Abstract
MicroRNAs (miRNAs) are increasingly implicated in regulating cancer initiation and progression. In this study, two miRNAs, miR-25 and -32, are identified as p53-repressed miRNAs by p53-dependent negative regulation of their transcriptional regulators, E2F1 and MYC. However, miR-25 and -32 result in p53 accumulation by directly targeting Mdm2 and TSC1, which are negative regulators of p53 and the mTOR (mammalian target of rapamycin) pathway, respectively, leading to inhibition of cellular proliferation through cell cycle arrest. Thus, there is a recurrent autoregulatory circuit involving expression of p53, E2F1, and MYC to regulate the expression of miR-25 and -32, which are miRNAs that, in turn, control p53 accumulation. Significantly, overexpression of transfected miR-25 and -32 in glioblastoma multiforme cells inhibited growth of the glioblastoma multiforme cells in mouse brain in vivo. The results define miR-25 and -32 as positive regulators of p53, underscoring their role in tumorigenesis in glioblastoma.
Glioblastoma multiforme (GBM) is by far the most common and aggressive tumor of the CNS. Despite recent improvements in surgery, radiation therapy, and cytotoxic chemotherapy, the prognosis for GBM remains grim, with median survival time <1 y after diagnosis. Of all glial tumors, GBM seems to exhibit the greatest number of genetic changes (1). The TP53 tumor suppressor gene, a transcription factor for numerous genes involved in cell cycle control, DNA repair, apoptosis, and angiogenesis (2, 3), is one of the most frequently mutated genes in human cancer. Given its profound effects in either inhibiting cell proliferation or inducing apoptosis, the expression levels of the TP53 gene product, p53, are tightly controlled through a feedback loop involving the p53 downstream target gene, Mdm2, which negatively regulates p53 through Mdm2-mediated ubiquitination of p53 (4). As such, even modest changes in the Mdm2 level can perturb the p53 protein level and affect the tumorigenesis process.
MicroRNAs (miRNAs), small noncoding RNAs of ∼22 nt that mediate posttranscriptional silencing of specific target mRNAs, are being increasingly recognized as an important determinant of tumor development and progression (5). Deregulated miRNAs were suggested to exert their function in cancer through silencing of key cell fate regulators by directly binding their 3′ UTR (6, 7). Furthermore, miRNAs cooperatively function with certain transcription factors (TFs) in the regulation of mutual sets of target genes, allowing the coordinated modulation of gene expression both transcriptionally and posttranscriptionally. Specifically, it has been revealed that there is a recurring network motif in which a TF regulates the miRNA with which it cooperates in regulating a common set of targets (8).
Several studies have implicated p53 in the regulation of miRNAs expression (9–11). However, most miRNAs studied so far are positively correlated with p53 expression, whereas miRNAs repressed by this tumor suppressor have rarely been studied. Here, we report the identification of a set of miRNAs repressed by p53 through transcriptional repression of two TFs, E2F1 and MYC, and we show the antioncogenic potential of these miRNAs that induces cell cycle arrest and inhibition of cellular proliferation and tumor growth in vivo. Finally, we delineate a network architecture that includes two transcriptional factors, E2F1 and MYC, and miR-25 and -32, which directly or indirectly coregulate mutual genes in a p53-dependent manner in GBM.
Results
Identification of p53-Regulated miRNAs in Human GBM.
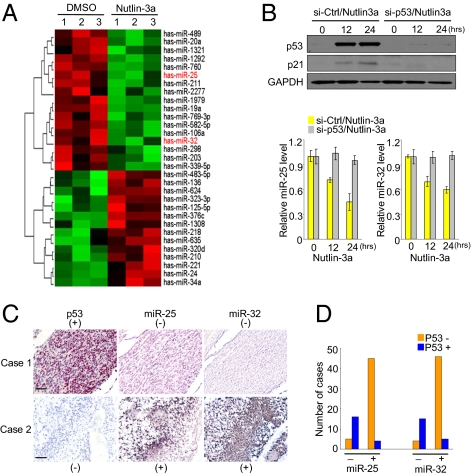
To identify p53-regulated miRNAs in GBM cells, we performed NanoString nCounter analysis of miRNA expression, a direct global profiling of individual miRNAs in a single reaction without amplification, in TP53 WT U87 cells treated with or without Nutlin-3a, which inhibits the formation of the Mdm2/p53 complex and results in activation of p53 (12). Unexpectedly, the predominant consequence of p53 induction in this model system was widespread repression of miRNAs expression (17 p53-repressed miRNAs of 31 total p53-responsive miRNAs; P < 0.05) (Fig. 1A and Table S1). Among the p53-repressed miRNAs, miR-25, the most strongly down-regulated in response to p53 activation (median fold change = −2.23), stood out as an attractive candidate for a role in p53-related functions. Interestingly, one of the p53 down-regulated miRNAs (median fold change = −1.5), miR-32, has the same seed sequence as miR-25, a conserved heptameric sequence, indicating that they are able to target the same transcripts (Fig. S1). These observations led us to pursue miR-25 and -32 as interesting targets for additional studies. To first validate the profile data, we performed stem loop quantitative RT-PCR (qRT-PCR) analysis in cells with activated p53; miR-25 and -32 were significantly down-regulated on treatment with Nutlin-3a in p53-activated U87 cells, whereas miR-34a, a direct target of p53 (9), was up-regulated (Fig. S1). Both mRNA and protein levels of p21, a downstream gene of p53, were also up-regulated (Fig. S1). Additionally, expression of two other miRNAs of the cluster with miR-25 (miR-106b and miR-93) displayed similar down-regulation (Fig. S1), confirming that expression of the entire miR-106/93/25 cluster is attenuated (7, 13). In particular, we found no effect of Nutlin-3a treatment of the p53-silenced cells on expression of miR-25 and -32, whereas the down-regulation in the control cells (Fig. 1B) provided direct evidence that p53 was involved in miR-25 and -32 repression.
