Abstract
The question of whether a single hematopoietic stem cell (HSC) gives rise to all of the B-cell subsets [B-1a, B-1b, B-2, and marginal zone (MZ) B cells] in the mouse has been discussed for many years without resolution. Studies here finally demonstrate that individual HSCs sorted from adult bone marrow and transferred to lethally irradiated recipients clearly give rise to B-2, MZ B, and B-1b, but does not detectably reconstitute B-1a cells. These findings place B-2, MZ, and B-1b in a single adult developmental lineage and place B-1a in a separate lineage derived from HSCs that are rare or missing in adults. We discuss these findings with respect to known developmental heterogeneity in other HSC-derived lymphoid, myeloid, and erythroid lineages, and how HSC developmental heterogeneity conforms to the layered model of the evolution of the immune system that we proposed some years ago. In addition, of importance to contemporary medicine, we consider the implications that HSC developmental heterogeneity may have for selecting HSC sources for human transplantation.
The hematopoietic stem cell (HSC) derived from adult bone marrow (BM) is commonly thought to have multilineage potential, meaning that the HSC is considered capable of reconstituting all lymphoid, myeloid, and erythroid lineages of the immune system (1, 2). Indeed, HSCs from BM readily replenish B, T, myeloid, and erythroid cells in irradiated recipients (3, 4). However, more detailed examination of the reconstituted B cells derived from HSCs taken at different times during development reveals differences in reconstitution efficiency for the four currently recognized murine B-cell subsets, [i.e., B-1a, B-1b, B-2, and marginal zone B (MZ)] (5–7).
Transferring adult BM into lethally irradiated recipients readily reconstitutes B-2 and MZ, which represent the majority of the B cells in spleen and other lymphoid organs but only poorly reconstitute B-1 cells in the same recipients. In contrast, transferring neonatal BM, liver, or spleen to similar irradiated recipients fully reconstitutes B-1 (B-1a and B-1b), B-2, and MZ. Thus, at least with respect to B cells, the multilineage potential of the HSC population in adults is more limited than the multilineage potential of the HSC population in neonates (5, 7–12). These differences in B-cell reconstitution capabilities of adult versus neonatal BM underlie the idea that B-1 and B-2 belong to distinct developmental lineages derived from distinct HSCs (13).
Recent studies by Dorshkind and colleagues (5) confirm and extend the earlier findings. By sorting and transferring highly enriched HSC populations from adult BM and neonatal sources, these investigators demonstrate that the HSC population sorted from adult BM principally reconstitutes B-2 and B-1b and only poorly reconstitutes B-1a (5). In contrast, B-1a cells are relatively well reconstituted by transfers of HSC populations sorted from “neonatal” BM (2.5 wk of age), although the sorted cells still predominantly reconstitute B-2 and B-1b (5). These findings demonstrate clearly that the commitment to give rise to develop into B-1a occurs at or before the HSC development and that BM HSC populations collectively lose the potential to give rise to B-1a as the animal ages.
Importantly, however, because the Dorshkind studies are based on transfers of sorted HSC populations (roughly 1,000 sorted cells per recipient), they are not informative with respect to the potential of individual HSCs in the transferred population to give rise to each of the B-cell subsets (B-1a, B-1b, B-2, and MZ). In studies here, we close this gap by definitively demonstrating that individual HSCs sorted from adult BM fully reconstitute B-2, MZ, and some B-1b but do not reconstitute B-1a. These findings place B-2, MZ, and at least some B-1b in a single adult developmental lineage and place B-1a in a separate lineage derived from HSCs that are rare or missing in adults.
We discuss these findings with respect to known developmental heterogeneity in other HSC-derived lymphoid and myeloid lineages in the mouse (14, 15) and how/whether HSC developmental heterogeneity conforms to the layered model of the evolution of the immune system that we proposed some years ago (13, 16). In addition, of importance to contemporary medicine, we consider the implications HSC developmental heterogeneity for selecting HSC sources for human transplantation.
Results
Adult Bone Marrow Transfers Poorly Reconstitute B-1a in Irradiated Recipients.
