Abstract
Cytoplasmic dynein is a microtubule-based molecular motor that participates in a multitude of cell activities, from cell division to organelle transport. Unlike kinesin and myosin, where different tasks are performed by highly specialized members of these superfamilies, a single form of the dynein heavy chain is utilized for different functions. This versatility demands an extensive regulation of motor function. Using an improved application of an optical trap, we were now able to demonstrate that cytoplasmic dynein can generate a discrete power stroke as well as a processive walk in either direction; i.e., towards the plus- or towards the minus-end of a microtubule. Thus, dynein’s motor functions can be described by four basic modes of motion: processive and nonprocessive movement, and movement in the forward and reverse directions. Importantly, these four modes of movement can be controlled by two switches. One switch, based on phosphate, determines the directionality of movement. The second switch, depending on magnesium, converts cytoplasmic dynein from a nonprocessive to a processive motor. The two switches can be triggered separately or jointly by changing concentrations of phosphate and magnesium in the local environment. The control of four modes of movement by two switches has major implications for our understanding of the cellular functions and regulation of cytoplasmic dynein. Based on recent studies of dynein’s structure we are able to draw new conclusions on cytoplasmic dynein’s stepping mechanism.
Keywords: molecular motors, motor mechanics, single molecule
In eukaryotic cells almost all organelle transport is performed by the three families of cytoskeleton-based molecular motors, myosin, kinesin, and dynein (1–3). While myosin and kinesin have evolved into large families with multiple members, each of which specialized for different tasks, a single dynein heavy chain performs all of the dynein related activities in the cytoplasm of eukaryotic cells (1).
One key function of cytoplasmic dynein is to power cargo transport over long distances. Here, motor processivity is required if long distance transport is to be achieved by a single motor molecule. To facilitate cargo delivery processivity must be switched off, when the target area is reached. Regulation becomes even more important when multiple motors of the same or even different type act together. For example, a model situation for the cooperation of different motors occurs during axonal transport driven by kinesin-1 and cytoplasmic dynein. During long distance travel, frequent changes in direction are observed. It is generally thought that the reversals of direction result from counteracting motor activity (4, 5). Though, a simple tug-of-war model in which only the winner determines the direction of movement appears rather inefficient to deliver cargo to specific locations. Moreover, it has been demonstrated in vitro and in vivo that dynein’s activity dominates over kinesin (6, 7). Therefore, being able to modulate dynein’s processivity and directionality would benefit proper targeting of cargo as previously proposed (8).
Cytoplasmic dynein interacts with several regulating cofactors (9–11) but the organization and complexity of the dynein heavy chain allows an intrinsic motor regulation as well. The heavy chain is comprised of a ring of six AAA+ domains, four of which contain discrete nucleotide-binding sites (AAA+ domains 1 to 4). Nucleotide-binding in AAA1 serves as the primary catalytic site that couples nucleotide hydrolysis to force generation and microtubule affinity. The three sites in AAA2-4 also bind nucleotides and their disruption has varied effects on motor activity. These results indicate that the three additional AAA sites contribute to dynein’s function (12–15), but there is little information on how they modulate motor behavior.
Despite dynein’s structural complexity, its ATPase cycle appears to share some similarities with myosin (16, 17). Several myosins have been shown to be sensitive to physiological changes in free Mg2+ (18, 19) and phosphate (Pi) ion concentrations (20), and thus raise the possibility that these ions can also modulate dynein’s activity. In the present study we directly tested whether the motor activity of cytoplasmic dynein is sensitive to changes in the concentrations of Mg2+ and inorganic phosphate (Pi).
