Abstract
Growing evidence suggests serotonin's role in anxiety and depression is mediated by its effects on learned fear associations. Pharmacological and genetic manipulations of serotonin signaling in mice alter the retention of fear extinction learning, which is inversely associated with anxious temperament in mice and humans. Here, we test whether genetic variation in serotonin signaling in the form of a common human serotonin transporter polyadenylation polymorphism (STPP/rs3813034) is associated with spontaneous fear recovery after extinction. We show that the risk allele of this polymorphism is associated with impaired retention of fear extinction memory and heightened anxiety and depressive symptoms. These STPP associations in humans mirror the phenotypic effects of serotonin transporter knockout in mice, highlighting the STPP as a potential genetic locus underlying interindividual differences in serotonin transporter function in humans. Furthermore, we show that the serotonin transporter polyadenylation profile associated with the STPP risk allele is altered through the chronic administration of fluoxetine, a treatment that also facilitates retention of extinction learning. The propensity to form persistent fear associations due to poor extinction recall may be an intermediate phenotype mediating the effects of genetic variation in serotonergic function on anxiety and depression. The consistency and specificity of these data across species provide robust support for this hypothesis and suggest that the little-studied STPP may be an important risk factor for mood and anxiety disorders in humans.
Serotonin is implicated in the etiology of anxiety and depression; however, its precise role in these disorders is unclear. An early “chemical hypothesis” that depression stems from a serotonin deficiency was belied by observations that reducing serotonin levels does not induce depression in healthy individuals (1) and that inhibition of serotonin reuptake fails to yield rapid antidepressant effects (2). These findings suggest that necessary intervening processes mediate serotonin's role in anxiety and depression. The “network hypothesis” proposes that serotonin fosters neural plasticity that supports adaptive processing of affective information (3). Under this view, anxiety and depression stem from aberrant integration of salient environmental information due to altered serotonergic function. Consistent with this notion, a prominent theory posits the dysregulation of learned fear associations as a central underlying mechanism by which serotonin contributes to mood and anxiety disorders (4).
Extinction learning is a primary means of regulating conditioned fear responses (5). During extinction, fear expression decreases, reflecting new learning that a once-threatening stimulus now signals safety. The efficacy of extinction at reducing fear depends on the ability to retrieve an extinction memory upon subsequent encounters with a conditioned stimulus. However, failure to recall extinction learning may occur with the passage of time, resulting in “spontaneous recovery” of the original fear memory (6). Research suggests that the degree of extinction retention is a relatively stable individual trait with corresponding neurobiological substrates (7–9). Recent reports in humans that spontaneous recovery of fear is associated with heightened anxiety (10, 11) suggest that modulation of extinction memory may underlie serotonin's effects on anxiety and depression.
Research in animal models provides strong evidence for this hypothesis. Genetic knockout of the serotonin transporter (5-HTT) increases anxiety and depression-related behavior in the mouse (12). Furthermore, during fear conditioning, 5-HTT knockout mice show normal acquisition and initial extinction learning, but exhibit a selective deficit in extinction recall (13, 14) accompanied by abnormal neuronal morphology in regions that support extinction retention (9, 13). These data suggest that 5-HTT down-regulation impairs extinction retention, resulting in persistent fear memories that may contribute to anxiety and depression. However, no study has yet examined whether normal variation in serotonergic function modulates extinction memory in humans.
The 5-HTT is expressed as two mRNA species that differ in the use of two polyadenylation signals (15, 16). Polydenylation is a posttranscriptional modification of the 3′ end of the transcript that occurs in the majority of protein-coding mRNAs. Alternative polyadenylation forms are common in the brain and can lead to diversity in the regulation of gene expression (17). The two reported 5-HTT polyadenylation forms differ by a 123-bp element, and the human gene contains a common T/G single nucleotide polymorphism (rs3813034) in the more distal of the two polyadenylation signals. We have termed rs3813034 the serotonin transporter polyadenylation polymorphism (STPP) because it alters the use of the polyadenylation signal in which it occurs, influencing the balance of the two polyadenylation forms in the brain (15, 16). G-allele carriers have a reduced fraction of 5-HTT mRNA containing the distal polyadenylation sequence (distal polyadenylation fraction; DPF), and also exhibit increased risk for panic disorder (15). Of functional interest, the distal polyadenylation sequence element is positively correlated with the steady-state level of 5-HTT mRNA (15) and occurs adjacent to a microRNA (miR-16) binding site (18), suggesting that binding of regulatory proteins to the distal sequence element may modulate miR-16 binding (19) and alter 5-HTT translation (18), as has been described in miR-16–mediated regulation of cyclooxygenase 2 gene expression.