Fig. 1.
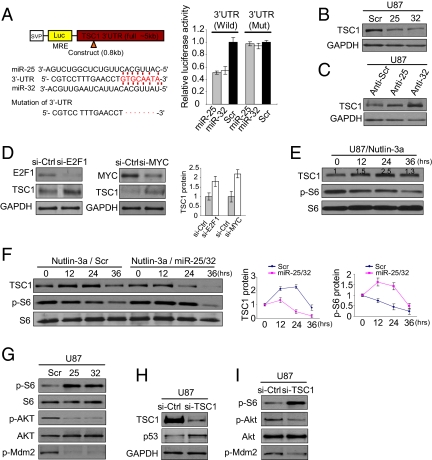
Identification of p53-regulated miRNAs in human GBM. (A) Overview of NanoString assay with U87 cells treated with 10 μM Nutlin-3a overnight (biological triplicate) and DMSO (biological triplicate). miRNAs sorted by P value of the univariate test (BRB tools) at the nominal 0.05 level of the univariate. (B) p53 and p21 protein levels (Upper) and miR-25 and -32 expression levels (Lower) in response to Nutlin-3a treatment (10 μM) of p53-silenced (si-p53) and control U87 cells (si-Ctrl). Data are presented as mean ± SD. We performed three biological experiments in triplicate. (C) Representative cases from 70 glioblastoma specimens in tissue microarrays were analyzed by immunohistochemical staining (p53; red) and ISH (miR-25 and -32; blue). (Scale bar: 40 μm.) (D) Graphs summarizing χ2 analysis of immunohistochemical and ISH staining results.
As additional evidence of the functional connection between p53 and miR-25 and -32, we performed a correlation analysis of p53 and miR-25 and -32 levels using immunohistochemistry and in situ hybridization (ISH), respectively, in glioblastoma tissue microarrays consisting of 70 brain tumor samples (Fig. 1 C and D and Table S2). Consistently, it was observed that miR-25 and -32 were rarely expressed in tumors in which p53 was highly expressed, whereas the tumors with low signal of p53 showed high expression of miR-25 and -32, confirming the inverse correlation (Fig. 1 C and D). In addition, we found a strong positive correlation between the expression of p53 and p21 in coexpression assay (Fig. S2). These data further support the finding that miR-25 and -32 are p53-repressed miRNAs in vivo.
E2F1 and MYC Transcriptionally Activate miR-25 and -32.
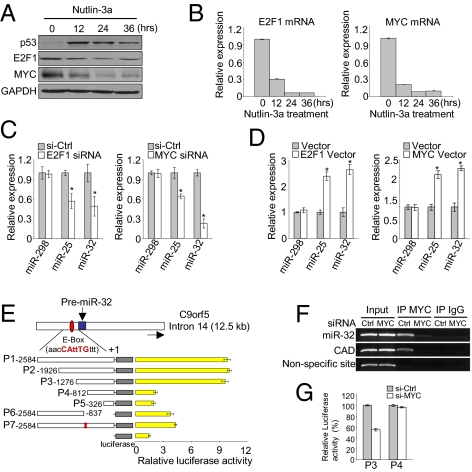
Next, we were interested in the mechanism of p53 repression of miR-25 and -32 expression. It has been reported that E2F1 regulates the expression of the miR-106b-25 cluster, which is located in intron 13 of the MCM7 host gene (13, 14). MYC-dependent regulation of the miR-106b-25 cluster has also been observed (13). In fact, E2F1 can activate MYC transcription and vice versa, and they cooperatively modulate the expression of miRNAs (15–18). Therefore, we decided to test whether E2F1 and MYC could also be responsible for the transcriptional activation of miR-32, which is located in intron 14 of the host gene C9orf5. We first investigated the correlation between miR-25 and -32 and E2F1/MYC expression in p53-induced cells. Consistent with previous data showing that p53 transcriptionally represses MYC expression through the binding to its promoter (19), MYC mRNA and protein expression were significantly down-regulated in p53-induced U87 cells (Fig. 2 A and B). E2F1 mRNA and protein expression were also strongly reduced (Fig. 2 A and B) along with significant reduction of miR-25 and -32 (Fig. S1).