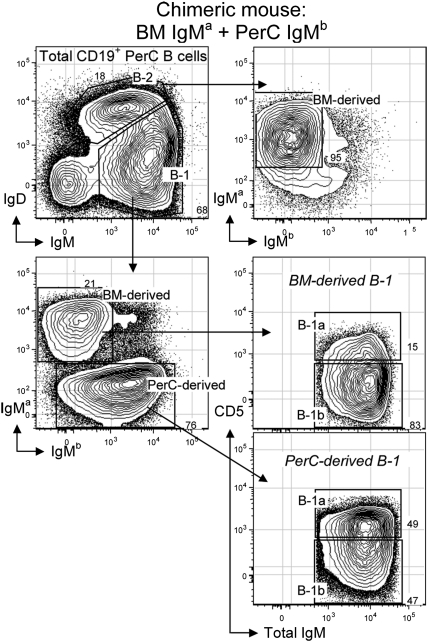
Multiple studies show that B-1 cells, which represent the majority of the B cells in the peritoneal cavity (PerC), are only poorly reconstituted by adult bone marrow transfers that readily reconstitute B-2 cells in the PerC and elsewhere in irradiated recipients (7, 8, 10–12). However, B-1 are readily reconstituted by transfers of mature B-1 from adult PerC to the same irradiated recipients. Similarly, we show here that B-1 are poorly reconstituted by transfers of 3 × 106 adult BALB/c (IgHa allotype) BM cells to sublethally irradiated (3.25 Gy) RAG1−/− recipient mice (Fig. 1). Cotransfer of 3 × 106 adult PerC cells from CB.17 (IgHb allotype) congenic mice reconstitutes only B-1 in the same animals (Fig. 1).
Fig. 1.
Reconstitution of recipient peritoneal cavity (PerC) B cells after cotransfer of bulk bone marrow (BM) and PerC. Donor cells (3 × 106 BALB/c BM and 3 × 106 CB.17 PerC) were injected i.v. to sublethally irradiated RAG1−/− recipients. Two months after transfer, B-cell reconstitution was analyzed in recipient PerC as shown. Donor BM (IgMa) cells readily reconstituted B-2 (IgDhi, IgMlo) and B-1b (IgDlo, IgMhi, CD5–) in PerC of recipient mice. In contrast, donor PerC (IgMb) cells mainly reconstituted B-1a (IgDlo, IgMhi, and CD5+) and B-1b.
At the B-cell subset level, adult BM (IgHa) transfers reconstitute B-2 and a small percentage of B-1b (CD5– B-1) but only very few B-1a (CD5+ B-1). The extent of this minimal B-1a and B-1b reconstitution decreases with the number of BM cells transferred, more so for B-1a than B-1b (Table 1). Thus, B-1a reconstitution falls below detectability at 2 × 105 transferred BM cells, whereas this number of transferred BM still reconstitutes B-1b (CD5– B-1) to a reasonable extent (Table 1). These findings are consistent with distinctive origins for B-1a and B-2 and raise questions about the developmental relationship between B-1a and B-1b. Studies that follow address these issues.
Table 1.
The extent of B-1 (B-1a and B-1b) reconstitution changes with the number of donor bone marrow (BM) transfers
| PerC B-1 cells derived from donor BM cells, % |
||
| Amount of donor BM | B-1a | B-1b |
| 3 × 106 total cells | 12–20 | 80–88 |
| 2 × 105 total cells | 2–5 | 95–98 |
| Single HSC | 0.2–1 | 99–99.8 |
B-1a (CD5+ B-1) reconstitution falls below detectability at 2 × 105 transferred BM cells, whereas B-1b (CD5– B-1) are still reasonably reconstituted.
Single HSCs Sorted from Adult BM and Transferred to Lethally Irradiated Recipients Provide Long-Term Reconstitution of All Hematopoietic Cells.
