Abstract
Tumor necrosis factor (TNF) is an important inflammatory cytokine and induces many cellular responses, including inflammation, cell proliferation, apoptosis, and necrosis. It is known that receptor interacting protein (RIP) kinases, RIP1 and RIP3, are key effectors of TNF-induced necrosis, but little is known about how these two RIP kinases mediate this process, although reactive oxygen species (ROS) generation and JNK activation have been suggested to be two downstream events of RIP kinases. Here we report the identification of mixed lineage kinase domain-like, MLKL, as a key RIP3 downstream component of TNF-induced necrosis. Through screening a kinase/phosphatase shRNA library in human colon adenocarcinoma HT-29 cells, we found that knockdown of MLKL blocked TNF-induced necrosis. Our data suggest that MLKL functions downstream of RIP1 and RIP3 and is recruited to the necrosome through its interaction with RIP3. Finally, we found that MLKL is required for the generation of ROS and the late-phase activation of JNK during TNF-induced necrosis. However, because these two events are not involved in TNF-induced necrosis in HT-29 cells, the target of MLKL during TNF-induced necrosis remains elusive. Taken together, our study suggests that MLKL is a key RIP3 downstream component of TNF-induced necrotic cell death.
Tumor necrosis factor (TNF) is a pleiotropic inflammatory cytokine and plays a critical role in diverse cellular events, including cell proliferation, differentiation, apoptosis, and necrosis (1, 2). TNF is also a major mediator of both inflammation and immunity and is involved in many pathological conditions and autoimmune diseases, such as rheumatoid arthritis and Crohn disease (3). Since its tumoricidal activity was discovered, the TNF pathway has been one of the most studied signaling pathways (4). In almost all types of cells treated with TNF, the transcription factor NF-κB and three MAP kinases, ERK, JNK, and p38, are activated and, occasionally, apoptotic or necrotic cell death can be induced as well (5, 6).
The molecular mechanisms of TNF signaling have been significantly worked out. It is known that the binding of TNF homotrimer to TNF-receptor 1 (TNF-R1) initiates the formation of TNF-R1 signaling complex by recruiting several adaptor/effector proteins. TRADD (TNF-R1–associated death domain protein) is the first protein to interact with the receptor through its death domain and recruits other effector proteins, such as RIP1 (receptor interacting protein) and TRAF2 (TNFR-associated factor 2) to form the TNF-R1 signaling complex leading to the activation of several pathways, including NF-κB and MAP kinases (1, 2). Both TRAF2 and RIP1 are necessary for the activation of NF-κB and MAP kinase pathways through recruiting IKK (IκB kinase) and MAP3Ks to the complex (7). Under certain conditions, the complex of TRADD, RIP1, and TRAF2 proteins dissociates from the receptor and recruits other proteins to form different secondary complexes to mediate apoptosis and necrosis (8, 9). Apoptosis is primarily initiated through the recruitment of the death domain protein FADD (Fas-associated death domain protein), whereas necrosis needs the presence of RIP3 (receptor interacting protein 3) in these secondary complexes respectively (5, 10). FADD recruits and induces dimerization and activation of the autocatalytic activation of the initiator cysteine proteases caspases 8 and 10, which drive apoptosis (11, 12).
Although the mechanism of TNF-induced apoptotic cell death is well elucidated, the signaling events that lead to TNF-initiated necrotic death, which has recently been named necroptosis (13, 14), are still largely unknown. Caspase-independent necrotic cell death has been proposed to involve the generation of reactive oxygen species (ROS) derived from mitochondria (9, 15–18). ROS may mediate necrotic cell death through inactivating MAPK phosphatases, which leads to sustained JNK activation (19). The protein RIP1 is necessary for the generation of ROS by TNF and is required for the initiation of necrotic cell death (20). RIP1 is cleaved by caspase 8 during the apoptotic process (21), which may limit its ability to activate the pathway(s) of ROS generation. More recently, RIP3 has been found to be essential for TNF-induced necrotic cell death (10, 22, 23). Through its interaction with RIP1, RIP3 is needed for TNF-induced necrosis, but not for other TNF-induced signaling pathways (23). It has been found that the kinase activity of both RIP1 and RIP3 is essential for TNF-induced necrosis, but the downstream target(s) of these kinases are still unknown, although RIP1 and RIP3 are phosphorylated through their interaction (10).
In the current study, we report the identification of mixed lineage kinase domain-like (MLKL) as a key RIP3 downstream component of TNF-induced necrosis. Through screening a kinase/phosphatase shRNA library in human colon adenocarcinoma HT-29 cells, we found that knockdown of MLKL blocked TNF-induced necrosis. MLKL functions downstream of RIP3 because its knockdown does not affect the interaction of RIP1 and RIP3 and the phosphorylation of these two kinases. We also found that MLKL interacts with RIP3, but not RIP3 kinase dead mutant, and is recruited to the necrotic signaling complex. Finally, we found that MLKL is required for the generation of ROS and the late-phase activation of JNK during TNF-induced necrosis. Thus, our study suggests that MLKL is a key RIP3 downstream component of TNF-induced necrotic cell death.
