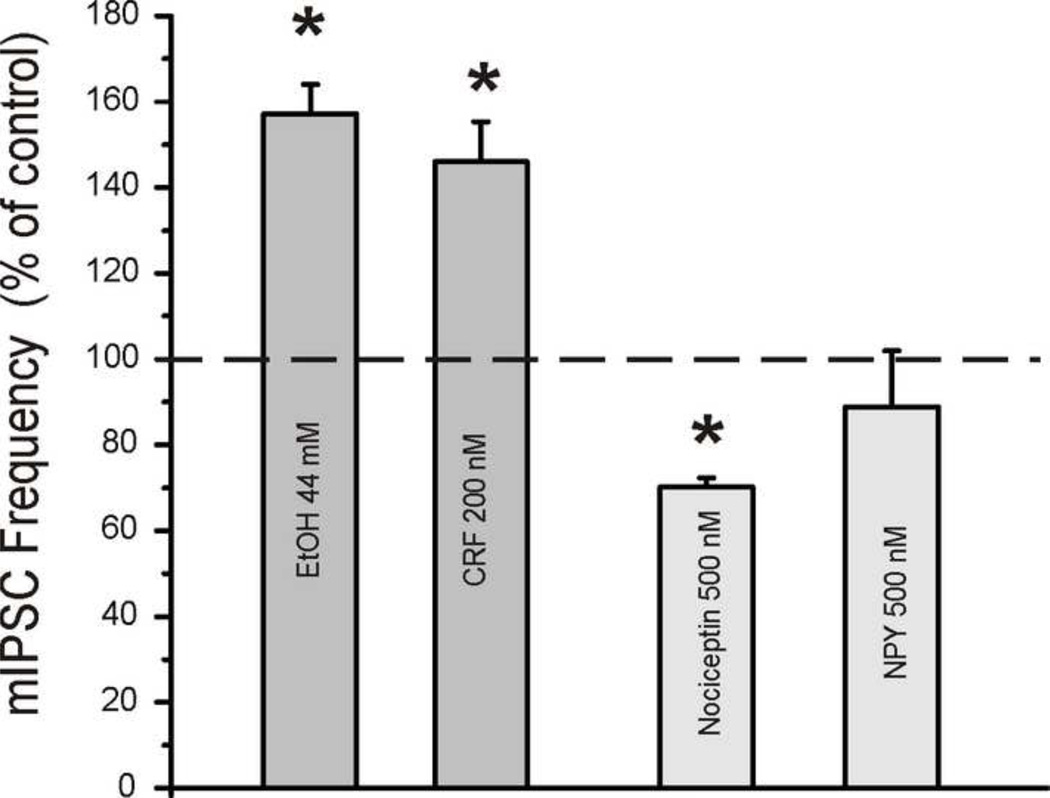
Figure 3.
Histogram summarizing the effects of ethanol (44 mM), CRF (200nM), nociceptin (500 nM) and NPY (500 nM) on miniature IPSC frequencies in medial CeA. Ethanol and CRF facilitate mIPSC frequency, indicative of increased pre-synaptic GABA release. Nociceptin decreases mIPSC frequency, indicative of decreased GABA release. NPY alone produces a non-significant tendency toward decreased mIPSC frequency, although a Y2R antagonist produces decreases in paired-pulse ratio of GABA IPSPs (not shown), indicative of increased GABA release. Together, these data suggest that ethanol and these neuromodulators have a pre-synaptic site of action in medial CeA.
Although the precise mechanism(s) by which alcohol potentiates GABA release have yet to be identified, past studies have examined the role of intracellular signaling pathways in the facilitatory effect of acute alcohol on GABAergic transmission. It is well established that activation of adenylyl cylase (AC) or protein kinase C (PKC) potentiates transmission at synapses throughout the nervous system (Cruz et al., 2011; Leenders & Sheng, 2005; Nguyen et al., 2000; Kelm et al., 2010; Kelm et al., 2011). It is logical to speculate that these signaling molecules might play a role in acute alcohol-induced facilitation of GABAergic release from pre-synaptic terminals since, for example, the enhancing effect of alcohol on GABA release is impaired in CeA of mice that lack PKC epsilon (PKCε; Bajo et al., 2008), suggesting that PKCε facilitates vesicular GABA release in the presence of alcohol. It is important to note that basal GABA release is increased in PKCε knockout mice prior to alcohol application, suggesting that under normal conditions in wild-type neurons, PKCε limits spontaneous GABA release. Because developmental changes or compensatory effects of other gene products may confound studies in gene-targeted mice, we pharmacologically confirmed the role of PKCε in regulating GABA release from CeA neurons by using a PKCε inhibitor peptide, Tat-εV1–2 (Qi, et al., 2007). Superfusion of the inhibitor alone onto slices from PKCε wild type mice increased the mean evoked IPSP amplitude and decreased the PPF of IPSPs in CeA neurons. These results suggest a constitutive role for PKCε in tonically inhibiting GABA release at CeA synapses that may account, in part, for increased basal GABAergic transmission seen in PKCε knockout mice. Moreover, pretreatment of CeA neurons with the Tat-εV1–2 peptide blocked the ethanol and CRF-induced GABA release. These results resemble those obtained in CeA neurons from PKCε knockout mice and support the conclusion that PKCε mediates ethanol-induced GABA release in CeA. Therefore, PKCε serves at least two roles in the CeA: (1) limit baseline GABA release, and (2) mediate alcohol-stimulated release of GABA.
3.3 Chronic Alcohol Effects on Pre-Synaptic GABA Release in CeA
Chronic alcohol exposure affects pre-synaptic release of GABA, and most of these data come from in vitro brain slice preparations, an approach that allows for multiple methods of detecting pre-synaptic changes in transmitter release (for review, see Siggins et al., 2005; Weiner and Valenzuela, 2006). Although the current review focuses on alcohol effects in the amygdala, the effects of alcohol on GABA transmission in other brain regions has been reviewed elsewhere (Criswell and Breese, 2005; Siggins et al., 2005; Weiner and Valenzuela, 2006). Our lab has shown that chronic in vivo alcohol exposure facilitates GABA release in the CeA, mainly via actions at pre-synaptic GABAergic terminals (Roberto et al., 2004a, 2010). Relative to alcohol-naïve controls, alcohol-dependent rats exhibit larger baseline amplitudes of evoked IPSP/Cs, smaller baseline paired-pulse facilitation (PPF) of GABAA IPSCs, and higher baseline frequency of mini IPSCs in CeA neurons, suggesting augmented GABA release in CeA following chronic alcohol exposure. Interestingly, acute alcohol increases IPSCs, decreases PPF ratio of IPSCs, and increases mIPSC frequency similarly in alcohol-dependent and alcohol-naïve rats, suggesting a lack of tolerance for the acute effects of alcohol (Roberto et al., 2004a). This unaltered sensitivity of GABAARs to acute alcohol in vitro following chronic in vivo alcohol exposure is confirmed by recordings from BLA neurons of monkeys with a long history of alcohol self-administration (Anderson et al., 2007). Although animals develop tolerance to many of the behavioral effects of alcohol in vivo, the observed lack of tolerance to the acute effects of alcohol on GABA release in CeA of alcohol-dependent rats is not necessarily surprising in light of the highly region-specific effects of acute and chronic alcohol on synaptic transmission.
Consistent with in vitro electrophysiological results, in vivo microdialysis studies have measured a four-fold increase of baseline dialysate GABA concentrations in the CeA of alcohol-dependent rats relative to alcohol-naïve controls, as well as lack of tolerance for alcohol-induced increases in dialysate GABA levels in alcohol-dependent rats (Roberto et al., 2004a). These results strongly suggest that both acute and chronic alcohol alter pre-synaptic components of GABAergic synapses in the CeA, although it is not clear at this point whether the microdialysis data are attributable to changes in release or uptake or both. Future studies should determine the molecular mechanisms responsible for chronic alcohol-induced adaptations in CeA neurons, and the behavioral implications of these neuroadaptations in the alcohol-dependent and/or alcohol-withdrawn organism. For example, acute and chronic alcohol each produce changes in histone acetylation in CeA (in opposite directions) that have been functionally linked to some of the peptide systems discussed below (Pandey et al., 2008). Future studies will help to elucidate the mechanism(s) responsible for reductions in alcohol withdrawal-related hyperexcitability produced by GABAmimetic drugs (Breese et al., 2006; McCown et al., 1985; Roberto et al., 2008; Ticku and Burch, 1980), and may have implications for treatment of pathological excessive alcohol-drinking behaviors.
3.4 Chronic Alcohol Effects on Post-Synaptic GABAAR Transmission in CeA
Chronic alcohol exposure produces tolerance to many behavioral effects of the drug, including the anxiolytic, sedative, ataxic, and positive reinforcing effects of alcohol (Kumar et al., 2004; Kumar et al., 2009). Chronic alcohol also produces physical and motivational dependence, and alcohol withdrawal is associated with increased neuronal excitability (Kliethermes, 2005; Weiner and Valenzuela, 2006). These chronic alcohol effects are thought to reflect, at least in part, compensatory adaptations to the facilitatory effects of alcohol on GABAergic synapses (Siggins et al., 2005; Weiner and Valenzuela, 2006). Using in vitro brain slice preparations, we have shown that evoked IPSCs in CeA slices from alcohol-dependent rats are significantly increased relative to alcohol-naive controls (Roberto et al., 2004a). This result differs, for example, from the GABAAR tolerance seen in BLA of monkeys following long-term alcohol consumption (Floyd et al., 2004). Slices from alcohol-dependent rats also exhibit increased mIPSC amplitude relative to naïve rats, indicative of a post-synaptic effect of chronic alcohol. Substantial evidence suggests that alcohol-induced behavioral and neural adaptations are attributable to marked changes in GABAAR subunit assembly rather than decreases in the number of GABAARs (Devaud et al., 1995; Eckardt et al., 1998; Grobin et al., 1998; Kumar et al., 2004; Kumar et al., 2009; Morrow et al., 1992). That said, there is considerable ambiguity in the literature (both within and across species) regarding which GABAAR subunits are most critical for mediating alcohol actions (e.g., Borghese & Harris, 2007; Hemby et al., 2006; Korpi et al., 2007), and the critical subunits are likely to vary across brain regions (Grobin et al., 2000).
3.5 Acute and Chronic Alcohol Effects on GABABR Transmission in CeA
It has been suggested that the ability of alcohol to facilitate GABAergic neurotransmission is limited by GABA feedback onto pre-synaptic GABABRs (Ariwodola and Weiner, 2004). For example, acute alcohol facilitates GABAergic transmission in hippocampus (Ariwodola and Weiner, 2004; Wan et al., 1996; Wu and Saggau, 1994) and nucleus accumbens (Nie et al., 2000) only if GABAB receptors are blocked. However, in the CeA, GABAB receptor blockade is not required for the enhancement of IPSPs by acute alcohol nor does it potentiate this effect (Roberto et al., 2003). Thus, the involvement of GABAB receptors in alcohol-induced GABA release may be brain region-specific, and may depend on the presence or absence of pre-synaptic GABAB receptors in particular brain regions (Ariwodola and Weiner, 2004).