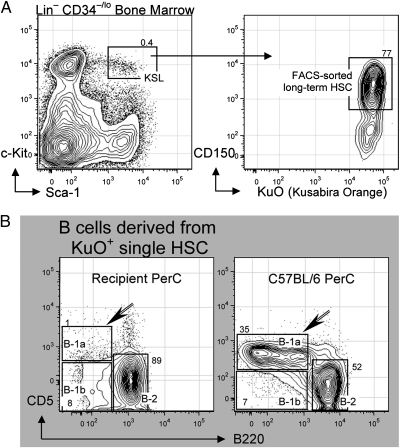
For these studies, we FACS sorted and transferred a single HSC obtained from adult BM of transgenic mice expressing Kusabira Orange (KuO+), a readily detectable fluorescent marker, and identified as Lin– cells that express c-Kit+, Sca-1+, CD150+ but not CD34 (17) (Fig. 2A). Each individual HSC was transferred i.v. to lethally irradiated mice along with 2 × 105 “competitor” congenic BM cells. Mice were bled monthly to check for the level of chimerism, i.e., percentage of immune cells derived from the single HSC (KuO+) versus percentage of immune cells derived from the competitor congenic BM. Preliminary analysis demonstrated significant hematopoietic reconstitution (i.e., multilineage reconstitution) in 17/80 recipients of sorted HSC. Here, we examined the five recipients that had the highest chimerism in the B-cell compartment (10–80% of total B cells in blood derived from sorted KuO+ HSCs).
Fig. 2.
Reconstitution of recipient peritoneal cavity (PerC) B cells after single HSC transfer. Individual HSCs were isolated from adult (9 wk) bone marrow (BM) of KuO+ mice and injected i.v. to lethally irradiated C57BL/6 recipients. (A) HSC in adult BM was identified as Lin–, c-Kit+, Sca-1+, CD150+, and CD34–/lo. (B) Recipient PerC was analyzed 27 wk after single HSC (KuO+) transfer. Total HSC-derived B cells in recipient PerC were identified by CD19+ and KuO+ and analyzed for surface expression level of CD5 and B220. Sorted and transferred individual KuO+ HSC failed to reconstitute B-1a (CD5+, B220lo, and CD19hi) but readily reconstituted B-2 (CD5–, B220hi, and CD19lo) and some B-1b (CD5–, B220lo, and CD19hi).
Despite the difference in its ability to reconstitute B-1a versus B-2 (Results), the individually transferred HSCs studied here were fully multipotent, at least by the current definition of multipotency, i.e., they stably reconstituted platelets, erythrocytes, myeloid cells, T cells, and B cells in all recipient mice. These reconstituted hematopoietic cells were still readily detectable when the mice were killed and the organs harvested at 30 wk posttransfer.
Individual HSCs Sorted from Adult BM Give Rise to B-2 and B-1b, but Not to B-1a: Reconstitution in Recipient PerC.
Transfers of individual HSCs to irradiated recipients are well known to reconstitute B-2 cells, which typically predominate in spleen and peripheral blood and are commonly taken as a measure of B-cell reconstitution (4). We similarly find that B-2 cells are readily reconstituted by transfers of single HSCs from adult bone marrow to irradiated recipients and that B-1b are reconstituted to about half-normal level (Fig. 2B). However, even 30 wk after transplantation, individual HSCs sorted and transferred from adult bone marrow do not replenish the B-1a compartment in the otherwise fully reconstituted recipients (Fig. 2B).
This selective B-1a developmental failure cannot be explained by a lack of support for B-1a development in the adult recipient environment. Earlier transfer studies have clearly shown that B-1a cells are readily reconstituted when early progenitors (fetal liver, neonatal spleen, and BM) are transferred to adult recipients (11). Moreover, Dorshkind et al. (5) have shown directly that bulk-sorted and transferred neonatal HSCs readily reconstitute B-1a cells in adult recipients. These and other similar findings demonstrate clearly that the adult environment readily supports development of B-1a. Therefore, the failure of the HSCs in our study to give rise to B-1a demonstrates that adult BM contains HSCs that are restricted developmentally to giving rise only to B-2 and some B-1b.
