Abstract
B-1a cells are primarily thought of as natural antibody-producing cells. However, we now show that appropriate antigenic stimulation induces IgM and IgG B-1a antibody responses and long-lived T-independent antigen-specific B-1a memory that differs markedly from canonical B-2 humoral immunity. Thus, we show here that in the absence of inflammation, priming with glycolipid (FtL) from Francisella tularensis live vaccine strain induces splenic FtL-specific B-1a to mount dominant IgM and activation-induced cytidine deaminase-dependent IgG anti-FtL responses that occur within 3–5 d of FtL priming and fade within 1 wk to natural antibody levels that persist indefinitely in the absence of secondary FtL immunization. Equally surprising, FtL priming elicits long-term FtL-specific B-1a memory cells (IgM>>IgG) that migrate rapidly to the peritoneal cavity and persist there indefinitely, ready to respond to appropriately administrated secondary antigenic stimulation. Unlike B-2 responses, primary FtL-specific B-1a responses and establishment of persistent FtL-specific B-1a memory occur readily in the absence of adjuvants, IL-7, T cells, or germinal center support. However, in another marked departure from the mechanisms controlling B-2 memory responses, rechallenge with FtL in an inflammatory context is required to induce B-1a secondary antibody responses. These findings introduce previously unexplored vaccination strategies for pathogens that target the B-1a repertoire.
Keywords: B-1, memory B cells, vaccine
B-1a lymphocytes mainly develop de novo during fetal/neonatal life and are maintained thereafter by self-replenishment (1–3). Although they and their plasma cell progeny are well known as natural antibody-producing cells, they are not commonly viewed as participating antigen-stimulated antibody responses. However, in this and a companion article (see ref. 4), we demonstrate that immunization with a glycolipid (FtL) isolated from Francisella tularensis live-vaccine strain (Ft LVS) readily induces splenic FtL-specific B-1a to produce T-independent antigen-specific IgM and IgG (IgM>>IgG) primary antibody responses, to develop long-term antigen-specific memory, and to produce secondary antibody responses when appropriately rechallenged with the antigen. Strikingly, although the B-1a memory responses that we identify share many of the properties of B-2 memory responses, they nonetheless differ in key ways that make the B-1a responses more suitable for the functional niche they occupy.
B-1 lymphocytes represent 1–5% of total B cells in adult mice. They are the principal B cells in peritoneal (PerC) and pleural cavities, are present at low but detectable frequencies in spleen and intestine, and are very rare in bone marrow (BM) and lymph nodes (1, 3). B-1a, which express low levels of CD5, predominate among PerC B-1, but B-1b, which do not express CD5, are present at much lower frequencies in the PerC (2, 3). Functionally, B-1a are well known to produce natural antibodies (2, 5–8) and to up-regulate the antibody production in response to Toll-like receptor (TLR) stimulation (9–11). Consistent with this function, our recent studies show that stimulation with Salmonella typhimurium LPS, a TLR4 agonist, nonspecifically induces PerC B-1a to migrate to spleen, where they join with resident splenic B-1a to augment polycolonal antibody production (10). In addition, intranasal influenza infection has been shown to induce B-1a migration, in this case to respiratory tract lymphoid organs where, without undergoing clonal expansion, the migrants produce IgM that includes virus-neutralizing natural IgM antibodies (12).
These findings fuel the prevailing view that B-1a do not mount antigen-induced antibody responses (13). However, B-1a are clearly known to produce specific antibody responses to certain antigens, including phosphorylcholine (14–16) and α1,3 dextran (17, 18). Most recently, foreshadowing studies presented here, we have shown that immunization with FtL, an atypical LPS isolated from Ft LVS, induces B-1a with FtL-binding IgM receptors to appear in spleen and to produce anti-FtL IgM primary antibody responses that protect against lethal Ft LVS challenge (19, 20). Consistent with B-1a mediating this protection, FtL priming does not similarly protect Bruton's tyrosine kinase (Btk)-mutant (xid) mice, which have B-1b but lack B-1a (21–23).