Results
Although RIP1 and RIP3 are known key regulators of TNF-induced necrosis, little is known about how these two kinases mediate the process. To identify additional components involved in TNF-induced necrosis, we made use of a powerful retroviral short hairpin RNA (shRNA)-mediated genetic screen to identify genes leading to loss-of-function phenotypes. Our approach was to use several available shRNA libraries including a kinase/phosphatase shRNA library (24) to knock down a large number of genes and then screen for shRNAs that protect cells from TNF-induced-necrosis. Because these shRNA libraries were designed for knocking down human genes (24), we chose human colon adenocarcinoma HT-29 cells, which are sensitive to TNF-induced necrosis (23), to carry out our screening. To screen the kinase/phosphatase shRNA library that has 2,951 different shRNAs, HT-29 cells were infected with the retroviral shRNA library and were then treated to undergo necrosis by the combination of TNF, Smac mimetic, and caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (z-VAD-fmk) (TSZ) (Fig. S1). Surviving clones were selected for confirmation of necrotic resistance and for identification of the corresponding shRNAs by PCR and DNA sequencing (24). Among the 50 clones selected for sequencing, 6 had two different shRNAs targeting the gene, mlkl.
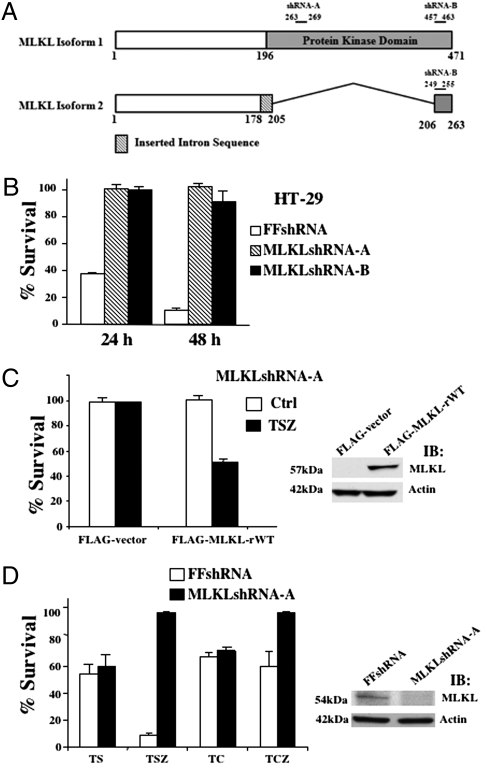
MLKL is predicted as a kinase-like protein and has two transcript variants produced by alternative splicing. MLKL variant 1 is the longer transcript and encodes the full length isoform of a 471-amino-acid protein and the variant 2 lacks exons 4–8 and encodes the short isoform of a 263-amino-acid protein, although the two isoforms of MLKL have the same N- and C termini (25). The full length MLKL, but not the shorter isoform of the gene, has one putative protein kinase domain (25). As shown in Fig. 1A, five of the six MLKL shRNA clones have MLKLshRNA-A, which targets the full-length MLKL, and the other clone has MLKLshRNA-B, which targets both MLKL transcript variants. Compared to control firefly (FF)shRNA clones, all of these six MLKL shRNA clones are resistant to necrotic death (Fig. 1B). Because there is no difference in sensitivity to necrosis among these six clones, clone 1-3-2 containing MLKLshRNA-A is used as a representative in the rest of the experiments involving MLKL shRNA knockdown cells, whereas the results were verified in other clones as well. To rule out the off-target effect of the MLKLshRNA-A on TNF-induced necrosis, a MLKLshRNA-A–resistant MLKL mutant, MLKL-rWT, was introduced back into MLKLshRNA-A HT-29 cells. As shown in Fig. 1C, the ectopic expression of MLKL-rWT restored the sensitivity of cells to TNF-induced necrosis, indicating that the insensitivity of MLKLshRNA-A cells to necrosis is specifically due to the knockdown of MLKL. To examine whether MLKL is specific for necrosis, we treated MLKLshRNA-A and FFshRNA cells with TNFα and Smac mimetic (TS) or TNFα and cycloheximide (TC) for 48 h to induce apoptosis (23). Cells were also induced to undergo necrosis in treatments with TSZ or TNFα, cycloheximide, and z-VAD-fmk (TCZ). Whereas MLKLshRNA-A cells were resistant to TSZ or TCZ-induced necrosis, the sensitivity of these cells to apoptosis was not altered by MLKL knockdown compared with FFshRNA cells (Fig. 1D). The specific involvement of MLKL in TNF-induced necrosis was further confirmed by siRNA knockdown experiments as shown in Fig. S2, in which knockdown of MLKL with MLKL siRNA only blocked TNF-induced necrosis, but had no effect on TNF-induced apoptosis. The efficiency of MLKLshRNA and siRNA knockdown was examined by Western blotting and shown on the Right of Fig. 1D and Fig. S2. In addition, when MLKL was overexpressed in HEK293 cells, it caused cell death that could not be blocked by caspase inhibitor (Fig. S3). Thus, these results suggest that MLKL is specifically involved in necrosis but not apoptosis by TNF treatment.