Our lab has reported neuroadaptations in CeA GABABRs following chronic alcohol exposure (Roberto et al., 2008). More specifically, the sensitivity of GABA IPSCs to GABABR agonists and antagonists is decreased following chronic alcohol, suggesting downregulation of this system. In alcohol-naïve rats, a GABABR antagonist significantly increases the amplitude of evoked IPSCs and decreases the PPF ratio of IPSCs in CeA, suggesting a tonic activation of pre-synaptic GABABRs. Conversely, a GABABR agonist markedly depresses evoked IPSC amplitudes and increases the PPF ratio of IPSCs in the CeA of alcohol-naïve rats, indicative of decreased pre-synaptic GABA release. These effects of GABABR agonists and antagonists are either absent or greatly attenuated in the CeA of alcohol-dependent rats, suggesting a chronic alcohol-induced downregulation of the GABABR system that may help to explain the increased GABAergic tone observed in the CeA of alcohol-dependent rats (Roberto et al., 2008). These alcohol dependence-induced neuroadaptations of the GABABR system may account for chronic alcohol-induced changes in gabapentin effects on inhibitory transmission in CeA. Gabapentin, a structural analogue of GABA (Sills, 2006), increases the amplitude of evoked IPSCs in CeA neurons from non-dependent rats (an effect that is blocked by a GABABR antagonist), but decreases IPSC amplitude in CeA of alcohol-dependent rats. Likewise, gabapentin infused directly into the CeA reverses alcohol dependence-induced increases in operant alcohol responding, but tends to increase alcohol drinking by non-dependent rats (Roberto et al., 2008).
4. Excitatory (Glutamatergic) Transmission in Amygdala & Alcohol
4.1 Glutamatergic Transmission & Alcohol-Related Behaviors
Glutamate, the major excitatory neurotransmitter in the brain, has long been implicated in the acute reinforcing actions of alcohol. Glutamate receptors include three major classes of ionotropic receptors (iGluRs) that are cation-permeable ion channels, with varying ratios of selectivity for Na+, K+ and Ca2+. The ionotropic glutamate receptors include α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), N-Methyl-D-aspartate receptors (NMDARs), and kainate receptors (KARs). In contrast, the various sub-classes of metabotropic glutamate receptors (mGluRs) are G protein-coupled receptors (GPCRs).
In contrast to its effects on GABA, alcohol inhibits glutamatergic neurotransmission in the brain (for review, see Lovinger & Roberto, in press) and in fact, animals generalize the interoceptive cues (i.e., feelings) associated with alcohol to NMDA receptor antagonists (Colombo & Grant, 1992). Dysregulation of brain glutamate systems is thought to contribute to hyperexcitability and craving associated with alcohol withdrawal (Pulvirenti & Diana, 2001). Acamprosate, a pharmacotherapeutic approved for treatment of alcoholic patients, modulates glutamatergic transmission via actions at NMDA receptors and/or metabotropic glutamate receptors (Blednov & Harris, 2008; Mann et al., 2008). Acamprosate dampens increased glutamate levels in abstinent alcoholics as measured by magnetic resonance spectroscopy (Umhau et al., 2010) and reduces excessive alcohol drinking in those individuals, presumably by reducing craving and negative affect (for review, see Littleton, 2007). AMPARs are another category of ionotropic glutamate receptors that appear to be important in regulating relapse-like behaviors without having a central role in alcohol consumption per se (Sanchis-Segura et al., 2006).
Excitatory transmission in the CeA appears to mediate some of the aversive aspects of withdrawal from drugs of abuse (e.g., morphine; Watanabe et al., 2002). In the CeA of P rats, long-term consumption of high quantities of alcohol produces increased levels of metabotropic glutamate receptors, NMDA receptor subunits, and a scaffolding protein that regulates expression of these receptors on the cell membrane (Obara et al., 2009). It has also been suggested that Group II metabotropic glutamate receptors block stress-induced and cue-induced reinstatement of alcohol-seeking behavior via neuronal activation in CeA or BNST (Zhao et al., 2006).
4.2 Acute Alcohol Effects on Excitatory Transmission in CeA
Acute alcohol (2–100 mM) exerts inhibitory effects on ionotropic glutamate receptor function, the best characterized of which may be inhibition of NMDARs (Dildy and Leslie, 1989; Hoffman et al., 1989; Lima-Landman and Albuquerque, 1989, Lovinger et al., 1989) and reduction of NMDAR-mediated synaptic responses (Lovinger et al., 1990, Morrisett and Swartzwelder, 1993, Wang et al., 2007; Roberto et al., 2004b). Low concentrations (5–50 mM) of alcohol also inhibit AMPAR function (Akinshola et al., 2001; Dildy-Mayfield and Harris, 1992, Moykkynen et al., 2003; Wirkner et al., 2000). Our lab has shown that acute alcohol (5–66 mM) decreases excitatory post-synaptic potentials (EPSPs) and currents (EPSCs) in the CeA, and that these effects are mediated by both NMDAR and non-NMDAR mechanisms (Roberto et al., 2004b). In the BLA, high concentrations of acute alcohol suppress NMDAR-mediated EPSCs, but lower concentrations suppress basal and evoked EPSCs mediated by kainate receptors (Läck et al., 2008). In contrast to alcohol facilitation of GABA release, the vast majority of studies indicate that acute alcohol either has no effect on or inhibits glutamate release (for review, see Siggins et al., 2005).
4.3 Chronic Alcohol Effects on Excitatory Transmission in CeA
Chronic alcohol exposure produces neuroadaptive changes in glutamatergic synaptic transmission. Specifically, chronic alcohol produces upregulation of NMDAR function and NMDAR-mediated glutamatergic synaptic transmission (Cebers et al., 1999; Grover et al., 1998; Gulya et al., 1991, Läck et al., 2007, Smothers et al. 1997). In the context of increased basal NMDAR-mediated glutamatergic transmission following chronic alcohol exposure, acute alcohol produces similar or greater suppression of NMDAR function in multiple brain regions including CeA, relative to alcohol-naïve rats (Floyd et al., 2003; Roberto et al., 2006; Roberto et al., 2004b, but see Grover et al., 1998; Miyakawa et al., 1997). Specifically, acute alcohol produces greater suppression of NMDAR-mediated EPSPs and EPSCs in the CeA of alcohol-dependent rats relative to alcohol-naïve controls. With local applications of NMDA, acute alcohol elicits a greater inhibition of NMDA currents in slices taken from alcohol-dependent rats, suggesting that alcohol dependence sensitizes NMDA receptors to ethanol (Roberto et al., 2004b, Roberto et al., 2006).
Severe alcoholism in humans affects synthesis and expression of multiple NMDAR subunits (e.g., in cortex; Ridge et al., 2008). In rodent studies, the NR2B subunit appears to be very sensitive to chronic alcohol exposure, particularly in limbic brain regions (Carpenter-Hyland et al., 2004; Floyd et al., 2003, Kash et al., 2009; Roberto et al., 2004b). In the CeA and BNST, chronic alcohol exposure produces increases in NR2B mRNA and/or protein levels (Kash et al., 2009; Roberto et al., 2006), although alcohol does not produce such increases in all brain regions (Cebers et al., 1999, Floyd et al., 2003, Läck et al., 2005). The molecular mechanisms that underlie NR2B subunit changes are not yet well understood, and it also not yet clear what portion of alcohol-induced increases in NMDAR function is attributable to changes in NR2B subunit expression.
Microdialysis experiments indicate that chronic alcohol exposure produces increases in extracellular glutamate levels in brain, especially after single or repeated withdrawals (Dahchour & DeWitte, 1999, 2003; Roberto et al., 2004b; Rossetti & Carboni 1995). These increases may be due to chronic alcohol-induced decreases in glutamate uptake (Melendez et al., 2005), or perhaps changes in cystine/glutamate exchange (Kalivas, 2009). In the amygdala, several studies have reported increases in glutamate release following chronic alcohol exposure (at 2–8 hrs withdrawal in CeA, [Roberto et al., 2004b]; at 24 hrs withdrawal in BLA, [Läck et al., 2007]). In particular, our lab showed that prior chronic alcohol exposure unmasks the ability of acute alcohol to increase pre-synaptic glutamate release (Roberto et al., 2004b), and that this effect persists two weeks into abstinence (Roberto et al., 2006). Collectively, these results suggest that multiple factors contribute to increased extracellular glutamate levels and increased glutamatergic transmission following chronic alcohol exposure and withdrawal.
5. Central Amygdala Neuromodulators & Alcohol Dependence
The extended amygdala is densely populated by pro-stress and anti-stress neuropeptides. Each of the neuropeptides described below has robust effects on anxiety-like and alcohol-drinking behaviors. These peptides play particularly prominent roles in regulating such behaviors in individuals that are either alcohol-dependent, genetically vulnerable to develop excessive alcohol drinking (e.g. selective breeding), repeatedly cycled through periods of alcohol withdrawal and/or innately anxious. It is interesting that pro-stress peptides (e.g., CRF, dynorphin) and anti-stress peptides (e.g., nociception, NPY) exhibit a high degree of neuroanatomical overlap, and opposite behavioral profiles (Koob, 2008). The effects of these peptides on anxiety-like and alcohol-related behaviors have been localized to the amygdala and neighboring regions, and are likely attributable to their modulation of excitatory and inhibitory transmission in those regions (see Table 1).
Table 1.