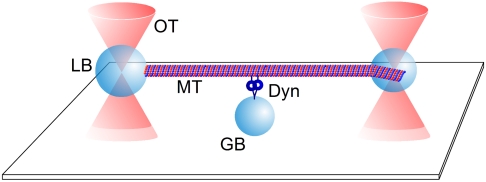
Cytoplasmic dynein has primarily been considered a processive motor, a property demonstrated at the single molecule level by total internal reflection fluorescence (TIRF) microscopy (21) and by a single bead optical trap approach (22–24). However, because of their limited spatial/time resolution these methods do not allow the detection of nonprocessive, single interactions between dynein and microtubules. In order to examine single interactions between dynein and microtubules, we have adapted an improved two bead filament dumbbell approach previously used to study myosin motors (25). By employing an end-on link of a microtubule to a latex microsphere (bead) surface we significantly improved the stiffness of the microtubule-bead link and thereby the detection of binding events (26) (Fig. 1). This set-up allows a simultaneous detection of processive and nonprocessive interactions of a motor with its filamentous track. Our improved application of optical trapping revealed that the dynein motor, by itself is capable of multiple modes of operation, and that switching between these modes can be triggered by modulating ionic conditions in the environment.
Fig. 1.
Diagram of the dumbbell set-up. To distinguish polarity of the MT dumbbell and to reduce compliance of the MT-bead link the minus-end of a MT was attached end-on to a 1 μm bead (LB) using an antibody specific for the minus-end of α-tubulin. A slightly smaller bead (0.8 μm) was attached laterally near the plus-end using the avidin/biotin chemistry as has been described for actin-bead dumbbells (26). Each bead was held in a weak optical trap (OT). The dumbbell was presented over a dynein molecule (Dyn), which was attached nonspecifically or via an antibody to the top surface of a 1.5 μm glass bead (GB) immobilized on a microscope cover slip.
Results
The Processivity of Cytoplasmic Dynein Is Regulated by Magnesium or Magnesium-Free ATP.
Cytoplasmic dynein behaves as a nonprocessive motor at 1 μM ATP and 1 mM Mg2+. Under these conditions, dynein produces single discrete microtubule binding events in optical trap experiments (Fig. 2A). The events could be identified by a reduction of the thermal fluctuation of the dumbbell. Using Molloy’s definition (27) of a working stroke as the difference in the mean positions of the dumbbell when free vs. bound to a motor, we determined an approximately 8 nm apparent working stroke for cytoplasmic dynein towards the minus-end of the microtubule (Fig. S1 A and B, S2). Interestingly, the -8 nm shift of the histogram distribution of the binding events corresponds to the 8 nm periodicity of the tubulin heterodimer within a microtubule.
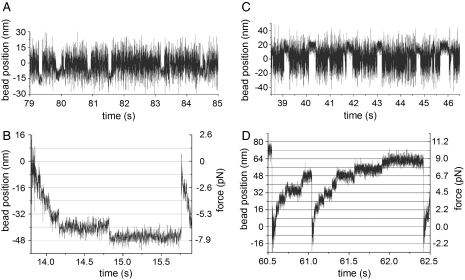
Fig. 2.
Four modes of movement of cytoplasmic dynein at a constant ATP concentration of 1 μM. (A) Single binding events of a single dynein molecule at 1 mM Mg2+. (B) Reducing the concentration of Mg2+ to 0.1 mM resulted in repetitive, staircase-like, processive runs with 8 nm steps towards the minus-end of the microtubule. (C) The addition of 1 mM of inorganic Pi reversed the directionality of the apparent working stroke at 1 mM Mg2+ (for more details see Fig. S1). (D) At 0.1 mM Mg2+ plus 0.5 mM Pi cytoplasmic dynein again underwent processive runs with steps of 8 nm; however, directed towards the plus-end of the microtubule. All experiments were carried out at low ionic strength (35 mM Pipes-KOH).
Using 1 μM ATP but with a Mg2+ concentration reduced from 1.0 to 0.1 mM we observed a switch from a binding/release behavior to processive stepping along a microtubule (Fig. 2 A and B). The step size throughout these processive runs was -8 nm, independent of the restoring force generated by the optical trap; thus, at our experimental conditions the step size of cytoplasmic dynein was not load dependent. The restoring force did, however, affect the life time of the steps (Fig. 2B).