Fig. 1.
Loss of MLKL protects against cell death in necrotic conditions but not apoptosis. (A) MLKL shRNA clones target full-length MLKL variant (shRNA-A) or the short isoform (shRNA-B). (B) HT-29 clones infected with either control shRNA (FFshRNA) or MLKLshRNA-A (clone shMLKL 1–3-2) or MLKLshRNA-B (clone shMLKL 1–3-1), were treated with TNF (30 ng/mL), Smac mimetic (SM-164 10 nM), and z-VAD-fmk (20 μM) (TSZ) for 24 or 48 h. Cell survival was determined by MTT. (C, Left) MLKLshRNA-A cells cotransfected with pCMV-LacZ and either control FLAG-vector or FLAG-MLKL-rWT(MLKL shRNA-resistant mutation). After 24 h, cells were either untreated (control) or treated with TSZ for 24 h. Cell survival was determined by counting LacZ+ cells and normalized to untreated cells. (Right) Immunoblot analysis of MLKLshRNA-A cells transfected with either control FLAG-vector or FLAG-MLKL using anti-FLAG or antiactin antibodies. (D) HT-29 clone MLKLshRNA-A cells were treated with apoptotic conditions [TNF (30 ng/mL) and Smac mimetic (SM-164 10 nmol) (TS) or TNF (30 ng/mL) and CHX (10 μg/mL) (TC)] or necrotic conditions (TSZ or TCZ) for 48 h. Cell survival was determined by MTT. (Right) Immunoblot analysis of HT-29 clones infected with FFshRNA or MLKLshRNA-A with anti-MLKL or anti-Actin antibodies.
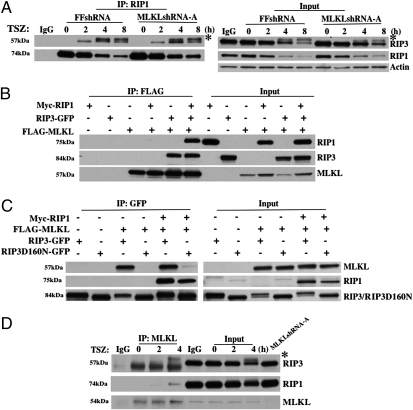
Recent studies found that the formation of RIP1 and RIP3 necrosome is essential for TNF-induced necrosis and this RIP1–RIP3 interaction results in RIP3 phosphorylation (23). To determine whether MLKL functions upstream or downstream of RIP1 and RIP3, we tested whether the knockdown of MLKL disrupts the RIP1–RIP3 interaction and alters RIP3 phosphorylation. To examine the RIP1–RIP3 interaction during TNF-induced necrosis, we did immunoprecipitation experiments with a RIP1-specific antibody in FFshRNA and MLKLshRNA-A cells and then examined the recruitment of RIP3. As shown in Fig. 2A, knockdown of MLKL does not block RIP1–RIP3 interaction because RIP3 was coprecipitated with RIP1 in MLKLshRNA-A cells as efficiently as it was in FFshRNA cells. In addition, because RIP3 phosphorylation will cause RIP3 mobility decrease and result in a slightly larger band compared to nonphosphorylated RIP3, the results shown in Fig. 2A indicated that knockdown of MLKL does not affect RIP3 phosphorylation during TNF-induced necrosis. Therefore, MLKL most likely functions downstream of RIP1 and RIP3. After we identified MLKL as a key component of TNF-induced necrosis, we searched the literature for clues about MLKL function, but found no report except a patent (25) on the potential function of MLKL. In the patent, the inventors claimed that MLKL interacts with RIP3 without showing any data. To find out whether MLKL interacts with RIP1, RIP3, or both, we first expressed Myc-RIP1, RIP3-GFP with/out FLAG-MLKL in HEK293 cells and examined the potential interaction between MLKL and RIP1 or RIP3 by immunoprecipitation of MLKL with a specific anti-FLAG antibody. As shown in Fig. 2B, RIP3, but not RIP1, was coprecipitated with MLKL. Interestingly, RIP1 was only pulled down with MLKL in the presence of RIP3 (Fig. 2B). These data suggest that MLKL and RIP3 interact and that RIP3 bridges RIP1 and MLKL in the same complex. Because it has been shown that RIP3 kinase activity is essential for its ability to mediate TNF-induced necrosis (10, 22, 23), we next examined whether the kinase activity of RIP3 is necessary for its interaction with MLKL. To do so, we performed similar immunoprecipitation experiments as described in Fig. 2B except we included the RIP3 kinase dead mutant, RIP3D160N-GFP (10) and examined the interactions between these proteins by immunprecipitation of RIP3 or RIP3D160N with a GFP-specific antibody. As shown in Fig. 2C, unlike the WT RIP3, which interacts with both RIP1 and MLKL, the kinase dead mutant of RIP3 only interacts with RIP1, but not MLKL. These results indicated that the kinase activity of RIP3 is critical for its interaction with MLKL. Finally, we examined whether endogenous MLKL was recruited to RIP1/RIP3 necrosome following TSZ treatment. As shown in Fig. 2D, both RIP1 and RIP3 (phosphorylated RIP3) were coprecipitated with MLKL when an anti-MLKL antibody was used for immunoprecipitation. Therefore, MLKL is a key component of the necrosome.