In contrast, B-1b generate protective antibody responses and provide long-lasting immunity against Borrelia hermsii infection such that transferring sorted PerC B-1b from B. hermsii infected mice intravenously to Rag1−/− mice confers long-term protection (24). Confirming that B-1b rather than B-1a mediate this protection, B. hermsii immunization also protects the Btk-mutant xid mice mentioned above, which lack B-1a (25). Thus, B-1a and B-1b have distinct repertoires and response properties.
Studies here and in a companion article (4) together show that the B-1a–mediated protection that FtL priming induces against lethal Ft LVS challenge (19) is accompanied by induction of anti-FtL B-1a primary responses and, importantly, by induction of anti-FtL B-1a memory cells that persist indefinitely in PerC (but not elsewhere) and remain ready to respond to FtL rechallenge under appropriate conditions. Activation-induced cytidine deaminase (AID)-dependent isotype switching occurs during development of a proportion of these FtL-specfic B-1a memory cells. However, unlike B-2 memory, B-1a memory cells develop in the absence of T-cell or germinal center (GC) influence. Furthermore, the induction of antigen-specific B-1a memory cells is inhibited when the antigen is initially encountered in an inflammatory context but their production of secondary antibody responses requires rechallenge with priming antigen presented in just such a context (TLR4 stimulation). Collectively, these findings open a view on previously unsuspected immune memory mechanisms and thereby introduce previously unexplored vaccination strategies likely to be suitable for immunization with pathogen-associated antigens that target B-1a repertoire.
Results
FtL Immunization Induces Antigen-Specific Isotype Switching and IgG Plasma Cell Development in Splenic B-1a.
In addition to inducing IgM anti-FtL production (19), FtL priming induces anti-FtL (FtL-binding Igκ+) B-1a in the spleen to undergo IgG isotype switching and to differentiate to plasma cells producing either IgG1 or IgG3 anti-FtL. Class-switched anti-FtL B-1a (including plasma cells) account for roughly 20% of total anti-FtL B-1a in spleen by day 5 after FtL priming (Fig. S1A); they express either IgG1 or IgG3 receptor (Fig. S1A) but not IgG2a, IgG2b, IgE, or IgA. Interestingly, IgG1 is expressed more in BALB/c mice than IgG3, but IgG3 predominates in C57BL/6 mice.
The class-switched plasma cells in spleens from FtL-primed animals are readily visualized as IgM–IgD– anti-FtL cells that express a typical plasma cell phenotype (CD19+CD138+CD23–CD5–). These cells arise slightly later (day 4, rather than day 3) than IgM+ anti-FtL plasma cells, and increase until day 5. Then, like their IgM counterparts, their numbers fall rapidly to below FACS-detectability by day 7 (Fig. S1B).
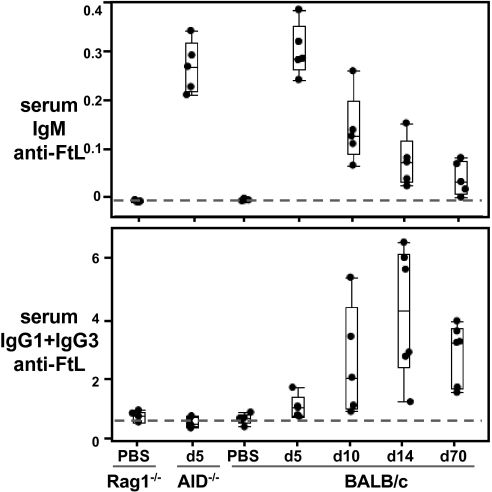
Consistent with the plasma-cell development kinetics in spleen, anti-FtL antibodies are not present in the natural antibody pool before FtL immunization. Instead, after being induced and reaching peak within 1–2 wk after FtL immunization, anti-FtL antibodies ultimately fall and merge into the natural antibody pool in FtL-primed mice. Thus, serum IgM and IgG anti-FtL antibodies are barely detectable before FtL immunization, rise quickly in FtL-immunized animals, and peak at days 5–7 for IgM and days 10–14 for IgG (Fig. 1). Notably, long after primary response resolves, both IgM and IgG anti-FtL persist indefinitely (>70 d) at levels slightly above background (Fig. 1).