Here, we genotyped participants (SI Appendix, Table S1) in a two-day fear conditioning paradigm (Fig. 1A) for the STPP to determine whether genetic variation in serotonergic function might influence extinction retention. During fear acquisition, a colored square (the conditioned stimulus +, or CS+) was paired with a mild electric shock on one-third of presentations, whereas another square, conditioned stimulus − (CS-), was never paired with shock. Differential skin conductance response (SCR) to the two stimuli (CS+ minus CS-) served as our measure of the conditioned fear response (CR). Acquisition was immediately followed by extinction, during which the CS+ was no longer paired with shock. A day later, a second extinction phase enabled evaluation of whether participants exhibited spontaneous fear recovery (defined as the increase in the CR from the final block of day one extinction to the initial block of extinction recall). After conditioning, participants completed questionnaires assessing anxiety and depressive symptoms (20, 21). We found that the STPP showed a dose-dependent association with the spontaneous recovery of fear, anxious temperament, and depressive symptoms.
Fig. 1.
Fear conditioning paradigm and summary conditioned response data. (A) Experimental design. (B) Summary graphs illustrating group conditioned response (mean differential SCR to the CS+ versus the CS-) during late acquisition (final two blocks) and late extinction (final two blocks). Participants acquired a conditioned fear response that was significantly decreased via extinction learning. (C) Conditioned responses increased (P = 0.063) from the final block of extinction to the first block of extinction recall (spontaneous recovery). *****P < 0.00001. Error bars ± SEM.
To further relate the 5-HTT distal polyadenylation fraction to anxiety-related emotional processing, we treated mice with the selective serotonin reuptake inhibitor (SSRI) fluoxetine, a treatment that facilitates extinction retention (22–24), alleviates anxiety and depressive symptoms (25), and is the most commonly used pharmacological treatment for panic disorder. Chronic fluoxetine increased the 5-HTT DPF in mouse brain, suggesting that biological factors that increase the 5-HTT DPF are anxiolytic and those that decrease it are anxiogenic.
Collectively, these data suggest that the 5-HTT DPF is associated with extinction retention and that common genetic variation in 5-HTT polyadenylation may modulate the retention of fear extinction memory in humans, influencing the risk of anxiety disorders and depression.
Results
Genetic Variation in 5-HTT Polyadenylation Selectively Modulates Extinction Retention in Humans.
Conditioned responses during late acquisition and late extinction for participants who exhibited successful discriminative fear acquisition (mean CS+ SCR > mean CS- SCR during the final two blocks of acquisition; n = 110) are shown in Fig. 1B. Because extinction and spontaneous recovery cannot be assessed in those who do not show initial acquisition, these individuals were excluded from the physiological analyses. A paired Student's t test revealed a decrease in conditioned fear from late acquisition to late extinction (t = 4.868, df = 109, P = 0.0000038). As a group, participants showed modest spontaneous fear recovery (differential CR from the first block extinction recall minus the final block of extinction) the following day (Fig. 1C) (one-sample t test; t = 1.88, df = 109, P = 0.063).
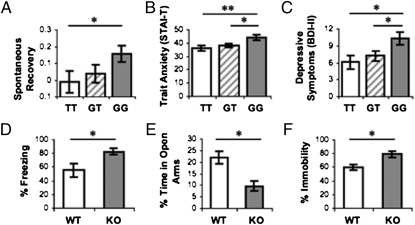
To ascertain whether STPP genotype influenced fear expression, we conducted linear regressions with each participant's number of putative “risk” (G) alleles as an independent variable, and fear acquisition, extinction, and spontaneous recovery measures as dependent variables. We made the a priori prediction that the STPP G allele, which has been associated with a lower 5-HTT DPF and increased risk of panic disorder (15), would be associated with impaired extinction retention. We found that the number of G alleles showed a dose-dependent linear relationship with the magnitude of spontaneous fear recovery (β = 0.191, P = 0.046) (Fig. 2A, see SI Appendix, Fig. S1 for scatterplot). This association was unaffected by participants’ age, sex, and race (SI Appendix, Tables S2 and S3). Demonstrating the selectivity of this effect, there was no relationship between the number of G alleles and either fear acquisition (β = −0.077, P = 0.422) or extinction (β = −0.105, P = 0.266) (SI Appendix, Fig. S2). This STPP association is consistent with the selective effect of 5-HTT knockout in mice on extinction retention (13, 14) (Fig. 2B).
Fig. 2.