Fig. 2.
MLKL interacts with RIP3. (A) HT-29 clones FFshRNA and MLKLshRNA-A were treated with TSZ for the indicated times; cell lysates were immunoprecipitated with anti-RIP1 antibody (IP:RIP1) and analyzed by immunoblot with anti-RIP3, anti-RIP1, or anti-Actin antibodies. Input, 1% of extract before immunoprecipitation (control). * indicates phosphorylated RIP3. (B) HEK293 cells were transfected with Myc-RIP1, RIP3-GFP, or FLAG-MLKL as indicated. After 24 h, cell lysates were immunoprecipitated with anti-FLAG antibody (IP:FLAG) and analyzed by immunoblot with anti-Myc, anti-GFP, or anti-FLAG antibodies. Input, 3% of extract before immunoprecipitation (control). (C) HEK293 cells were transfected with Myc-RIP1, FLAG-MLKL, RIP3-GFP, or RIP3D160N-GFP as indicated. After 24 h, cell lysates were immunoprecipitated with anti-GFP antibody (IP:GFP) and analyzed by immunoblot with anti-FLAG, anti-Myc, or anti-GFP antibodies. Input, 3% of extract before immunoprecipitation (control). (D) HT-29 cell lysates were immunoprecipitated with anti-MLKL antibody (IP:MLKL) and analyzed by immunoblot with anti-RIP3, anti-RIP1, and anti-MLKL antibodies. Input, 1.5% of extract before immunoprecipitation (control). * indicates phosphorylated RIP3.
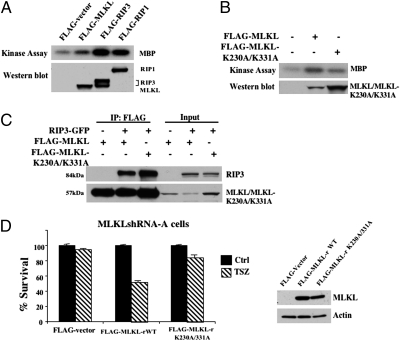
Because MLKL is predicted as a kinase-like protein and the kinase activity of MLKL is suggested in the patent (25), we then tested whether MLKL can function as a kinase using myelin basic protein (MBP) as the substrate. To do so, empty vector and FLAG-tagged MLKL were used to transfect HEK293 cells and after immunoprecipitation of MLKL with an anti-FLAG antibody, in vitro kinase assays were performed. The FLAG-RIP1 and FLAG-RIP3 were used as positive controls. As shown in Fig. 3A, MLKL modestly phosphorylated MBP compared with the efficient phosphorylation of MBP by RIP1 and RIP3. These results indicated that MLKL may be an atypical kinase with weak kinase activity. Because RIP3's kinase activity is essential for its interaction with MLKL, we next wanted to know whether the kinase activity of MLKL is needed for its interaction with RIP3. Therefore, we generated MLKL mutant by changing both lysine residues within the MLKL ATP binding domain, K230 and K331, to alanines. As shown in Fig. 3B, the MLKL double mutant, MLKL-K230A/K331A, lost its kinase activity. We then used the MLKL double mutant to test the necessity of its kinase activity for its interaction with RIP3. We expressed GFP-RIP3 with either FLAG-MLKL or FLAG-MLKL-K230A/K331A in HEK293 cells and performed immunoprecipitation with anti-FLAG antibody. As shown in Fig. 3C, both WT and mutant MLKL pulled down RIP3 efficiently, indicating that the kinase activity of MLKL is not critical for its interaction with RIP3. We next examined whether this kinase mutant of MLKL is still able to mediate TSZ-induced necrosis by cotransfecting MLKLshRNA-A cells with MLKLshRNA-A resistant MLKL-rWT, or MLKL-rK230A/K331A and LacZ plasmids while using empty vector and LacZ as a control. As shown in Fig. 3D, the expression of the mutant MLKL, MLKL-rK230A/K331A, did not restore the sensitivity of the cells to TNF-induced necrosis, whereas MLKL-rWT did. In addition, overexpression of this kinase mutant of MLKL failed to induce necrotic cell death in HEK293 cells (Fig. S4). Therefore, this mutation abolished MLKL's ability to mediate necrosis.