Fig. 2.
miR-25 and -32 are transcriptional targets of E2F1 or MYC. (A and B) E2F1/MYC protein and mRNA levels in p53-activated U87 cells were measured by Western blot and qRT-PCR assays, respectively. (C and D) Relative expression of the miR-25 and -32 in E2F1/MYC-silenced and overexpressed U87 cells, respectively. (E) Identification of MYC interacting region by using luciferase reporter-containing promoter regions of pre–miR-32; +1 position corresponds to the 5′ terminus of miR-32 hairpin. Putative MYC responsive sequences (E-box) are indicated in the red box, and the miR-32 sequence is in blue. Deletion of E-box is shown by the red X, showing abolition of the promoter activity. (F) Chip assay after 48 h of MYC knockdown. CAD (carbamoyl phosphate synthetase 2, aspartate transcarbamylase, dihydroorotase) was used as a positive MYC target control, whereas nonspecific site served as the negative control. (G) Luciferase activity of P3 (with E-box) and P4 (without E-box) reporter constructs after knockdown of MYC. Luciferase activities were normalized by Renilla luciferase activities. (B–E and G) Data are presented as mean ± SD (*P < 0.05). We performed three biological experiments in triplicate.
To determine if E2F1 and MYC expressions are essential for regulation of miR-25 and -32, we specifically silenced E2F1 or MYC by RNAi. Consistent with previous reports that they transactivate each other (15, 16), their transcriptional levels were decreased or increased in response to the knockdown or overexpression of each other, respectively (Fig. S3). The knockdown of E2F1 or MYC resulted in a reduction of miR-25 and -32 levels, whereas miR-298, one of p53-repressed miRNAs in profile data (Fig. 1A), was not changed (Fig. 2C). Conversely, overexpression of the individual TFs induced miR-25 and -32 (Fig. 2D). The levels of miR-93 and -106b expression were also decreased or increased in response to knockdown or overexpression of E2F1 and MYC, respectively (Fig. S3).
As mentioned above, miR-25 and -32 are intragenic and located in introns of MCM7 and C9orf5, respectively. In p53-activated U87 cells, MCM7 mRNA was markedly decreased after p53 induction as was miR-25 expression (Fig. S1), whereas C9orf5 expression did not change, although miR-32 was decreased (Fig. S3). We also observed that MCM7 levels were reduced in E2F1- or MYC-silenced cells but not C9orf5 (Fig. S3). These data suggest that MCM7 and miR-25 are cotranscribed as previously reported (7, 13), whereas C9orf5 and miR-32 are not; miR-32 expression is expressed independently of its host gene, and it might have its own promoter. Furthermore, we observed that there was not any luciferase activity with plasmid containing ∼3 kb upstream of the C9orf5 locus. Thus, we cloned ∼2.6 kb upstream from the 5′ terminus of miR-32 hairpin structure into the pGL3 reporter (Fig. 2E) for a luciferase assay. We found that an MYC-responsive element, a noncanonical E-box (CANNTG), is indeed associated with MYC transcriptional activation of miR-32 using a construct mutated for this sequence (Fig. 2E). Notably, we did not observe any luciferase activity in response to E2F1, suggesting that E2F1 might indirectly regulate miR-32 expression through E2F1-transactivated MYC expression. Because we noticed that the mir-32 promoter region was responsive to MYC, MYC-ChIP specificity was confirmed by using MYC siRNA, which resulted in reduced MYC occupation on the miR-32 promoter (Fig. 2F). In addition, knockdown of MYC led to a reduction of ∼50% in luciferase activity for the luciferase reporter construct containing the E-box region (P3) (Fig. 2G). Collectively, these data confirm that E2F1 and MYC function as transcriptional activators of miR-25 and -32.
miR-25 and -32 Stabilize p53 Protein to Induce Cell Cycle Arrest and Inhibit Cell Proliferation.
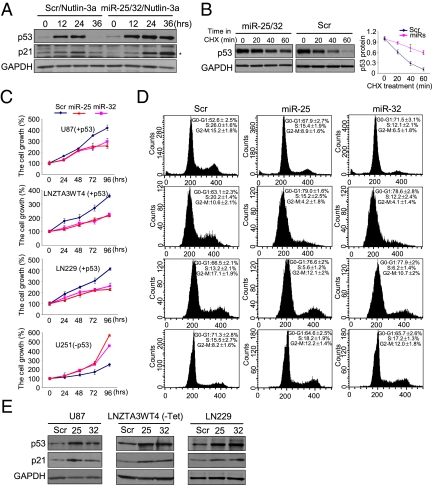
It has been reported that miR-25 targets p53 by directly binding to its 3′ UTR, resulting in the reduction of both protein and mRNA levels (20). This finding led us to test whether p53 could be a direct target of miR-25 and -32 in glioblastoma; we, thus, introduced miR-25 and -32 into cells with induced p53 expression to determine if these miRNAs can suppress the elevated p53. Interestingly and inconsistently with the previous report, p53 accumulated in the presence of miR-25 and -32 compared with scrambled cells, whereas p53 mRNA levels did not change (Fig. 3A and Fig. S4). To clarify the basis of p53 protein accumulation in the presence of exogenously expressed miR-25 and -32, we determined the half-life of p53 protein after treatment with cycloheximide, which blocks protein synthesis, in LNZTA3WT4 (GBM) cells (Fig. 3B), a p53 tetracycline-inducible cell line in which p53 is produced in the absence of the antibiotic but not in its presence (Fig. S4). The turnover rate of p53 is normally high in the glioblastoma cells, but in cells expressing miR-25 and -32, p53 became more stable. Of note, p53 mRNA levels did not change on miR-25 and -32 expression (Fig. S4). These results indicate that miR-25 and -32 are able to stabilize p53.