The absolute Mg2+ concentration does not appear to be the sole factor in regulating processivity. While we observed nonprocessive movement of cytoplasmic dynein at 1 mM Mg2+ and 1 μM ATP, we observed processivity at 1 mM Mg2+ and a ATP concentration of 0.1 mM. At this elevated ATP concentration processivity was again abolished by raising the [Mg2+], e.g., to 10 mM (Table 1, Fig. S3), implying that it could actually also be the decrease in Mg2+-free ATP or an increase in the ratio between free Mg2+ and the Mg2+-free ATP that abolishes processivity.
Table 1.
Modulating processivity with ATP and Mg2+ (nd, not determined)
| 0.1 mM Mg2+ | 1 mM Mg2+ | 10 mM Mg2+ | |
| 1 μM ATP | processive | single event | nd |
| 100 μM ATP | nd | processive | single event |
The Direction of Cytoplasmic Dynein Can Be Regulated by Phosphate.
For myosins it has been shown that motor function can also be influenced by Pi, which is a product of ATP hydrolysis. We have therefore investigated whether Pi can affect the motor function of cytoplasmic dynein. At low Pi concentrations (0 to 0.2 mM Pi in assay buffer), dynein produced an apparent working stroke with a displacement of approximately 8 nm towards the microtubule (MT) minus-end (-8 nm) (Fig. 3), a result consistent with other single molecule measurements (21–24). However, to our surprise, addition of elevated Pi (0.5 to 1 mM Pi) to our assay buffer with 1 μM ATP and 1 mM Mg2+ reversed the direction of the apparent working stroke of cytoplasmic dynein from a minus-end direction (-8 nm) to a plus-end direction (+8 nm) (Fig. 2C, Fig. 3, Fig. S4). To confirm this result we checked the reliability of our indicator for the polarity of the MT dumbbell; i.e., the minus-end specific coupling of our 1 μm bead using an antibody specific for the minus-end of α-tubulin. Using these bead-coupled MTs in a MT gliding assay over a kinesin-1-coated surface we confirmed that 97% (74 out of 76) of the bead-MT links had the expected orientation, that is, beads coated with the α-tubulin specific antibody were indeed bound to the minus-end of the examined MTs (Movie S1).
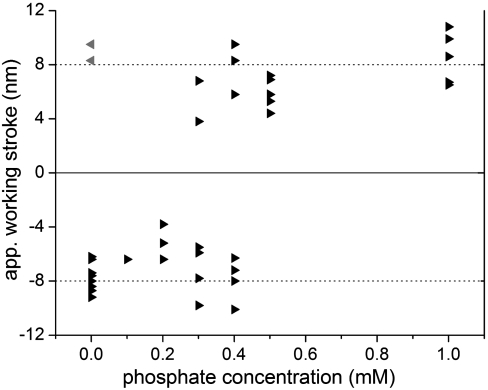
Fig. 3.
Influence of Pi on the directionality of the apparent working stroke of dynein. To obtain nonprocessive single binding events optical trap experiments were carried out at 1 μM ATP and 1 mM Mg2+. Changing the Pi concentration affected the direction of the apparent working stroke. Each triangle represents the apparent working stroke of a single motor at the given Pi concentration. The apparent working strokes of 38 single motors were determined using the Gaussian fit of the displacement histograms. At low [Pi] (up to 0.2 mM) the apparent working stroke was approximately -8 nm. At high [Pi] (0.5–1 mM) all motors produced an apparent working stroke of approximately +8 nm. At a Pi concentration of 0.3–0.4 mM a given dynein molecule produced an apparent working stroke either towards the minus- or the plus-end of the MT. No switching of direction could be recorded. Note, two out of 21 motors (gray triangles) did not perform as expected; i.e., they generated an 8 nm apparent working stroke in opposite direction. The data from these two motors were obtained with the same dumbbell. Because the polarity of our dumbbells has an 3% error margin (see SI Text), we assume that this dumbbell had a polarity opposite than expected.