Fig. 3.
MLKL kinase activity is required for necrosis. (A) HEK293 cells were transfected with either FLAG-vector, FLAG-MLKL, FLAG-RIP3, or FLAG-RIP1. After 24 h, cell lysates were immunoprecipitated with anti-FLAG antibody and used for in vitro kinase assay using MBP as substrate. Lysates were analyzed by Western blot with anti-FLAG antibody. (B) HEK293 cells were transfected with either FLAG-vector, or FLAG-MLKL, or FLAG-MLKL-K230A/K331A mutant. After 24 h, cell lysates were immunoprecipitated with anti-FLAG antibody and used for in vitro kinase assay using MBP as substrate. Lysates were analyzed by Western blot with anti-FLAG antibody. (C) HEK293 cells were transfected with FLAG-MLKL, RIP3-GFP, or FLAG-MLKL-K230A/K331A as indicated. After 24 h, cell lysates were immunoprecipitated with anti-FLAG antibody (IP:FLAG) and analyzed by immunoblot with anti-FLAG or anti-GFP antibodies. Input, 5% of extract before immunoprecipitation (control). (D, Left) MLKLshRNA-A cells were cotransfected with pCMV-LacZ plasmid and either control FLAG-vector or MLKL shRNA-resistant plasmids, FLAG-MLKL-rWT, or FLAG-MLKL-rK230A/K331A for 24 h followed by treatment with TSZ for 24 h. Survival was determined by counting LacZ+ cells and normalized to untreated cells. (Right) Western blot analysis of MLKLshRNA-A cells cotransfected with pCMV-LacZ plasmid and either control FLAG-vector, or FLAG-MLKL-rWT, or FLAG-MLKL-rK230A/K331A. After 24 h, cell lysates were immunoblotted with anti-FLAG and antiactin.
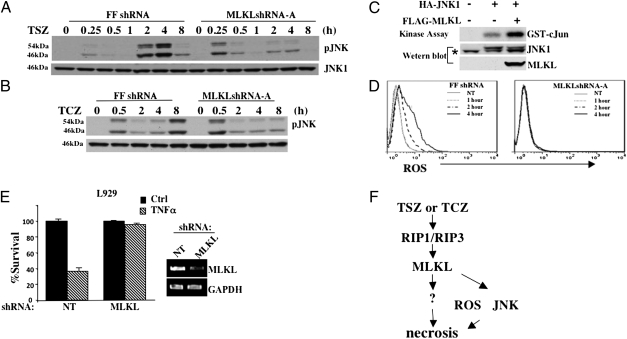
It has been suggested that the prolonged JNK activation and the induction of ROS are important events in TNF-induced necrosis, although the requirement of prolonged JNK activation and ROS generation may be tissue/cell-type specific (7, 19, 23). To investigate whether the knockdown of MLKL affects the late-phase JNK activation, we examined JNK activation in the control FFshRNA and MLKLshRNA-A cells at varying time points under necrotic conditions. As shown in Fig. 4A, JNK was activated at two different phases following TSZ treatment in control FFshRNA HT-29 cells: the immediate activation and the late-phase activation. However, in MLKLshRNA-A HT-29 cells, JNK was only activated at the early phase, indicating that MLKL may be required for the late-phase JNK activation. This observation was further confirmed when cells were induced to undergo necrosis by TCZ treatment. As shown in Fig. 4B, knockdown of MLKL clearly affects the phosphorylation of JNK at 8 h post-TCZ treatment. Slower kinetics of JNK activation under TCZ condition is consistent with the fact that TCZ induces a slower necrotic response in these cells. In addition, knockdown of MLKL does not have any effect on other TNF-activated pathways, including NF-κB activation (Fig. S5). To further prove MLKL plays a role in the late-phase JNK activation, we tested whether MLKL overexpression leads to JNK activation. To do so, HA-tagged JNK1 was cotransfected with the empty vector or MLKL plasmid and JNK in vitro kinase assays were performed with GST-cJun (1–79) as the substrate. As shown in Fig. 4C, the presence of MLKL increased JNK kinase activity, indicating MLKL functions upstream of JNK. Next, we determined whether the loss of MLKL affects ROS production under necrosis. We measured ROS production with 5(6)-chlormethyl-2-7-dichlorodihydrofluorescene diacetate (DCFDA) staining in TSZ-treated control FFshRNA and MLKLshRNA HT-29 cells at varying time points. Flow cytometry analysis showed that ROS production was dramatically increased at as early as 2 h in control FFshRNA cells, whereas no increase in ROS was detected in MLKLshRNA-A cells (Fig. 4D), suggesting MLKL is required for ROS production during necrosis as well. However, because the late-phase JNK activation and ROS generation are not essential for TNF-induced necrosis in HT-29 cells (23), we further tested the role of MLKL in mouse fibroblasts and L929 cells, in which both JNK inhibition and ROS scavenging have a protective effect on TNF-induced necrosis (Fig. S6) (7). Similarly to what we found in HT-29 cells, knockdown of MLKL by MLKL shRNA had an inhibitory effect on TNFα-induced necrosis in L929 cells (Fig. 4E). Also, siRNA knockdown of MLKL in mouse embryonic fibroblasts (MEFs) had a protective effect (Fig. S7). These data suggest that the role of MLKL in TNF-induced necrosis is a general phenomenon, regardless of cell types and whether JNK activation and ROS generation are involved.