Fig. 3.
miR-25 and -32 stabilize p53 to induce cell cycle arrest and inhibit cell proliferation. (A) p53 and p21 protein levels in cells treated with Nutlin-3a in the presence of miR-25 and -32. Human U87 cells were transfected with miRNA oligonucleotides (100 nM) combined with treatment of Nutlin-3a (10 μM). The protein levels of p53 and p21 were measured at every 12 h after treatment of Nutlin-3a by Western blot assays. *p21 protein. (B) Western blot in LNZTA3WT4 cells transfected with miRNAs and treated with cycloheximide (CHX) for the indicated time. Before treatment with miRNAs and CHX, LNZTA3WT4 cells were grown without tetracycline for 48 h to activate p53. Note that the p53 blot for scrambled oligonucleotides (Scr) was exposed longer than the blot of miR-25 and -32 to achieve equivalent zero points. The p53 protein band intensities were quantified and normalized to GAPDH intensities. (C) MTS assay performed in U87, LNZTA3WT4, and U251 cell lines. Cells were transfected with miR-25 and -32 and scrambled sequence (Scr), and they were harvested at 24, 48, 72, and 96 h after transfection. (D) Flow cytometry analysis in U87, LNZTA3WT4, and U251 cell lines at 48 h after transfection with miR-25 or -32 or scrambled oligonucleotides. (E) Western blot analysis in U87 and LNZTA3WT4 cells transfected with miR-25 or -32 or scrambled oligonucleotides. (B–D) Data are presented as mean ± SD. We performed three biological experiments in triplicate.
To examine the relevance of p53-mediated regulation of miR-25 and -32 in glioblastoma, we tested whether ectopic expression of these miRNAs affected the biology of glioblastoma cells. As shown in Fig. 3C, rapid growth proliferation was observed only in U251 cells harboring inactivating mutations in p53, whereas significant growth arrest was shown after transfection with miR-25 and -32 in U87, LNZTA3WT4, and LN229 containing functional p53. Subsequent experiments indicated that expression of miR-25 and -32 induced a consistent G0/G1 arrest in U87, LNZTA3WT4, and LN229 but not U251 cells (Fig. 3D). The levels of p53 protein were increased by miR-25 and -32 compared with scrambled sequence in U87, LNZTA3WT4, and LN229 cells (Fig. 3E). Levels of p21, a p53 downstream transcriptional target, were also increased (Fig. 3E). Altogether, these data suggest that miR-25 and -32 expression causes p53 accumulation, which induces cell cycle arrest and inhibits cell proliferation in cells with functional p53.
miR-25 and -32 Target Mdm2.
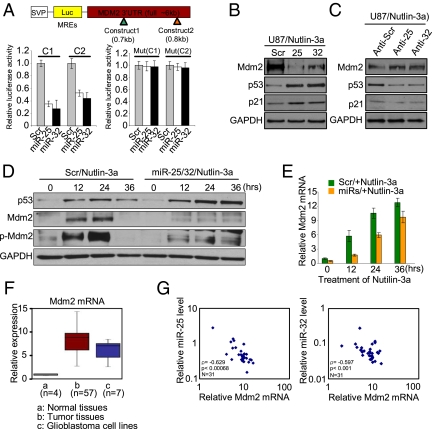
To define molecular mechanisms by which miR-25 and -32 cause accumulation of p53, the RNA22 target prediction program was used to discover targets of these miRNAs; we found that the 3′ UTR of the Mdm2 gene, a negative regulator of p53, has two predicted miRNA-responsive elements containing regions that matched the seed sequences of miR-25 and -32 (Fig. S5). To verify that Mdm2 is a direct target of miR-25 and -32, Mdm2 3′ UTR containing miRNA-responsive elements was cloned into the pGL3 construct downstream of the luciferase ORF. Cotransfection of this construct with pre–miR-25 and -32 oligonucleotides decreased that luciferase activity compared with the scrambled oligonucleotides, whereas the reporter with a mutated seed region did not (Fig. 4A). In addition, ectopic expression of miR-25 and -32, combined with 24 h of Nutlin-3a treatments, led to significantly decreased levels of endogenous Mdm2 compared with scrambled cells and increased p53 protein (Fig. 4B). Because Mdm2 protein is rapidly autoubiquitinated and degraded through the proteasome pathway (21), p53 induction is necessary for its detection in glioblastoma cells. In contrast, knockdown of miR-25 and -32 by 2′-O-me anti–miR-25 and -32 increased the protein levels of Mdm2 and decreased p53 protein (Fig. 4C). These findings led us to speculate whether introduction of miR-25 and -32 suppresses p53-dependent Mdm2 activation in p53-activated cells. We observed that Mdm2 protein levels were significantly reduced in presence of miR-25 and -32 followed by Nutlin-3a treatment in U87 cells, resulting in accumulation of p53 protein (Fig. 4D). Furthermore, Mdm2 mRNA was strongly reduced in the miR-25 and -32 transfected cells (Fig. 4E). These results indicate that miR-25 and -32 induce the degradation of Mdm2 mRNA, confirming that they regulate both protein and RNA levels. We also found that Mdm2 mRNA was significantly up-regulated in patient tissues and glioblastoma cell lines compared with normal brain tissues (Fig. 4F), consistent with previous reports (22, 23). Notably, using nonparametric test analysis, we found a significant inverse correlation between miR-25 or -32 and Mdm2 mRNA in glioblastoma patient tissues (Fig. 4G). Together, these data indicate that miR-25 and -32 contribute to p53 accumulation through the direct silencing of Mdm2.
