Abstract
In the retinal binding pocket of rhodopsin, a Schiff base links the retinal ligand covalently to the Lys296 side chain. Light transforms the inverse agonist 11-cis-retinal into the agonist all-trans-retinal, leading to the active Meta II state. Crystal structures of Meta II and the active conformation of the opsin apoprotein revealed two openings of the 7-transmembrane (TM) bundle towards the hydrophobic core of the membrane, one between TM1/TM7 and one between TM5/TM6, respectively. Computational analysis revealed a putative ligand channel connecting the openings and traversing the binding pocket. Identified constrictions within the channel motivated this study of 35 rhodopsin mutants in which single amino acids lining the channel were replaced. 11-cis-retinal uptake and all-trans-retinal release were measured using UV/visible and fluorescence spectroscopy. Most mutations slow or accelerate both uptake and release, often with opposite effects. Mutations closer to the Lys296 active site show larger effects. The nucleophile hydroxylamine accelerates retinal release 80 times but the action profile of the mutants remains very similar. The data show that the mutations do not probe local channel permeability but rather affect global protein dynamics, with the focal point in the ligand pocket. We propose a model for retinal/receptor interaction in which the active receptor conformation sets the open state of the channel for 11-cis-retinal and all-trans-retinal, with positioning of the ligand at the active site as the kinetic bottleneck. Although other G protein-coupled receptors lack the covalent link to the protein, the access of ligands to their binding pocket may follow similar schemes.
Keywords: G protein-coupled-receptor, regeneration, signal transduction
The photoreceptor rhodopsin is a prototypical member of the superfamily of seven transmembrane (7 TM) helix or G protein-coupled receptors (GPCRs). Rhodopsin consists of the apoprotein opsin and the covalently bound chromophoric ligand 11-cis-retinal, which acts as a powerful inverse agonist and holds the receptor in its inactive conformation. Absorption of a photon isomerizes the chromophore to the agonist all-trans-retinal which in turn triggers conformational changes of the protein leading to the active, G protein-binding form metarhodopsin II (Meta II). In rod cells Meta II decays within minutes by hydrolysis of the Schiff base and release of all-trans-retinal. The regeneration of the rhodopsin dark state by uptake of new 11-cis-retinal effectively suppresses the basal activity of opsin and primes it at the same time for photoactivation (1, 2).
In the rhodopsin dark state, 11-cis-retinal is buried in its binding pocket in the core of the 7 TM bundle. The side chain of Lys296 in TM7 protrudes into the pocket and provides the active site for the protonated Schiff base linkage between ligand and protein. The protonated Schiff base is stabilized by a salt-bridge with its counterion, Glu113, and by residues in the second extracellular loop, which is folded deeply into the core of the protein. The closed helix bundle effectively shields the Schiff base from bulk solvent, making Schiff base hydrolysis in the dark state exceedingly slow (3). In the Meta II state, the Schiff base is still intact but deprotonated, leading to a strong shift of the absorption maximum to 380 nm. Meta II forms in milliseconds and remains in a pH- and temperature dependent equilibrium with its predecessor Meta I (4). In the sequence of isochromic Meta II species, the late subform Meta IIb (which is the dominant form under the conditions of this study) has a more open conformation with a reorganized helix bundle and an outward tilt of TM6 (1, 5), allowing the G protein transducin (Gt) to bind. In Meta II, bulk water has access to the Schiff base (6), which then hydrolyzes within minutes. The remaining opsin apoprotein is orders of magnitude less active towards Gt than Meta II (7). However, at low pH an active form of opsin (Ops*) could be spectroscopically identified, which is in equilibrium with the inactive opsin (Ops) form (8). It is likely that, analogous to Meta I/ Meta II (9), the Ops/ Ops* equilibrium is in detergent solution further shifted towards Ops*. The opsin conformation in crystals obtained at low pH was identified with Ops*, because it shows the helix tilt and cocrystallizes with the C-terminal fragment of the Gtα-subunit (CTα peptide), similar to the all-trans-retinal bound Meta II-like forms (10, 11). The CTα peptide mimics the key binding site of Gtα-subunit (12, 13).
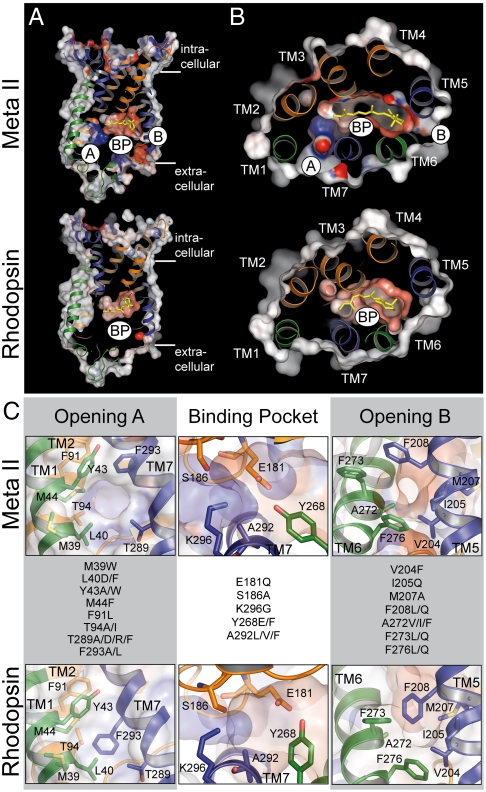
In the crystal structures of opsin and Meta II, the reorganized 7 TM bundle displays not only the cytoplasmic crevice-like binding site for CTα. The reorganization of the transmembrane bundle also provides two openings into the hydrophobic membrane layer, namely opening A between TM1 and TM7, and opening B between TM5 and TM6, respectively (Fig. 1) (10, 11, 14, 15). Using computational methods, a continuous retinal channel through the protein of ca. 70 Å length was identified that connects these two nonpolar openings and comprises in its central part the more polar retinal binding pocket (16). Besides this ligand channel, yet another channel structure has been suggested by recent radiolysis studies (17). This channel connects the open cytoplasmic crevice with the ligand binding pocket and is thought to allow the access of water to the retinal Schiff base site (6). Recent work suggests that this solvent channel is so narrow that water and hydroxylamine but not its alkylated derivatives can pass it (18). Our study starts from these two putative channel structures, the retinal channel which traverses the protein in parallel to the membrane plane and the water channel which runs perpendicular to it. The results strongly support that these two channel structures indeed exist and are functionally important.