Fig. 4.
Loss of MLKL affects JNK phosphorylation. (A) HT-29 clones FFshRNA and MLKLshRNA-A were treated with TSZ for the indicated times; cell lysates were analyzed by immunoblot with anti-pJNK or anti-JNK1 antibodies. (B) HT-29 clones FFshRNA and MLKLshRNA-A were treated with TCZ for the indicated times; cell lysates were analyzed by immunoblot with anti-pJNK or anti-JNK1 antibodies. (C) HEK293 cells were transfected with either FLAG-vector, or FLAG-MLKL, and/or HA-JNK1 as indicated. After 24 h, cell lysates were immunoprecipitated with anti-HA antibody and used for in vitro kinase assay using GST-cJun (1–79) as substrate. Lysates were analyzed by immunoblot with anti-FLAG and anti-HA antibodies. * indicates nonspecific band. (D) HT-29 clones FFshRNA and MLKLshRNA-A were treated with TSZ for the indicated times, stained with DCFDA, and analyzed by flow cytometry. Gray line (NT) represents background ROS in untreated samples. (E) L929 cells were infected with lentivirus, nontargeting (NT) or MLKL shRNA for 24 h followed by selection with puromycin for 48 h. Cells were then untreated or treated with TNFα (30 ng/mL) for 8 h. Cell survival was determined by MTT assay. (Right) RT-PCR of L929 cells infected with NT or MLKL shRNA with MLKL or GAPDH primers. (F) Diagram of the role of MLKL in RIP1/RIP3-dependent necrosis.
Discussion
Most recently, necrosis has been defined as a controlled and programmed death, which is both physiologically and pathologically relevant. The events leading to necrosis triggered by TNF are just starting to be understood and, however, are still largely elusive. Here, we describe the identification of MLKL protein belonging to the protein kinase superfamily as the critical RIP3 downstream mediator of TNF-induced necrosis. Through screening a kinase/phosphatase shRNA library in human colon adenocarcinoma HT-29 cells, we found that knockdown of MLKL completely blocked TNF-induced necrosis. Our data suggest that MLKL functions downstream of RIP1 and RIP3 and is recruited to the necrosome through its interaction with RIP3. Finally, we found that overexpression of MLKL is able to activate JNK, and MLKL is required for the generation of ROS and the late-phase activation of JNK during TNF-induced necrosis. However, because these two events are not involved in TNF-induced necrosis in HT-29 cells, the target of MLKL during TNF-induced necrosis remains elusive. Thus, our study suggests that MLKL is a key RIP3 downstream component of TNF-induced necrotic cell death.
Recent studies identify RIP3 as a key component of necrotic cell death (10, 22, 23). However, it is not known how RIP3 induces the necrotic machinery after it forms the necrosome with RIP1. Therefore, to further understand the molecular mechanism of necrosis, it is critical to identify some downstream components of RIP3-mediated necrosis. MLKL appears to be one of the downstream mediators of RIP3 in TNF-induced necrosis because we found that (i) knockdown of MLKL completely blocked TNF-induced necrosis, and the loss of MLKL had a minimal effect on the formation of RIP1/RIP3 necrosome and the subsequent phosphorylation of RIP3; and (ii) MLKL is able to induce necrotic cell death in HEK293 cells, which are RIP3-deficient cells. Whereas MLKL functions downstream of RIP3, it is recruited to the RIP1/RIP3 necrosome through its interaction with RIP3. Because the kinase dead RIP3 fails to interact with MLKL (Fig. 2C), one explanation is that the kinase activity of RIP3 is needed for RIP3 to interact with MLKL. Further study is necessary to dissect how RIP3 and MLKL interact and to investigate the role of RIP3 kinase activity in this interaction.
MLKL has two isoforms generated by alternative splicing and the longer isoform contains the kinase domain. Our screening of the kinase/phosphatase shRNA library indicated that the longer form of MLKL is needed for TNF-induced necrosis. This conclusion is further supported by our finding that the kinase mutant of MLKL lost its ability to mediate necrosis, and by the fact that the short isoform of MLKL does not have the putative kinase domain (Figs. 1A and 3D and Figs. S2 and S3). Although the protein sequence of MLKL predicts that it may be a pseudokinase, MLKL apparently has some kinase activity because it phosphorylates MBP modestly and the mutant MLKL, MLKL-K230A/K331A, lost the ability dramatically (Fig. 3 A and B). However, even though there is no RIP3 in HEK293 cells, our current data do not rule out the possibility that the phosphorylation of MBP observed in Fig. 3 A and B may be achieved by another kinase(s) interacting with MLKL. Currently we do not have any evidence to suggest that RIP3 regulates MLKL function and it is important to investigate whether MLKL is activated when it is recruited to the necrosome.