While high and low Pi concentrations resulted in apparent working strokes of approximately 8 nm either towards or away from the MT plus-end (+8 nm or -8 nm), respectively, we also investigated intermediate concentrations of Pi (0.3 to 0.4 mM [Pi] range). At this range of [Pi] the motors showed either an apparent working stroke of -8 nm or of +8 nm. We did not observe an intermediate stroke size. The observation that cytoplasmic dynein had either a “positive” or “negative” working stroke was also demonstrated by data obtained from two dumbbells, where a given dumbbell supported displacements in plus and minus direction (Fig. S4A). In this case one motor generated an apparent working stroke of approximately -8 nm and a second motor attached to a different bead a working stroke of approximately +8 nm. Although different motors moved in different directions at 0.3–0.4 mM Pi, no actual switching of direction could be recorded for an individual motor. This observation suggests that switching must be a rare event. While the concentration of Pi could change the direction of the working stroke, it had no effect on the rate of ATP-induced release of cytoplasmic dynein from MTs. At 1 μM ATP the life time of the nonprocessive binding events was approximately 100 ms independent of the directionality of the motor (Fig. S4B).
Having observed a [Pi]-dependent directionality of cytoplasmic dynein at the level of a single motor, we wanted to know whether Pi would also affect the directionality of a motor ensemble. Thus, under the same buffer conditions, we investigated the Pi-dependent reversal of directionality in a MT gliding assay (motor ensemble). Interestingly, a Pi-induced reversal of directionality could not be observed (Movies S2 and S3).
Dynein’s Modes of Motion Are Controlled by Two Independent Switches.
Using the single molecule approach, we have identified two switches governing the motor activity of cytoplasmic dynein: Pi which controls the direction of movement and Mg2+ or possibly Mg2+-free ATP which controls the extent of processivity. Because processivity can be reduced gradually (28), we believe that the Mg2+/ATP switch acts as a dimmer switch rather than an on/off switch. In order to examine whether the two switches act independently of each other, we tested the last of the four possible combinations; i.e., whether the addition of Pi also changes directionality of processively moving cytoplasmic dynein. As expected for two independent switches, we again observed processive runs at 1 μM ATP and 0.1 mM Mg2+ but in the opposite direction when 0.5 mM Pi was added to the assay buffer (Fig. 2D). The step size appeared not to be altered by Pi.
Discussion
With our polarity defined dumbbell-assay we found that a single cytoplasmic dynein can display (i) a discrete power stroke either towards the plus-end or towards the minus-end of a MT and (ii) processive walking in either direction. The directional switch could not be observed in a gliding assay. A similar effect has been described for kinesin Cin8 (29). Here the switch in direction was observed when the motor changed from a motor ensemble to single motor complex. Our observations suggest that the core motor activity of a single cytoplasmic dynein is fundamentally sensitive to regulation by at least two independent switches, governed by ionic conditions. The size of the apparent working stroke and the stepping kinetics generated during processive walks appear to be independent of the direction of movement. Thus, a back and forth movement of cargo in vivo, thought to result from a tug-of-war between different motors could in principle also be the result of dynein’s action on its own, via an ability to change direction and modify stepping behavior. In the following we will discuss what might be the structural and mechanochemical bases for our observations.
Mechanochemical Control of Processivity.
In a previous study, we showed that cytoplasmic dynein is capable of switching from nonprocessive to processive stepping by increasing the concentration of ATP (28). In the present study we further demonstrate that processivity at a fixed concentration of ATP can be varied by changing the level of Mg2+. Variation in the amount of Mg2+ will also affect the concentration of Mg-free ATP. Thus, the first switch which controls processivity could be governed by either Mg2+ or Mg-free ATP. The effect of Mg2+ could be explained by the presence of multiple, multifunctional nucleotide binding sites. The dynein motor core is a ring-shaped structure with multiple AAA+ modules that differentially bind and hydrolyze nucleotide (30, 31). The first AAA domain serves as the primary ATP hydrolytic site essential for motor activity (13–15). Unlike kinesin or myosin, dynein’s filament binding domain is located at a considerable distance from the ATP-hydrolysis site, as it emerges from the ring at a position opposite AAA1 (30, 32). Conformational changes produced by nucleotide hydrolysis at AAA1 must therefore propagate around the ring structure to effect affinity changes within the MT-binding domain. Thus any structural alterations that enhance or retard this conformational coupling will have an effect on how the motor operates.