Fig. 1.
Comparison of the inactive rhodopsin and active Meta II crystal structures and location of the residues mutated in this study. (A) longitudinal and (B) coplanar (cytoplasmic view) cuts through Meta II (top; PDB accession 3PXO) and rhodopsin (bottom; PDB accession 1U19). In both boxes opening A (A) between TM1 and TM7, the retinal binding pocket (BP), and opening B (B) between TM 5 and TM 6 are assigned. Electrostatic surface potentials are contoured at ± 30 kT/e with negatively and positively charged surface areas in red and blue, respectively. (C) Close-ups of openings A and B (side views) and of the retinal binding pocket (top view) with the amino acid residues mutated in this study as indicated.
The geometry of the retinal channel was mapped using skeleton search and flexible docking analysis (16). In agreement with molecular dynamics simulations (19), constrictions for retinal passage were identified which include bulky residues at openings A and B, and Lys296 within the retinal pocket. The proposed constrictions have guided the selection of amino acids for the present study, in which we have attempted to analyze the role of individual amino acids in retinal uptake and release. Residues located at or close to these constrictions were replaced. The salient result is that the specific local effects on the interactions between retinal and opsin during retinal passage are surprisingly small. The data rather reflect an impact on protein dynamics that affects the switch between active and inactive conformations of the receptor and thus between open and closed states of the channel.
Results
Characterization of the Mutants.
In this study, 20 residues lining the channel were individually replaced with side chains of different bulkiness and polarity (Fig. 1). All mutants were constructed on the background of the thermally stable rhodopsin mutant N2C/D282C (control), to avoid the known rapid denaturation of opsin in detergent solution (20). Consistent with a previous FTIR study (21), comparison of the wild type and the thermally stable control pigment showed that the N2C/D282C background has no significant effect on the light-induced structural changes under the conditions of the experiments (Fig. S1). All mutants formed rhodopsin-like pigments with λmax between 475 and 510 nm (Fig. S2A). Most purified mutants exhibited spectral ratios (A280/Aλmax) below 2.2, with the exception of M44F, Y268E, and M207A (Fig. S2B). All mutants formed a Meta II-like photointermediate upon illumination. However, mutants Y43A, Y268E/F, A272V/I/F, M207A, and V204F showed an additional Meta I—like species with λmax ∼ 480 nm immediately after illumination (Figs. S3 and S4) as previously reported for Y268F (3). We selected eight mutants for FTIR spectroscopy. These mutants displayed the overall normal difference spectrum of light-activation (Fig. S1). 15 mutants were tested for G protein activation and showed only a small effect on the light-induced activation after regeneration with 11-cis-retinal (Fig. S5A), while the basal opsin activity was more distinctly affected (Fig. S5B). An example is K296G, which showed the reported constitutive opsin activity (22, 23).
Effect of Mutations on Uptake of 11-cis-Retinal into Opsin.
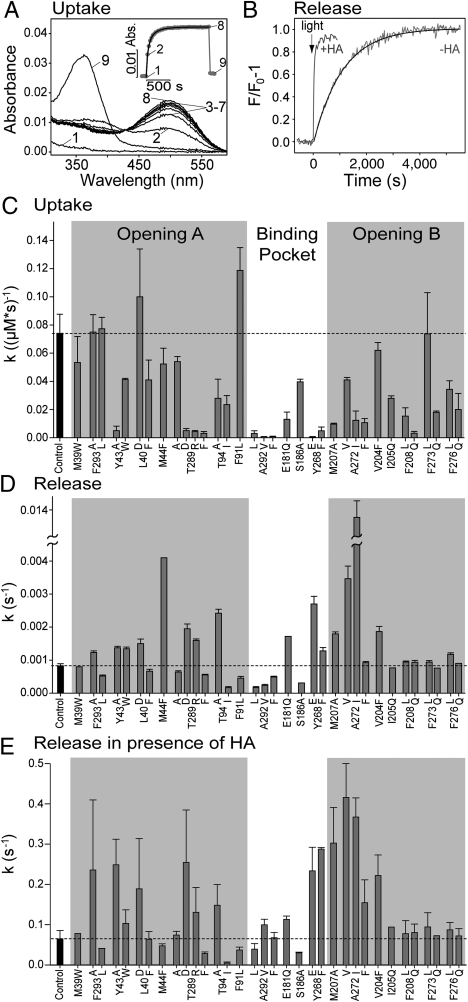
Uptake of 11-cis-retinal by opsin was measured by the increase of the 500 nm (or respective λmax) absorption of the rhodopsin dark state, using purified opsin mutants solubilized in 0.03% n-dodecyl-β-D-maltopyranoside (DDM) (Fig. 2A). Under the conditions of the experiment (0.5 μM opsin and 1 μM 11-cis-retinal) the regeneration of the mutants followed a bimolecular time course. For the control (N2C/D282C background) a rate constant of 0.074 ± 0.014 μM-1 s-1 was obtained, which is in good agreement with previous results obtained under similar conditions (24). The respective rate constants for the regeneration of the mutants are summarized in Fig. 2C. There is only one mutation (F91L, located at a constriction close to K296) that slightly, but significantly, accelerated regeneration, while the majority of the mutants slowed down regeneration. In opening A, the effect is most pronounced for mutations of Y43, T289, or T94. The slowed uptake of 11-cis-retinal by the night blindness mutant T94I was not observed in a previous study (24). In opening B, most mutations slowed retinal uptake, in particular mutations of M207, A272, I205, F208, F273, and F276. All mutations within the retinal binding pocket, namely of A292, E181, S186, and Y268, significantly slowed regeneration. Interestingly, all mutants of residue A292 used in this study (A292L/V/F) dramatically slowed retinal uptake, while the night blindness mutant A292E was reported to rapidly regenerate under similar conditions (24).