The effects of neuropeptides/neuromodulators on GABAergic transmission in the central nucleus of the amygdala (CeA) as assessed in our lab using slice electrophysiology techniques. Up arrows indicate a facilitatory effect on inhibitory transmission in CeA and down arrows indicate a suppression of inhibitory transmission in CeA. The table shows the effects of neuropeptides in CeA of alcohol-naïve and chronic alcohol-exposed rats, and also alone and in combination with acute alcohol application. The last two columns describe the site (pre- vs. post-synaptic) and mechanism (receptor subtype) of action for each of these neuropeptides.
| Alcohol- Naïve |
Chronic Alcohol |
Pre vs Post |
Mechanism | |||
|---|---|---|---|---|---|---|
| Basal | Acute Alcohol |
Basal | Acute Alcohol |
|||
| ---- | ↑ | ↑ | ↑ | |||
| CRF | ↑ | ↑↑ | ↑↑ | ↑↑ | PRE | CRF1Rs |
| NPY | ---- | Block | ↓ | Block | PRE | Y2Rs |
| Nociceptin | ↓ | Block | ↓↓ | Block | PRE | NOP |
| Dynorphin | ↓ | Partial Block | Not known | Not known | PRE | KOR |
| Galanin | ||||||
| Population 1 | ↑ | ↑↑ | Not known | Not known | POST? | Gal3R |
| Population 2 | ↓ | Block | POST? | Gal2R | ||
| eCBs | ↓ | Block | Not known | Not known | PRE | CB1R |
A wealth of evidence points to the CeA as a major point of convergence for peptide neuromodulators of excitatory and inhibitory transmission, and amygdalar stress systems are heavily recruited during the development of alcohol dependence. The CeA is the major output center for the amygdala but is not a homogeneous neuroanatomical structure. It can be subdivided into lateral and medial aspects that differ in terms of their neuropeptide content, origin of incoming afferents, and target sites of efferent projections (for review of amygdala anatomical organization, see Pitkänen, 2000). The lateral portion of the CeA contains a much higher density of neuropeptides (e.g., CRF; Cassell et al., 1999; Cassell et al., 1986; Shimada et al., 1989; Veening et al., 1984) than the medial CeA, receives input from cortical areas and thalamus, and projects to the substantia innominata and quite heavily to the lateral portion of the BNST. The medial portion of the CeA, on the other hand, receives prominent inputs from other amygdaloid nuclei, and sends dense projections to effector regions such as hypothalamus and brainstem nuclei (Pitkänen, 2000), although medial CeA also projects to BNST (Sun et al., 1991). Medial CeA projection neurons receive excitatory inputs from BLA as well as inhibitory inputs from lateral CeA and intercalated GABA cells, although it is not well understood at this point which synaptic connections govern emotion- and alcohol-related behavior (Ehrlich et al., 2009; Pape & Pare, 2010). In general, the amygdala receives strong inputs about the external environment and projects lateromedially (both between nuclei and within the CeA) to convert sensory information into appropriate behavioral and physiological responses. In our electrophysiological studies, we use local stimulation in the medial subdivision of CeA and we record from neurons in the same subdivision.
5.1 Pro-Stress Neuromodulators
5.1.1. CRF Effects on Alcohol-Related Behavior
CRF is a peptide that plays a central role in arousal as well as the hormonal, sympathetic, and behavioral responses to stress. The CeA, BNST, and BLA are abundant in CRF neurons and receptors (de Souza et al., 1984; Sakanaka, 1986). Hyperfunction of CRF systems in the CeA, BLA, and BNST produce increases in anxiety-like behavior (Lee et al., 2008; Rainnie et al., 2004; Sajdyk et al., 1999a). Extracellular CRF levels in CeA are elevated following exposure to stress and also following development of alcohol dependence (Merlo-Pich et al., 1995; Zorrilla et al., 2001). Alcohol withdrawal produces increases in CRF synthesis and release in CeA (Funk et al., 2006; Roberto et al., 2010a; Sommer et al., 2008) and BNST (Olive et al., 2002), the latter of which is normalized by alcohol consumption. CRF receptor antagonists suppress alcohol dependence-induced increases in alcohol drinking during acute withdrawal and protracted abstinence (Valdez et al., 2002), and also block the increased sensitivity to stress-induced anxiety during protracted abstinence from chronic alcohol (Valdez et al., 2003). CRF repeatedly administered into the CeA, BLA, and dorsal (but not ventral) BNST “kindles” or exaggerates the increases in anxiety-like behavior produced by alcohol withdrawal, and this effect is due to CRF action at CRF1 receptors (CRF1Rs; Huang et al., 2010). Conversely, antagonism of CRF receptors in the CeA attenuates the increases in anxiety-like behavior observed in rats during withdrawal from chronic high-dose alcohol exposure (Rassnick et al., 1993b).
Recent research has highlighted the role of CRF1Rs in mediating the effects of limbic CRF on anxiety-like behavior and alcohol drinking. CRF1R antagonists block the anxiogenic effects of many stressors (including alcohol withdrawal) in tests of open field exploration, elevated plus-maze, light-dark box, and defensive withdrawal (Arborelius et al., 2000; Zorrilla et al., 2007). CRF1R antagonists also block increases in alcohol self-administration produced by stressors and alcohol withdrawal (Funk et al., 2007; Gehlert et al., 2007; Hansson et al., 2006; Lowery et al., 2008; Marinelli et al., 2007; Richardson et al., 2008), and chronic antagonism of CRF1Rs abolishes dependence-induced escalation of alcohol drinking in rats chronically exposed to high doses of alcohol (Roberto et al., 2010a). Likewise, stressors and alcohol withdrawal produce increases in CRF1R synthesis and expression in limbic brain regions (Aguilar-Valles et al., 2005; Sommer et al., 2008). Similarly, rats selectively bred for high alcohol preference exhibit increased anxiety-like behavior and CRF1R levels (Ciccocioppo et al., 2006), and CRF1R knockout (KO) mice exhibit decreased anxiety-like behavior (Muller et al., 2003), as well as decreased alcohol drinking following withdrawal from chronic high-dose alcohol exposure (Chu et al., 2007).
5.1.2 CRF Effects on Synaptic Transmission in CeA
Our lab has shown that CRF produces robust increases in GABAergic transmission in CeA of rats and mice (Nie et al., 2004; Roberto et al., 2010a; see also Figures 2 & 3). Pre-synaptic GABA release is increased by CRF and decreased by antagonism of CRF1Rs, the latter of which reflects a tonic facilitation of GABA release by CRF in the CeA. CRF1R antagonists also block the ability of acute alcohol to augment GABAergic transmission in CeA (Figure 2). In some CeA neurons from alcohol-naïve rats, CRF and acute alcohol produce additive increases in evoked IPSC amplitudes (Roberto et al., 2005). The ability of CRF and acute alcohol to augment GABAergic transmission in CeA is contingent on the integrity of PKCε intracellular signaling pathways (Bajo et al., 2008). Alcohol-dependent rats exhibit heightened sensitivity to the effects of CRF and CRF1R antagonists on GABA release in CeA, suggesting an upregulation of the CRF-CRF1R system. These electrophysiological findings are further corroborated by increased CRF and CRF1R mRNA levels in the CeA of alcohol-dependent rats, as well as reversal of alcohol dependence–induced elevations in amygdalar GABA dialysate by a CRF1R antagonist (Roberto et al., 2010b).
CRF also increases GABAergic transmission in the BNST, likely via actions at post-synaptic CRF1Rs (Kash and Winder, 2006). The juxtacapsular nucleus of the anterior division of the BNST (jcBNST) receives robust glutamatergic and dopaminergic projections from BLA and midbrain (Hasue & Shammah-Lagnado, 2002; Larriva-Sahd, 2004), and sends GABAergic projections to the medial division of the CeA (Dong et al., 2000). Protracted withdrawal from chronic alcohol and repeated CRF treatment each produce impairments in long-term potentiation of the intrinsic excitability (LTP-IE) of jcBNST neurons in response to high-frequency stimulation (HFS), and these effects are dependent on the functionality of CRF1Rs but not CRF2Rs (Francesconi et al., 2009). Induction of LTP-IE of jcBNST neurons is dependent on the presence of intact D1R function (Francesconi et al., 2009), a result that is especially interesting in light of the finding that integrity of CRF1Rs is essential for enhancement of glutamatergic transmission in BNST produced by either exogenous dopamine application or history of cocaine exposure (Kash et al., 2008). Therefore, disruption of excitatory transmission in the BNST during protracted abstinence from alcohol may be dependent on specific and concurrent activation CRF and DA signaling in that region. A possible downstream effect of impaired intrinsic neuronal plasticity in the jcBNST might be reduced inhibition of the CeA and the emergence of a negative affective state during abstinence following the development of alcohol dependence (Francesconi et al., 2009).
5.1.3 Dynorphin/Kappa Opioid Receptor (KOR) Effects on Alcohol-Related Behavior
Dynorphin is an opioid peptide that acts primarily at KORs. Dynorphin/KOR systems generally promote anxiety-like behavior and may have implications for addictive behaviors (Bruchas et al., 2010; Wee & Koob, 2010). Dynorphin and KORs are abundantly expressed throughout the extended amygdala (Mansour et al., 1996; Marchant et al., 2007), and acute alcohol injection produces increases in extracellular concentrations of dynorphin in both CeA (Lam et al., 2008) and NAc (Marinelli et al., 2006). Variation in the gene that encodes for the KOR is associated with alcohol dependence in humans (Beadles-Bohling et al., 2000; Edenberg et al., 2008; Xuei et al., 2006). KOR knockout mice consume less ethanol than wild-type mice (Kovacs et al., 2005). The KOR antagonist, nor-BNI, selectively suppresses alcohol drinking by alcohol-dependent rats, but does not affect alcohol drinking by non-dependent rats (Walker et al., 2010). Finally, brain dynorphin and CRF systems appear to work together to produce the aversive effects (e.g., anxiety, dysphoria) associated with stress exposure and drug withdrawal, but it is not yet clear precisely how these systems interact since there is evidence that CRF systems drive dynorphin systems and vice-versa (e.g., Land et al., 2008; Valdez et al., 2007).
5.1.4 Dynorphin/KOR Effects on and Synaptic Transmission in CeA
Our lab has recently presented findings that the dynorphin/KOR system modulates GABAergic transmission in the CeA of alcohol-naïve rats (Gilpin et al., unpublished data). Dynorphin decreases GABAAR-mediated synaptic transmission and attenuates acute alcohol-induced increases in GABAergic transmission in CeA by reducing pre-synaptic GABA release. Furthermore, antagonism of KORs augments GABAergic transmission in CeA, suggesting a tonic inhibitory effect of endogenous dynorphin on inhibitory transmission in CeA (Gilpin et al., unpublished data). These results correlate well with recent findings that dynorphin decreases GABAergic transmission in BNST by reducing pre-synaptic GABA release, an effect that is mimicked by a KOR agonist and blocked by a KOR antagonist (Kash et al., 2009). It is not yet clear what implications these findings have for in vivo effects of dynorphin/KOR manipulations on anxiety- and alcohol-related behaviors.
5.2 Anti-Stress Neuromodulators
5.2.1 Nociceptin/Orphanin FQ Effects on Alcohol-Related Behavior
Nociceptin is a peptide that structurally resembles endogenous opioids (Meunier et al., 1995; Reinscheid et al., 1995) and acts at opioid-like (NOP) receptors, although nociceptin does not appear to bind at endogenous opioid receptors and endogenous opioids do not bind to the NOP receptor. Nociceptin is abundantly expressed in the CeA and BNST (Neal et al., 1999) and has been described as a functional CRF antagonist (Ciccocioppo et al., 2004a). Nociceptin and NOP receptor agonists have a general anxiolytic-like profile in animal studies (Jenck et al., 1997, 2000). Nociceptin knockout (KO) mice exhibit increased anxiety-like behavior (Koster et al., 1999) and are more sensitive to social stress (Ouagazzal et al., 2003). Nociceptin KO mice also exhibit higher basal and post-stress corticosterone levels, indicating that the nociceptin system tonically inhibits activity of the HPA stress axis (Koster et al., 1999). Nociceptin also tonically inhibits norepinephrine (NE) release from afferents arriving in the amygdala, which may constitute an important site of entry for stress/arousal inputs into the extended amygdala circuitry (Kawahara et al., 2004).