STPP modulates extinction retention, anxiety, and depressive symptoms in humans and parallels reported effects of 5-HTT knockout in the mouse. Rodent data adapted from refs. 13 and 33. (A) Participants showed a linear increase (β = 0.191, P = 0.046) in spontaneous fear recovery as a function of number of STPP G alleles (TT vs. GG: t = 2.109, df = 61, P = 0.039). Trait anxiety (B) and depressive symptoms (C) increased linearly (STAI-T: β = 0.320, P = 0.00011; BDI-II: β = 0.293, P = 0.0004) as a function of number of STPP G alleles [GG vs. TT: STAI-T: t(79) = 3.576, P = 0.0006; BDI-II: t(79) = 3.264, P = 0.002; GG vs. GT: STAI-T: t(105) = 3.468, df =, P = 0.0007; BDI-II: t(105) = 2.673, P = 0.009]. (D) 5-HTT knockout mice (KO) show higher levels of freezing during extinction recall (first block) compared with wild-type mice (WT) (E) Percentage of time spent in open arms of the elevated plus maze task was reduced in KO versus WT mice. (F) Percentage of time spent immobile during the second day of exposure to the forced swim task was greater in KO versus WT mice. *P < 0.05, **P < 0.01, ***P < 0.001. Error bars ± SEM.
For comparison purposes, we also genotyped samples at the highly studied 5-HTTLPR, which has been inconsistently associated with 5-HTT expression (26, 27) and a variety of behavioral phenotypes (28–32). There was no significant relationship between 5-HTTLPR genotype and fear expression during any phase of conditioning (all P > 0.05) (SI Appendix, Fig. S3 A and B and Table S6), and there were no STPP-5-HTTLPR genotype interactions upon any of our fear conditioning measures (all P > 0.05).
Genetic Variation in 5-HTT Polyadenylation Is Associated with Individual Differences in Anxiety and Depressive Symptoms.
Consistent with previous studies linking poor extinction retention to heightened anxiety (10, 11), participants’ degree of spontaneous fear recovery was positively correlated with self-reported state anxiety [State-Trait Anxiety Inventory (STAI)-S subscale; mean (M) = 38.61, SD = 9.95] (r = 0.193, P = 0.043) and showed a trending relationship with trait anxiety (STAI-T subscale; M = 39.76, SD = 10.86) (r = 0.159, P = 0.096), independent of STPP genotype.
We then examined whether individual differences in trait anxiety and depressive symptomatology (BDI-II; M = 8.38; SD = 7.05) were related to STPP genotype. Participants’ (n = 141) number of STPP G alleles showed linear associations with both trait anxiety (β = 0.320, P = 0.00011) (Fig. 2B; see SI Appendix, Fig. S4 for scatterplot) and depressive symptoms (β = 0.293, P = 0.0004) (Fig. 2C; see SI Appendix, Fig. S5 for scatterplot), which were unaffected by age, sex, and race (SI Appendix, Tables S4 and S5) and remained significant after excluding subjects reporting high levels of depressive symptoms and trait anxiety (SI Appendix, SI Text). The number of G alleles also showed a trending relationship with state anxiety (STAI-S; β = 0.166, P = 0.083).
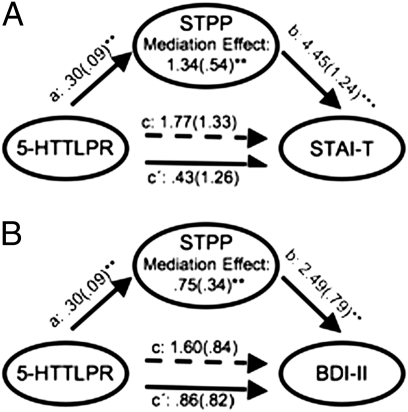
By comparison, participants’ number of 5-HTTLPR S′ (triallelic “short”, S and LG) alleles showed a correlation with depressive symptoms (β = 0.170, P = 0.044) (SI Appendix, Fig. S6A), and a trending association with trait anxiety (β = 0.121, P = 0.153) (SI Appendix, Fig. S6B) (see SI Appendix, Tables S7 and S8 for demographic controls). There were no STPP-5-HTTLPR interactions upon trait anxiety or depressive symptoms (both P > 0.05). However, in our sample, participants’ number of STPP G and 5-HTTLPR S′ alleles were correlated (r = 0.296, P = 0.00037), a phenomenon referred to in population genetics as linkage disequilibrium. We hypothesized that the observed associations of the 5-HTTLPR with trait anxiety and depressive symptoms might be mediated by the correlation of its alleles with those of the STPP, which was more strongly predictive of these measures. Including participants’ allele counts for both polymorphisms in a multiple regression to control for their correlation revealed that only number of STPP G alleles was a significant predictor of both trait anxiety (β = 0.311, P = 0.0003) and depressive symptoms (β = 0.266, P = 0.0021). A bootstrap mediation analysis (SI Appendix, SI Text) confirmed that the apparent relationship between the 5-HTTLPR and these phenotypes was mediated by the STPP (STAI-T: z = 3.26, P = 0.0011; BDI-II: z = 3.13, P = 0.0017) (Fig. 3 A and B). Reversing the position of the STPP and 5-HTTLPR in this mediation does not yield a significant reduction in the direct effect of the STPP (STAI-T: P = 0.71; BDI-II: P = 0.92).