Earlier reports suggested that the prolonged JNK activation and the induction of ROS are important events in TNF-induced necrosis, although the requirement of these two events may be tissue/cell-type specific. JNK has different functions in cell death because of its biphasic activation: the prolonged JNK activation contributes to TNF-induced cell death and the transient JNK activation protects cells against death (7, 26). Inhibition of JNK blocks TNF-induced necrotic cell death in L929 cells and MEFs (7). Thus, sustained JNK activation is thought to be one of the key events in necrotic cell death induced by TNFα. In this study, although the late-phase JNK activation is not involved in TNF-induced necrosis in HT-29 cells, we found that this event is dependent on the presence of MLKL. The result that overexpression of MLKL leads to JNK activation (Fig. 4A) suggests that MKL functions upstream of the late-phase JNK activation either directly as an upstream MAP3K or indirectly through inducing necrosis. Also, because ROS has been shown to play a role in the prolonged JNK activation through inactivating phosphatases (19), and MLKL is also required for ROS generation during TNFα-induced necrosis (Fig. 4D), it is necessary to examine whether MLKL mediates the late-phase JNK activation through inducing ROS generation. Our preliminary study indicates that blocking ROS generation with ROS scavengers has limited effect on TSZ/TCZ-induced late-phase JNK activation in HT-29 and MEFs (Fig. S8). Currently, we are investigating whether MLKL-mediated late-phase JNK activation is a direct effect of MLKL or an outcome of MLKL-induced necrosis.
Whereas our study provides evidence that MLKL is the molecular connection between RIP3 and the late-phase JNK activation/ROS generation in necrotic cell death, it also raises the question: what is (are) the other target(s) of MLKL as these two downstream events of MLKL play a limited role in certain types of cells including HT-29 cells (Fig. 4F)? Because knockdown of MLKL in HT-29 cells completely blocks TNF-induced necrosis, but inhibition of JNK activation and ROS generation has no effect on cell death, MLKL must mediate this process mainly through another target(s) in these cells. Therefore, searching for the unknown target(s) of MLKL will be critical for us to fully understand the machinery of necrosis. Nevertheless, uncovering MLKL as a key RIP3 downstream mediator of TNF-induced necrosis brings us one step closer to this goal.
Materials and Methods
shRNA Library Screening.
shRNA screening was performed using a retroviral shRNA library targeting human kinases, phosphatases, genes involved in protein ubiquination, and genes implicated in cancer as described (24). HT-29 cells cultured in 15-cm dishes were infected using 8 μg/mL polybrene (Millipore). Two independent infections were carried out. At 24 h after the infection, media were replaced with 1.3 μg/mL puromycin. After a 4-d selection, cells were treated with TSZ for 48 h and grown in regular media that contained puromycin. Two days later, cells were treated with TSZ for an additional 48 h. The colonies were picked and the genomic DNA was isolated for PCR and sequencing. Two different shRNA sequences (A: 5′-GCGTATATTTGGGATTTGCAT-3′, B: 5′-CCTCTGTGGATGAAATCTTAA-3′) targeting human mlkl gene were identified. The firefly luciferase shRNA (FFshRNA) served as a negative shRNA control.
Reagents.
TNFα and z-VAD-fmk were purchased from R&D. SP600125 and CHX were from Calbiochem. Smac mimetic was a gift from S. Wang (University of Michigan, Ann Arbor, Michigan). Antibodies were from commercial sources: anti-pJNK from Invitrogen; anti-JNK1 from PharMingen; anti-FLAG (M2), antiactin, and anti-GFP from Sigma; anti-RIP1 from BD Transduction Laboratories; anti–c-Myc from BD Bioscience; anti-MLKL from Novus Biologicals and Abcam; and anti-RIP3 from Abcam.
Cell Culture and Treatment.
HT-29, HEK293, and MEF cell lines were cultured in DMEM with 10% FBS (vol/vol), 2 mM l-glutamine, and 100 units/mL penicillin/streptomycin.
Necrosis was induced by TNFα (30 ng/mL), Smac mimetic (10 nM), and z-VAD-fmk (20 μM) or TNFα (30 ng/mL), CHX (10 μg/mL), and z-VAD-fmk (20 μM). Apoptosis was induced by TNFα (30 ng/mL) and Smac (10 nM) or TNFα (30 ng/mL) and CHX (10 μg/mL).
Plasmid.