Fig. 2.
Kinetics of retinal uptake and release in rhodopsin mutants. (A) Regeneration of N2C/D282C opsin (control) with 11-cis-retinal monitored by UV/visible spectroscopy: spectrum of 0.5 μM opsin (1), 30 s after addition of 1 μM 11-cis-retinal (2) and spectra recorded subsequently every 60 s (3–8). Spectrum 9 was taken after addition of 25 mM hydroxylamine and illumination of the sample. The inset shows a plot of the absorbance change at 500 nm as a function of time (numbers identify respective spectra). The solid line is a fit to the data points with a bimolecular rate equation (see SI Text: SI Materials and Methods). (B) Fluorescence spectroscopic analysis of light-induced all-trans-retinal release from N2C/D282C rhodopsin (control) in the absence (−HA) and in the presence of 25 mM hydroxylamine (+HA). Reactions were initiated by 15 s illumination with orange light. Reaction rates were determined by a monoexponential fit to the data points (−HA, solid line) or by normalizing the initial slope of the fluorescence change to the maximum amplitude (+HA). (C) Regeneration rates of opsin mutants measured as illustrated in (A). Light-induced all-trans-retinal release rates of rhodopsin mutants measured in the absence (D) or in the presence of HA (E) as illustrated in (B). The dashed lines in (C–E) refer to the values obtained for N2C/D282C rhodopsin (control) and error bars display SD of five (control) or three (all other mutants) independent measurements. All measurements were performed at pH 6.0, 20 °C in 0.03% DDM.
Effect of Mutations on Release of All-trans-Retinal from Light-Activated Rhodopsin.
The release of all-trans-retinal from light-activated rhodopsin was monitored by the accompanying increase in intrinsic opsin Trp fluorescence (25). As expected, all mutants displayed a monoexponential decay reaction. The half time of retinal release was 13.9 min for the N2C/D282C control (Fig. 2B), which is comparable with reported values (3, 25). The effect of the mutations on light-induced all-trans-retinal release is summarized in Fig. 2D. Replacements of residues located at the rims of the two openings (namely M39, Y43, F293 in opening A, and I205, F208, F273, and F276 in opening B) had only a minor if any effect on the release rate. In contrast, most substitutions of residues located in the retinal binding site or within the adjacent inner part of the openings considerably affected the reaction rate. Mutants M44F, Y268E, A272V and particularly A272I exhibited a significantly faster release rate than the control, while the mutations of A292 and S186 (near K296) slowed the release significantly. In some cases (e.g., T289 and T94) substitution of residues resulted in either accelerated (T289D/R and T94A) or slowed (T289A/F and T94I) retinal release. The effects seen with mutants T94I, Y268F, E181Q, and S186A are in agreement with a previous report (3).
In order to discriminate between the effect of the mutations on Schiff base hydrolysis and on subsequent release of retinal from its binding site, we employed hydroxylamine, which has long been used to accelerate cleavage of the retinal Schiff base. In the presence of 25 mM hydroxylamine the light induced retinal release of the control was approximately 80 times accelerated (Fig. 2B). With the exception of only two of the mutants analyzed (M44F and A292V) the relative effect of the different mutations remained similar, although the overall reaction was much faster (Fig. 2E). For example, replacements of Thr94 by Ile or Ala yielded the same opposite effect.
The Effect of K296G Mutation on Uptake and Release.