Single molecule TIRF experiments (21) and gliding assays (33) performed over a wide range of ATP concentrations indicated that more than one bound ATP molecule is necessary for processive dynein movement. A mutation in the P-loop of AAA3 that prevents nucleotide binding at this site, led to an ATP-insensitive, rigor-like MT interaction of Drosophila dynein (12). Thus, there is clear evidence that the nucleotide binding activity at sites other than the primary catalytic domain do indeed modulate motor activity. Furthermore, the velocity of single mouse dynein-dynactin cocomplexes was significantly increased by the addition of 1 mM of the nonhydrolysable nucleotide AMP-PNP to 100 μM ATP (21). The authors hypothesized that binding of a nonhydrolyzed nucleotide at a regulatory nucleotide binding site may act as an allosteric activator for the ATPase activity in AAA1. This finding was confirmed by experiments with single dynein molecules from yeast where mutations that blocked ATP hydrolysis in the AAA4 domain increased dynein’s processivity by increasing the binding affinity to MTs (15). Latter findings imply that the nucleotide bound state at AAA3 and AAA4 influences motor processivity. Thus, a Mg2+/ATP-based switch could act via domains AAA3/4, by modulating the occupancy of the sites or their steric coupling to the adjacent AAA domains. The directed switch from processive to nonprocessive stepping is tantamount to a controlled release of the motor from the MT, thus, it could be crucial in positioning and anchoring as well as transport of cargo.
Mechanochemical and Structural Basis for the Switch in Directionality.
Cytoplasmic dynein primarily drives MT minus end-directed transport. Nevertheless, dynein movement in the opposite direction, toward the MT plus-end, have previously been demonstrated (21,34, 35). Ross, et al. (21) showed that single native dynein-complexes were able to walk towards either MT end, but in a manner that required dynactin. It was suggested that the change in direction may be triggered by running into physical obstacles along the MT surface (e.g., MT associated proteins) (34). A first hint at a mechanical reverse in direction, in the absence of dynactin, was provided by force-induced measurements (24). The authors applied a backward load to a single bead assay, revealing that under these conditions dynein is able to step backwards along the MT. Our results show, that the dynein complex itself is capable of moving in either direction; i.e., even in the absence of dynactin or applied force. Our data also reveal that there may be an underlying Pi-dependent biochemical and or physiochemical basis to this reversal. The fact that Pi does not alter dynein’s MT detachment-rate (Fig. S4B) indicates that the switch in directionality is caused by a conformational change rather than altered enzyme kinetics. Such a conformational change can theoretically be located in the stalk or converter (linker) domains connecting the motor to the tail.
Pi may play a critical role in providing a directional bias to force production, by altering the relative orientation of the two motor domains upon MT binding. Electron microscopic studies illustrate that the two motor domains of dynein can be in close contact with each other (36, 37). Thus, a head-head interaction and/or relative head-head orientation could determine which of the two heads binds first, thereby influencing the binding position of the second motor domain and thus the directionality.
A recent atomic structure of the Dictyostelium cytoplasmic dynein dimer presents the two motor domains arranged in an asymmetric back-to-back orientation (38). The two heavy chains termed A and B each have a distinct stalk orientation. Perhaps due to flexibility of the stalk, the electron density was insufficient to identify the tip domain in molecule B, though the coiled-coil of the stalk shows a rotation of approximately 90° compared to molecule A. This rotation of the stalk might be sufficient for a parallel orientation of the binding domains, and has been discussed previously (39) as a result of slight shifts within the coiled-coil of the stalk (40). Two distinct binding conformations would further agree with a study by Gibbons, et al. (41) that describes two stalk conformations with different MT affinity. Furthermore, electron microscopic images of sea urchin outer arm dynein showed no significant change in the angle of stalk attachment between different nucleotide states (37) indicating that, during stepping, the stalk conformation within one motor domain remains stable.