Of course, adult BM may also contain HSCs capable of giving rise to all B-cell subsets, including B-1a. Indeed, Dorshkind and colleagues obtained some B-1a reconstitution when they transferred 500–1,000 sorted HSCs from adult BM to irradiated recipients (5). In any event, because we failed to obtain B-1a in 5/5 HSC recipients, the data we present here (Fig. 2B) clearly demonstrate that a sizable proportion of HSCs in adult BM cannot reconstitute B-1a.
Surprisingly, given the close historical and notational relationship between B-1a and B-1b (9, 11), we find that all of the individually sorted and transferred adult HSCs replenish a substantial proportion of the B-1b compartment (Fig. 2B). Thus, we additionally conclude that adult HSCs are committed to give rise to B-2 and a proportion of B-1b cells.
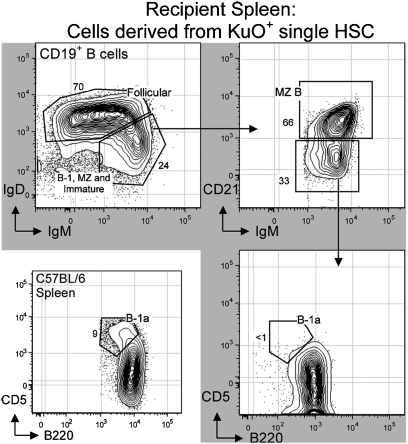
Individual HSCs Sorted from Adult BM Give Rise to Follicular (B-2) and MZ B Cells, but Not B-1a: Reconstitution in Recipient Spleen.
Consistent with data for PerC reconstitutions presented above, individually sorted adult BM-derived HSCs do not reconstitute detectable numbers of B-1a cells (B220lo, CD5+) in spleen (Fig. 3). As expected, these HSCs efficiently reconstitute splenic follicular B-2 (IgDhi and IgMlo) cells and MZ (CD21hi) B cells. Moreover, the percentage of CD21hi MZ cells among total B cells derived from the sorted HSCs is similar to that found in the spleen of control animals.
Fig. 3.
Reconstitution of recipient spleen B cells after single HSC transfer. Individual HSCs were isolated from adult (9 wk) bone marrow (BM) of KuO+ mice and injected i.v. to lethally irradiated C57BL/6 recipients. Recipient spleen was analyzed 27 wk after single HSC (KuO+) transfer. Total HSC-derived B cells in recipient spleen were identified by the expression of CD19+ and KuO+. Sorted and transferred individual KuO+ HSCs readily reconstituted follicular B-2 cells (IgDhi, IgMlo, and B220hi), marginal zone B (MZ) cells (CD21hi, IgDlo, and IgMhi), and immature B cells (IgMhi, IgDlo, CD21–, and CD5–) at appropriate proportions. However, sorted and transferred individual KuO+ HSCs failed to reconstitute B-1a (CD5+, B220lo, and CD19hi) in recipient spleen, even after 27 wk of HSC transplantation.
Discussion
We have shown that individual HSCs, FACS sorted from adult BM and transferred to lethally irradiated recipients, give rise to B-2, MZ B, and B-1b cells but do not give rise to B-1a. These findings confirm the longstanding hypothesis that B-1a and B-2 originate from distinct progenitors in adults and hence belong to distinct developmental lineages (6, 7, 10, 11, 18–24). Further, these findings unexpectedly place a proportion of B-1b and MZ in the same developmental lineage as B-2.
Laying the groundwork for these findings, Dorshkind et al. (5) have shown that 500–1,000 bulk-sorted HSCs from adult BM principally reconstitute B-2 in lethally irradiated recipients. In addition, small but detectable numbers of B-1a (roughly 6% of total B in PerC) were reconstituted by the 500–1,000 bulk-sorted BM cells that were transferred (5). In our studies, transfers of individual HSCs also fully reconstituted B-2. However, these transfers failed to detectably reconstitute B-1a (only a few scattered “dots” constituting at best 0.2–1% of total B in PerC were visible in FACS analyses) (Figs. 2B and 3). The difference can be explained if rare HSCs capable of giving rise to B-1a were present in the HSC bulk sorted from adult BM in the previous study.