Human MLKL transcript variant 1 (NM_152649) was cloned into the mammalian expression vector pcDNA3.1/HisA, p3XFLAG-CMV.7.1, and pEGFP-C3. Fidelity of sequence was confirmed by DNA sequencing. MLKL shRNA-resistant mutation was generated by an 18-base replacement from 5′-CGTATATTTGGGATTTGC′-3–5′-AGA ATC TTC GGCATC TGT′-3 at position 789–806. The mutated base pairs are underlined.
Transfection of Plasmid and siRNA.
HEK293 cells were transfected with indicated plasmids using Lipofectamine-PLUS reagent (Invitrogen). After 24–36 h, cells were collected and used for the immunoprecipitation experiment. For transfection of siRNA, HT-29 cells were plated in six-well plates, and cells were transfected with 50 pmol of MLKL or nontargeting siRNA (human ON-TARGETplus siRNA pools of four oligos or mouse ON-TARGET plus siRNA pool of two oligos; Dharmacon) using Lipofectamine RNAiMAX (Invitrogen). After 48 h, cells were treated under apoptotic or necrotic conditions.
Lentivirus Infection.
HEK293T cells were cotransfected with pCMV-VSV-G and pCMV-dr8.2-dvpr and either nontargeting or MLKL-shRNA plasmids (SIGMA). After 24 h, supernatant was collected and this lentiviral preparation was used to infect cells. After 24 h of infection, cells were selected with puromycin for an additional 48 h.
Western Blot Analysis.
Cells were collected and lysed in M2 buffer (20 mM Tris at pH 7, 0.5% Nonidet P-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM DTT, 0.5 mM PMSF, 20 mM β-glycerol phosphate, 1 mM sodium vanadate, and 1 mg/mL leupeptin). Cell lysates were separated by SDS/PAGE and analyzed by immunoblot. The proteins were visualized by enhanced chemiluminescence (Pierce).
Immunoprecipitations.
Cells were collected in M2 buffer. Lysates were precipitated with antibody and protein G-agarose beads by incubation at 4 °C overnight. Beads were washed four to six times with 1 mL M2 buffer, and the bound proteins were removed by boiling in SDS buffer and resolved in 4–20% SDS-polyacrylamide gels for Western blot analysis.
Cytotoxicity Assay.
Cell death was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). MTT absorbance was read at 570 nM. Survival ratio was calculated by comparison of TSZ to untreated cells or TCZ to CHX-treated cells.
Kinase Assays.
Cell lysates were prepared by lysis in M2 buffer. FLAG-tagged vectors or HA-JNK1 was immunoprecipitated with FLAG or HA antibodies overnight at 4 °C. After washing with 3× each of M2 buffer and kinase buffer [20 mM Hepes (pH 7.5), 10 mM MgCl2, 1 mM MnCl2, 1 mM DTT, 5 mM NaF, 100 μM Na3VO4, 20 mM β-glycerophosphate], the beads were incubated in hot kinase buffer (10 mM PnPP, 60 μM ATP, 10 μCi [32P] γ-ATP) and supplemented with 5 μg of MBP (Sigma) or GST-cJun (1–79), respectively, for 30 min at 30 °C. Samples were resolved on 10% gels and exposed to autoradiographic films.
LacZ Staining Cell Death Assays.
Cells were cotransfected with pCMV-LacZ reporter plasmid and the plasmids as indicated in Figs. 1C and 3D for 24 h and then treated with TSZ for an additional 24 h. All X-Gal–stained blue cells were counted and averaged from five different randomly chosen fields. Cell survival was determined by normalizing blue cell numbers from TSZ-treated plates to the control plates. Data represent three independent experiments.
Note Added in Proof.
While this paper was under revision, Sun, L. et al. reported MLKL mediates necrotic signaling downstream of the kinase RIP3 (27).
Supplementary Material
Acknowledgments
We thank Drs. Francis Ka Ming Chan and Jiahuai Han for the RIP3 plasmids. This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200012109/-/DCSupplemental.
References
- 1.Chen G, Goeddel DV. TNF-R1 signaling: A beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 2.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 3.Tracey KJ, Cerami A. Tumor necrosis factor: An updated review of its biology. Crit Care Med. 1993;21(10, Suppl):S415–S422. [PubMed] [Google Scholar]
- 4.Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 5.Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: Apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 6.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 7.Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 9.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: Signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Galluzzi L, Kroemer G. Necroptosis: A specialized pathway of programmed necrosis. Cell. 2008;135:1161–1163. doi: 10.1016/j.cell.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Degterev A, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 15.Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci USA. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakon S, et al. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y, et al. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–10828. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- 18.Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr, Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004;18:2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamata H, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 23.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Luo J, et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137:835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollenhauer J, et al. Use of mixed lineage like kinase polypeptides (mlkl polypeptides) in cancer therapy. International Patent A61K38/45; C12N9/12. 2010 [Google Scholar]
- 26.Ventura JJ, et al. Chemical genetic analysis of the time course of signal transduction by JNK. Mol Cell. 2006;21:701–710. doi: 10.1016/j.molcel.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Sun L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2011;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.