Fig. 1.
FtL priming establishes persistent long-term production of serum IgM and IgG anti-FtL at natural antibody levels. IgM and IgG anti-FtL levels measured in sera from WT (BALB/c) and syngenic mutant (Rag1−/− and AID−/−) mice primed with FtL or injected with PBS, as indicated. Each dot represents a single mouse; n = 5 per group. Horizontal lines in “quartile box plots” indicate the 25th, median, and 75th percentiles. The dashed line shows background levels (Rag−/− and AID−/− sera). Values are expressed as microliter equivalents of a standard serum pool from 5-d FtL primed BALB/c mice (Fig. S1 shows FACS data).
As the persistent low-level serum anti-FtL production would predict, ELISPOT assays of spleen cells from long-term FtL-immunized animals reveal small numbers of plasma cells producing anti-FtL IgM (∼2,000 per spleen) or IgG (IgG1+IgG3) (∼300 per spleen) (Table S1). Of note, comparable analysis of BM from the same animals do not reveal either IgM or IgG anti-FtL plasma cells (Table S1), even though long-lived plasma cells originating from GC responses typically reside in BM (26, 27). Therefore, these anti-FtL antibody-secreting cells in spleen provide the sole source for the sustained low-level production of anti-FtL antibodies that we detect in serum from FtL-immunized animals.
Antigen-Induced Isotype Switching in B-1a Requires AID; T Cells and GCs Are Not Required.
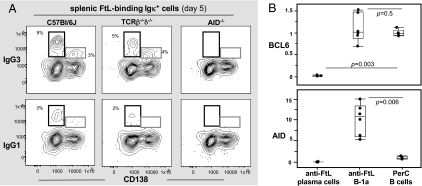
Primary IgM anti-FtL responses are comparable in WT, T-cell–deficient (TCRβ−/−δ−/−), and AID−/− mice (Fig. S2). As expected, IgG anti-FtL response fails in AID−/− mice (Fig. 2A). Consistently, AID expression is induced in anti-FtL B-1a cells (Fig. 2B). These findings demonstrate that FtL-induced isotype switching in B-1a requires AID. However, neither T-cell help nor GC formation is required for these responses. Thus, anti-FtL IgG responses proceed normally in TCRβ−/−δ−/− mice (Fig. 2A). GL7, a GC marker (27, 28), is not detected on the anti-FtL B-1a (CD138–). Furthermore, anti-FtL B-1a disappear from the spleen within 1 wk of immunization (19), clearly before the time when GC usually starts developing (27–30). Finally, B-cell lymphoma 6 (BCL6) up-regulation, a hallmark for GC B cells (26, 31, 32), is not detected on anti-FtL B-1a (Fig. 2B). Consistent with its down-regulation on plasma cells (33), BCL6 expression is turned off in anti-FtL plasma cells (CD138+) (Fig. 2B).
Fig. 2.
FtL-induced isotype switching in anti-FtL B-1a requires AID but occurs without T-cell help or GC support. (A) Live FtL-binding Igκ+ cells from spleen of C57BL6/J, TCRβ−/−δ−/−, and AID−/− syngenic mice 5 d after FtL immunization were gated to reveal CD138 and IgG expression. Boxes show IgG+ CD138− and IgG+CD138+ plasma cells. One of four experiments is shown. (B) AID and BCL6 expression in sorted anti-FtL B-1a (CD138–) and anti-FtL plasma cells (CD138+) from spleen of day 5 FtL-primed C57BL6/J mice. Data for each gene is shown as fold-change relative to expression level (dashed line) in PerC B cells from nonimmunized mice. Each dot represents data for 100 sorted cells of each subset, n = 6. One of three experiments is shown.