Dorshkind et al. have already shown that B-1a are clearly, albeit not fully, reconstituted by HSC bulk sorted from 2.5-wk-old neonatal BM (5). Thus, it is reasonable to expect that a small number of neonatal HSCs are present among sorted adult HSC populations. These HSCs could either reconstitute both B-1a and B-2 (plus MZ and B-1b) or they could be committed to reconstitute only B-1a. There is no data at present to decide between these alternatives. In any event, the current data collectively demonstrate that a high proportion (most or all) adult HSCs are committed to reconstitute only B-2, MZ, and some B-1b.
Fetal liver and neonatal (2 d–2 wk) spleen, of course, have long been known to fully reconstitute B-1 and all other B-cell subsets in irradiated recipients (11). As in intact animals, the number of B-1a is greater than B-2 and B-1b (B-1a > B-2 > B-1b) in PerC in these recipients. In contrast, B-2 > B-1b > B-1a in PerC in recipients of the HSC populations sorted and transferred from 2.5 wk BM, although B-1a reconstitution by the sorted neonatal cells is still substantially greater than B-1a reconstituted from HSC bulk sorted from adult BM (5). Thus, either HSCs gradually lose their ability to reconstitute B-1a as animals age or the HSCs in adults never had this ability, i.e., they are developmentally distinct from the HSCs that predominate during fetal and neonatal life (6, 13, 21). Again, there are no data available to distinguish these possibilities. See Table 2 for a comprehensive description of B-cell reconstitution potential of several in vivo cell transfer studies (2, 5, 11, 22–26).
Table 2.
B-cell subset reconstitution after in vivo cell transfer studies
| Cells transferred to lethally irradiated recipients |
Progeny B cells |
|||||||
| Source (reference) | Donor age | Isolation | Type | Phenotype | B-1a | B-1b | MZ | B-2 |
| Yolk sac* (22) | E9–9.5 | Culture* | Hemogenic endothelial cells | VE-cadherin+ CD41− | ++ | ++ | ++ | None |
| Yolk sac (24) | E12–13 | Whole tissue | Total cells | +++ | +++ | +++ | +++ | |
| Fetal liver (2, 11) | E13-prior birth | Whole tissue | Total cells | ++ | +++ | (?) | +++ | |
| Neonatal BM (5) | 2.5 wk | Bulk sort | HSC | KSL CD150+ | ++ | +++ | (?) | +++ |
| Adult BM | >8 wk | Single cell | HSC | KSL CD34−CD150+ | None | ++ | +++ | +++ |
The amount of reconstitution of each B-cell subset (B-1a, B-1b, marginal zone, and B-2) in lethally irradiated recipients is directly dependent on the source of donor cells used in these transfer studies.
*Isolated at indicated day and cultured for 5–11 d before transfer.
In any event, the differences in B-cell development potential between neonatal and adult HSCs indicate that evolution has crafted a developmental strategy for differentially populating the B-cell compartment to gradually enrich it for functionally relevant B cells as the animal ages (13, 16). This strategy would appear to extend to all hematopoietic lineages in the mouse. Differences between fetal and adult erythrocytes are well known. Further, early work from the Weissman and Allison laboratories defined a “first wave” of T-cell development, which occurs during fetal life and principally generates γδ T cells and later wave(s) that generate the αβ T cells that ultimately predominate in adult life (27). In this construction, B-1a would be located alongside γδ T cells in the earliest lymphoid development wave(s), whereas B-2 would colocate with αβ T cells.
The development of the hematopoietic system in man similarly changes with age (28). The differences between fetal/neonatal and adult erythroid development are well established. In addition, McCune et al. have shown that human fetal and adult T cells belong to distinct subsets originating from HSCs that are present in different stages during development (29). Moreover, they show that the fetal T-cell lineage is biased toward immune tolerance, providing a plausible mechanistic explanation for the unique tolerogenic properties of the immune system during ontogeny (29). Finally, findings from the de la Hera and Rothstein laboratories report phenotypic and functional shifts in the B-cell compartment during development (30–32) that, as in the mouse, distinguish the B-cell development pathway in children from the well-known adult pathway.