Fig. 4.
Mdm2 is a direct target of miR-25 and -32. (A) Mdm2 3′ UTR contains two predicted miR-25 and -32 binding sites. Reporter constructs, containing a WT (Left) or mutated (Right) Mdm2 3′ UTR, were assayed. (B) Mdm2, p53, and p21 protein levels in U87 at 24 h after transfection with miR-25 and -32 combined with treatment of Nutlin-3a. (C) Mdm2, p53, and p21 protein levels in cells transfected with antisense oligonucleotides against miR-25 and -32 in U87 cells. (D) Mdm2 and p53 expression levels in U87 cells treated with Nutlin-3a after transfected with miR-25 and -32. (E) Mdm2 mRNA expression normalized for GAPDH by qRT-PCR. (F) Mdm2 mRNA relative expression in glioblastoma tissues (n = 57), cell lines (n = 7), and normal brain samples (n = 4) was determined by qRT-PCR assay. The relative expression values were used to design box and whisker plots. (G) Graphic of the negative Spearman correlation coefficient (ρ = −0.629 or −0.597) corresponding to a decreasing monotonic trend between log of Mdm2 mRNA relative expression and log of miR-25 or -32 relative expression (P < 0.00068, n = 31 or P < 0.001, n = 31). (A and E) Data are presented as mean ± SD. We performed three biological experiments in triplicate.
miR-25 and -32 Directly Target TSC1.
In a preliminary survey, we used several computational algorithms, including TargetScan and PicTar, to search for other target genes of miR-25 and -32; this search revealed TSC1 as a predicted target gene of the two miRNAs (Fig. 5A). Recently, it has been shown that an active mTOR pathway can suppress PI3K-Akt signaling, which affects p53 activity through Akt-mediated phosphorylation of Mdm2 (24–29). Thus, it is possible that TSC1 suppression by miR-25 and -32 could enhance mTOR activity and induce p53 accumulation in glioblastoma. To verify this possibility, we first examined whether miR-25 and -32 target TSC1 directly by generating luciferase reporters containing its 3′ UTR. The reporter activity was markedly suppressed by the presence of the 3′ UTR of TSC1, which reversed when the 3′ UTR was mutated (Fig. 5A). In addition, we found that, in the presence of miR-25 and -32, TSC1 protein levels decreased in U87 and LNZTA3WT4 cells (Fig. 5B and Fig. S5). However, inhibition of endogenous miR-25 and -32 using antisense oligonucleotides led to increased TSC1 levels (Fig. 5C and Fig. S5). In particular, TSC1 protein was increased in knockdown of MYC or E2F1 (Fig. 5D), which might occur as a result of miR-25 and -32 repression. Next, we wondered if p53-driven down-regulation of miR-25 and -32 would increase the level of TSC1 protein. To this end, we performed Western blot to see TSC1 protein levels in U87 with activated p53. Interestingly, TSC1 was dramatically increased in both U87 and LNZTA3WT4 cells in response to the elevated p53 (Fig. 5E and Fig. S5) without changing mRNA levels (Fig. S5). The increased levels of TSC1 were associated with gradual reduction of S6 phosphorylation, a marker of mTOR activation (Fig. 5E and Fig. S5). Conversely, we observed that miR-25 and -32 were sufficient to reduce the elevated TSC1 on p53 activation when introduced into p53-activated U87 cells (Fig. 5F) along with the increase of mTOR activity (Fig. 5F). Taken altogether, these data suggest that TSC1 is a direct target gene of miR-25 and -32 in glioblastoma.
Fig. 5.
mir-25 and -32 target TSC1. (A) TSC1 3′ UTR contains one predicted miR-25 and -32 binding site. The reporter assays were performed three times with essentially identical results. (B) The levels of TSC1 proteins in U87 cells at 48 h after transfection with miR-25 and -32 and scrambled oligonucleotides. (C) The levels of TSC1 protein in cells transfected with antisense oligonucleotides against miR-25 and -32 and scrambled oligonucleotides. (D) TSC1 expression levels at 48 h after knockdown of E2F1 or MYC in U87 cells, respectively. (E) The levels of TSC1 protein and mTOR activity (p-S6) in response to p53 induction in U87 cells. (F) TSC1 protein levels and mTOR activity in U87 cells treated with Nutlin-3a and transfected with miR-25 and -32. (G) Effects of miR-25 and -32 on mTOR and Akt activities at 24 h after transfection with miR-25 and -32 in U87 cells. (H) Fifty-three protein levels at 24 h after knockdown of TSC1. (I) mTOR and Akt activities in TSC1-silenced cells (U87). (A, D, and F) Data are presented as mean ± SD. We performed three biological experiments in triplicate.