Fig. 3.
STPP polymorphism mediates apparent relationship between 5-HTTLPR and anxiety and depressive symptoms. Bootstrap mediation analyses indicate that the influence of 5-HTTLPR on trait anxiety (A) and depressive symptoms (B) is mediated by the STPP. Path a shows the estimated coefficient of the relationship between the 5-HTTLPR and STPP. Path b shows the coefficient for the relationship between STPP and each self-report measure. Paths c and c′ show coefficients for the total (dashed line) and direct (solid line) effects of 5-HTTLPR on each measure. All coefficients are unstandardized, SEM in parentheses. **P < 0.01, ***P < 0.001 (Mediation Effects: STAI-T: P = 0.0011; BDI-II: P = 0.0017).
Chronic Fluoxetine Alters 5-HTT Polyadenylation in the Mouse Brain.
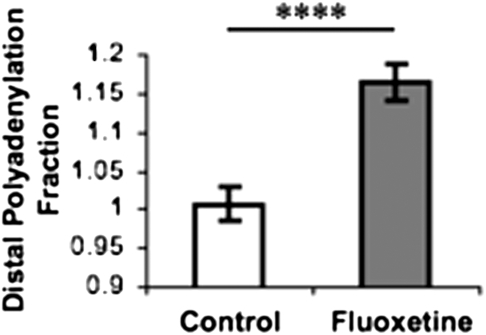
Administration of SSRIs is a common pharmacological approach to the treatment of mood and anxiety disorders including panic disorder. Recent studies indicate that chronic administration of the SSRI fluoxetine improves extinction retention (22–24), suggesting a potential mechanism underlying its anxiolytic and antidepressant effects (25). Here, we test whether fluoxetine alters the 5-HTT DPF, which would further implicate the 5-HTT DPF as a mediator of both altered extinction retention and the therapeutic effects of SSRIs. We found that brains of mice after 3 wk of fluoxetine administration (n = 5) had a significantly higher DPF than those of drug-free control animals (n = 5) [Fig. 4; t(8) = 5.17 ; P = 0.000012], suggesting that the therapeutic effects of fluoxetine may stem from changes in 5-HTT polyadenylation.
Fig. 4.
Fluoxetine increases 5-HTT distal polyadenylation fraction in mouse brain. Brain tissue of fluoxetine-treated mice had a higher fraction of 5-HTT mRNA containing the distal polyadenylation sequence than that of control animals. Values for fluoxetine group are normalized to controls. ****P < 0.0001.
Discussion
Our results suggest that the STPP selectively influences the recall of fear extinction memory in humans. Consistent with the hypothesis that poor extinction retention confers vulnerability to anxiety and fear-related affective disorders (11, 12), the STPP was also associated with trait anxiety and depressive symptoms. Fig. 2 highlights the striking parallel between these STPP associations and reported behavioral effects of genetic knockout of 5-HTT in the mouse. In both species, extinction retrieval, but not initial fear acquisition or extinction, is selectively impaired (13, 14) (Fig. 2D). Furthermore, the modulation of anxiety and depressive symptoms by STPP genotype in humans parallels the behavioral impairments of knockout mice in the elevated plus maze and forced swim tests (13, 33), rodent assays of anxiety, and depression-related behavior (Fig. 2 E and F). We note that gene knockout may not fully recapitulate the effects of the STPP because the two are very different forms of genetic variation. Nonetheless, the consistency and specificity of the phenotypes reported here across species lend confidence to the validity of the STPP associations and support the theory that 5-HTT contributes to the development of anxiety and depression via modulation of extinction recall.
Our findings suggest that extinction retention may be an endophenotype mediating the effects of genetic variability in 5-HTT function on anxiety disorders and depression. An endophenotype is a heritable and quantifiable trait that genetically cosegregates with a psychiatric illness, but manifests independently of the presence of disease (34). Both fear acquisition and initial extinction learning show moderate heritability (35). Although no large-scale twin study has quantified the heritability of extinction retention, specific interstrain behavioral differences in extinction recall (8) suggest that genetic background also influences extinction retention. Here, we identify a common genetic factor associated with extinction retention and subclinical anxiety and depressive symptoms. Although the STPP is associated with risk for panic disorder (15), it is not known whether individuals with this illness show impaired extinction recall. A more robust future validation of this endophenotype hypothesis would require a demonstration of a genetically driven impaired extinction retention in a clinical population.