Interestingly, the findings discussed above were predicted by the “layered immune system” model proposed by our laboratory over two decades ago (13, 16). In essence, this model suggests that as species evolved, layers were added to the immune system to perform functions necessary for the survival of more advanced species. In addition, consistent with the idea that ontogeny recapitulates phylogeny, the model proposes that more advanced functions (e.g., affinity maturation) were associated with immune system components that evolved to be expressed later in development, when more diverse and directed functionality is required.
B-1 and B-2, with their specialized functions and their differential development, engendered this layered immune system model (13, 21). However, the proposed model generated more heat than light for many years, largely because better tools were needed to clearly define the developmental origins of B-1 and B-2. The HSC transfer data demonstrate clearly that adult BM contains HSCs that do not give rise to B-1a, but clearly give rise to B-2, MZ, and B-1b. Thus, B-1a emerges as a distinct developmental lineage whose early development, which occurs and is largely complete before the emergence of the B-cell lineages that predominate in adults, is consistent with the layered immune system model.
These findings have direct implications for BM human transplantation protocols (33). HSCs from adult BM have widely been considered to be capable of reconstituting all hematopoietic cells. However, data from both mouse and man now indicate that HSC populations in adult BM (and perhaps in cord blood) may be heterogeneous and may be limited with respect to the potential for regenerating the various hematopoietic cells present in adults (17, 34). If so, then technologies will have to be developed to enable the full reconstitution of the adult immune system.
Materials and Methods
Mice and Tissue Preparation.
BALB/c (IgHa allotype), CB.17 (IgHb allotype), and RAG1−/− mice (8–10 wk old) were purchased from Jackson Labs or bred at the Stanford Medical School Animal Care Facility. C57BL/6-Ly5.2 and Ly5.1/Ly5.2-F1 recipient mice (8–9 wk old) were bred at the Institute of Medical Science, University of Tokyo, Tokyo, Japan, and a transgenic mouse line expressing the fluorescent marker humanized Kusabira Orange (huKO) was a generous gift from Masafumi Onodera (National Center for Child Health and Development, Tokyo, Japan). All experiments were conducted with institutional animal care and use committee approval. PerC cells were harvested by injecting 7 mL of staining medium [deficient RPMI plus 3% (vol/vol) newborn calf serum] into PerC. Spleen was disrupted and resuspended to obtain single cell suspensions. BM from femurs and tibias was washed multiple times using a syringe to obtain single cell suspensions. All cell samples were filtered and resuspended at 25 × 106 cells/mL using custom RPMI medium 1640 deficient in biotin, l-glutamine, phenol red, riboflavin, and sodium bicarbonate (Invitrogen).
FACS.
Cell suspensions were preincubated with anti-CD16/CD32 mAb to block FcγRII/III and stained on ice for 30 min with the following fluorochrome-conjugated mAb in an 11-color staining combination: FITC-labeled anti-CD21 (7G6) or anti-Ig κ- and λ-light chains (187.1 and R26-46, respectively); PE-labeled anti-CD43 (S7) or anti-IgMa (DS-1); PECy5-labeled anti-CD5 (53-7.3); PECy5.5-labeled anti-CD19 (1D3); PECy7-labeled anti-IgM (331); APC-labeled anti-B220 (RA3-6B2) or anti-CD23 (B3B4); Alexa700-labeled anti-IgD (11–28) or anti-IgMb (AF6-78.25); APCCy7-labeled anti-CD19 (1D3), anti-B220 (RA3-6B2), or anti-CD11b (M1/70); Pacific Blue-labeled (Dump channel) anti-F4/80 (BM8), anti-Gr-1 (RB6-8C5), and anti-CD11b (M1/70); biotin-labeled anti-Ig κ- and λ-light chains (187.1 and R26-46, respectively) or anti-CD23 (B3B4). Cells were then washed and stained again on ice for 15 min with streptavidin Qdot 605 (Invitrogen) to reveal biotin-coupled antibodies. Antibodies were either purchased (Invitrogen and BD Pharmingen) or conjugated in our laboratory at Stanford University. After washing, stained cells were resuspended in 10 μg/mL propidium iodide (PI), idenitified on PE-Texas Red channel), to exclude dead cells (i.e., PI+ cells). Cells were analyzed and sorted on Stanford FACS facility instruments (Becton Dickinson LSRII or FACSAria). Data were collected for 0.2–1 × 106 cells. Staining protocols were designed with CytoGenie software (Woodside Logic); data were analyzed with FlowJo software (TreeStar). To distinguish autofluorescent cells from cells expressing low levels of individual surface markers, we established upper thresholds for autofluorescence by staining samples with fluorescence-minus-one control stain sets in which a reagent for a channel of interest is omitted.