In summary, we have shown that dynein is a complex, finely controlled universal motor capable of bidirectional processive and nonprocessive movement. In this context magnesium and Pi ions appear to play critical roles. At the cellular level, magnesium is important for ATPase function as well as a number of other cellular processes. The observation of a [Mg2+] gradient from a high concentration near the cell center to a low concentration at the cell periphery implies that its intracellular levels are regulated (42) and thus may make Mg2+ a suitable control mechanism for dynein function; e.g., (i) to maintain a high level of processivity and anchor function (43, 44) at the cell periphery and (ii) to modulate dynein’s processivity, when the cargo is near the cell center. Phosphate on the other hand is a byproduct of high ATPase activity leading to localized increase of Pi concentrations. Neither of these mechanisms negates the possibility that physical confrontation with obstacles could temporarily force the motor into a reverse orientation for “short range manoeuvres” (21), but do provide an underlying basis by which these reversals could occur. The mechanisms by which the two switches act on dynein require further detailed investigations, particularly regarding the relationship between nucleotide states in all four ATP-binding sites and motor activity. Our improved optical trap approach which allows detection of processive as well as nonprocessive motor activity will help address these questions.
Methods
Protein Isolation and Purification.
Cytoplasmic dynein was isolated largely as described by Toba and Toyoshima (45). Tubulin was isolated from fresh porcine brain as described by Castoldi and Popov (46) and labeled following protocols of the Mitchison laboratory*. A more detailed description is given in SI Text.
Optical Trap Experiments.
Optical trap experiments were carried out as described previously (26, 28). Details are provided in SI Text.
Supplementary Material
Acknowledgments.
We thank Petra Uta for technical support and Dr. Julie Hodgkinson for critically reading of this manuscript. This work has been supported by the German Research Foundation (STE 1697/1; www.dfg.de) to W.S. and the National Science Foundation (MCB-1051612; www.nsf.gov) to M.P.K. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116315109/-/DCSupplemental.
References
- 1.Vallee RB, Williams JC, Varma D, Barnhart LE. Dynein: an ancient motor protein involved in multiple modes of transport. J Neurobiol. 2004;58:189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- 2.Sellers JR. Myosins: a diverse superfamily. Biochim Biophys Acta. 2000;1496:3–22. doi: 10.1016/s0167-4889(00)00005-7. [DOI] [PubMed] [Google Scholar]
- 3.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 4.Kural C, et al. Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science. 2005;308:1469–1472. doi: 10.1126/science.1108408. [DOI] [PubMed] [Google Scholar]
- 5.Soppina V, Rai AK, Ramaiya AJ, Barak P, Mallik R. Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes. Proc Natl Acad Sci USA. 2009;106:19381–19386. doi: 10.1073/pnas.0906524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muresan V, Godek CP, Reese TS, Schnapp BJ. Plus-end motors override minus-end motors during transport of squid axon vesicles on microtubules. J Cell Biol. 1996;135:383–397. doi: 10.1083/jcb.135.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuster M, Lipowsky R, Assmann MA, Lenz P, Steinberg G. Transient binding of dynein controls bidirectional long-range motility of early endosomes. Proc Natl Acad Sci USA. 2011;108:3618–3623. doi: 10.1073/pnas.1015839108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallik R, Gross SP. Molecular motors: strategies to get along. Curr Biol. 2004;14:R971–R982. doi: 10.1016/j.cub.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 9.King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- 10.Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10:854–865. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenney RJ, Vershinin M, Kunwar A, Vallee RB, Gross SP. LIS1 and NudE induce a persistent dynein force-producing state. Cell. 2010;141:304–314. doi: 10.1016/j.cell.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silvanovich A, Li MG, Serr M, Mische S, Hays TS. The third P-loop domain in cytoplasmic dynein heavy chain is essential for dynein motor function and ATP-sensitive microtubule binding. Mol Biol Cell. 2003;14:1355–1365. doi: 10.1091/mbc.E02-10-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kon T, Nishiura M, Ohkura R, Toyoshima YY, Sutoh K. Distinct functions of nucleotide-binding/hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry. 2004;43:11266–11274. doi: 10.1021/bi048985a. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi Y, Edamatsu M, Toyoshima YY. Multiple ATP-hydrolyzing sites that potentially function in cytoplasmic dynein. Proc Natl Acad Sci USA. 2004;101:12865–12869. doi: 10.1073/pnas.0403429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho C, Reck-Peterson SL, Vale RD. Regulatory ATPase sites of cytoplasmic dynein affect processivity and force generation. J Biol Chem. 2008;283:25839–25845. doi: 10.1074/jbc.M802951200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson KA. Pathway of the microtubule-dynein ATPase and the structure of dynein: a comparison with actomyosin. Annu Rev Biophys Biophys Chem. 1985;14:161–188. doi: 10.1146/annurev.bb.14.060185.001113. [DOI] [PubMed] [Google Scholar]
- 17.Hackney DD. The kinetic cycles of myosin, kinesin, and dynein. Annu Rev Physiol. 1996;58:731–750. doi: 10.1146/annurev.ph.58.030196.003503. [DOI] [PubMed] [Google Scholar]
- 18.Dürrwang U, et al. Dictyostelium myosin-IE is a fast molecular motor involved in phagocytosis. J Cell Sci. 2006;119:550–558. doi: 10.1242/jcs.02774. [DOI] [PubMed] [Google Scholar]
- 19.Taft MH, et al. Dictyostelium myosin-5b is a conditional processive motor. J Biol Chem. 2008;283:26902–26910. doi: 10.1074/jbc.M802957200. [DOI] [PubMed] [Google Scholar]
- 20.Caremani M, Dantzig J, Goldman YE, Lombardi V, Linari M. Effect of inorganic phosphate on the force and number of myosin cross-bridges during the isometric contraction of permeabilized muscle fibers from rabbit psoas. Biophys J. 2008;95:5798–5808. doi: 10.1529/biophysj.108.130435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross JL, Wallace K, Shuman H, Goldman YE, Holzbaur ELF. Processive bidirectional motion of dynein-dynactin complexes in vitro. Nat Cell Biol. 2006;8:562–570. doi: 10.1038/ncb1421. [DOI] [PubMed] [Google Scholar]
- 22.Reck-Peterson SL, et al. Single-molecule analysis of dynein processivity and stepping behavior. Cell. 2006;126:335–348. doi: 10.1016/j.cell.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toba S, Watanabe TM, Yamaguchi-Okimoto L, Toyoshima YY, Higuchi H. Overlapping hand-over-hand mechanism of single molecular motility of cytoplasmic dynein. Proc Natl Acad Sci USA. 2006;103:5741–5745. doi: 10.1073/pnas.0508511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gennerich A, Carter AP, Reck-Peterson SL, Vale RD. Force-induced bidirectional stepping of cytoplasmic dynein. Cell. 2007;131:952–965. doi: 10.1016/j.cell.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finer JT, Simmons RM, Spudich JA. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994;368:113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- 26.Steffen W, Smith D, Simmons R, Sleep J. Mapping the actin filament with myosin. Proc Natl Acad Sci USA. 2001;98:14949–14954. doi: 10.1073/pnas.261560698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molloy JE, Burns JE, Kendrick-Jones J, Tregear RT, White DC. Movement and force produced by a single myosin head. Nature. 1995;378:209–212. doi: 10.1038/378209a0. [DOI] [PubMed] [Google Scholar]