The deficit in extinction recall associated with the STPP G allele could stem from a failure to either consolidate or retrieve extinction learning. These processes depend on interaction between the amygdala, the ventromedial prefrontal cortex (vmPFC), and the hippocampus (9), regions that receive serotonergic innervation (36, 37) and are functionally modulated by 5-HTT expression (12). Morphological abnormalities of neurons in the basolateral amygdala and the infralimbic cortex of 5-HTT knockout mice implicate these regions as potential substrates of the associated impairment in extinction retention (13, 14). Successful retention of extinction in humans is associated with increased cortical thickness in the vmPFC (7, 38), a putative human homolog of the rodent infralimbic region thought to facilitate fear inhibition during extinction retrieval (9). Because no studies have yet examined differences in brain structure or function associated with the STPP, further research is needed to clarify the mechanisms underlying its effects on spontaneous fear recovery.
Anxiety disorders are commonly treated with extinction-based exposure therapy (39). Extinction retention across exposure sessions predicts the degree of reduction in anxiety symptoms, highlighting its importance for persistent fear reduction (40). Our data suggest that individuals who may be most at risk for anxiety disorders might benefit the least from this therapeutic approach, because reduced extinction retention would limit its efficacy. Administration of SSRIs is another common approach to treating clinical anxiety and depression. Recent reports that chronic SSRI administration reduces spontaneous recovery (22, 24), contextual renewal (22), and reinstatement (23) of extinguished fear while leaving fear acquisition intact provide further evidence for the specificity of the effects of serotonin on extinction recall. In conjunction with our present finding that chronic fluoxetine ameliorates the polyadenylation risk profile associated with reduced extinction retention in humans (15), these data suggest that the therapeutic efficacy of SSRIs may stem from molecular alterations that facilitate consolidation and retrieval of extinction learning.
The mechanism by which the distal polyadenylation sequence alters 5-HTT function is unknown. The steady state level of total 5-HTT mRNA expression is correlated with the presence of the distal polyadenylation sequence (15), suggesting that it may stabilize 5-HTT mRNA. Furthermore, a recent report that miR-16 binding to a site directly adjacent to the distal polyadenylation sequence causes translational repression of the 5-HTT suggests that an RNA binding protein may modulate the effects of miR-16 on 5-HTT expression, as has been reported for the cyclooxygenase2 gene (19).
The 5-HTTLPR is presently the most widely studied polymorphism in human genetics. This common polymorphism has been reported to alter transcription of 5-HTT (26, 28) and has been associated with a range of phenotypes including anxious temperament (28), frontoamygdala responses to emotional stimuli (41–43), and risk of depression in response to stressful life events (29). These associations have not been consistently replicated and even recent large-scale meta-analyses of several of these phenotypes present conflicting evidence for the 5-HTTLPR's role in behavioral variability (30–32) These inconsistent findings suggest that the link between the 5-HTTLPR and anxiety-related phenotypes may be complex, with its detection dependent upon the methodologies used as well as factors including alternative genetic variants such as the STPP (44, 45). In our data, we find that the 5-HTTLPR does not phenocopy the effects of 5-HTT knockout in mice on extinction retention and we do not replicate an earlier report of a 5-HTTLPR association with human fear acquisition (46). Furthermore, an apparent relationship of the 5-HTTLPR to trait anxiety and depressive symptoms is explained by correlation of its alleles with those of the STPP, which was more strongly associated with these phenotypes. Thus, inconsistent associations of the 5-HTTLPR with anxiety and depression related phenotypes might be due to variable correlation in experimental samples between the two polymorphisms. Given the history of nonreplication in the field of behavioral genetics and the limited statistical power of our sample, the conclusions we draw from our data are speculative. We encourage future studies to test this linkage disequilibrium hypothesis by genotyping behavioral samples for both the 5-HTTLPR and the STPP, clarifying their relative phenotypic effects and attempting to resolve the conflicts in the present literature. We strongly suggest the use of pooled genetic samples, consistent phenotypic criteria, and a priori hypotheses in such efforts.
A shortcoming of our study was the failure to fully control for psychiatric history and drug use of participants. Although our subjects were not presently taking psychiatric medication and reported subclinical symptoms of depression, they were not formally characterized as free of psychiatric disorders. Given the high lifetime prevalance of anxiety disorders and depression (47), undiagnosed or unmedicated individuals with these disorders were likely included in our sample. Furthermore, our sample might include subjects taking nonpsychiatric medications known to influence memory consolidation (e.g., beta blockers). These confounds may have influenced our reported effects and should be controlled for in future studies.