Nociceptin suppresses alcohol drinking and prevents relapse-like behavior in rats (Ciccocioppo et al., 2004b; Kuzmin et al., 2007). High doses of buprenorphine also suppress alcohol drinking via activation of nociceptin receptors (Ciccocioppo et al., 2007). Nociceptin reverses stress-induced anorexia (Ciccocioppo et al., 2002), and blocks reinstatement of alcohol-seeking behavior produced by footshock stress (Martin-Fardon et al., 2000; a behavior mediated by brain CRF systems) and cues predictive of alcohol availability (Ciccocioppo et al., 2004b). Nociceptin also prevents the development and expression of conditioned place preference for alcohol in rats and mice (Ciccocioppo et al., 1999; Kuzmin et al., 2003). In general, rats selectively bred for high alcohol intake exhibit heightened sensitivity to the suppressive effects of nociceptin on alcohol drinking and related behaviors (Economidou et al., 2008). Relative to genetically heterogeneous rats, alcohol-preferring rats have higher levels of nociceptin in CeA and BNST, and nociceptin transmission in CeA appears to be most important in regulating alcohol drinking in these rats (Economidou et al., 2008). Human alcoholics exhibit decreased levels of mRNA for the nociceptin OPRL1 receptor in the CeA (Kuzmin et al., 2009), suggesting that the CeA is a critical brain site for nociceptin effects on alcohol-related behaviors.
5.2.2 Nociceptin Effects on Synaptic Transmission in CeA
Our lab has shown that nociceptin dose-dependently and reversibly reduces GABAAR-mediated IPSCs in CeA (Roberto & Siggins, 2006). Nociceptin increases the PPF ratio of IPSCs and decreases the frequency of mIPSCs in CeA (Figure 3), suggesting decreased pre-synaptic GABA release in that brain region. Interestingly, nociceptin both prevents (when applied before acute alcohol; Figure 2) and reverses (when applied after acute alcohol) the ability of acute alcohol to increase evoked IPSC amplitudes and mIPSC frequencies, and decrease PPF ratio by opposing alcohol-induced increase in GABA release in CeA. Furthermore, the ability of nociceptin to decrease GABAergic transmission in CeA is augmented following a history of alcohol dependence, suggesting that nociceptin systems in the CeA undergo neuroadaptations during chronic alcohol exposure (Roberto and Siggins, 2006). It has yet to be determined whether these effects also occur in other regions of the extended amygdala.
5.2.3 NPY Effects on Alcohol-Related Behavior
The amygdala contains a wealth of NPY fibers and receptors (Allen et al., 1984; de Quidt & Emson, 1986; Dumont et al. 1990; Gustafson et al. 1997; Migita et al., 2001). NPY exerts robust anxiolytic effects that are mediated by amygdala, and it appears that both the CeA (Heilig et al., 1993) and BLA (Sajdyk et al., 1999b) contribute to this effect. Rats selectively bred for high alcohol preference exhibit low levels of NPY mRNA and NPY in CeA that are restored by voluntary alcohol consumption (Pandey et al., 2005). Alcohol-withdrawn rats exhibit increases in anxiety-like behavior and decreased amygdalar NPY, perhaps via decreases in histone acetylation (i.e., gene transcription; Pandey et al., 2008; Roy & Pandey, 2002). It has therefore been hypothesized that rescue of impaired histone acetylation in amygdala might block withdrawal-related increases in alcohol consumption and anxiety-like behavior via restoration of NPY levels (Pandey et al., 2008; Zhao et al., 2007).
Of particular relevance to this review, it has been suggested that the ability of ICV NPY administration to block stress-induced reinstatement of alcohol-seeking behavior is mediated by activation of inhibitory neuronal populations in CeA (Cippitelli et al., 2010), a hypothesis that is supported by data showing that NPY activation in the CeA suppresses alcohol consumption in alcohol-dependent rats and abstinent alcohol-preferring rats (Gilpin et al., 2008a,b), as well as rats that exhibit innately high levels of anxiety-like behavior (Primeaux et al., 2006). Both post-synaptic Y1 and pre-synaptic Y2 receptors have been implicated in the effects of NPY on anxiety-like behavior and alcohol consumption. Early studies described roles for both Y1 (Heilig et al., 1993) and Y2 receptors (Sajdyk et al., 2002) in amygdala in anxiety-like behavior. Studies in mice have indicated that Y1 receptors are responsible for mediating the suppressive effects of NPY on alcohol drinking (Eva et al., 2006; Sparta et al., 2004; Thiele et al., 2002). Likewise, acute stress and alcohol withdrawal produce increases in amygdalar Y1 receptor expression in rodents (Eva et al., 2006).
5.2.4 NPY Effects on Synaptic Transmission in CeA
Our lab has shown that NPY prevents and reverses acute alcohol-induced increases in evoked GABAergic transmission in CeA (Gilpin et al., 2011; Figure 2). NPY blocks alcohol-induced decreases in PPF ratio and increases in mIPSC frequency, suggesting that the ability of NPY to block alcohol effects is due to suppression of pre-synaptic GABA release. Pharmacological probes with Y1R and Y2R antagonists confirmed the pre-synaptic site of action and suggested that NPY blocks alcohol effects via actions at pre-synaptic Y2Rs. NPY alone does not suppress GABAergic transmission in CeA unless post-synaptic Y1Rs are blocked, suggesting that functional Y1Rs in CeA buffer the effects of NPY at pre-synaptic Y2Rs. NPY also normalizes alcohol dependence-induced increases in GABA release in CeA, suggesting that chronic alcohol exposure produces neuroadaptations in NPY systems that affect inhibitory transmission in that brain region. These results are consistent with findings that NPY modulates GABA release in BNST via activation of pre-synaptic Y2Rs (Kash and Winder, 2006), supporting the notion that Y2Rs function not only as autoreceptors regulating NPY release (Chen et al., 1997), but also as heteroceptors regulating the release of other neurotransmitters (Greber et al., 1994). These findings concerning NPY-GABA circuitry in the CeA are supported by recent data from our lab that infusion of a Y2R antagonist into CeA selectively increases alcohol drinking in alcohol-dependent rats (Gilpin et al., unpublished data), as well as findings that infusion of a Y1R antagonist into CeA reduces alcohol self-administration in rats (Schroeder et al., 2003).
5.3 Other Neuromodulators
5.3.1 Endocannabinoids
have recently been implicated in regulation of alcohol-related behavior, particularly via their actions at cannabinoid type I receptors (CB1Rs; Colombo et al., 2002, 2005; Hungund et al., 2003; Wang et al., 2003). Agonists and antagonists of CB1Rs produce neuronal activation in CeA (Patel et al., 2005) and activation of CB1Rs produces decreases in anxiety-like behavior that are dependent on the integrity of brain opioid systems (Zarrindast et al., 2008). Recently, our group showed that activation of CB1Rs in the CeA produces dose-dependent decreases in GABAAR-mediated transmission, and also blocks acute alcohol-induced increases in GABAAR-mediated transmission by modulating GABA release (Roberto et al., 2010b). Conversely, CB1R antagonists augment GABAAR-mediated IPSPs and this effect is additive with acute alcohol-induced increases, suggesting a tonic inhibitory effect of endocannabinoids and non-CB1R site of action for alcohol.
5.3.2 Galanin
like the opioid peptides, is widespread in diverse brain regions, including amygdala where it is co-localized and co-released with vasopressin (Han & de Vries, 1999; Miller et al., 1993) and norepinephrine (Hokfelt et al., 1998; Skofitsch & Jacobowitz, 1985). High levels of Gal1, Gal2 and Gal3 receptors are expressed in amygdala (Branchek et al., 2000; Waters et al., 2000). Galanin administered into the ventricles is anxiolytic (Bing et al., 1993) and galanin infused directly into CeA reduces stress-induced anxiety (Khoshbouei et al., 2002; Barrera et al., 2006; but see Moller et al., 1999). Stressful stimuli release galanin (Weiss et al., 2005) and increase galanin mRNA in CeA (Palkovits et al., 2000). Recent electrophysiological data from our group (Bajo et al, in press) suggest that galanin has differential effects in mouse CeA neurons (see also Sharkey et al., 2008) according to the presence of galanin receptor subtypes. Galanin produces an early increase in IPSP amplitudes in one subpopulation of CeA neurons, but a later and more pronounced IPSP decrease in other CeA neurons, all in the absence of robust effects on GABA release. Augmented GABA transmission is likely mediated by post-synaptic GalR3 receptors, whereas depression of IPSPs is likely mediated by GalR2 receptors (Bajo et al, in press). Of particular relevance to the current review, galanin and alcohol produces additive facilitatory effects on IPSP amplitudes. More specifically, alcohol attenuates the ability of galanin to decrease IPSP amplitudes, but potentiates the ability of galanin to augment IPSP amplitudes, suggesting that alcohol and galanin act via different mechanisms. Future studies will clarify the behavioral relevance of the complex interaction between galanin and alcohol in CeA.
5.4 Neuropeptide Interactions
5.4.1 CRF-Nociceptin
Nociceptin in the brain opposes the actions of CRF and antagonizes the pro-anxiety effects of CRF. Nociceptin opposes the anorexia induced by stress and CRF (Ciccocioppo et al., 2001, 2004a) and this effect has been localized to the BNST (Ciccocioppo et al., 2003). Nociceptin infused directly into the BNST blocks the anxiogenic effects of CRF as measured on the elevated plus maze (Rodi et al., 2008).
Because excessive alcohol drinking is selectively suppressed by intra-CeA infusion of both CRF receptor antagonists and nociceptin (Funk et al., 2006; Economidou et al., 2008), there has been recent interest in potential nociceptin-CRF interactions in the CeA. Recent data from our lab show that nociceptin prevents and reverses CRF-induced increases in pre-synaptic GABA release via effects on pre-synaptic adenylate cyclase (Cruz et al., submitted). That study also confirms previous findings that nociceptin and CRF effects are each augmented in the CeA of alcohol-dependent rats, raising the possibility that the interaction between these peptides in CeA is enhanced following chronic alcohol exposure.