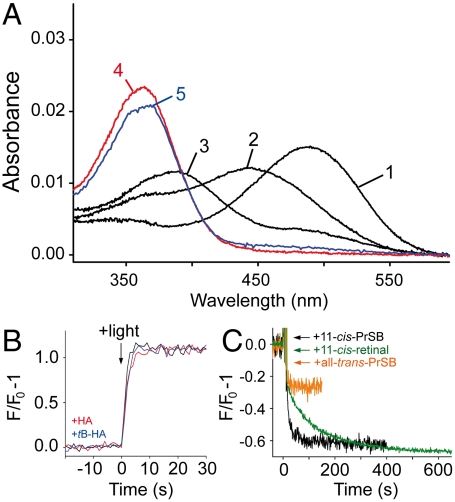
We also studied mutant K296G, in which the active site lysine is removed. It is known that K296G forms a stable rhodopsin-like pigment with 11-cis-PrSB, a retinoid formed by a Schiff base between n-propylamine and 11-cis-retinal (23, 26). Illumination of K296G regenerated with 11-cis-PrSB resulted in a 100 times faster fluorescence change as compared to the 11-cis-retinal regenerated control pigment (Fig. 3B). To confirm the interpretation of this phenomenon as a fast release of the ligand (22) we applied an additional test with alkylated hydroxylamine (18). UV-visible spectra of K296G/11-cis-PrSB taken immediately after illumination showed an absorption band at 440 nm which corresponds to protonated all-trans-PrSB (Fig. 3A). The band decayed slowly to a 380 nm product, consistent with a slow formation of free retinal. Illumination of K296G/11-cis-PrSB in the presence of hydroxylamine or o-tert-butylhydroxylamine (tB-HA) resulted in a very rapid formation of retinal oxime (within the 30 s dead time of the instrument). Because tB-HA cannot enter the binding pocket (18), this finding provides the evidence that Schiff base hydrolysis indeed occurs after a rapid release of all-trans-PrSB upon light-activation of K296G.
Fig. 3.
Characterization of mutant K296G. (A) UV/visible spectrum of K296G opsin regenerated with 11-cis-PrSB in the dark (1), 30 s (2) and 160 min (3) after illumination of the sample. Spectra (4) and (5) were recorded 30 s after illumination of samples containing 25 mM hydroxylamine or tB-HA, respectively. (B) Light-induced retinal release from K296G monitored by fluorescence spectroscopy in the absence (black trace), and in the presence of 25 mM hydroxylamine (red trace) or tB-HA (blue trace), respectively. (C) Fluorescence change induced by addition of 1 μM 11-cis-PrSB (black trace) or all-trans-PrSB (orange trace) to 0.5 μM K296G opsin. Addition of 1 μM 11-cis-retinal to control opsin (green trace). All measurements were performed at pH 6.0, 10 °C in 0.03% DDM.
In contrast to regeneration of the control pigment with 11-cis-retinal, regeneration of K296G opsin with 11-cis-PrSB was too fast to be monitored by UV/visible spectroscopy. Therefore retinal uptake at 10 °C was analyzed using a fluorescence assay (27). With control opsin, addition of 11-cis-retinal or all-trans-retinal resulted in a rapid initial fluorescence decrease. Only with 11-cis-retinal, the initial jump was followed by a slower component that likely reflects retinal uptake into the binding pocket. Consistently, the second-order rate constant of the slow component (k = 0.014 μM-1 s-1) matches that of regeneration monitored by UV/visible spectroscopy. Addition of 11-cis-PrSB to K296G opsin also resulted in a biphasic fluorescence decrease. Analysis of the slow component (k = 0.375 μM-1 s-1) indicates that in K296G the regeneration proceeds about 30 times faster than in the control. The fast uptake and release of the retinal Schiff base was also confirmed by the finding that the light induced G-protein activity is rapidly quenched in the presence of excess 11-cis-PrSB (Fig. S5).
Discussion
The controlled uptake and release of the ligand is of pivotal importance for the functioning of receptor proteins. For the GPCR rhodopsin, a channel has been identified that connects the retinal ligand binding pocket with the hydrophobic environment of the membrane protein (16). This retinal channel is open in active Meta II and Ops* apoprotein conformations (10, 11, 14, 15) but closed in the 11-cis-retinal bound rhodopsin dark state (28). For the inactive Ops conformation, which prevails at neutral pH and in the native membrane (8), the structure could so far not be determined. Available evidence argues for a closed, more dark state-like, conformation in Ops (see below).
In the present study, it was investigated how retinal passage is affected by replacements of amino acids along the retinal channel. We find pronounced effects of the mutations on both the uptake of 11-cis-retinal during regeneration of the light sensitive dark state and the release of all-trans-retinal during decay of the active Meta II state. The question arises what factors are responsible for the site-dependent slowing or acceleration of rhodopsin regeneration and Meta II decay.
Amino Acid Residue Replacements have Nonlocal Effects.
It would be an obvious assumption that the mutations act as local probes of retinal passage. Such an explanation may apply in the case of T289F, which shows a strong slowing effect on uptake but much less on release. The mutation T289A has a much smaller effect. In agreement with previous assignments of the opening A as an uptake gate (16, 19), this would be consistent with a mechanism in which the phenyl side group in T289F provides a hindrance for the passage of 11-cis-retinal during uptake. However, local probing fails to explain the data in the majority of cases. For example, all the replacements of the Phe residues near opening B (F208, F273, and F276) also slow the uptake, while mutants of F293, the residue which shows the largest change of conformation between the rhodopsin and Ops* structures (14), do not show any significant effect on uptake or release. Moreover, most other replacements have an effect on both uptake and release, and in several cases the uptake is slowed down and the release accelerated. This finding suggests first of all that uptake and release do not proceed through the same opening. Although these results argue for an unidirectional mechanism of retinal passage (16, 27), a firm assignment of openings A and B to retinal uptake and release paths, respectively, is not yet possible.
Taken together, the data show that the effect of the mutations is not restricted to the mutated site. Although a pathway that mediates the nonlocal effect of the mutations could not be identified in the present study, residues along the channel may belong to an interacting network, in which replacements have a long-ranging effect. Because the effect of the alterations is the greater the nearer to the binding pocket, the focal point of such a network would reside in the binding pocket itself.
A Solvent Channel May Be Coupled to the Retinal Channel.