Anti-FtL Primary Responses Require Btk but Not IL-7.
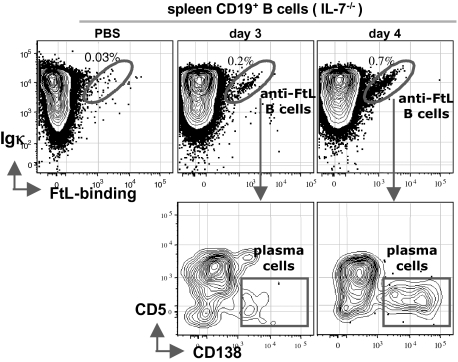
Although B-2 development fails entirely in IL-7−/− mice, substantial numbers of B-1a develop and are readily detectable in adult spleen and PerC (34, 35). Similar to WT mice, immunizing IL-7−/− mice with FtL induces the development of splenic anti-FtL B-1a (CD5+B220low) and anti-FtL plasma cells (CD5–B220–CD138+) (Fig. 3 and Fig. S3A). Isotype switching also occurs normally (Fig. S3B) and serum anti-FtL IgM levels rise after FtL priming (Fig. S3C). However, the overall anti-FtL titers are about four-times lower than that in WT mice (Fig. S3C), which likely reflects the smaller spleens and the consequently lower numbers of anti-FtL B-1a in IL-7−/− mice (Table S2). These data suggest that IL-7 is not (or minimally) required either for the development of anti-FtL B-1a or for their responsiveness to FtL immunization.
Fig. 3.
FtL immunization induces splenic anti-FtL B-1a responses in IL-7−/− mice. Live splenic CD19+ B cells from IL-7−/− mice injected with PBS or immunized with FtL for 3–4 d were gated to reveal FtL-binding Igκ+ B cells (“diagonal” in circled population, Upper), and further gated to distinguish anti-FtL plasma cells (CD138+CD5–) (boxed population, Lower) from CD138– cells, >95% of which are CD5+. (For serum responses, see Fig. S3C).
As expected, B-1a anti-FtL responses fail entirely in xid mice, which lack functional Btk and show lack of B-1a (21, 22). Thus, FtL immunization of xid mice does not result in expanded anti-FtL B-1a (<0.01% of splenic and PerC B cells) and does not induce production of serum anti-FtL. This response failure is consistent with our previous demonstration that FtL immunization of xid mice does not protect against subsequent lethal Ft LVS challenge (19).
Spleen, Not PerC, from Naïve Animals Contains Cells That Give Rise to Adoptive Primary Anti-FtL B-1a Responses.
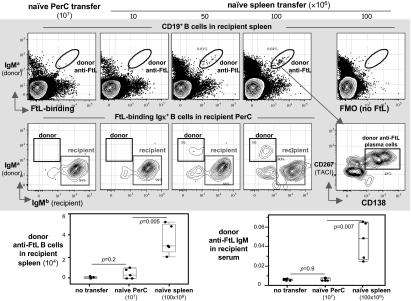
Intravenous transfers of spleen cells from naïve donors to naïve allotype-congenic recipients results in the appearance of donor anti-FtL B-1a in recipient spleen (and PerC) shortly after the recipients are challenged with FtL (Fig. 4). However, similar transfers of 107 PerC cells harvested from one to two naïve donors give rise to minimal numbers of donor anti-FtL B-1a (Fig. 4). Consistent with these findings, donor anti-FtL antibody titers rise significantly in the serum of FtL-challenged recipients transferred with naïve donor spleen but not naïve donor PerC (Fig. 4). Thus, although we have shown that FtL priming triggers the appearance of anti-FtL B-1a in the PerC and spleen at approximately the same time in animals (day 3) (19), only the spleen from naïve animals contains cells that can give rise to primary anti-FtL responses in adoptive recipients.