5.4.2 CRF-NPY
CRF and NPY have long been considered to exist in an opposing relationship, particularly because of their high degree of neuroanatomical overlap and their opposite behavioral profiles. CRF produces decreases in feeding behavior (Levine et al., 1983) via effects in the PVN (Krahn et al., 1988), increases in arousal (Koob et al., 1984), perhaps via actions in amygdala (Lee & Tsai, 1989; Liang et al., 1992) and/or interactions with noradrenergic neurons in the locus coeruleus (Butler et al., 1990), and increases in anxiety-like behavior (Koob & Thatcher-Britton, 1985) via effects in amygdala (Rassnick et al., 1993b). Conversely, NPY produces robust increases in feeding behavior (Levine & Morley, 1984) via effects in the PVN (Gilpin et al., 2004a; Stanley & Leibowitz, 1985), increases in sedation (Gilpin et al., 2004b; Heilig & Murison, 1987) via effects in the posterior hypothalamus (Naveilhan et al., 2001), and decreases in anxiety-like behavior (Heilig et al., 1989, 1992) via effects in the amygdala (Heilig et al., 1993; Sajdyk et al., 2002). It has long been hypothesized that CRF and NPY interact in a complex way to regulate emotion (Heilig et al., 1994; Sajdyk et al., 2004) and modulate alcohol dependence-related behaviors (Cowen et al., 2004; Valdez & Koob, 2004), an interaction that may occur in the amygdala.
CRF facilitates GABAergic transmission in CeA (Roberto et al., 2010b) and BNST (Kash and Winder, 2006) via actions at CRF1Rs, whereas NPY opposes GABAergic transmission in CeA (Gilpin et al., 2011) and BNST (Kash and Winder, 2006) via actions at pre-synaptic Y2Rs. In the CeA, CRF1R antagonists and NPY block the effects of acute alcohol on inhibitory transmission, and neuroadaptations occur in both CRF and NPY systems during the development of alcohol dependence (Gilpin et al., 2011; Roberto et al., 2010a), reflecting recruitment or upregulation of those systems. The fact that CRF and NPY effects on inhibitory transmission are similar in multiple sub-regions of the extended amygdala suggests that these neuropeptide systems may converge on GABA neurons in CeA and BNST to affect anxiety- and alcohol-related behaviors.
6. Disinhibition Model of CeA Output
Most neurons in the CeA are GABAergic inhibitory projection neurons or interneurons that co-transmit GABA and one of several neuromodulators (see Figure 1). It may seem initially counterintuitive that pro-anxiety pro-alcohol-drinking peptides (e.g., CRF) increase GABAergic transmission in CeA, whereas anti-anxiety anti-alcohol-drinking peptides (e.g., nociceptin, NPY) decrease GABAergic transmission in the same region. However, in our slice preparation, electrical stimulation and recording occur locally in the CeA and recordings of GABAergic transmission reflect the activity of inhibitory CeA interneurons and/or afferent projection neurons from other brain regions to the CeA. Therefore, observed increases in GABAergic transmission from CeA interneurons/afferents (e.g., following application of acute alcohol or CRF) inhibit the activity of GABAergic neurons projecting out of CeA. Conversely, observed decreases in GABAergic transmission from CeA interneurons/afferents (e.g., following application of nociceptin or NPY) reduce inhibition of GABAergic neurons projecting out of CeA, thereby facilitating the release of GABA onto downstream targets. As such, recorded increases in GABAergic transmission reflect a disinhibition of downstream target regions (e.g., BNST, hypothalamus, periaqueductal gray), whereas recorded decreases in GABAergic transmission reflect a net inhibition of downstream target regions. Therefore, increases and decreases in inhibitory output from the CeA to downstream effector regions may produce decreases and increases in anxiety-like behavior, respectively (see also Davis et al., 2010; Paré et al., 2004; Tye et al., 2011).
There are two apparent exceptions to the otherwise consistent relationship between the effects of a compound on anxiety-related behavior and inhibitory transmission in CeA. First, acute alcohol, a presumed anxiolytic, produces increases in GABAergic transmission in CeA. However, even acute doses of alcohol can produce anxiogenic-like dysphoric-like effects during and after elimination of alcohol from the blood following the peak of blood-alcohol levels (e.g., see Roberto et al., 2008). Furthermore, alcohol has profound effects on excitatory transmission in CeA, particularly via effects at NMDA receptors (Roberto et al., 2004b; Roberto et al., 2006). NMDA receptors may act as cellular ‘toxicity stressors’ and/or contribute to the behavioral effects of acute alcohol and alcohol withdrawal, lending at least partial resolution to this conundrum. Finally, alcohol may alter the release of local opioids (e.g., Lam et al., 2008) and/or cannabinoids (Roberto et al., 2010b) in CeA that in turn leads to increased GABA-mediated inhibition of downstream target areas. A second exception to the relationship between anxiety-related behavior and inhibitory transmission in CeA is that dynorphin, a presumed endogenous anxiogenic, produces decreases in GABAergic transmission in CeA. In the brain, dynorphin appears to interact in a complex way with CRF, which may account for this counterintuitive result. It is not yet clear what the implications of this finding are for which brain region(s) mediate dynorphin/KOR effects on anxiety- and alcohol-related behaviors.
7. CeA is an Important Locus for Alcohol-Related Neuroplasticity
The data summarized here support the hypothesis that the CeA is a critical locus of neuroadaptation during the transition to alcohol dependence. Alcohol has persistent effects, particularly on inhibitory transmission, in the CeA of alcohol-dependent animals. Neuropeptides are present in high quantities in the CeA and these peptides profoundly alter inhibitory transmission, and potentially excitatory transmission. The ability of these neuropeptides to affect neurotransmission in the CeA either alone or in combination with alcohol is often up-regulated in the alcohol-dependent organism. Although manipulation of many of these systems affects alcohol drinking exclusively in the alcohol-dependent organism, it is not surprising that these neuropeptides affect basal neurotransmission in alcohol-naïve animals, particularly because the activity of all these peptides in the CeA contributes to anxiety-related behavior independent of alcohol exposure history. This final point also contributes to our understanding of why these neuropeptide systems are recruited and/or up-regulated during the transition to alcohol dependence, a dynamic disease state defined largely by a negative emotional state in the absence of the drug.
Electrophysiological approaches have contributed substantially to our understanding of the neuroadaptations that occur during the transition to alcohol dependence. Multiple neuropeptide and neuromodulator systems appear to be recruited in the alcohol-dependent organism, and these systems may converge on GABA circuitry in the CeA to produce alcohol-related behaviors (e.g., self-administration and anxiety-related behavior). Manipulation of neural systems that are recruited and/or functionally up-regulated during the transition to alcohol dependence produces more robust effects on both post-dependent CeA neurotransmission and alcohol-related behaviors. Future studies will unravel the complex interaction effects of these neuropeptides on synaptic transmission in this brain region, and more closely examine the downstream effects of modulating these peptidergic inputs to GABAergic transmission in the CeA.
Highlights.
CeA mediates alcohol-related behaviors and chronic alcohol-induced plasticity
Pro-stress and anti-stress peptides alter GABAergic transmission in CeA
Chronic alcohol alters the effects of peptides on GABAergic transmission in CeA
Peptides that affect GABA in CeA also affect anxiety and excessive alcohol drinking
Acknowledgements
This work was supported by National Institute of Alcoholism grants AA018400, AA015566, AA06420, AA016985, AA017447. The authors would like to thank Drs. Floyd Bloom, George Koob, Roberto Melendez, Michal Bajo, Melissa Herman, Maureen Cruz, and Marsida Kallupi for critical comments and discussions on the manuscript. This is manuscript number 21331 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar-Valles A, Sánchez E, de Gortari P, Baldera I, Ramírez-Amaya V, Bermúdez-Rattoni F, Joseph-Bravo P. Analysis of the stress response in rats trained in the water-maze: differential expression of corticotropin-releasing hormone, CRHR1, glucocorticoid receptors and brain-derived neurotrophic factor in limbic regions. Neuroendocrinol. 2005;82:306–319. doi: 10.1159/000093129. [DOI] [PubMed] [Google Scholar]
- Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. GABA(A) receptors as molecular sites of ethanol action. Direct or indirect actions? Curr Top Med Chem. 2002;2:869–885. doi: 10.2174/1568026023393426. [DOI] [PubMed] [Google Scholar]
- Akinshola BE, Stewart RR, Karvonen LL, Taylor RE, Liesi P. Involvement of non-NMDA receptors in the rescue of weaver cerebellar granule neurons and sensitivity to ethanol of cerebellar AMPA receptors in oocytes. Brain Res Mol Brain Res. 2001;93:8–17. doi: 10.1016/s0169-328x(01)00152-8. [DOI] [PubMed] [Google Scholar]
- Allen YS, Roberts GW, Bloom SR, Crow TJ, Polak JM. Neuropeptide Y in the stria terminalis: evidence for an amygdalofugal projection. Brain Res. 1984;321:357–362. doi: 10.1016/0006-8993(84)90193-8. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Anderson NJ, Daunais JB, Friedman DP, Grant KA, McCool BA. Long-term ethanol self-administration by the nonhuman primate, Macaca fascicularis, decreases the benzodiazepine sensitivity of amygdala GABAA receptors. Alcohol Clin Exp Res. 2007;31:1061–1070. doi: 10.1111/j.1530-0277.2007.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arborelius L, Skelton KH, Thrivikraman KV, Plotsky PM, Schulz DW, Owens MJ. Chronic administration of the selective corticotropin-releasing factor 1 receptor antagonist CP-154,526: behavioral, endocrine and neurochemical effects in the rat. J Pharmacol Exp Ther. 2000;294:588–597. [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci USA. 2008;105:8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Madamba SG, Lu X, Sharkey LM, Bartfai T, Siggins GR. Receptor Subtype-Dependent Galanin Actions on GABAergic Neurotransmission and Ethanol Responses in the Central Amygdala. Addict Biol. doi: 10.1111/j.1369-1600.2011.00360.x. (in press) (EPub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera G, Hernandez A, Poulin JF, Laforest S, Drolet G, Morilak DA. Galanin-mediated anxiolytic effect in rat central amygdala is not a result of corelease from noradrenergic terminals. Synapse. 2006;59:27–40. doi: 10.1002/syn.20208. [DOI] [PubMed] [Google Scholar]
- Beadles-Bohling AS, Crabbe JC, Wiren KM. Elevated prodynorphin expression associated with ethanol withdrawal convulsions. Neurochem Intl. 2000;37:463–472. doi: 10.1016/s0197-0186(00)00056-5. [DOI] [PubMed] [Google Scholar]
- Bing O, Möller C, Engel JA, Söderpalm B, Heilig M. Anxiolytic-like action of centrally administered galanin. Neurosci Lett. 1993;164:17–20. doi: 10.1016/0304-3940(93)90846-d. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Harris RA. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008;11:775–793. doi: 10.1017/S1461145708008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Harris RA. Studies of ethanol actions on recombinant δ-containing γ –aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41:155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle AE, Segal R, Smith BR, Amit Z. Bidirectional effects of GABAergic agonists and antagonists on maintenance of voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1993;46:179–182. doi: 10.1016/0091-3057(93)90338-t. [DOI] [PubMed] [Google Scholar]
- Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends Pharmacol Sci. 2000;21:109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- Breese GR, Criswell HE, Carta M, Dodson PD, Hanchar HJ, Khisti RT, Mameli M, Ming Z, Morrow AL, Olsen RW, et al. Basis of the gabamimetic profile of ethanol. Alcohol Clin Exp Res. 2006;30:731–744. doi: 10.1111/j.0145-6008.2006.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Weiss JM, Stout JC, Nemeroff CB. Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. J Neurosci. 1990;10:176–183. doi: 10.1523/JNEUROSCI.10-01-00176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- Cebers G, Cebere A, Wagner A, Liljequist S. Prolonged inhibition of glutamate reuptake down-regulates NMDA receptor functions in cultured cerebellar granule cells. J Neurochem. 1999;72:2181–2190. doi: 10.1046/j.1471-4159.1999.0722181.x. [DOI] [PubMed] [Google Scholar]
- Chen X, DiMaggio DA, Han SP, Westfall TC. Autoreceptor-induced inhibition of neuropeptide Y release from PC-12 cells is mediated by Y2 receptors. J Am Physiol. 1997;273:H1737–H1744. doi: 10.1152/ajpheart.1997.273.4.H1737. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Biondini M, Antonelli L, Wichmann J, Jenck F, Massi M. Reversal of stress- and CRF-induced anorexia in rats by the synthetic nociceptin/orphanin FQ receptor agonist, Ro 64-6198. Psychopharmacol. 2002;161:113–119. doi: 10.1007/s00213-002-1020-7. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Cippitelli A, Economidou D, Fedeli A, Massi M. Nociceptin/orphanin FQ acts as a functional antagonist of corticotropin-releasing factor to inhibit its anorectic effect. Physiol Behav. 2004a;82:63–68. doi: 10.1016/j.physbeh.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addiction Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, et al. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacol. 2004b;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Rimondini R, Sommer W, Massi M, Heilig M. Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol Psychiatry. 2007;61:4–12. doi: 10.1016/j.biopsych.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 2003;23:9445–9451. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F, Massi M. Nociceptin/orphanin FQ inhibits stress- and CRF-induced anorexia in rats. Neuroreport. 2001;12:1145–1149. doi: 10.1097/00001756-200105080-00019. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacol. 1999;141:220–224. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, et al. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacol. 2010;208:417–426. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- Clarke TK, Treutlein J, Zimmermann US, Kiefer F, Skowronek MH, Rietschel M, Mann K, Schumann G. HPA-axis activity in alcoholism: examples for a gene-environment interaction. Addict Biol. 2008;13:1–14. doi: 10.1111/j.1369-1600.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Grant KA. NMDA receptor complex antagonists have ethanol-like discriminative stimulus effects. Ann NY Acad Sci. 1992;654:421–423. doi: 10.1111/j.1749-6632.1992.tb25986.x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, et al. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacol. 2002;159:181–187. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Vacca G, Carai MAM, Gessa GL. Endocannabinoid system and alcohol addiction: pharmacological studies. Pharmacol Biochem Behav. 2005;81:369–380. doi: 10.1016/j.pbb.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Cooper BR, Viik K, Ferris RM, White HL. Antagonism of the enhanced susceptibility to audiogenic seizures during alcohol withdrawal in the rat by gamma-aminobutyric acid (GABA) and "GABA-mimetic" agents. J Pharmacol Exp Ther. 1979;209:396–403. [PubMed] [Google Scholar]
- Cowen MS, Chen F, Lawrence AJ. Neuropeptides: implications for alcoholism. J Neurochem. 2004;89:273–285. doi: 10.1111/j.1471-4159.2004.02394.x. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacol. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Cruz MT, Bajo M, Magnoli EM, Tabakoff B, Siggins GR, Roberto M. Type 7 Adenylyl Cyclase is Involved in the Ethanol and CRF Sensitivity of GABAergic Synapses in Mouse Central Amygdala. Front Neurosci. 2011;4:1–7. doi: 10.3389/fnins.2010.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effect of repeated ethanol withdrawal on glutamate microdialysate in the hippocampus. Alcohol Clin Exp Res. 1999;23:1698–1703. doi: 10.1111/j.1530-0277.1999.tb04063.x. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur J Pharmacol. 2003;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacol. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Quidt ME, Emson PC. Distribution of Neuropeptide Y-like immunoreactivity in the rat central nervous system-II. Immunohistochemcial analysis. Neurosci. 1986;18:545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Perrin MH, Insel TR, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors in rat forebrain: autoradiographic identification. Science. 1984;224:1449–1451. doi: 10.1126/science.6328656. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Morrow AL, Criswell HE, Breese GR, Duncan GE, Simson PE, Knapp DJ, McCown TJ. Regional differences in the effects of chronic ethanol administration on [3H]zolpidem binding in rat brain. Alcohol Clin Exp Res. 1995;19:910–914. doi: 10.1111/j.1530-0277.1995.tb00966.x. [DOI] [PubMed] [Google Scholar]
- Dildy-Mayfield JE, Harris RA. Comparison of ethanol sensitivity of rat brain kainate, DL-alpha-amino-3-hydroxy-5-methyl-4-isoxalone proprionic acid and N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1992;262:487–494. [PubMed] [Google Scholar]
- Dildy JE, Leslie SW. Ethanol inhibits NMDA-induced increases in free intracellular Ca2+ in dissociated brain cells. Brain Res. 1989;499:383–387. doi: 10.1016/0006-8993(89)90789-0. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Swanson LW. Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res. 2000;859:1–14. doi: 10.1016/s0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Fournier A, St-Pierre S, Schwartz TW, Quirion R. Differential distribution of Y1 and Y2 receptors in the rat brain. Eur J Pharmacol. 1990;191:501–503. doi: 10.1016/0014-2999(90)94189-5. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, et al. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry. 2008;64:211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Wang J, Tian H, Pochareddy S, Xuei X, Wetherill L, et al. A regulatory variation in OPRK1, the gene encoding the κ-opioid receptor, is associated with alcohol dependence. Human Mol Genetics. 2008;17:1783–1789. doi: 10.1093/hmg/ddn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, June HL. Blockade of GABA(A) receptors within the extended amygdala attenuates D(2) regulation of alcohol-motivated behaviors in the ventral tegmental area of alcohol-preferring (P) rats. Neuropharmacol. 2007;52:1570–1579. doi: 10.1016/j.neuropharm.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva C, Oberto A, Mele P, Serra M, Biggio G. Role of brain neuroactive steroids in the functional interplay between the GABAA and the NPY-Y1 receptor mediated signals in the amygdala. Pharmacol Biochem Behav. 2006;84:568–580. doi: 10.1016/j.pbb.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Therapeutic. 2004;311:1071–1079. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Jung KY, McCool BA. Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J Pharmacol Exp Ther. 2003;307:1020–1029. doi: 10.1124/jpet.103.057505. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Koob GF, Sanna PP. Intrinsic neuronal plasticity in the juxtacapsular nucleus of the bed nuclei of the stria terminalis (jcBNST) Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1347–1355. doi: 10.1016/j.pnpbp.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Characterization of susceptibility to audiogenic seizures in ethanol-dependent rats after microinjection of gamma-aminobutyric acid (GABA) agonists into the inferior colliculus, substantia nigra or medial septum. J Pharmacol Exp Ther. 1983;227:663–670. [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee M-J, Rice KC, Koob GF. Corticotropin-releasing factor-1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Lê AD, Hipskind PA, Hamdouchi C, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M. Neuropeptide Y Opposes Alcohol Effects on Gamma-Aminobutyric Acid Release in Amygdala and Blocks the Transition to Alcohol Dependence. Biol Psychiatry. 2011;69:1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced decreases in alcohol drinking. Pharmacol Biochem Behav. 2008a;90:475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN. Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res. 2009;33:2113–2123. doi: 10.1111/j.1530-0277.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Badia-Elder NE. Neuropeptide Y administration into the amygdala suppresses ethanol drinking in alcohol-preferring (P) rats following multiple deprivations. Pharmacol Biochem Behav. 2008;90:470–474. doi: 10.1016/j.pbb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Elder RL, Kho Y, Murphy JM, Li TK, Badia-Elder NE. Sedative and motor-impairing effects of neuropeptide Y and ethanol in selectively bred P and NP rats. Pharmacol Biochem Behav. 2004b;78:65–73. doi: 10.1016/j.pbb.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Badia-Elder NE. Effects of neuropeptide Y in the paraventricular nucleus of the hypothalamus on ethanol intake in HAD1 and LAD1 rats depend on availability of food. Alcohol Clin Exp Res. 2004a;28:1492–1498. doi: 10.1097/01.alc.0000141813.27875.d5. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li TK, Lumeng L. Limited access alcohol drinking in high- and low-alcohol preferring selected lines of mice. Alcohol Clin Exp Res. 1999;23:1015–1022. [PubMed] [Google Scholar]
- Greber S, Schwarzer C, Sperk G. Neuropeptide Y inhibits potassium-stimulated glutamate release through Y2¬ receptors in rat hippocampal slices in vitro. Br J Pharmacol. 1994;113:737–740. doi: 10.1111/j.1476-5381.1994.tb17055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacol. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Papadeas ST, Morrow AL. Regional variations in the effects of chronic ethanol administration on GABAA receptor expression: potential mechanisms. Neurochem Intl. 2000;37:453–461. doi: 10.1016/s0197-0186(00)00058-9. [DOI] [PubMed] [Google Scholar]
- Grover CA, Wallace KA, Lindberg SA, Frye GD. Ethanol inhibition of NMDA currents in acutely dissociated medial septum/diagonal band neurons from ethanol dependent rats. Brain Res. 1998;782:43–52. doi: 10.1016/s0006-8993(97)01001-9. [DOI] [PubMed] [Google Scholar]
- Gulya K, Grant KA, Valverius P, Hoffman PL, Tabakoff B. Brain regional specificity and time-course of changes in the NMDA receptor-ionophore complex during ethanol withdrawal. Brain Res. 1991;547:129–134. [PubMed] [Google Scholar]
- Gustafson EL, Smith KE, Durkin MM, Walker MW, Gerald C, Weinshank R, Branchek TA. Distribution of the neuropeptide Y Y2 receptor mRNA in rat central nervous system. Mol Brain Res. 1997;46:223–235. doi: 10.1016/s0169-328x(97)00017-x. [DOI] [PubMed] [Google Scholar]
- Han TM, De Vries GJ. Neurogenesis of galanin cells in the bed nucleus of the stria terminalis and centromedial amygdala in rats: a model for sexual differentiation of neuronal phenotype. J Neurobiol. 1999;38:491–498. [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Nat Acad Sci. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17:80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacol. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Koob GK, Britton KT. Anxiolytic-like effect of neuropeptide Y (NPY), but not other peptides in an operant conflict test. Regul Pept. 1992;41:61–69. doi: 10.1016/0167-0115(92)90514-u. [DOI] [PubMed] [Google Scholar]