Next, to investigate whether TSC1, as a target gene of miR-25 and -32, is involved in p53 accumulation through the PI3K-Akt pathway in glioblastoma, we first examined the expression levels of genes involved in this mechanism, such as S6, Akt, Mdm2, and p53, in miRNAs-transfected cells. As a result of the constitutive activation of downstream signal transduction of the mTOR pathway, mTOR-mediated phosphorylation of S6 was highly activated in the presence of miR-25 and -32 (Fig. 5G). In contrast, Akt and Mdm2 activities were markedly reduced, which was assessed by phosphorylation (Fig. 5G), indicating that mTOR activation by miR-25 and -32 may induce the attenuation of Akt and Mdm2 activity and then, p53 accumulation. To further examine whether TSC1 is essential for miRNAs-mediated p53 activation, we used RNAi against TSC1 and assayed p53 protein. We observed that the level of p53 protein was increased in response to knockdown of TSC1 and was associated with reduced p-Akt and p-Mdm2 levels (Fig. 5 H and I). Thus, miR-25 and -32 can stabilize p53 through activation of mTOR by targeting TSC1.
miR-25 and -32 Suppress Tumorigenicity in Vivo.
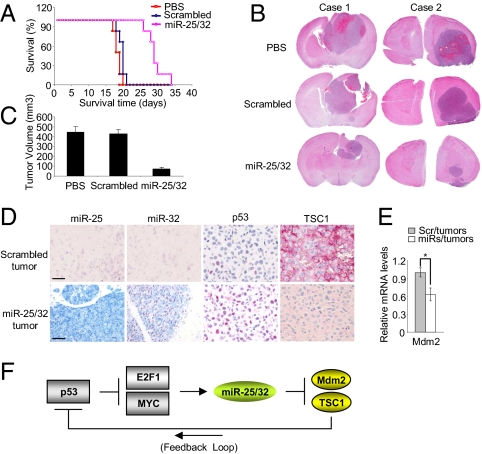
To provide physiological evidence for miR-25 and -32 regulation of tumor suppression in glioblastoma, we compared the antitumor efficacy of miR-25 and -32 in mice bearing intracranial glioma cells U87 Δepidermal growth factor receptor (EGFR), a mutant cell line that harbors amplification in the EGFR gene (30). U87 ΔEGFR cells have been widely used in studies of brain tumors, because intracranial xenografts of these cells grow much more stably and faster than U87 parental cells, U87 (31). These cells were transfected with miR-25 and -32 pools and scrambled or treated with PBS as negative control; 2.5 × 105 U87 ΔEGFR cells after the three treatments were implanted into mouse brain, and the three mouse groups were observed in vivo. The survival of mice in each group (n = 6/group) was analyzed by Kaplan–Meier curve (Fig. 6A). Although control mice treated with PBS died of tumor burden with a median survival of 18 d, the miRNAs-transfected group showed a significant improvement in survival compared with the scrambled group, showing median survival of 28 vs. 19 d (Fig. 6A). In a separate experiment, mice in the three groups were killed 14 d after cell implantation in mouse brain, and the brains were preserved for immunohistochemistry and histopathological analysis. We observed that the miRNAs-transfected group had significantly smaller tumors compared with the scrambled or PBS group, indicating that miR-25 and -32 suppressed the tumor growth (Fig. 6 B and C). Next, to determine if our model of regulation of p53 by miR-25 and -32 was reflected in vivo, mouse brains were stained by immunohistochemistry. We observed that p53 was activated in the miRNAs-transfected group with highly expressed miR-25 and -32 compared with the scrambled group (Fig. 6D). In addition, TSC1 was significantly repressed in the presence of miR-25 and -32 (Fig. 6D). The expression levels of Mdm2 mRNA were markedly reduced in the miRNAs-transfected group compared with the scrambled group (Fig. 6E), consistent with the finding that miR-25 and -32 target Mdm2 mRNA in vitro. In particular, we found coexpression of miR-25 or -32 and p53 in the miRNAs-transfected tumor group (Fig. S6). Altogether, the data indicate that miR-25 and -32 suppress tumor growth by causing accumulation of p53 protein in vivo.
Fig. 6.
miR-25 and -32 suppress tumorigenicity in vivo. (A) Kaplan–Meier survival curve of mice implanted with intracranial U87 ΔEGFR cells treated with PBS, scrambled, and miR-25 and -32 (n = 6/group). (B) In vivo effect of miR-25 and -32 on tumor growth of glioblastoma cells transfected with miR-25 and -32 or scrambled oligonucleotides (100 nM) by H&E staining. Case 1, coronal section; case 2, horizontal section. (C) Tumor volume in mice implanted with intracranial U87 ΔEGFR cells. Data are presented as mean ± SD (n = 5). (D) miR-25 and -32, TSC1, and p53 expression in tumor tissues using ISH and immunohistochemistry. (Scale bar: 20 μm.) (E) qRT-PCR to represent Mdm2 mRNA levels in miRNAs or scrambled tumors. Data are presented as mean ± SD (*P < 0.05). We performed three biological experiments in triplicate. (F) A model for a feedback regulatory loop including miRNAs, MYC, E2F1, and p53.