Fig. 4.
Naïve spleen, but not naïve PerC, produce anti-FtL primary responses to FtL challenge when transferred intravenously to naïve recipients. (Top) 107 PerC (from one to two naïve mice) or indicated number of spleen cells from naïve Igha mice were transferred intravenously to naïve congenic Ighb recipients immunized with FtL the next day. Donor and recipient anti-FtL responses were measured 5 d later. (Middle) Live B cells from indicated recipient spleen were gated to identify donor anti-FtL B-1a (IgMa+) (circled population, Top), which are then further gated to show donor anti-FtL plasma cells (CD267hiCD138+, boxed population, Rightmost Middle). CD267, transmembrane activator, and calcium signal-modulating cyclophilin ligand interactor (TACI) is a marker induced during plasma cell differentiation. The boundary for FtL binding is determined from the fluorescence-minus-one (FMO) (40) staining control in which fluorochrome-labeled FtL was omitted from the stain-set (Rightmost Top). Live PerC anti-FtL B-1a were gated to distinguish donor or recipient-derived cells (boxed populations, Middle). (Bottom) Numbers of donor (IgMa) anti-FtL B-1a in recipient spleen (Left); donor-derived (IgMa) anti-FtL in sera of recipients (Right). Each point represents data from an individual mouse (n = 5, per group). Values are expressed as microliter equivalents of a standard serum pool from 5-d FtL-primed Igha mice.
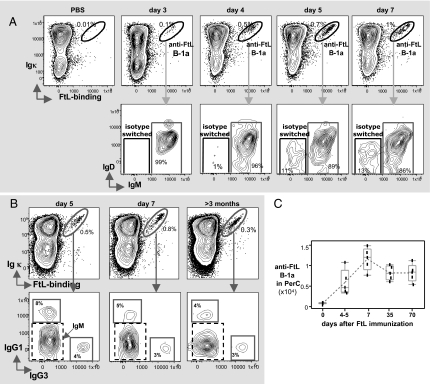
PerC, Not Spleen, Is the Long-Term Reservoir for Anti-FtL B-1a in FtL-Primed Animals.
Like anti-FtL B-1a in spleen, all of the anti-FtL B-1a in PerC express IgM at day 3 after FtL priming (Fig. 5A); by day 5, roughly 12% of anti-FtL B-1a in PerC have switched to IgG1 or IgG3 (Fig. 5 A and B). After 1 wk, however, the majority of anti-FtL B-1a disappear from the spleen (19), leaving the PerC as the long-term reservoir of these cells in primed animals (Fig. 5). Of note, although most of anti-FtL B-1a in the PerC have divided at least once (>90% BrdU+) at day 5, their cell division rate drops precipitously (i.e., about 20% are BrdU+ at day 7 but <5% are BrdU+ at day 15) (Fig. S4). Thus, after the initial FtL-induced expansion, anti-FtL B-1a cells exit the cell cycle and persist in the PerC as quiescent cells in the absence of further antigenic stimulation.
Fig. 5.
FtL priming induces the development of anti-FtL B-1a (IgM>>IgG) populations that persist long-term in the PerC. (A) Anti-FtL B-1a (tight diagonal, Upper) in gated live BALB/c PerC B at indicated days after FtL immunization. IgM or isotype-switched (IgM–) anti-FtL B-1a are shown in boxes (Lower). (B) Anti-FtL B-1a (circled population, Upper) gated from PerC B cells from FtL-immunized BALB/c for indicated days were further gated to reveal anti-FtL subsets expressing IgG1 or IgG3 (gray line boxed populations, Lower). The remaining cells (IgG1–IgG3–, dashed line boxed population) express IgM anti-FtL. (C) Numbers of PerC anti-FtL B-1a from BALB/c mice at indicated days after FtL immunization. Each point represents data from an individual mouse (n = 5, per group). Mean value for each group is connected by dashed line.