- Heilig M, Murison R. Intracerebroventricular neuropeptide Y suppresses open field and home cage activity in the rat. Regul Pept. 1987;19:221–231. doi: 10.1016/0167-0115(87)90278-3. [DOI] [PubMed] [Google Scholar]
- Heilig M, Söderpalm B, Engel JA, Widerlöv E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacol. 1989;98:524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid G. Piecing together the puzzle of basal forebrain anatomy. In: Napier TC, Kalivas PW, Hanin I, editors. The Basal Forebrain: Anatomy to Function (series title: Advances in Experimental Medicine and Biology, vol 295) New York: Plenum Press; 1991. pp. 1–42. [DOI] [PubMed] [Google Scholar]
- Hemby SE, O’Connor JA, Acosta G, Floyd D, Anderson N, McCool BA, et al. Ethanol-induced regulation of GABAA subunit mRNAs in prefrontal fields of cynomolgus monkeys. Alcohol Clin Exp Res. 2006;30:1978–1985. doi: 10.1111/j.1530-0277.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcohol Clin Exp Res. 1995;19:1486–1493. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Xu ZQ, Shi TJ, Holmberg K, Zhang X. Galanin in ascending systems. Focus on coexistence with 5-hydroxytryptamine and noradrenaline. Ann N Y Acad Sci. 1998;863:252–263. doi: 10.1111/j.1749-6632.1998.tb10700.x. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Rabe CS, Moses F, Tabakoff B. N-methyl-D-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J Neurochem. 1989;52:1937–1940. doi: 10.1111/j.1471-4159.1989.tb07280.x. [DOI] [PubMed] [Google Scholar]
- Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J Pharmacol Exp Ther. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Jr, Nothacker HP, Civelli O. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci USA. 1997;94:14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, Lundstrom K, Cesura AM, Poli SM, Roever S, Kolczewski S, Adam G, Kilpatrick G. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci USA. 2000;97:4938–4943. doi: 10.1073/pnas.090514397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kash TL. Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2009. Kappa opioid receptor regulation of inhibitory transmission in the bed nucleus of the stria terminalis. Program No. 423.15. Online. [Google Scholar]
- Kash TL, Baucum AJ, 2nd, Conrad KL, Colbran RJ, Winder DG. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacol. 2009;34:2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacol. 2006;51:1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Hesselink MB, van Scharrenburg G, Westerink BH. Tonic inhibition by orphanin FQ/nociceptin of noradrenaline neurotransmission in the amygdala. Eur J Pharmacol. 2004;485:197–200. doi: 10.1016/j.ejphar.2003.11.061. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Ethanol-enhanced GABA release: a focus on G protein-coupled receptors. Brain Res Rev. 2011;65:113–123. doi: 10.1016/j.brainresrev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Weinberg RJ, Criswell HE, Breese GR. The PLC/IP 3 R/PKC pathway is required for ethanol-enhanced GABA release. Neuropharmacology. 2010;58:1179–1186. doi: 10.1016/j.neuropharm.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Dove S, Javors M, Morilak DA. Behavioral reactivity to stress: amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacol Biochem Behav. 2002;71:407–417. doi: 10.1016/s0091-3057(01)00683-9. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Wiedemann K. Neuroendocrine pathways of addictive behaviour. Addict Biol. 2004;9:205–212. doi: 10.1080/13556210412331292532. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56 Suppl 1:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Swerdlow N, Seeligson M, Eaves M, Sutton R, Rivier J, Vale W. Effects of alpha-flupenthixol and naloxone on CRF-induced locomotor activation. Neuroendocrinol. 1984;39:459–464. doi: 10.1159/000124021. [DOI] [PubMed] [Google Scholar]
- Koob GF, Thatcher-Britton K. Stimulant and anxiogenic effects of corticotropin releasing factor. Prog Clin Biol Res. 1985;192:499–506. [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Debus F, Linden A-M, Malécot C, Leppa E, Vekovischeva O, et al. Does ethanol act preferentially via selected brain GABAA receptor subtypes? the current evidence is ambiguous. Alcohol. 2007;41:163–176. doi: 10.1016/j.alcohol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Köster A, Montkowski A, Schulz S, Stübe EM, Knaudt K, Jenck F, Moreau JL, Nothacker HP, Civelli O, Reinscheid RK. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc Natl Acad Sci USA. 1999;96:10444–10449. doi: 10.1073/pnas.96.18.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KM, Szakall I, O'Brien D, Wang R, Vinod KY, Saito M, Simonin F, Kieffer BL, Vadasz C. Decreased oral self-administration of alcohol in kappa-opioid receptor knock-out mice. Alcohol Clin Exp Res. 2005;29:730–738. doi: 10.1097/01.alc.0000164361.62346.d6. [DOI] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Levine AS, Morley JE. Behavioral effects of corticotropin-releasing factor: localization and characterization of central effects. Brain Res. 1988;443:63–69. doi: 10.1016/0006-8993(88)91598-3. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101:211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacol. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Bazov I, Sheedy D, Garrick T, Harper C, Bakalkin G. Expression of pronociceptin and its receptor is downregulated in the brain of human alcoholics. Brain Res. 2009;1305 Suppl:S80–S85. doi: 10.1016/j.brainres.2009.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Kreek MJ, Bakalkin G, Liljequist S. The nociceptin/orphanin FQ receptor agonist Ro 64–6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacol. 2007;32:902–910. doi: 10.1038/sj.npp.1301169. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Ariwodola OJ, Chappell AM, Weiner JL, McCool BA. Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacol. 2008;55:661–668. doi: 10.1016/j.neuropharm.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Floyd DW, McCool BA. Chronic ethanol ingestion modulates proanxiety factors expressed in rat central amygdala. Alcohol. 2005;36:83–90. doi: 10.1016/j.alcohol.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larriva-Sahd J. Juxtacapsular nucleus of the stria terminalis of the adult rat: extrinsic inputs, cell types, and neuronal modules: a combined golgi and electron microscopic study. J Comp Neurol. 2004;475:220–237. doi: 10.1002/cne.20185. [DOI] [PubMed] [Google Scholar]
- Lam MP, Marinelli PW, Bai L, Gianoulakis C. Effects of acute ethanol on opioid peptide release in the central amygdala: an in vivo microdialysis study. Psychopharmacol. 2008;201:261–271. doi: 10.1007/s00213-008-1267-8. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Fitz S, Johnson PL, Shekhar A. Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacol. 2008;33:2586–2594. doi: 10.1038/sj.npp.1301674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EH, Tsai MJ. The hippocampus and amygdala mediate the locomotor stimulating effects of corticotropin-releasing factor in mice. Behav Neural Biol. 1989;51:412–423. doi: 10.1016/s0163-1047(89)91052-2. [DOI] [PubMed] [Google Scholar]
- Leenders AG, Sheng ZH. Modulation of neurotransmitter release by the second messenger-activated protein kinases: implications for presynaptic plasticity. Pharmacol Ther. 2005;105:69–84. doi: 10.1016/j.pharmthera.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AS, Morley JE. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- Levine AS, Rogers B, Kneip J, Grace M, Morley JE. Effect of centrally administered corticotropin releasing factor (CRF) on multiple feeding paradigms. Neuropharmacol. 1983;22:337–339. doi: 10.1016/0028-3908(83)90249-6. [DOI] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Campeau S, Falls WA, Miserendino MJ, Davis M. Lesions of the central nucleus of the amygdala, but not the paraventricular nucleus of the hypothalamus, block the excitatory effects of corticotropin-releasing factor on the acoustic startle reflex. J Neurosci. 1992;12:2313–2320. doi: 10.1523/JNEUROSCI.12-06-02313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber C, DeCarli L. Ethanol dependence and tolerance: a nutritionally controlled experimental model in the rat. Res Comm in Chemical Path Pharmacol. 1973;6:983–991. [PubMed] [Google Scholar]
- Lima-Landman MT, Albuquerque EX. Ethanol potentiates and blocks NMDA-activated single-channel currents in rat hippocampal pyramidal cells. FEBS Lett. 1989;247:61–67. doi: 10.1016/0014-5793(89)81241-4. [DOI] [PubMed] [Google Scholar]
- Littleton JM. Acamprosate in alcohol dependence: implications of a unique mechanism of action. Journal of Addiction Medicine. 2007;1:115–125. doi: 10.1097/ADM.0b013e318156c26f. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Homanics GE. Tonic for what ails us? high-affinity GABAA receptors and alcohol. Alcohol. 2007;41:139–143. doi: 10.1016/j.alcohol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Roberto M. Synaptic Effects Induced by Alcohol. In: Spanagel R, Sommerm WH, editors. “Behavioural Neurobiology of Alcohol Addiction”, Current Topics in Behavioral Neurosciences. Berlin Heidelberg: Springer-Verlag; (in press) [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci. 1990;10:1372–1379. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Sparrow AM, Breese GR, Knapp DJ, Thiele TE. The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcohol Clin Exp Res. 2008;32:240–248. doi: 10.1111/j.1530-0277.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Kiefer F, Spanagel R, Littleton J. Acamprosate: recent findings and future research directions. Alcohol Clin Exp Res. 2008;32:1105–1110. doi: 10.1111/j.1530-0277.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- Mansour A, Burke S, Pavlic RJ, Akil H, Watson SJ. Immunohistochemical localization of the cloned kappa 1 receptor in the rat CNS and pituitary. Neuroscience. 1996;71:671–690. doi: 10.1016/0306-4522(95)00464-5. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Densmore VS, Osborne PB. Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J Comp Neurol. 2007;504:702–715. doi: 10.1002/cne.21464. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacol. 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2006;30:982–990. doi: 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- Martz A, Deitrich RA, Harris RA. Behavioral evidence for the involvement of gamma-aminobutyric acid in the actions of ethanol. Eur J Pharmacol. 1983;89:53–62. doi: 10.1016/0014-2999(83)90607-6. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- McCool BA, Christian DT, Diaz MR, Läck AK. Glutamate plasticity in the drunken amygdala: the making of an anxious synapse. Int Rev Neurobiol. 2010;91:205–233. doi: 10.1016/S0074-7742(10)91007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ, Frye GD, Breese GR. Evidence for site specific ethanol actions in the CNS. Alcohol Drug Res. 1985;6:423–429. [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Merlo-Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob G, Weiss F. Increase of extracellular corticotrophin-releasing factor-like immunoreactivity levels in the amygdale of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Migita K, Loewy AD, Ramabhadran TV, Krause JE, Waters SM. Immunohistochemical localization of the neuropeptide Y Y1 receptor in rat central nervous system. Brain Res. 2001;889:23–37. doi: 10.1016/s0006-8993(00)03092-4. [DOI] [PubMed] [Google Scholar]