Discussion
In the current study, we identified two miRNAs, miR-25 and -32, as p53-repressed miRNAs through p53-dependent negative regulation of their transcriptional regulators, E2F1 and MYC. Our study provides compelling evidence that expression of these miRNAs causes tumor suppression through mechanisms that lead to accumulation of p53 protein, resulting in growth arrest in glioblastoma cells. Furthermore, miR-25 and -32 significantly inhibit tumor growth in vivo. In this process, we revealed that there is a feedback regulatory loop that includes two transcriptional factors, E2F1 and MYC, and miR-25 and -32, which coregulate mutual genes in a p53-dependent manner.
Recently, it has been reported that E2F1-inducible miRNAs function as tumor suppressors to suppress cell growth and induce apoptosis (32). However, E2F1- or MYC-dependent miRNAs were involved in cell proliferation and survival by targeting several tumor suppressors (7, 14, 17, 33). In fact, concomitant with promoting cell growth, proliferation, and survival in response to various external stimuli, E2F1 or MYC inhibits terminal differentiation of most cell types and sensitizes cells to cell cycle arrest and apoptosis in a p53-dependent manner (34–38). Thus, if the p53 pathway is genetically altered and nonfunctional, the imbalance between proliferation and cell death can lead to tumor development. It is, therefore, not surprising that this pathway is ablated in many human cancers. Along the same line, we observed that MYC-dependent miR-25 and -32 promote cell proliferation in U251 cells harboring nonfunctional p53 (Fig. 3C). Thus, the opposing roles of E2F1- and MYC-dependent miR-25 and -32 in tumorigenesis seem to be dependent on p53 status; in many human cancers with mutated or strongly down-regulated p53, miR-25 and -32 might result in a failure to stimulate enough p53 activity to inhibit tumor development and contribute instead to promotion of cellular proliferation and survival. Furthermore, our observations that miR-25 and -32 target Mdm2 and TSC1 in glioblastoma suggest the possibility that those miRNAs render the cells more susceptible to p53-dependent responses. Taken together, our data propose an indirect feedback circuitry of miRNAs/TP53 that includes an intermediary between miRNAs and TP53 (Fig.6F) in addition to the direct feedback proposed in ref. 39.
It has been recently reported that Nutlin-3a induces p53-dependent cell cycle arrest, apoptosis, and cellular senescence in U87 cells (40). This finding suggests that a combination of miR-25 and -32 and Nutlin-3a could be a successful therapeutic strategy for cancer therapy through the synergistic effects on p53 activity. Our study provides important insights into the central roles of miRNAs in a well-known tumor suppressor network, the p53 pathway, which may provide a route to therapeutic miRNA intervention in cancer.
Materials and Methods
In Vivo Experiments.
Animal experiments were performed according to the Subcommittee on Research Animal Care of the Ohio State University guidelines and have been approved by the Institutional Review Board. Athymic nude mice that were 5–6 wk old (Charles River Laboratories) were used for all studies. In the intracranial tumor study, nude mice were fixed in stereotactic apparatus, and a burr hole was drilled at 2 mm lateral to the bregma to a depth of 3 mm. In vivo assay and U87 (ΔEGFR) cells were used; 2 wk after tumor cell implantation of U87 (ΔEGFR) cells (2 × 105) transfected with scrambled oligonucleotides, PBS, or miR-25 and -32 oligonucleotides (1:1 ratio; 100 nM each miRNA), three mice from each treatment were euthanized for immunohistochemistry and ISH assay. Six mice were used in the survival test.
MTS [3-(4,5-Dimethylthiazol-2-yl)-5-(3-Carboxymethoxyphenyl)-2-(4-Sulfophenyl)] Assay.
Cells were plated in 96-well plates in triplicate and incubated at 37 °C in a 5% CO2 incubator. Cell viability was examined with 3-(4,5-dimethylthiazol-2-yl)-2, 5-dipheniltetrazolium bromide Cell Titer 96AQueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer's protocol. Metabolically active cells were detected by adding 20 μL 3-(4,5-dimethylthiazol-2-yl)-2, 5-dipheniltetrazolium bromide to each well. After 1 h incubation, the plates were analyzed in a Multilabel Counter (Bio-Rad Laboratories).
Supplementary Material
Acknowledgments
We thank Dr. Kay Huebner (Ohio State University) for careful editing of this paper and Sharon Palko for administrative support.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202465109/-/DCSupplemental.