Sorting and Transfer of Single HSC.
Total BM cells from 9-wk-old huKO transgenic mice were stained with biotin-labeled lineage markers mAb (anti-CD4, CD8, Gr-1, Ter-119, B220, and IL-7R) on ice for 30 min. Cells were then washed and stained again on ice for 90 min with the following fluorochrome-conjugated mAb: PECy7-labeled anti-CD117 (c-Kit 2B8); APC-labeled anti-CD150 (SLAM); Alexa700-labeled anti-CD34 (RAM34); Pacific Blue-labeled anti–Sca-1 (Ly-6A/E D7) and with streptavidin-APCCy7 to reveal biotin-coupled mAb. After washing, stained cells were resuspended in 10 μg/mL PI to exclude dead (PI+) cells. KuO+ HSCs were identified as Lin–CD34–/loc-Kit+Sca-1+CD150+ and individually sorted in 96-well plates. Individual KuO+ HSCs were transferred i.v. to 80 lethally irradiated (two doses of 4.9 Gy delivered 4 h apart) C57BL/6 recipients along with 2 × 105 whole BM competitor cells from 8-wk-old Ly5.1/Ly5.2-F1 mice. After 30 wk, recipient PerC and spleen cells were harvested and KuO+ HSC-derived B cells were analyzed as described above. BM-derived HSCs were sorted on a FACSAriaII (Becton Dickinson).
Chimerism.
In this study, 80 mice received a single HSC i.v. along with 2 × 105 congenic BM cells. Recipient mice were bled monthly to check for the level of chimerism, i.e., percentage of immune cells derived from the single HSC (KuO+) versus percentage of immune cells derived from the competitor congenic BM (KuO–). A total of 17/80 recipient mice showed some level of chimerism in one, or all, hematopoietic lineages analyzed in blood (erythrocytes, platelets, myeloid cells, T cells, and B cells). Here, we chose to examine the five recipients that had chimerism in all hematopoietic lineages and yet had the highest chimerism in the B-cell compartment (10–80% of total B cells in blood derived from sorted KuO+ HSCs). These reconstituted hematopoietic cells were still readily detectable in blood when the mice were killed and tissues (PerC and spleen) harvested at 30 wk posttransfer.
Note Added in Proof.
While this manuscript was under review, Yuan et al. (35) reported that adult HSC can be reprogrammed to express the fetal HSC developmental profile, including the ability to develop into B-1a. The authors interpret these findings, as we interpret the HSC developmental difference reported here, as support for the Layered Immune System model that we first proposed in the late 1980s.
Acknowledgments
We thank Megan Phillips for excellent technical help; P. Sadate-Ngatchou, Motohito Okabe, and Makoto Otsu for helpful discussion; Masafumi Onodera for providing huKO transgenic mice; and John Mantovani and Claudia Weber for administrative support. This work was supported by National Institutes of Health Grant AI076434 and by grants from the Japanese Ministry of Education, Culture, Sport, Science and Technology.
Footnotes
The authors declare no conflict of interest.