- Miller MA, Kolb PE, Raskind MA. Extra-hypothalamic vasopressin neurons coexpress galanin messenger RNA as shown by double in situ hybridization histochemistry. J Comp Neurol. 1993;329:378–384. doi: 10.1002/cne.903290308. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H. Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA-receptor function. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- Moller C, Sommer W, Thorsell A, Heilig M. Anxiogenic-like action of galanin after intra-amygdala administration in the rat. Neuropsychopharmacol. 1999;21:507–512. doi: 10.1016/S0893-133X(98)00102-X. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Swartzwelder HS. Attenuation of hippocampal long-term potentiation by ethanol: a patch-clamp analysis of glutamatergic and GABAergic mechanisms. J Neurosci. 1993;13:2264–2272. doi: 10.1523/JNEUROSCI.13-05-02264.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Herbert JS, Montpied P. Differential effects of chronic ethanol administration on GABA(A) receptor alpha1 and alpha6 subunit mRNA levels in rat cerebellum. Mol Cell Neurosci. 1992;3:251–258. doi: 10.1016/1044-7431(92)90045-4. [DOI] [PubMed] [Google Scholar]
- Moykkynen T, Korpi ER, Lovinger DM. Ethanol inhibits alpha-amino-3-hydyroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor function in central nervous system neurons by stabilizing desensitization. J Pharmacol Exp Ther. 2003;306:546–555. doi: 10.1124/jpet.103.050666. [DOI] [PubMed] [Google Scholar]
- Muller M, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, et al. Limbic corticotrophin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Canals JM, Valjakka A, Vartiainen J, Arenas E, Ernfors P. Neuropeptide Y alters sedation through a hypothalamic Y1-mediated mechanism. Eur J Neurosci. 2001;13:2241–2246. doi: 10.1046/j.0953-816x.2001.01601.x. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999;406:503–547. [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000;7:170–179. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. Ethanol enhances gamma-aminobutyric acid responses in a subpopulation of nucleus accumbens neurons: role of metabotropic glutamate receptors. J Pharmacol Exp Ther. 2000;293:654–661. [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, Szumlinski KK. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33:1924–1934. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouagazzal AM, Moreau JL, Pauly-Evers M, Jenck F. Impact of environmental housing conditions on the emotional responses of mice deficient for nociceptin/orphanin FQ peptide precursor gene. Behav Brain Res. 2003;144:111–117. doi: 10.1016/s0166-4328(03)00066-4. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Stress-induced expression of co-localized neuropeptides in hypothalamic and amygdaloid neurons. Eur J Pharmacol. 2000;405:161–166. doi: 10.1016/s0014-2999(00)00549-5. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadeas S, Grobin AC, Morrow AL. Chronic ethanol consumption differentially alters GABA(A) receptor alpha1 and alpha4 subunit peptide expression and GABA(A) receptor-mediated 36 Cl(−) uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res. 2001;25:1270–1275. [PubMed] [Google Scholar]
- Pape H-C, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Patel S, Cravatt BF, Hillard CJ. Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacol. 2005;30:497–507. doi: 10.1038/sj.npp.1300535. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala. Oxford University Press; 2000. [Google Scholar]
- Primeaux SD, Wilson SP, Bray GA, York DA, Wilson MA. Overexpression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self administration in “anxious” rats. Alcohol Clin Exp Res. 2006;30:791–801. doi: 10.1111/j.1530-0277.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Diana M. Drug dependence as a disorder of neural plasticity: focus on dopamine and glutamate. Rev Neurosci. 2001;12:141–158. doi: 10.1515/revneuro.2001.12.2.141. [DOI] [PubMed] [Google Scholar]
- Qi ZH, Song M, Wallace MJ, Wang D, Newton PM, McMahon T. Protein kinase Cε regulates γ-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of γ2 subunits. J Biol Chem. 2007;282:33052–33063. doi: 10.1074/jbc.M707233200. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008 Jan;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, D'Amico E, Riley E, Koob GF. GABA antagonist and benzodiazepine partial inverse agonist reduce motivated responding for ethanol. Alcohol Clin Exp Res. 1993a;17:124–130. doi: 10.1111/j.1530-0277.1993.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993b;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Fekete EM, Zhao Y, Funk CK, Zorrilla EP, Koob GF. A novel small molecule antagonist of the corticotropin-releasing factor type 1 receptor (CRF1) is a potent anxiolytic and reduces excessive alcohol intake in dependent male rats. Pharmacol Biochem Behav. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge JP, Ho AM, Innes DJ, Dodd PR. The expression of NMDA receptor subunit mRNA in human chronic alcoholics. Ann NY Acad Sci. 2008;1139:10–19. doi: 10.1196/annals.1432.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacol. 2006;31:988–996. doi: 10.1038/sj.npp.1300840. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacol. 2010b;35:1962–1972. doi: 10.1038/npp.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010a;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, O'Dell LE, Cruz MT, Morse AC, Siggins GR, Koob GF. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 2008;28:5762–5771. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004a;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004b;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Siggins GR. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc Natl Acad Sci USA. 2006;103:9715–9720. doi: 10.1073/pnas.0601899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, P s, Madamba SG, Nie Z, Siggins GR. Ethanol-CRF interactions at GABAergic synapses in rat central amygdala. Alcohol Clin. Exp. Res. Suppl. 2005;29:26. [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, Polidori C. Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacol. 2008;196:523–531. doi: 10.1007/s00213-007-0985-7. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advatnages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin Exp Res. 2002;26:796–803. [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacol. 2002;43:1165–1172. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999a;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999b;368:143–147. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Borchardt T, Vengeliene V, Zghoul T, Bachteler D, Gass P, Sprengel R, Spanagel R. Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse. J Neurosci. 2006;26:1231–1238. doi: 10.1523/JNEUROSCI.4237-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey LM, Madamba SG, Siggins GR, Bartfai T. Galanin alters GABAergic neurotransmission in the dorsal raphe nucleus. Neurochem Res. 2008;33:285–291. doi: 10.1007/s11064-007-9524-5. [DOI] [PubMed] [Google Scholar]
- Shimada S, Inagaki S, Kubota Y, Ogawa N, Shibasaki T, Takagi H. Coexistence of peptides (corticotropin releasing factor/neurotensin and substance P/somatostatin) in the bed nucleus of the stria terminalis and central amygdaloid nucleus of the rat. Neuroscience. 1989;30:377–383. doi: 10.1016/0306-4522(89)90259-5. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6:108–113. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6:509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- Smothers CT, Mrotek JJ, Lovinger DM. Chronic ethanol exposure leads to a selective enhancement of N-methyl-D-aspartate receptor function in cultured hippocampal neurons. J Pharmacol Exp Ther. 1997;283:1214–1222. [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala Crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Fee JR, Hayes DM, Knapp DJ, MacNeil DJ, Thiele TE. Peripheral and central administration of a selective neuropeptide Y Y1 receptor antagonist suppresses ethanol intake by C57BL/6J mice. Alcohol Clin Exp Res. 2004;28:1324–1330. doi: 10.1097/01.ALC.0000139829.67958.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc Natl Acad Sci USA. 1985;82:3940–3943. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;15:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Koh MT, Pedrazzini T. Voluntary alcohol consumption is controlled via the neuropeptide Y Y1 receptor. J Neurosci. 2002;22:RC208. doi: 10.1523/JNEUROSCI.22-03-j0006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticku MK, Burch T. Alterations in gamma-aminobutyric acid receptor sensitivity following acute and chronic ethanol treatments. J Neurochem. 1980;34:417–423. doi: 10.1111/j.1471-4159.1980.tb06612.x. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, et al. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry. 2010;67:1069–1077. doi: 10.1001/archgenpsychiatry.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav. 2004;79:671–689. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Rüedi-Bettschen D, Spealman RD. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Intrinsic and extrinsic connections of the rat central extended amygdala: an in vivo electrophysiological study of the central amygdaloid nucleus. Brain Res. 1998;794:188–198. doi: 10.1016/s0006-8993(98)00228-5. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The γ-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2008;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic κ-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addiction Biol. 2010;16:116–119. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan FJ, Berton F, Madamba SG, Francesconi W, Siggins GR. Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proc Natl Acad Sci USA. 1996;93:5049–5054. doi: 10.1073/pnas.93.10.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu H, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci USA. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol. 2002;88:399–406. doi: 10.1254/jjp.88.399. [DOI] [PubMed] [Google Scholar]
- Waters SM, Krause JE. Distribution of galanin-1-2 and -3 receptor messenger RNAs in central and peripheral rat tissues. Neurosci. 2000;95:265–271. doi: 10.1016/s0306-4522(99)00407-8. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: The view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacol. 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Boss-Williams KA, Moore JP, Demetrikopoulos MK, Ritchie JC, West CH. Testing the hypothesis that locus coeruleus hyperactivity produces depression-related changes via galanin. Neuropeptides. 2005;39:281–287. doi: 10.1016/j.npep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982;232:255–270. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Eberts C, Poelchen W, Allgaier C, Illes P. Mechanism of inhibition by ethanol of NMDA and AMPA receptor channel functions in cultured rat cortical neurons. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:568–576. doi: 10.1007/s002100000262. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International Classification of Diseases. 10th revision. 2007 Retrieved from http://apps.who.int/classifications/apps/icd/icd10online/
- Wu LG, Saggau P. Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J Neurosci. 1994;14:645–654. doi: 10.1523/JNEUROSCI.14-02-00645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuei X, Dick D, Flury-Wetherill L, Tian H-J, Agrawal A, Bierut L, et al. Association of the κ-opioid system with alcohol dependence. Mol Psychiatry. 2006;11:1016–1024. doi: 10.1038/sj.mp.4001882. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Sarahroodi S, Arzi A, Khodayar MJ, Taheri-Shalmani S, Rezayof A. Cannabinoid CB1 receptors of the rat central amygdala mediate anxiety-like behavior: interaction with the opioid system. Behav Pharmacol. 2008;19:716–723. doi: 10.1097/FBP.0b013e3283123c83. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96:433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacol. 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Zhao Y, Koob GF. Anti-CRF. In: Fink G, editor. Encyclopedia of Stress. Vol 1 A–E. Amsterdam: Elsevier; 2007. pp. 206–214. [Google Scholar]