References
- 1.Nigro JM, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989;342:705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- 2.Shangary S, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci USA. 2008;105:3933–3938. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shats I, et al. p53-dependent down-regulation of telomerase is mediated by p21waf1. J Biol Chem. 2004;279:50976–50985. doi: 10.1074/jbc.M402502200. [DOI] [PubMed] [Google Scholar]
- 4.Xirodimas DP, Stephen CW, Lane DP. Cocompartmentalization of p53 and Mdm2 is a major determinant for Mdm2-mediated degradation of p53. Exp Cell Res. 2001;270:66–77. doi: 10.1006/excr.2001.5314. [DOI] [PubMed] [Google Scholar]
- 5.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 6.Johnson SM, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Petrocca F, et al. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–286. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Shalgi R, Lieber D, Oren M, Pilpel Y. Global and local architecture of the mammalian microRNA-transcription factor regulatory network. PLoS Comput Biol. 2007;3:e131. doi: 10.1371/journal.pcbi.0030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang TC, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pichiorri F, et al. Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell. 2010;18:367–381. doi: 10.1016/j.ccr.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Raver-Shapira N, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26:731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Dickens MP, Fitzgerald R, Fischer PM. Small-molecule inhibitors of MDM2 as new anticancer therapeutics. Semin Cancer Biol. 2010;20:10–18. doi: 10.1016/j.semcancer.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Petrocca F, Vecchione A, Croce CM. Emerging role of miR-106b-25/miR-17-92 clusters in the control of transforming growth factor β signaling. Cancer Res. 2008;68:8191–8194. doi: 10.1158/0008-5472.CAN-08-1768. [DOI] [PubMed] [Google Scholar]
- 14.Brosh R, et al. p53-Repressed miRNAs are involved with E2F in a feed-forward loop promoting proliferation. Mol Syst Biol. 2008 doi: 10.1038/msb.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leone G, DeGregori J, Sears R, Jakoi L, Nevins JR. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature. 1997;387:422–426. doi: 10.1038/387422a0. [DOI] [PubMed] [Google Scholar]
- 16.Matsumura I, Tanaka H, Kanakura Y. E2F1 and c-Myc in cell growth and death. Cell Cycle. 2003;2:333–338. [PubMed] [Google Scholar]
- 17.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 18.Woods K, Thomson JM, Hammond SM. Direct regulation of an oncogenic micro-RNA cluster by E2F transcription factors. J Biol Chem. 2007;282:2130–2134. doi: 10.1074/jbc.C600252200. [DOI] [PubMed] [Google Scholar]
- 19.Ho JSL, Ma W, Mao DY, Benchimol S. p53-Dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol Cell Biol. 2005;25:7423–7431. doi: 10.1128/MCB.25.17.7423-7431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar M, et al. Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene. 2011;30:843–853. doi: 10.1038/onc.2010.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marine JC, Lozano G. Mdm2-mediated ubiquitylation: p53 and beyond. Cell Death Differ. 2010;17:93–102. doi: 10.1038/cdd.2009.68. [DOI] [PubMed] [Google Scholar]
- 22.He J, Reifenberger G, Liu L, Collins VP, James CD. Analysis of glioma cell lines for amplification and overexpression of MDM2. Genes Chromosomes Cancer. 1994;11:91–96. doi: 10.1002/gcc.2870110205. [DOI] [PubMed] [Google Scholar]
- 23.Korkolopoulou P, et al. MDM2 and p53 expression in gliomas: A multivariate survival analysis including proliferation markers and epidermal growth factor receptor. Br J Cancer. 1997;75:1269–1278. doi: 10.1038/bjc.1997.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen ML, et al. The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/- mice. Genes Dev. 2006;20:1569–1574. doi: 10.1101/gad.1395006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington LS, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manning BD. Balancing Akt with S6K: Implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning BD, et al. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 2005;19:1773–1778. doi: 10.1101/gad.1314605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabatini DM. mTOR and cancer: Insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 30.Wikstrand CJ, Reist CJ, Archer GE, Zalutsky MR, Bigner DD. The class III variant of the epidermal growth factor receptor (EGFRvIII): Characterization and utilization as an immunotherapeutic target. J Neurovirol. 1998;4:148–158. doi: 10.3109/13550289809114515. [DOI] [PubMed] [Google Scholar]
- 31.Nishikawa R, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lizé M, Pilarski S, Dobbelstein M. E2F1-inducible microRNA 449a/b suppresses cell proliferation and promotes apoptosis. Cell Death Differ. 2010;17:452–458. doi: 10.1038/cdd.2009.188. [DOI] [PubMed] [Google Scholar]
- 33.He L, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evan GI, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 35.Henriksson M, Selivanova G, Lindström M, Wiman KG. Inactivation of Myc-induced p53-dependent apoptosis in human tumors. Apoptosis. 2001;6:133–137. doi: 10.1023/a:1009644716727. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22:9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- 37.Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in β cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–334. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka H, et al. E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kappaB activity that facilitates MnSOD-mediated ROS elimination. Mol Cell. 2002;9:1017–1029. doi: 10.1016/s1097-2765(02)00522-1. [DOI] [PubMed] [Google Scholar]
- 39.Fabbri M, et al. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA. 2011;305:59–67. doi: 10.1001/jama.2010.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villalonga-Planells R, et al. Activation of p53 by nutlin-3a induces apoptosis and cellular senescence in human glioblastoma multiforme. PLoS One. 2011;6:e18588. doi: 10.1371/journal.pone.0018588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.