References
- 1.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 2.Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci USA. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao YA, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci USA. 2004;101:221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R, Neff NF, Quake SR, Weissman IL. Tracking single hematopoietic stem cells in vivo using high-throughput sequencing in conjunction with viral genetic barcoding. Nat Biotechnol. 2011;29:928–933. doi: 10.1038/nbt.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barber CL, Montecino-Rodriguez E, Dorshkind K. Reduced production of B-1–specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proc Natl Acad Sci USA. 2011;108:13700–13704. doi: 10.1073/pnas.1107172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber CL, Montecino-Rodriguez E, Dorshkind K. Developmental relationships between B-1 and B-2 progenitors. Cell Cycle. 2011;10(22):3810–3811. doi: 10.4161/cc.10.22.18190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci USA. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Adoptive transfer of murine B-cell lineages. Ann N Y Acad Sci. 1992;651:168–169. doi: 10.1111/j.1749-6632.1992.tb24610.x. [DOI] [PubMed] [Google Scholar]
- 9.Stall AM, Adams S, Herzenberg LA, Kantor AB. Characteristics and development of the murine B-1b (Ly-1 B sister) cell population. Ann N Y Acad Sci. 1992;651:33–43. doi: 10.1111/j.1749-6632.1992.tb24591.x. [DOI] [PubMed] [Google Scholar]
- 10.Herzenberg LA, Kantor AB. B-cell lineages exist in the mouse. Immunol Today. 1993;14:79–83, discussion 88–90. doi: 10.1016/0167-5699(93)90063-Q. [DOI] [PubMed] [Google Scholar]
- 11.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 12.Kantor AB, Stall AM, Adams S, Watanabe K, Herzenberg LA. De novo development and self-replenishment of B cells. Int Immunol. 1995;7:55–68. doi: 10.1093/intimm/7.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Herzenberg LA, Kantor AB, Herzenberg LA. Layered evolution in the immune system. A model for the ontogeny and development of multiple lymphocyte lineages. Ann N Y Acad Sci. 1992;651:1–9. doi: 10.1111/j.1749-6632.1992.tb24588.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He S, Kim I, Lim MS, Morrison SJ. Sox17 expression confers self-renewal potential and fetal stem cell characteristics upon adult hematopoietic progenitors. Genes Dev. 2011;25:1613–1627. doi: 10.1101/gad.2052911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herzenberg LA, Herzenberg LA. Toward a layered immune system. Cell. 1989;59:953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- 17.Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med. 2010;207:1173–1182. doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu LS, et al. Identification of a germ-line pro-B cell subset that distinguishes the fetal/neonatal from the adult B cell development pathway. Proc Natl Acad Sci USA. 2002;99:3007–3012. doi: 10.1073/pnas.052715399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tung JW, Mrazek MD, Yang Y, Herzenberg LA, Herzenberg LA. Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse. Proc Natl Acad Sci USA. 2006;103:6293–6298. doi: 10.1073/pnas.0511305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tung JW, Herzenberg LA. Unraveling B-1 progenitors. Curr Opin Immunol. 2007;19:150–155. doi: 10.1016/j.coi.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 23.Ghosn EE, Sadate-Ngatchou P, Yang Y, Herzenberg LA, Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci USA. 2011;108:2879–2884. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshimoto M, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci USA. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7:213–219. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 26.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 27.Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 28.Mold JE, McCune JM. At the crossroads between tolerance and aggression: Revisiting the “layered immune system” hypothesis. Chimerism. 2011;2:35–41. doi: 10.4161/chim.2.2.16329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mold JE, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanz E, et al. Ordering human CD34+CD10-CD19+ pre/pro-B-cell and CD19- common lymphoid progenitor stages in two pro-B-cell development pathways. Proc Natl Acad Sci USA. 2010;107:5925–5930. doi: 10.1073/pnas.0907942107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70- J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffin DO, Rothstein TL. A small CD11b+ human B1 cell subpopulation stimulates T cells and is expanded in lupus. J Exp Med. 2011;208:2591–2598. doi: 10.1084/jem.20110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Czechowicz A, Weissman IL. Purified hematopoietic stem cell transplantation: The next generation of blood and immune replacement. Hematol Oncol Clin North Am. 2011;25:75–87. doi: 10.1016/j.hoc.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beerman I, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci USA. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012 doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]