The notion that properties of the binding pocket itself determine the rate of retinal passage, fits well to another line of evidence, which comes from the data obtained with active site mutant K296G and with hydroxylamine. Hydroxylamine replaces water in the Schiff base cleavage reaction. Previous results have shown that Meta II decay is accelerated by hydroxylamine according to a bimolecular reaction scheme (29) and with a hyperbolic saturation curve (18). The reaction is up to 300 times accelerated with hydroxylamine (18), demonstrating that the release of the retinaloxime formed is fast, as soon as the kinetic barrier of Schiff base cleavage has been overcome. Consistently, when K296 is mutated to glycine and the normal retinal is replaced with n-propyl-retinylidene (PrSB), the release (which in this case does not require Schiff base hydrolysis) becomes 400 times faster (22); i.e., similar to the wild type with saturating hydroxylamine. These results further support the notion that under physiological conditions hydrolysis of the retinal Schiff base limits the rate of retinal release (30).
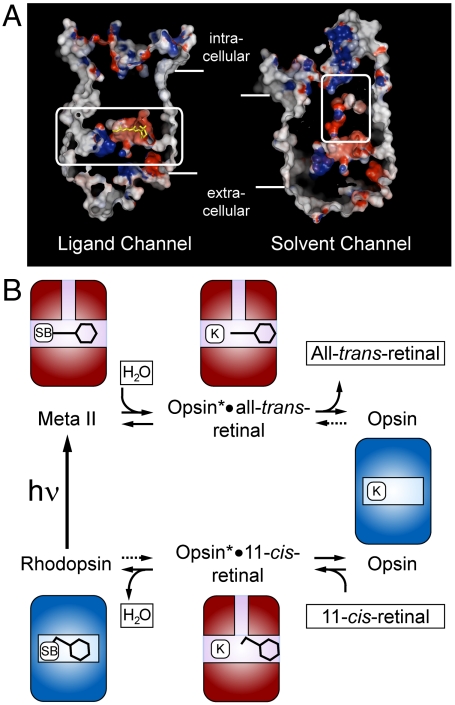
With regard to the uptake of the water, molecular dynamics simulations have suggested that it occurs early on the path towards Meta I formation and that the hydration change extends into the retinal pocket (31). Although the formation of Meta II is accompanied by a net loss of water (32, 33), it could be shown that bulk water (or hydroxylamine) enters the helical bundle from the cytoplasmic side (6). A solvent channel, which connects the cytoplasmic crevice of Ops* with the retinal binding pocket, was suggested to mediate the access of the nucleophiles (Fig. 4) (6, 17, 18). We speculate that this solvent channel is functionally coupled to the retinal channel, which may explain why even distant mutational alterations can affect the rate of Schiff base hydrolysis and thus retinal release. In the defined reaction environment of the hydrolysis reaction, any disturbance of the positioning of the Schiff base reactants, retinal and Lys296, must have an effect. Disturbances may be transmitted from the locus of a mutation to the Schiff base environment through the protein structure or through the ligand itself. The key observation in this regard is that, independent of the nucleophile (water or hydroxylamine), the action profile of the different mutations remains almost the same, although the overall reaction is much faster with hydroxylamine (Fig. 2). This finding indicates that the initial bimolecular step, which leads to the formation of a carbinolamine intermediate, is the one that is affected by the mutations (Fig. S6). The two partners in the bimolecular reaction are the Schiff base and the nucleophile. With hydroxylamine, the efficiency of the nucleophilic attack is increased, while with the mutational alterations of protein structure the Schiff base environment as the target of the attack is influenced. Eventually, the low steady state concentration of the carbinolamine formed determines the overall rate of Meta II decay.
Fig. 4.
Model of opening and closing of the retinal channel and its relation to the activation/deactivation cycle of rhodopsin. (A) Putative retinal (left) and solvent channel (right) found in the crystal structure of Meta II (PDB accession 3PXO). (B) The activation/deactivation cycle of rhodopsin. Both pathways, release of all-trans-retinal (decay of Meta II, top) and uptake of 11-cis-retinal (regeneration of rhodopsin, below) are based on switches between the Ops and Ops* conformations of the apoprotein. The ligands 11-cis- or all-trans-retinal are passing through the horizontal retinal channel and water enters or leaves through the vertically running solvent channel. The dark state rhodopsin and the photoactivated Meta II state appear as ligand-stabilized forms of Ops and Ops* [Schiff base (SB) bound Ops-11-cis-retinal and Ops*-all-trans-retinal, respectively]. Both pathways involve the noncovalent adducts, Ops*•11-cis-retinal and Ops*•all-trans-retinal, in which the Lys296 (K) is free. Light absorption (hν) closes the cycle by forming Meta II from rhodopsin. The uptake of 11-cis-retinal induces or selects the open Ops* conformation.
For a given residue, the resulting effect of different replacements can be quite complex. An example is A272, which is located at the β-ionone side of the retinal in the binding pocket. Here the replacement with the slightly bigger Val leads to faster release, the even bigger Ile shows a dramatic (14-fold) acceleration, while the biggest and rigid phenyl side group does not have any significant effect. Uptake however is slowed down, which is the more expressed the bigger the side group. Such complexity is expected in view of the quite different requirements for positioning the residues and reactants for forming or hydrolyzing the Schiff base.
Uptake of Reverse Agonist Requires the Active Receptor Conformation.