Depression and anxiety disorders show substantial heritability (48, 49) and high comorbidity (50), suggesting common pathophysiological mechanisms. Based on the broad efficacy of medications targeting 5-HTT and the marked behavioral effects of genetic knockout, the 5-HTT gene has been widely proposed as a candidate gene conferring vulnerability to these disorders (12, 37). Our present findings, as well as a previous association with panic disorder (15), indicate that the STPP may be an important genetic locus of risk for anxiety and depression. Furthermore, our data suggest that the retention of fear extinction memory may be a critical mediator of the influence of serotonin on individual vulnerability to these disorders in humans.
Methods
Participants.
One hundred forty-one volunteers, aged 18–35 (M = 21.1, SD = 3.5, 87 female), were recruited at New York University for a 2-d fear conditioning study. All participants gave informed consent and were paid for participation. Participants were not taking any psychiatric medication. Participants were racially diverse (63 Caucasian, 58 Asian, 3 Black/African-American, 6 Hispanic, 11 mixed race). Genotypes for both the STPP (34 TT, 60 GT, 47 GG) and the 5-HTTLPR (biallelic: 45 SS, 53 SL, 43 LL; triallelic, where S′ = S or LG and L′ = LA: 56 S′S′, 57 S′L′, 28 L′L′) were in Hardy Weinberg equilibrium within Caucasian (5-HTTLPR P = 0.7; STPP P = 0.14) and Asian (5-HTTLPR P = 0.33; STPP P = 0.28) ethnic groups, who collectively constituted 85.8% of the sample. Subject demographics by STPP genotype are shown in SI Appendix, Table S1.
Fear Conditioning.
During fear conditioning, participants saw a sequence of trials in which two colored squares were presented for 4 s each. During the fear acquisition phase, one square (the CS+) coterminated with a mild electric wrist shock on 33% of trials, whereas the other (the CS-) was never paired with shock. Acquisition was followed immediately by extinction, in which neither CS was paired with shock. The next day, a second extinction phase enabled assessment of whether participants retained extinction learning or exhibited spontaneous recovery of the conditioned fear response. Trials in each phase were grouped into “blocks.” In every block, the CS+ and CS- stimuli were presented four times each without shock. During acquisition, there were two additional trials per block in which the CS+ was paired with shock. Acquisition consisted of four blocks of trials (four CS+, four CS-, and two CS+ with shock per block), and each extinction phase consisted of five blocks of trials (four CS+ and four CS- per block) (Fig. 1A). Two randomized trial orders were counterbalanced across subjects. The first trial of the day 2 extinction session began with a CS+ for one-half of subjects and a CS- for the other. The skin conductance response (SCR) to each stimulus was assessed. Participants showing a greater mean SCR to the CS+ versus CS- during the final two blocks of acquisition were considered to have acquired a fear response (only CS+ presentations not paired with shock were analyzed). Our recovery measure was the increase in the CR from the final block of day 1 extinction to the initial block of extinction recall.
Shocks were delivered via a stimulator (Grass Instruments) connected to a bar electrode attached to the right wrist. Participants selected shock levels via a procedure in which the shock (200-ms duration) was gradually increased until it was deemed “uncomfortable, but not painful” (maximum shock level was 60 V). There were no genotype differences in shock level (SI Appendix, Table S1). SCR was recorded through shielded Ag-AgCl electrodes attached to the second and third fingers of the left hand by using a Biopac Systems module. AcqKnowledge software (Biopac Systems) was used to conduct offline analysis of the SCR waveforms. SCR data were low-pass filtered and smoothed. The greatest base to peak change in SCR in a 0.5- to 4.5-s window after each CS onset was assessed. These values were then square-root transformed to normalize the distribution and divided by the mean SCR to the shock to enable between-subject comparisons. Only unreinforced CS+ presentations were included in the analysis.
Eight participants were excluded from the physiological analysis because of experimental error in physiological recording or a failure to display a variable skin conductance signal. Another 23 participants were excluded because of failure to exhibit a discriminative CR (mean CS+ SCR > mean CS- SCR) during late acquisition. The remaining 110 participants (STPP: 28 TT, 47 GT, 35 GG; 5-HTTLPR: 22 L′L′, 50 S′L′, 38 S′S′) were included in the physiological analyses. χ2 tests showed no significant differences between the STPP and 5-HTTLPR genotypes of those who did not acquire a CR and the genotype frequencies of the complete sample (all P > 0.05).
Questionnaires.
After conditioning, participants completed the STAI (20), the Beck Depression Inventory II (BDI-II) (21), and the Life Experience Survey (LES) (51), assessing recent life experiences. There were no significant differences in STAI-T or BDI-II scores as a function of sex or age (all P > 0.05) or between Caucasian and Asian participants (both P > 0.05). There was no significant interaction of either genotype and negative life events (LES scores) upon anxiety and depressive symptoms, or any fear conditioning measures (all P > 0.05).