Overall, the frequency of anti-FtL B-1a that appear in the PerC rises from undetectable (<0.01%) before FtL priming to a persistent 3–5% of total PerC B cells (Fig. 5 B and C). These cells express the typical PerC B-1a phenotype (CD5+CD43+CD11b+B220low) (Fig. S5) and dominantly express IgM (Fig. 5B). Similar findings with FtL-primed IL-7−/− and TCRβ−/−δ−/− mice (Fig. S6) demonstrate that neither IL-7 nor T-cell support is required for the development or persistence of anti-FtL B-1a cells in PerC.
Importantly, despite their initial dividing and later quiescent persistence in PerC, anti-FtL B-1a cells do not differentiate to plasma cells at this location. Thus, ELISPOT does not reveal anti-FtL secreting cells in PerC from primed animals, even though such assays do reveal tiny numbers of anti-FtL plasma cells in spleen long after the primary response resolves (Table S1). Furthermore, PerC from FtL-immunized animals does not contain FACS-detectable anti-FtL plasma cells, defined as either CD138+ cells or cells containing intracellular Ig (Fig. S7).
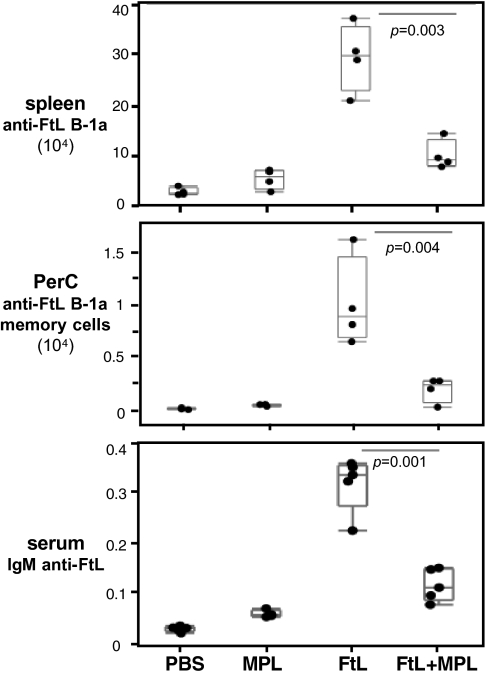
Priming with FtL in an Inflammatory Context Decreases, Rather than Augments, the Primary Anti-FtL Response.
Monophosphoryl lipid A (MPL) is well-known as a TLR4 agonist that readily induces inflammation (36, 37). It is commonly delivered with priming antigens as an adjuvant to enable/increase primary antibody responses (37, 39). Surprisingly, however, priming naïve animals with FtL plus MPL substantially dampens the anti-FtL primary responses and dramatically decreases the development of anti-FtL B-1a cells in the PerC (an index of memory cell generation; see companion article, ref. 4) (Fig. 6 and Fig. S8). Thus, adding this inflammation-inducing stimulus during FtL priming is counterproductive for anti-FtL primary responses.
Fig. 6.
MPL administered simultaneously with FtL priming dampens anti-FtL primary responses and inhibits development of FtL-specific B-1a memory in PerC. Absolute number of anti-FtL B-1a in spleen (Top) or in the PerC (Middle), and serum IgM anti-FtL antibodies levels (Bottom) in C57BL6/J mice at d 5 after PBS, MPL, FtL, or FtL plus MPL injection. Each dot represents data from an individual mouse (n = 4–5, per group). Values are expressed as microliter equivalents of a standard serum pool from 5-d FtL primed C57BL6/J mice.