In the wild type, the regeneration is slow but accelerates with more 11-cis-retinal, which was explained by binding of the retinal in a preequilibrium, followed by a quasi-irreversible transition into the rhodopsin dark state (34, 35). The simplest interpretation of this mechanism would be that the channel is always open in opsin (i.e., in Ops and Ops*) and the preequilibrium is determined by the partitioning of the retinal between the pocket and the bulk. However, this model is at odds with studies on intact functioning rods, which showed that addition of exogenous 11-cis-retinal leads to a transient activation of the phototransduction cascade (36). The authors proposed that 11-cis-retinal interacts noncovalently with free opsin and keeps it active. We can thus conclude that the uptake of 11-cis-retinal for regeneration requires the active Ops* conformation. We assume that the entire channel is in equilibrium between two conformations and assign Ops* and Ops to the open and closed states, respectively. The question whether 11-cis-retinal solely selects the preexisting fraction of Ops* [ca. 1/1,000 of opsin under physiological conditions (8)] or if it also generates the Ops* conformation by induced fit, cannot be answered with certainty. We do know, however, that increasing the amount of Ops* by low pH (37) or stabilizing CTα peptide (Fig. S7), does not accelerate the regeneration, arguing for a contribution of the induced fit branch to the process (38).
A Comprehensive Scheme for the Uptake and Release of the Retinal Ligand.
The discussion has shown that opening and closing of the retinal channel involves switches between the Ops and Ops* states of the receptor apoprotein and that the inactive and active states of the receptor generally correlate with closed and open retinal channel conformations. Indeed, inspection of the helix bundle structure immediately suggests that the repositioning of TM5 and TM6 in the active state enables the side chain rotations which open the retinal channel (Fig. 1). Once the channel is open in Ops*, the rate at which the ligand can enter or leave its binding pocket depends on the rate of Schiff base formation and hydrolysis, respectively, rather than on local properties of the openings. To propose a comprehensive scheme for the observations, we introduce the Ops and Ops* conformations of the apoprotein and their 11-cis-retinal (rhodopsin dark state) and all-trans-retinal (Meta II) stabilized forms. The role of the retinal channel in the pathways in Fig. 4 is to control the formation of the two adducts, Ops*•11-cis-retinal and Ops*•all-trans-retinal (and of the carbinolamines; Fig. S6) in a controlled way. Presumably, in both adducts, the retinal is not yet or no longer in its final position in the binding pocket. Both pathways are solely based on switches between the Ops and Ops* conformations and their ligand stabilized forms, rhodopsin and Meta II, respectively. The uptake goes through a sequence of two such switches, namely Ops/Ops* and Ops*/Ops-11-cis-retinal, while the release has just one switch, namely Ops*-all-trans-retinal/Ops. Light absorption closes the cycle by forming Meta II (Ops*-all-trans-retinal) from rhodopsin (Ops-11-cis-retinal). Under the conditions, both isomers, 11-cis and all-trans-retinal can enter the channel (10), but only 11-cis-retinal can trigger the ”mouse-trap” in which the protonated Schiff base snaps into the strong salt bridge with its counterion and forces the 7 TM bundle to refold into the rhodopsin dark state.
Conclusion
We have found that both the uptake and release of the retinal ligand are affected by single mutational replacements of residues along the retinal channel. The data suggest that the rate at which the ligand reaches or leaves Lys296 in the retinal binding pocket is not determined by the ease of passage through constrictions in the channel. The rate rather depends on the positioning of the retinal ligand in its pocket and the accessibility of bulk water during hydrolysis or formation of the Schiff base link to the apoprotein. The binding pocket and the constriction sites within the channel are the points at which protein-ligand interaction is most sensitive to alterations by amino acid replacements (16, 19). The effect of single mutations may be transmitted via interacting residues of the protein and/or via the long polyene chain of the ligand itself. Switches between the open and closed states of the channel correlate with transitions between the active and inactive states of the apoprotein, Ops* and Ops. A paradoxical result is that the inverse agonist 11-cis-retinal uses the active Ops* conformation of the receptor to enter the protein. Once positioned in the binding pocket, the ionic lock between the Schiff base proton and its glutamic acid counterion forces the protein into the inactive (but light-sensitive) Ops-11-cis-retinal dark state. After light-induced formation of Meta II, the photolyzed agonist all-trans-retinal leaves the protein through the open Ops* conformation, which then returns to the Ops conformation. Although other GPCRs do not bind their ligand covalently, the uptake of ligands and their positioning in the binding pocket may obey similar rules as described here for rhodopsin (39).
Materials and Methods
Materials.
All materials and purified anti-rhodopsin monoclonal antibody rho-1D4 were as described (40). 11-cis-PrSB and all-trans-PrSB, the Schiff base compounds of the respective retinal with n-propylamine, were prepared in ethanol as described (26). All measurements were performed in 10 mM bis-tris-propane (pH 6.0) and 0.03% (wt/vol) DDM.
Expression and Purification of Rhodopsin Mutants.
A modified synthetic opsin gene cloned into an eucaryotic expression vector was used to generate the opsin mutants with a N2C/D282C background using standard cloning techniques (see ref. 40 and references therein). The N2C/D282C mutant is a thermostable version of the rhodopsin wild type (20). The opsin genes were transiently expressed in COS-1 cells and in part reconstituted with 11-cis-retinal. The resulting opsin or rhodopsin mutants were purified by immunoaffinity adsorption essentially as described (40).
UV/Visible Spectroscopy.