- 28.Walter WJ, Brenner B, Steffen W. Cytoplasmic dynein is not a conventional processive motor. J Struct Biol. 2010;170:266–269. doi: 10.1016/j.jsb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Roostalu J, et al. Directional switching of the kinesin Cin8 through motor coupling. Science. 2011;332:94–99. doi: 10.1126/science.1199945. [DOI] [PubMed] [Google Scholar]
- 30.Samsó M, Koonce M. 25-Å resolution structure of a cytoplasmic dynein motor reveals a seven-member planar ring. J Mol Biol. 2004;340:1059–1072. doi: 10.1016/j.jmb.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 31.Roberts AJ, et al. AAA+ ring and linker swing mechanism in the dynein motor. Cell. 2009;136:485–495. doi: 10.1016/j.cell.2008.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gee MA, Heuser JE, Vallee RB. An extended microtubule-binding structure within the dynein motor domain. Nature. 1997;390:636–639. doi: 10.1038/37663. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu T, Toyoshima YY, Edamatsu M, Vale RD. Comparison of the motile and enzymatic properties of two microtubule minus-end-directed motors, ncd and cytoplasmic dynein. Biochemistry. 1995;34:1575–1582. doi: 10.1021/bi00005a013. [DOI] [PubMed] [Google Scholar]
- 34.Dixit R, Ross JL, Goldman YE, Holzbaur ELF. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Euteneuer U, Koonce MP, Pfister KK, Schliwa M. An ATPase with properties expected for the organelle motor of the giant amoeba, Reticulomyxa. Nature. 1988;332:176–178. doi: 10.1038/332176a0. [DOI] [PubMed] [Google Scholar]
- 36.Amos LA. Brain dynein crossbridges microtubules into bundles. J Cell Sci. 1989;93:19–28. doi: 10.1242/jcs.93.1.19. [DOI] [PubMed] [Google Scholar]
- 37.Ueno H, Yasunaga T, Shingyoji C, Hirose K. Dynein pulls microtubules without rotating its stalk. Proc Natl Acad Sci USA. 2008;105:19702–19707. doi: 10.1073/pnas.0808194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kon T, Sutoh K, Kurisu G. X-ray structure of a functional full-length dynein motor domain. Nat Struct Mol Biol. 2011;18:638–642. doi: 10.1038/nsmb.2074. [DOI] [PubMed] [Google Scholar]
- 39.Carter AP, Vale RD. Communication between the AAA+ ring and microtubule-binding domain of dynein. Biochem Cell Biol. 2010;88:15–21. doi: 10.1139/o09-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter AP, et al. Structure and functional role of dynein’s microtubule-binding domain. Science. 2008;322:1691–1695. doi: 10.1126/science.1164424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbons IR, et al. The affinity of the dynein microtubule-binding domain is modulated by the conformation of its coiled-coil stalk. J Biol Chem. 2005;280:23960–23965. doi: 10.1074/jbc.M501636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng C, Reynolds IJ. Subcellular localization of glutamate-stimulated intracellular magnesium concentration changes in cultured rat forebrain neurons using confocal microscopy. Neuroscience. 2000;95:973–979. doi: 10.1016/s0306-4522(99)00471-6. [DOI] [PubMed] [Google Scholar]
- 43.Dujardin DL, Vallee RB. Dynein at the cortex. Curr Opin Cell Biol. 2002;14:44–49. doi: 10.1016/s0955-0674(01)00292-7. [DOI] [PubMed] [Google Scholar]
- 44.Morris NR. Nuclear positioning: the means is at the ends. Curr Opin Cell Biol. 2003;15:54–59. doi: 10.1016/s0955-0674(02)00004-2. [DOI] [PubMed] [Google Scholar]
- 45.Toba S, Toyoshima Y. Dissociation of double-headed cytoplasmic dynein into single-headed species and its motile properties. Cell Motil Cytoskeleton. 2004;58:281–289. doi: 10.1002/cm.20018. [DOI] [PubMed] [Google Scholar]
- 46.Castoldi M, Popov AV. Purification of brain tubulin through two cycles of polymerization-depolymerization in a high-molarity buffer. Protein Expres Purif. 2003;32:83–88. doi: 10.1016/S1046-5928(03)00218-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.