Genotyping.
DNA was prepared from saliva samples by using standard procedures (Oragene; DNA Genotek). The STPP (rs3813034) was genotyped by using a commercially available TaqMan assay (Applied Biosystems; C__27504840_10). The 5-HTTLPR was genotyped by using a protocol that allows assignment of both the biallelic and triallelic genotype categorizations (52). Samples whose initial genotype was not clear were regenotyped in duplicate, and all such samples provided clear concordant genotypes on rerun.
Statistical Analysis.
All tests were two-tailed by using an α level of 0.05. Using SPSS 18.0 (IBM), we conducted simple linear and multiple regressions to assess the relationships between our fear conditioning measures, questionnaire scores, genotypes, and potential covariates. Independent samples t tests were used in pairwise genotype comparisons of conditioning measures and questionnaire scores, and comparison of DPF in control and fluoxetine treated brains. We conducted mediation analyses and bootstrap significance testing by using a custom Matlab toolbox (53, 54) (SI Appendix, SI Text).
Rodent Fluoxetine Distal Polyadenylation Analysis.
C57BL/6J mice were given 160 mg/L fluoxetine (n = 5) in their drinking water for 21 d, an approximate dose of 18 mg/kg per day under ad libitum access, which yields therapeutic plasma levels and anxiolytic behavioral effects (25). Controls (n = 5) received ad libitum access to tap water. Whole brain tissue was harvested and homogenized in Tri reagent (Molecular Research Center). Total RNA was isolated according to the manufacturer's standard protocol. Total RNA (2 μg) was treated with DNase I and heat-inactivated then reversed transcribed by using oligo dT primers and Moloney Murine Leukemia Virus reverse transcriptase (200 units). Total 5-HTT and distal sequence containing 5-HTT messages were quantified by using SYBR green-based quantitative PCR assays as described (15) except that the primers for the total 5-HTT assay targeting the proximal 3′ UTR were as follows: Forward: 5′-CCAAGCTGATGATGTAAGGTCTTT-3′, Reverse: 5′-GTCACCAGCTAATGTGGCAGTAA-3′. Standard curves for each assay were prepared by using serial dilutions of pooled cDNA samples to allow relative quantification. Distal polyadenylation fraction was calculated by dividing the relative amount of distal sequence containing 5-HTT messages by the relative amount of total 5-HTT message determined for each sample. All samples were reverse transcribed twice, and each cDNA sample was run in six replicates.
5-HTT Knockout Mouse Data.
Behavioral data from two published studies (13, 33) in the 5-HTT knockout mouse are included here to show cross-species phenotypic correspondence in fear conditioning (13) and anxiety and depression-like behavior [elevated plus maze (33) and forced swim (13) tasks, in the mouse]. Details of the experimental protocols and analyses appear in the methods sections of these manuscripts.
Supplementary Material
Acknowledgments
We thank A. Gorun and A. Richman for assistance with data collection and analysis. A. Izquierdo and A. Holmes collected the original 5-HTT knockout mouse data. This research was supported by the National Science Foundation (C.A.H.); the James S. McDonnell Foundation, and National Institutes of Health (NIH) Grants MH072279 and MH80758 (to E.A.P.); The National Institute on Alcohol Abuse and Alcoholism Intramural Research Program (A.H.); a generous gift by the Mortimer D. Sackler, MD family (to B.J.C.); NIH Grant MH079513 (to B.J.C. and C.E.G.); and The Hartwell Foundation, the Pritzker Neuropsychiatric Disorders Research Consortium, and NIH Grant MH091401 (to C.E.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202044109/-/DCSupplemental.