Absorption spectra were acquired with a Cary 50 spectrophotometer (Varian) with a resolution of 2 nm in a 90 μL cuvette. Samples were illuminated (15 s) with a 150-W fiber-optic light source equipped with a long-pass filter (GG 495; Schott) and a heat protection filter. Rhodopsin concentration was determined using ε500 = 40,600 M-1 cm-1 (41). Concentration of opsin mutants was determined using ε280 = 81,200 M-1 cm-1 (42).
Fluorescence Spectroscopy.
Decay of Meta II was measured essentially as described (43) using a SPEX Fluorolog 2 spectrofluorometer equipped with a 450 W xenon arc lamp. Protein fluorescence emission at 330 nm (excitation at 295 nm) was monitored in 2 s pulses at 2 s or 30 s intervals with the excitation shutter closed between acquisition pulses to minimize exposure to the measuring beam. Excitation and emission slit widths were 0.1 and 4 nm, respectively. Reactions were triggered with orange light using the same equipment as for UV/visible spectroscopy.
Supplementary Material
Acknowledgments.
We thank Alexander Rose for helpful discussions, and Brian Bauer and Ingrid Semjonow for technical assistance. This work was supported by Sfb 740 grants from the Deutsche Forschungsgemeinschaft (to P.W.H., M.H. and K.P.H.), an European Research Council Advanced Grant (to K.P.H.) and the Canada Excellence Research Chairs program (to O.P.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117268109/-/DCSupplemental.
References
- 1.Hofmann KP, et al. A G protein-coupled receptor at work: the rhodopsin model. Trends Biochem Sci. 2009;34:540–552. doi: 10.1016/j.tibs.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Janz JM, Farrens DL. Role of the retinal hydrogen bond network in rhodopsin Schiff base stability and hydrolysis. J Biol Chem. 2004;279:55886–55894. doi: 10.1074/jbc.M408766200. [DOI] [PubMed] [Google Scholar]
- 4.Parkes JH, Liebman PA. Temperature and pH dependence of the metarhodopsin I-metarhodopsin II kinetics and equilibria in bovine rod disk membrane suspensions. Biochemistry. 1984;23:5054–5061. doi: 10.1021/bi00316a035. [DOI] [PubMed] [Google Scholar]
- 5.Altenbach C, Kusnetzow AK, Ernst OP, Hofmann KP, Hubbell WL. High-resolution distance mapping in rhodopsin reveals the pattern of helix movement due to activation. Proc Natl Acad Sci USA. 2008;105:7439–7444. doi: 10.1073/pnas.0802515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jastrzebska B, Palczewski K, Golczak M. Role of bulk water in hydrolysis of the rhodopsin chromophore. J Biol Chem. 2011;286:18930–18937. doi: 10.1074/jbc.M111.234583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melia TJ, Jr, Cowan CW, Angleson JK, Wensel TG. A comparison of the efficiency of G protein activation by ligand-free and light-activated forms of rhodopsin. Biophys J. 1997;73:3182–3191. doi: 10.1016/S0006-3495(97)78344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel R, Siebert F. Conformations of the active and inactive states of opsin. J Biol Chem. 2001;276:38487–38493. doi: 10.1074/jbc.M105423200. [DOI] [PubMed] [Google Scholar]
- 9.König B, Welte W, Hofmann KP. Photoactivation of rhodopsin and interaction with transducin in detergent micelles. Effect of ‘doping’ with steroid molecules. FEBS Lett. 1989;257:163–166. doi: 10.1016/0014-5793(89)81811-3. [DOI] [PubMed] [Google Scholar]
- 10.Choe HW, et al. Crystal structure of metarhodopsin II. Nature. 2011;471:651–655. doi: 10.1038/nature09789. [DOI] [PubMed] [Google Scholar]
- 11.Standfuss J, et al. The structural basis of agonist-induced activation in constitutively active rhodopsin. Nature. 2011;471:656–660. doi: 10.1038/nature09795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamm HE, et al. Site of G protein binding to rhodopsin mapped with synthetic peptides from the alpha subunit. Science. 1988;241:832–835. doi: 10.1126/science.3136547. [DOI] [PubMed] [Google Scholar]
- 13.Scheerer P, et al. Structural and kinetic modeling of an activating helix switch in the rhodopsin-transducin interface. Proc Natl Acad Sci USA. 2009;106:10660–10665. doi: 10.1073/pnas.0900072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- 15.Scheerer P, et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 16.Hildebrand PW, et al. A ligand channel through the G protein coupled receptor opsin. PLoS ONE. 2009;4:e4382. doi: 10.1371/journal.pone.0004382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angel TE, Gupta S, Jastrzebska B, Palczewski K, Chance MR. Structural waters define a functional channel mediating activation of the GPCR, rhodopsin. Proc Natl Acad Sci USA. 2009;106:14367–14372. doi: 10.1073/pnas.0901074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piechnick R, Heck M, Sommer ME. Alkylated hydroxylamine derivatives eliminate peripheral retinylidene Schiff bases but cannot enter the retinal binding pocket of light-activated rhodopsin. Biochemistry. 2011;50:7168–7176. doi: 10.1021/bi200675y. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Duan Y. Retinal release from opsin in molecular dynamics simulations. J Mol Recognit. 2011;24:350–358. doi: 10.1002/jmr.1087. [DOI] [PubMed] [Google Scholar]