Discussion
The FtL-induced robust primary responses demonstrated here introduce key roles for B-1a in protective immunity and the production of “natural” (i.e., innate) antibodies in serum. Our findings demonstrate that B-cell receptor stimulation with a glycolipid (FtL) isolated from Ft LVS specifically triggers splenic FtL-binding B-1a to proliferate and, in a small proportion (about 15%), to undergo AID-dependent class-switching. These antigen-triggered events, which occur in the absence of accompanying adjuvant, T-cell support, or GC influence, result in the establishment of anti-FtL B-1a memory cells that migrate rapidly to the PerC and persist there indefinitely as a largely quiescent population in the absence of further antigenic stimulation.
We have also shown that FtL priming induces brief but robust development of anti-FtL B-1a plasma cells (IgM>>IgG) in spleen, but not in BM. These plasma cells largely disappear with a week of priming, although a few remain detectable as functional anti-FtL–secreting cells for months thereafter (essentially indefinitely). Consistent with these findings, the initial primary anti-FtL B-1a response is readily detectable in serum for 1–2 wk after FtL priming but fades rapidly thereafter to characteristic natural antibody levels that persist indefinitely as such in serum.
The anti-FtL B-1a memory cells (IgM>>IgG) that migrate to the PerC persist there as essentially quiescent cells similar in phenotype to the bulk of the B-1a in PerC. They do, however, divide occasionally, apparently as needed to replenish their numbers and feed the tiny persistent population of splenic anti-FtL–secreting cells that in turn feeds the serum anti-FtL pool. Thus, by likely analogy, B-1a memory emerges as the source of much of what is commonly referred to as “natural antibody” in serum.
In an accompanying article (4), we show that the typically quiescent anti-FtL B-1a memory cells in the PerC divide extensively and express a unique set of activation-associated signatures in response to FtL rechallenge. However, the FtL rechallenge must be administered in an inflammatory context (e.g., stimulation with a TLR4 agonist) to induce the migration of the PerC-based memory cells to the spleen and differentiate there to anti-FtL secreting cells. Thus, our findings suggest that initial encounter with a pathogen-associated antigen (e.g., FtL) under noninflammatory conditions establishes long-term low-level B-1a production of natural antibodies sufficient to provide a preexisting defense against a low level of pathogen re-encounter. To back up this minimal defense, the initial antigen encounter also induces long-term FtL-specific B-1a memory capable of rapidly ramping up anti-FtL production whenever the antigen is re-encountered in association with TLR4-stimulated or similar inflammation.
Materials and Methods
The methods for FACS staining, ELISPOT, real-time quantitative RT-PCR, serum anti-FtL antibody analysis, and the methods used for the cell transfer studies are all described in a companion article (see companion article, ref. 4), in which the same materials and methods are used. All of the animal experiment protocols are approved by Stanford Animal Care Review Board.
Supplementary Material
Acknowledgments
We thank Guang Qin, Megan Phillips, Claudia Weber, and John Mantovani for excellent assistance; Dr. Matthias Wabl (University of California) for generously providing AID−/− mice; and Dr. Yueh-Hsiu Chien and Dr. Xun Zeng for great help with the Fluidigm quantitative RT-PCR assay. This study was supported by National Institutes of Health Grants R01-AI076434 (to Leonore A. Herzenberg) and U54-AI157168 (to S.N.V.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121631109/-/DCSupplemental.