References
- 1.Booij L, Van der Does AJ, Riedel WJ. Monoamine depletion in psychiatric and healthy populations: Review. Mol Psychiatry. 2003;8:951–973. doi: 10.1038/sj.mp.4001423. [DOI] [PubMed] [Google Scholar]
- 2.Nestler EJ. Antidepressant treatments in the 21st century. Biol Psychiatry. 1998;44:526–533. doi: 10.1016/s0006-3223(98)00095-x. [DOI] [PubMed] [Google Scholar]
- 3.Castrén E. Is mood chemistry? Nat Rev Neurosci. 2005;6:241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- 4.Deakin JFW, Graeff FG. 5-HT and mechanisms of defence. J Psychopharmacol. 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- 5.Hartley CA, Phelps EA. Changing fear: The neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 7.Milad MR, et al. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci USA. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hefner K, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rauch SL, et al. Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport. 2005;16:1909–1912. doi: 10.1097/01.wnr.0000186599.66243.50. [DOI] [PubMed] [Google Scholar]
- 11.Milad MR, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy DL, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wellman CL, et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayanan V, et al. Social defeat: Impact on fear extinction and amygdala-prefrontal cortical theta synchrony in 5-HTT deficient mice. PLoS ONE. 2011;6:e22600. doi: 10.1371/journal.pone.0022600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyawali S, et al. Association of a polyadenylation polymorphism in the serotonin transporter and panic disorder. Biol Psychiatry. 2010;67:331–338. doi: 10.1016/j.biopsych.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battersby S, et al. Presence of multiple functional polyadenylation signals and a single nucleotide polymorphism in the 3′ untranslated region of the human serotonin transporter gene. J Neurochem. 1999;72:1384–1388. doi: 10.1046/j.1471-4159.1999.721384.x. [DOI] [PubMed] [Google Scholar]
- 17.Di Giammartino DC, Nishida K, Manley JL. Mechanisms and consequences of alternative polyadenylation. Mol Cell. 2011;43:853–866. doi: 10.1016/j.molcel.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: A new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- 19.Shanmugam N, Reddy MA, Natarajan R. Distinct roles of heterogeneous nuclear ribonuclear protein K and microRNA-16 in cyclooxygenase-2 RNA stability induced by S100b, a ligand of the receptor for advanced glycation end products. J Biol Chem. 2008;283:36221–36233. doi: 10.1074/jbc.M806322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists; 1983. [Google Scholar]
- 21.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 22.Karpova NN, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334:1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deschaux O, Spennato G, Moreau JL, Garcia R. Chronic treatment with fluoxetine prevents the return of extinguished auditory-cued conditioned fear. Psychopharmacology (Berl) 2011;215:231–237. doi: 10.1007/s00213-010-2134-y. [DOI] [PubMed] [Google Scholar]
- 24.Camp M, et al. Genetic strain differences in learned fear inhibition associated with variation in neuroendocrine, autonomic, and amygdala dendritic phenotypes. Neurophyschopharmocology. February 15, 2012 doi: 10.1038/npp.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 26.Heils A, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 27.Parsey RV, et al. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006;163:48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- 28.Lesch KP, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 29.Caspi A, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 30.Willis-Owen SA, et al. The serotonin transporter length polymorphism, neuroticism, and depression: A comprehensive assessment of association. Biol Psychiatry. 2005;58:451–456. doi: 10.1016/j.biopsych.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 31.Risch N, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- 34.Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hettema JM, Annas P, Neale MC, Kendler KS, Fredrikson MA. A twin study of the genetics of fear conditioning. Arch Gen Psychiatry. 2003;60:702–708. doi: 10.1001/archpsyc.60.7.702. [DOI] [PubMed] [Google Scholar]
- 36.Azmitia EC, Gannon PJ. The primate serotonergic system: A review of human and animal studies and a report on Macaca fascicularis. Adv Neurol. 1986;43:407–468. [PubMed] [Google Scholar]
- 37.Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartley CA, Fischl B, Phelps EA. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cereb Cortex. 2011;21:1954–1962. doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foa EB. Psychosocial therapy for posttraumatic stress disorder. J Clin Psychiatry. 2006;67(Suppl 2):40–45. [PubMed] [Google Scholar]
- 40.Berry AC, Rosenfield D, Smits JAJ. Extinction retention predicts social anxiety symptom reduction. Depress Anxiety. 2009;26:22–27. doi: 10.1002/da.20511. [DOI] [PubMed] [Google Scholar]
- 41.Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pezawas L, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 43.Heinz A, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- 44.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rutter M, Thapar A, Pickles A. Gene-environment interactions: Biologically valid pathway or artifact? Arch Gen Psychiatry. 2009;66:1287–1289. doi: 10.1001/archgenpsychiatry.2009.167. [DOI] [PubMed] [Google Scholar]
- 46.Lonsdorf TB, et al. Genetic gating of human fear learning and extinction: Possible implications for gene-environment interaction in anxiety disorder. Psychol Sci. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 47.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 48.Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- 49.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 50.Merikangas KR, et al. Zurich Cohort Study Longitudinal trajectories of depression and anxiety in a prospective community study: The Zurich Cohort Study. Arch Gen Psychiatry. 2003;60:993–1000. doi: 10.1001/archpsyc.60.9.993. [DOI] [PubMed] [Google Scholar]
- 51.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: Development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46:932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 52.Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 53.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davidson ML, Atlas LY, Lindquist MA, Bolger N, Wager TD. The M3 Toolbox: The Multi-level Mediation/Moderation Framework for Connectivity Analyses in fMRI Data. Davis, CA: Cognitive Neuroscience Society; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.