- 20.Xie G, Gross AK, Oprian DD. An opsin mutant with increased thermal stability. Biochemistry. 2003;42:1995–2001. doi: 10.1021/bi020611z. [DOI] [PubMed] [Google Scholar]
- 21.Standfuss J, Zaitseva E, Mahalingam M, Vogel R. Structural impact of the E113Q counterion mutation on the activation and deactivation pathways of the G protein-coupled receptor rhodopsin. J Mol Biol. 2008;380:145–157. doi: 10.1016/j.jmb.2008.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuyama T, Yamashita T, Imai H, Shichida Y. Covalent bond between ligand and receptor required for efficient activation in rhodopsin. J Biol Chem. 2010;285:8114–8121. doi: 10.1074/jbc.M109.063875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson PR, Cohen GB, Zhukovsky EA, Oprian DD. Constitutively active mutants of rhodopsin. Neuron. 1992;9:719–725. doi: 10.1016/0896-6273(92)90034-b. [DOI] [PubMed] [Google Scholar]
- 24.Gross AK, Xie G, Oprian DD. Slow binding of retinal to rhodopsin mutants G90D and T94D. Biochemistry. 2003;42:2002–2008. doi: 10.1021/bi020612r. [DOI] [PubMed] [Google Scholar]
- 25.Farrens DL, Khorana HG. Structure and function in rhodopsin. Measurement of the rate of metarhodopsin II decay by fluorescence spectroscopy. J Biol Chem. 1995;270:5073–5076. doi: 10.1074/jbc.270.10.5073. [DOI] [PubMed] [Google Scholar]
- 26.Zhukovsky EA, Robinson PR, Oprian DD. Transducin activation by rhodopsin without a covalent bond to the 11-cis-retinal chromophore. Science. 1991;251:558–560. doi: 10.1126/science.1990431. [DOI] [PubMed] [Google Scholar]
- 27.Schädel SA, et al. Ligand channeling within a G-protein-coupled receptor. The entry and exit of retinals in native opsin. J Biol Chem. 2003;278:24896–24903. doi: 10.1074/jbc.M302115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palczewski K, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann KP, Emeis D, Schnetkamp PP. Interplay between hydroxylamine, metarhodopsin II and GTP-binding protein in bovine photoreceptor membranes. Biochim Biophys Acta. 1983;725:60–70. doi: 10.1016/0005-2728(83)90224-4. [DOI] [PubMed] [Google Scholar]
- 30.Janz JM, Farrens DL. Engineering a functional blue-wavelength-shifted rhodopsin mutant. Biochemistry. 2001;40:7219–7227. doi: 10.1021/bi002937i. [DOI] [PubMed] [Google Scholar]
- 31.Grossfield A, Pitman MC, Feller SE, Soubias O, Gawrisch K. Internal hydration increases during activation of the G-protein-coupled receptor rhodopsin. J Mol Biol. 2008;381:478–486. doi: 10.1016/j.jmb.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell DC, Litman BJ. Effect of protein hydration on receptor conformation: decreased levels of bound water promote metarhodopsin II formation. Biochemistry. 1999;38:7617–7623. doi: 10.1021/bi990634m. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell DC, Litman BJ. Effect of ethanol and osmotic stress on receptor conformation. Reduced water activity amplifies the effect of ethanol on metarhodopsin II formation. J Biol Chem. 2000;275:5355–5360. doi: 10.1074/jbc.275.8.5355. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto H, Yoshizawa T. Existence of a beta-ionone ring-binding site in the rhodopsin molecule. Nature. 1975;258:523–526. doi: 10.1038/258523a0. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto H, Yoshizawa T. Rhodopsin regeneration is accelerated via noncovalent 11-cis retinal-opsin complex-A role of retinal binding pocket of opsin. Photochem Photobiol. 2008;84:985–989. doi: 10.1111/j.1751-1097.2008.00338.x. [DOI] [PubMed] [Google Scholar]
- 36.Kefalov VJ, Crouch RK, Cornwall MC. Role of noncovalent binding of 11-cis-retinal to opsin in dark adaptation of rod and cone photoreceptors. Neuron. 2001;29:749–755. doi: 10.1016/s0896-6273(01)00249-5. [DOI] [PubMed] [Google Scholar]
- 37.Henselman RA, Cusanovich MA. Characterization of the recombination reaction of rhodopsin. Biochemistry. 1976;15:5321–5325. doi: 10.1021/bi00669a019. [DOI] [PubMed] [Google Scholar]
- 38.Hammes GG, Chang YC, Oas TG. Conformational selection or induced fit: a flux description of reaction mechanism. Proc Natl Acad Sci USA. 2009;106:13737–13741. doi: 10.1073/pnas.0907195106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez A, Perez-Acle T, Pardo L, Deupi X. Molecular basis of ligand dissociation in beta-adrenergic receptors. PLoS One. 2011;6:e23815. doi: 10.1371/journal.pone.0023815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fritze O, et al. Role of the conserved NPxxY(x)5,6F motif in the rhodopsin ground state and during activation. Proc Natl Acad Sci USA. 2003;100:2290–2295. doi: 10.1073/pnas.0435715100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wald G, Brown PK. The molar extinction of rhodopsin. J Gen Physiol. 1953;37:189–200. doi: 10.1085/jgp.37.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Surya A, Foster KW, Knox BE. Transducin activation by the bovine opsin apoprotein. J Biol Chem. 1995;270:5024–5031. doi: 10.1074/jbc.270.10.5024. [DOI] [PubMed] [Google Scholar]
- 43.Heck M, et al. Signaling states of rhodopsin. Formation of the storage form, metarhodopsin III, from active metarhodopsin II. J Biol Chem. 2003;278:3162–3169. doi: 10.1074/jbc.M209675200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.