References
- 1.Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749–2754. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 2.Herzenberg LA. B-1 cells: The lineage question revisited. Immunol Rev. 2000;175:9–22. [PubMed] [Google Scholar]
- 3.Baumgarth N. The double life of a B-1 cell: Self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, et al. Antigen-specific memory in B-1a and its relationship to natural immunity. Proc Natl Acad Sci USA. 2012;109:5388–5393. doi: 10.1073/pnas.1121627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayakawa K, et al. Ly-1 B cells: Functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci USA. 1984;81:2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 7.Baumgarth N, et al. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci USA. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumgarth N, Choi YS, Rothaeusler K, Yang Y, Herzenberg LA. B cell lineage contributions to antiviral host responses. Curr Top Microbiol Immunol. 2008;319:41–61. doi: 10.1007/978-3-540-73900-5_3. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Tung JW, Ghosn EE, Herzenberg LA, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci USA. 2007;104:4542–4546. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol. 2005;17:230–236. doi: 10.1016/j.coi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 15.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 16.Masmoudi H, Mota-Santos T, Huetz F, Coutinho A, Cazenave PA. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int Immunol. 1990;2:515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- 17.Förster I, Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987;17:521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- 18.Jeanes A. Immunochemical and related interactions with dextrans reviewed in terms of improved structural information. Mol Immunol. 1986;23:999–1028. doi: 10.1016/0161-5890(86)90131-8. [DOI] [PubMed] [Google Scholar]
- 19.Cole LE, et al. Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proc Natl Acad Sci USA. 2009;106:4343–4348. doi: 10.1073/pnas.0813411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole LE, et al. Immunologic consequences of Francisella tularensis live vaccine strain infection: Role of the innate immune response in infection and immunity. J Immunol. 2006;176:6888–6899. doi: 10.4049/jimmunol.176.11.6888. [DOI] [PubMed] [Google Scholar]
- 21.Khan WN, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 22.Khan WN, Sideras P, Rosen FS, Alt FW. The role of Bruton's tyrosine kinase in B-cell development and function in mice and man. Ann N Y Acad Sci. 1995;764:27–38. doi: 10.1111/j.1749-6632.1995.tb55802.x. [DOI] [PubMed] [Google Scholar]
- 23.Knoops L, Louahed J, Renauld JC. IL-9-induced expansion of B-1b cells restores numbers but not function of B-1 lymphocytes in xid mice. J Immunol. 2004;172:6101–6106. doi: 10.4049/jimmunol.172.10.6101. [DOI] [PubMed] [Google Scholar]
- 24.Alugupalli KR, et al. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Alugupalli KR. A distinct role for B1b lymphocytes in T cell-independent immunity. Curr Top Microbiol Immunol. 2008;319:105–130. doi: 10.1007/978-3-540-73900-5_5. [DOI] [PubMed] [Google Scholar]
- 26.Tarlinton D. B-cell memory: Are subsets necessary? Nat Rev Immunol. 2006;6:785–790. doi: 10.1038/nri1938. [DOI] [PubMed] [Google Scholar]
- 27.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro-Shelef M, et al. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 29.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 30.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 31.Fukuda T, et al. Disruption of the Bcl6 gene results in an impaired germinal center formation. J Exp Med. 1997;186:439–448. doi: 10.1084/jem.186.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tangye SG, Tarlinton DM. Memory B cells: Effectors of long-lived immune responses. Eur J Immunol. 2009;39:2065–2075. doi: 10.1002/eji.200939531. [DOI] [PubMed] [Google Scholar]
- 33.Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol. 2003;21:205–230. doi: 10.1146/annurev.immunol.21.120601.141138. [DOI] [PubMed] [Google Scholar]
- 34.von Freeden-Jeffry U, et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/-) mice. J Exp Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alderson MR, McGowan P, Baldridge JR, Probst P. TLR4 agonists as immunomodulatory agents. J Endotoxin Res. 2006;12:313–319. doi: 10.1179/096805106X118753. [DOI] [PubMed] [Google Scholar]
- 37.Evans JT, et al. Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi.529. Expert Rev Vaccines. 2003;2:219–229. doi: 10.1586/14760584.2.2.219. [DOI] [PubMed] [Google Scholar]
- 38.Baldridge JR, et al. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther. 2004;4:1129–1138. doi: 10.1517/14712598.4.7.1129. [DOI] [PubMed] [Google Scholar]
- 39.Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19:103–107. doi: 10.1006/meth.1999.0834. [DOI] [PubMed] [Google Scholar]
- 40.Herzenberg LA, Tung J, Moore WA, Herzenberg LA, Parks DR. Interpreting flow cytometry data: A guide for the perplexed. Nat Immunol. 2006;7:681–685. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.